Abstract
Purpose of review:
To help clinicians optimize the conversion of a patient's Parkinson disease pharmacotherapy from immediate-release carbidopa/levodopa (IR CD/LD) to an extended-release formulation (ER CD/LD).
Recent findings:
Eleven movement disorders specialists achieved consensus positions on the modification of trial-based conversion guidelines to suit individual patients in clinical practice.
Summary:
Because the pharmacokinetics of ER CD/LD differ from those of IR CD/LD, modification of dosage and dosing frequency are to be expected. Initial regimens may be based on doubling the patient's preconversion levodopa daily dosage and choosing a division of doses to address the patient's motor complications, e.g., wearing-off (warranting a relatively high ER CD/LD dose, possibly at a lower frequency than for IR CD/LD) or dyskinesia (warranting a relatively low dose, perhaps at an unchanged frequency). Patients should know that the main goal of conversion is a steadier levodopa clinical response, even if dosing frequency is unchanged.
Half a century after its introduction as a replacement therapy for dopamine deficiency in Parkinson disease (PD), levodopa taken in combination with a peripheral dopa-decarboxylase inhibitor such as carbidopa remains the most effective oral treatment at all stages of the disease.1,2 Nevertheless, the immediate-release formulation of carbidopa/levodopa (IR CD/LD) provides a levodopa plasma half-life of only ∼1.5 hours,3 impeding the maintenance of a therapeutic drug concentration and contributing to long-term risks of motor fluctuations and dyskinesia,4 which are thought to eventually result from nonphysiologic intermittent stimulation of dopamine receptors.5,6 Strategies to enhance the effect of oral levodopa include the adjunctive administration of a monoamine oxidase B (MAO-B) or catechol-O-methyltransferase (COMT) inhibitor.7 Oral dosing of CD/LD as a controlled-release (CR) formulation may be problematic, owing to its erratic pharmacokinetic profile and inconsistent responses in advanced PD.1,8,9 For patients with severe fluctuations, continuous levodopa delivery via intestinal10,11 infusion is beneficial.
Extended-release (ER) CD/LD (marketed in the United States as Rytary)12 is an ER capsule formulation of oral CD/LD in a 1:4 ratio, designed to rapidly achieve therapeutic levodopa plasma concentrations and maintain them longer than IR CD/LD.13 Each capsule contains microbeads of IR and 2 types of ER CD/LD. The levodopa strengths are 95, 145, 195, and 245 mg per capsule. The formulation also includes tartaric acid, which appears to facilitate intestinal levodopa absorption distal to the jejunum.14,15 The clinical trials leading to US regulatory approval, granted in January 2015, utilized standardized procedures for treatment implementation. In early PD,16 ER CD/LD was uptitrated to fixed TID dose levels. In more advanced PD,17,18 the recommended initial regimens were based on dosage ranges of previous levodopa treatment (table 1). TID dosing was recommended for the initial conversion.
Table 1.
Conversion to extended release (ER) carbidopa/levodopa (CD/LD) in clinical trials: Suggested initial regimens and initial and final dosage conversion ratios among conversion completers
Subsequent clinical experience can now guide optimal use. In November 2015, Impax Laboratories, Inc. (Hayward, CA), the manufacturer of ER CD/LD, convened an advisory meeting devoted to a day-long exchange of views on conversion of patients from use of IR CD/LD among 11 movement disorders specialists highly experienced with ER CD/LD in clinical trials and clinical practice, involving more than 40 patients per expert. This article reports the resulting consensus positions.
Dose conversion in clinical practice
The conversion procedures utilized in the ER CD/LD clinical trials17,18 may be impractical in clinical practice. Roughly 60% of patients required a dosage higher than those that the trials' conversion tables had recommended for their initial regimens.19 In addition, the conversion tables listed only TID regimens (although dosing up to 5 times a day was allowed). At the end of conversion, the mean dosing frequency was higher than TID (e.g., 3.6 times/d17). Finally, the tables did not distinguish between patients taking evenly divided levodopa doses and those taking uneven doses (e.g., a high morning dose), either before or after conversion.
How can the ER CD/LD dosing guidelines based on clinical trials be modified to best suit the individual patient with PD? The advisory meeting considered various clinical scenarios.
Patients with no fluctuations
In early PD, all levodopa formulations may be expected to be effective, and a long-term advantage of any one of them over the others has not been empirically studied. Candidates for conversion to ER CD/LD might include patients prone to miss IR CD/LD doses and patients intolerant of dopamine agonists. The recommended initial dosage is 95 mg TID. If the response is suboptimal, the dosing can be uptitrated to 145 mg TID (approximating the levodopa exposure, but not the peak plasma level, produced by IR CD/LD administered TID as a 25/100-mg tablet). Further increases may be considered (e.g., for refractory tremor), by increments of 50 mg per ER CD/LD dose, on a weekly basis or slower.
Early wearing-off, without dyskinesia
As PD advances, the emergence of wearing-off may justify several treatment options supported by clinical data, such as increasing the IR CD/LD dose, decreasing the interdose interval, or adding a MAO-B or COMT inhibitor.1,2 Conversion from IR CD/LD to ER CD/LD is a novel option. If ER CD/LD is chosen, it may be desirable for the regimen to produce a peak levodopa plasma level (Cmax) resembling that of the patient's previous treatment, so as to provide similar “on” efficacy. Since the Cmax for levodopa administered in ER CD/LD is 30% of the Cmax for an equal dose of levodopa in IR CD/LD,13 each ER CD/LD dose would have to be approximately 3 times the patient's previous IR dose (3 × 30% = 90%; figure). But since the levodopa exposure produced by ER CD/LD (as measured pharmacokinetically by area under the curve [AUC]) is 70% of the AUC for IR CD/LD,13 the tripled ER CD/LD dose would approximately double the AUC of the IR dose (3 × 70% = 210%), permitting a substantial lengthening of the time between doses. In particular, it may permit a dosing-frequency reduction from QID to TID.
Figure. Diagrammatic levodopa pharmacokinetics after single doses of immediate release (IR) carbidopa/levodopa (CD/LD) and 3 strengths of extended release (ER) CD/LD.
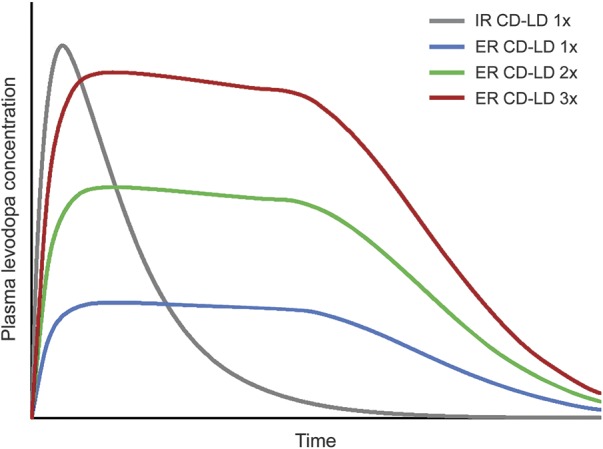
ER CD/LD is depicted as producing 30% of the peak levodopa plasma level (Cmax) and 70% of the levodopa exposure (area under the curve) produced by an equal dose of levodopa administered as IR CD/LD.12 If the IR CD/LD dose (×1) is 25/100 mg, the depicted ER CD/LD dose strengths (×1, ×2, and ×3) can be approximated by 1, 2, and 3 95-mg capsules (corresponding in levodopa Cmax to ∼32, ∼63, and ∼95 mg of IR CD/LD).
While a tripled ER CD/LD dose attains a levodopa Cmax similar to that of an individual IR CD/LD dose, tripling of the total ER CD/LD daily dosage would be excessive. Instead, a patient's initial ER CD/LD daily dosage can roughly double that of the patient's previous levodopa regimen, as supported by clinical data.17,18 The general idea is to add up the patient's daily doses of levodopa, multiply this total by 2, and divide the result by the number of daily doses in the intended ER CD/LD regimen. The outcome is the initial conversion dose. For example, an IR total dosage of 600 mg/d would prompt an initial ER total dosage of ∼1,200 mg/d, administered as 2 145-mg capsules QID or 2 195-mg capsules TID. If the patient has been using CR CD/LD (e.g., at bedtime), its contribution to the daily total can be multiplied by 0.7, in recognition that it has approximately 70% of the bioavailability of IR.20 Thus a regimen of IR 25/100 mg QID plus CR 50/200 mg QHS would total 100 × 4 plus 200 × 0.7, or 540 mg. If the patient has been using entacapone, each levodopa dose can be multiplied by 1.1, reflecting its enhanced bioavailability.21,22 CR CD/LD and entacapone may be discontinued upon conversion. Dopamine agonists and MAO-B inhibitors can be continued unchanged, at least initially (unless the goal is to achieve ER CD/LD monotherapy, which was not an intent in clinical trials and may not be needed in practice). Patients who continue to experience “off” episodes can be instructed to increase the initial ER CD/LD dose or decrease the interdose interval, depending on absence or presence of dyskinesia.
Wearing-off and dyskinesia
A patient's “off” periods may alternate, predictably or unpredictably, with “on” periods associated with troublesome dyskinesia. In consequence, a levodopa Cmax less than that of the patient's previous treatment may be desirable. Accordingly, each ER CD/LD dose might be 2 to 2.5 times the previous IR CD/LD dose, rather than 3 times the previous dose, even if dosing frequency is unchanged (e.g., for a patient who had been taking 5 or more IR doses per day).
Consider, for example, a patient taking 200 mg of IR CD/LD 6 times daily, who reports being “off” an hour before each dose and predictably dyskinetic half an hour after each dose. By the dosage-doubling formula described above, the patient's daily IR CD/LD dosage, 1,200 mg, would dictate an initial ER CD/LD daily dosage of 2,400 mg. The question then is how to divide it. Four doses per day would mean 600 mg per dose, which might still cause peak-dose dyskinesia yet not be sufficiently frequent to bridge the patient's “off” periods. A better alternative might be 480 mg 5 times a day. Each such dose could be taken as 2 245-mg capsules, or, as an exact match that includes 95-mg capsules to permit uptitration or downtitration, as one 195-mg capsule plus 3 95-mg capsules. A less formulaic alternative would be to dose ER CD/LD 5 times daily, but with the morning dose higher than subsequent doses (e.g., 585 mg [3 × 195 mg] for the morning dose, and 480 mg/dose [3 × 95 mg plus 1 × 195 mg] for the 4 subsequent doses). A third, conservative option would be to initially convert only the morning dose to ER CD/LD, and instruct the patient to take 200-mg IR CD/LD doses every 3 hours for the rest of the day, beginning when the ER CD/LD dose wears off. Complete conversion could subsequently be guided by the response to the morning dose.
In all of these strategies, the primary goal is not to dramatically decrease the levodopa dosing frequency, even though such a reduction may improve convenience and adherence23 and might also facilitate the timing of doses away from meals to reduce competition with dietary protein for intestinal absorption.24 The primary goal is to raise the troughs and lower the peaks in plasma levodopa level, so as to minimize motor fluctuations and peak-dose dyskinesia.
Undoubtedly, patients with complex, unpredictable fluctuations despite high doses of standard levodopa taken at very short dosing intervals are the most difficult to convert to ER CD/LD. In such cases, the hope is that the patient may improve enough to remain on oral PD management. It has been suggested that in these patients, ER CD/LD might best be initiated as a once-daily treatment each morning or perhaps each evening, with the initial aims of achieving a good response and observing how long it lasts, compared with IR CD/LD doses at other times. Opinion is divided on whether the conversion process might usefully end in a patient's maintenance on more than one levodopa formulation. The arguments favoring full conversion are that ER CD/LD incorporates levodopa in IR form, that the new regimen would be less complicated for the patient or caregiver, and that partial conversion is not evidence-based.
Some general strategies
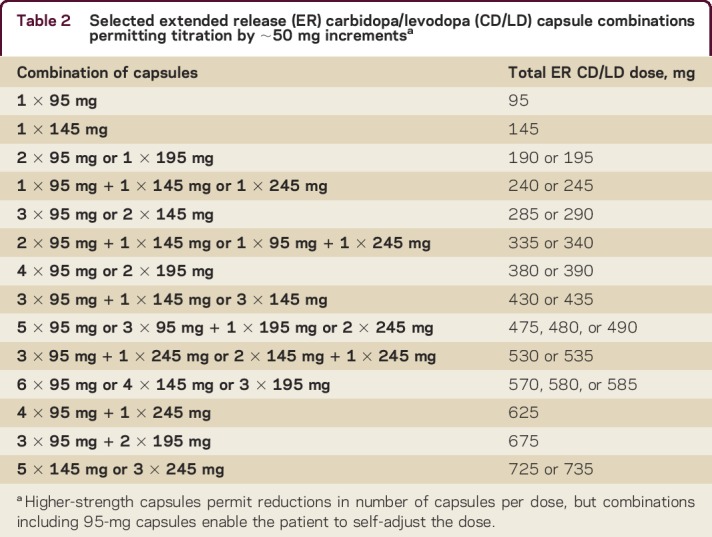
The various ER CD/LD capsules permit a physician to fine-tune a patient's doses in approximate 50-mg increments (table 2). Dosing may also be adjusted by the addition or subtraction of a 95-mg capsule (equivalent in Cmax to a highly conservative ∼32 mg of IR CD/LD; figure). Patients capable of self-adjusting their regimen can thus be forearmed with a contingency plan for doing so. For instance, their initial regimen can include one or more 95-mg capsules per dose, or can even consist entirely of 95-mg capsules. Patients who experience “off” episodes can add a 95-mg capsule to each dose, and patients who experience dyskinesia can remove a 95-mg capsule. (Some experts prefer using 145-mg capsules during uptitration and downtitration, equivalent in Cmax to ∼48 mg of IR CD/LD). Once a stable and optimal response has been achieved, the maintenance regimen may be a smaller, consolidated number of capsules (table 2)—an important aid for ensuring adherence. Alternatively, patients fearing “off” episodes can be instructed to take IR CD/LD as needed. Once a pattern of extra IR use has been defined, a corresponding increase in ER CD/LD dose can be calculated.
Table 2.
Selected extended release (ER) carbidopa/levodopa (CD/LD) capsule combinations permitting titration by ∼50 mg incrementsa
In all conversions, clear, understandable patient education is essential. Patients or caregivers should know that the main goal of the switch is a steadier levodopa clinical response (rather than a reduction in dosing frequency, however desirable that may be). They can be instructed that an oral IR CD/LD dose peaks rapidly but only lasts about 1.5 hours, whereas an ER CD/LD dose peaks rapidly and lasts several hours, filling the troughs associated with “off” episodes and smoothing the peaks associated with dyskinesia. It may also be helpful for patients or caregivers to see the physician perform their dose-conversion calculation. As part of their expectations for the conversion process, patients should understand that the initial regimen is an estimate of the optimal treatment, and that adjustment may be required. To facilitate optimization, fluctuators should be taught to reliably recognize “off” states as well as dyskinesia. Patients can then be asked to report the duration of the “on” state produced by their initial dose level, and whether they become dyskinetic. Clinicians and medical assistants should encourage such feedback, and be prepared to act on it.
Take-home points
ER CD/LD is an extended-release carbidopa/levodopa formulation designed to rapidly achieve and maintain therapeutic plasma levels.
Clinical experience accumulated since its US approval, in 2015, can now guide a patient's conversion from immediate-release (IR) CD/LD.
Initial ER CD/LD regimens may be based on doubling the patient's preconversion IR CD/LD daily dosage and choosing a division of doses to address the patient's motor complications.
Adjustment may be required to optimize response. Clinicians should encourage patient/caregiver feedback.
AUTHOR CONTRIBUTIONS
A.J. Espay: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision. F.L. Pagan: drafting/revising the manuscript. B.L. Walter: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. J.C. Morgan: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. L.W. Elmer: drafting/revising the manuscript, analysis or interpretation of data. C.H. Waters: drafting/revising the manuscript. P. Agarwal: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision. R. Dhall: drafting/revising the manuscript, contribution of vital reagents/tools/patients, study supervision. W.G. Ondo: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision. K.J. Klos: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. D.E. Silver: drafting/revising the manuscript, statistical analysis.
STUDY FUNDING
The advisory meeting from which this manuscript evolved was supported by Impax Laboratories, Inc. All participants received honoraria from Impax for their meeting participation. At the meeting and subsequently, the authors developed the manuscript's concepts and content. After several review cycles among the authors, Impax offered comments for author consideration. Decisions on final content were made by the authors, and all authors approved the final manuscript prior to submission.
DISCLOSURES
A.J. Espay serves on scientific advisory boards for AbbVie, Teva, Impax, Merz, Acadia, Cynapsus, Lundbeck, and US WorldMeds; serves as an Associate Editor for Journal of Clinical Movement Disorders and on the editorial board of Parkinsonism and Related Disorders; receives publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; serves as a consultant for AbbVie, Teva, Impax, Merz, Acadia, Cynapsus, Lundbeck, US WorldMeds Solvay, Abbott, Chelsea Therapeutics, Eli Lilly, and UCB; serves on speakers' bureaus for AbbVie, US WorldMeds, Lundbeck, Acadia, American Academy of Neurology, and Movement Disorders Society; and receives research support from NIH, CleveMed/Great Lakes Neurotechnologies, and Michael J Fox Foundation. F.L. Pagan serves as a consultant/speaker to Teva, Impax, Acadia, Cynapsus, Lundbeck, AbbVie, Merz Pharmaceuticals, and US WorldMeds and has received educational grants from Merz Pharmaceuticals and Medtronic and research grants from US WorldMeds and Teva. B.L. Walter has received grant support from the NIH (K23NS055000) and the Spitz Foundation; personal compensation as a consultant/scientific advisory board member for Teva, Impax, Lundbeck, UCB Pharma, US WorldMeds, and Medtronic; and honoraria from the American Academy of Neurology. J.C. Morgan has received honoraria for speaking for Impax and Teva. He has served as a consultant for AbbVie, Acadia, Acorda, Cynapsus, Impax, Lundbeck, National Parkinson Foundation, Teva, and UCB. He has also received compensation for serving as an expert witness in various neurologic legal cases. He has served as a site principal investigator or subinvestigator for clinical trials with AbbVie, Acadia, Acorda, Biotie, CHDI, Cynapsus, Impax, Kyowa, Lundbeck, NIH, NPF, PSG, and Serina. L.W. Elmer has received honoraria for speaking engagements from Lundbeck, Novartis, UCB Pharma, and Teva Neuroscience; has served as a paid consultant for Teva Neuroscience and UCB Pharma; has received honoraria as a member of advisory boards for Lundbeck, Teva Neuroscience, and UCB Pharma; and has received unrestricted educational grant support from Teva Neuroscience. C.H. Waters has been an investigator in Impax-sponsored clinical trials and has received honoraria for speaking engagements from Impax, Lundbeck, and Teva Pharmaceuticals. P. Agarwal has been a consultant for Impax, Teva, Lundbeck, UCB Pharma, and US WorldMeds, and has served as a speaker for Teva, US WorldMeds, Lundbeck, and Impax. R. Dhall has been an investigator in clinical trials sponsored by Impax Laboratories, Inc., and has been a consultant for Impax, Merz, Teva, and Acadia Pharmaceuticals. He has participated on speakers' bureaus for Impax, Lundbeck, Teva Pharmaceuticals, and UCB. W.G. Ondo has served as a consultant or an advisor for AbbVie, Acadia, Auspex, Avanir, Impax, Lundbeck, Merz, Teva, and UCB Pharma. K.J. Klos has been an investigator in clinical trials sponsored by Impax Laboratories, Intec Pharma, Ltd., Lundbeck, Merz, Auspex, US WorldMeds, Adamas Pharmaceuticals, Inc., Biotie Therapeutics, Inc., UCB Biosciences, Inc., Teva, Neuraltus Pharmaceuticals, Inc., XenoPort, Inc., and Chelsea Therapeutics. He has served as a consultant and speaker for Lundbeck, Allergan, UCB Pharma, AbbVie, and Impax Pharma. D.E. Silver has received research grants from Impax Pharmaceuticals and has been a principal investigator in the ADVANCE trial for Rytary. He has served as a consultant or advisor for Accera Pharma, Boehringer-Ingelheim, Forest, GlaxoSmithKline, Impax Pharmaceuticals, Innovex Pharmaceuticals, Ipsen, Johnson & Johnson, Kyowa Pharmaceuticals, MDS Pharma Services, Novartis Pharmaceuticals, Pfizer Inc., Teva Neuroscience, UCB Biosciences, and US WorldMeds. Full disclosure form information provided by the authors is available with the full text of this article athttp://cp.neurology.org/lookup/doi/10.1212/CPJ.0000000000000316.
ACKNOWLEDGMENT
Michael Feirtag of The Curry Rockefeller Group, LLC, Tarrytown, NY, provided editorial assistance with editing, formatting, and referencing for the development of this publication, which was supported by Impax Laboratories, Inc. He did not participate in drafting or revising the manuscript for intellectual content.

REFERENCES
- 1.Pahwa R, Factor SA, Lyons KE, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:983–995. [DOI] [PubMed] [Google Scholar]
- 2.LeWitt PA. Levodopa therapy for Parkinson's disease: pharmacokinetics and pharmacodynamics. Mov Disord 2015;30:64–72. [DOI] [PubMed] [Google Scholar]
- 3.Cedarbaum JM. Clinical pharmacokinetics of anti-parkinsonian drugs. Clin Pharmacokinet 1987;13:141–178. [DOI] [PubMed] [Google Scholar]
- 4.Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001;16:448–458. [DOI] [PubMed] [Google Scholar]
- 5.Chase TN, Baronti F, Fabbrini G, Heuser IJ, Juncos JL, Mouradian MM. Rationale for continuous dopaminometic therapy of Parkinson's disease. Neurology 1989;39:7–10. [PubMed] [Google Scholar]
- 6.Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson's disease: scientific rationale and clinical implications. Lancet Neurol 2006;5:677–687. [DOI] [PubMed] [Google Scholar]
- 7.Gershanik OS. Improving L-dopa therapy: the development of enzyme inhibitors. Mov Disord 2015;30:103–113. [DOI] [PubMed] [Google Scholar]
- 8.Ahlskog JE, Muenter MD, McManis PG, Bell GN, Bailey PA. Controlled-release Sinemet (CR-4): a double-blind crossover study in patients with fluctuating Parkinson's disease. Mayo Clin Proc 1988;63:876–886. [DOI] [PubMed] [Google Scholar]
- 9.Stocchi F, Quinn NP, Barbato L, et al. Comparison between a fast and a slow release preparation of levodopa and a combination of the two: a clinical and pharmacokinetic study. Clin Neuropharmacol 1994;17:38–44. [DOI] [PubMed] [Google Scholar]
- 10.Nyholm D, Askmark H, Gomes-Trolin C, et al. Optimizing levodopa pharmacokinetics: intestinal infusion versus oral sustained-release tablets. Clin Neuropharmacol 2003;26:156–163. [DOI] [PubMed] [Google Scholar]
- 11.Nyholm D, Nilsson Remahl AI, Dizdar N, et al. Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease. Neurology 2005;64:216–223. [DOI] [PubMed] [Google Scholar]
- 12.Rytary Full Prescribing Information. Hayward, CA: Impax Laboratories; 2015. Available at: documents.impaxlabs.com/rytary/pi.pdf. Accessed December 7, 2015. [Google Scholar]
- 13.Hauser RA, Ellenbogen AL, Metman LV, et al. Crossover comparison of IPX066 and a standard levodopa formulation in advanced Parkinson's disease. Mov Disord 2011;26:2246–2252. [DOI] [PubMed] [Google Scholar]
- 14.Fraga S, Serrão MP, Soares-da-Silva P. The L-3,4-dihydroxyphenylalanine transporter in human and rat epithelial intestinal cells is a type 2 hetero amino acid exchanger. Eur J Pharmacol 2002;44:127–135. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi M, Tomita M. Mechanistic analysis for drug permeation through intestinal membrane. Drug Metab Pharmacokinet 2007;22:67–77. [DOI] [PubMed] [Google Scholar]
- 16.Pahwa R, Lyons KE, Hauser RA, et al. Randomized trial of IPX066, carbidopa/levodopa extended release, in early Parkinson's disease. Parkinsonism Relat Disord 2014;20:142–148. [DOI] [PubMed] [Google Scholar]
- 17.Hauser RA, Hsu A, Kell S, et al. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson's disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol 2013;12:346–356. [DOI] [PubMed] [Google Scholar]
- 18.Stocchi F, Hsu A, Khanna S, et al. Comparison of IPX066 with carbidopa-levodopa plus entacapone in advanced PD patients. Parkinsonism Relat Disord 2014;20:1335–1340. [DOI] [PubMed] [Google Scholar]
- 19.Nausieda PA, Hsu A, Elmer L, et al. Conversion to IPX066 from standard levodopa formulations in advanced Parkinson's disease: experience in clinical trials. J Parkinsons Dis 2015;5:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh KC, August TF, Bush DF, et al. Pharmacokinetics and bioavailability of Sinemet CR: a summary of human studies. Neurology 1989;39(suppl 2):25–38. [PubMed] [Google Scholar]
- 21.Heikkinen H, Nutt JG, LeWitt PA, Koller WC, Gordin A. The effects of different repeated doses of entacapone on the pharmacokinetics of L-dopa and on the clinical response to L-dopa in Parkinson's disease. Clin Neuropharmacol 2001;24:150–157. [DOI] [PubMed] [Google Scholar]
- 22.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 23.Schapira AH, Barone P, Hauser RA, et al. Patient-reported convenience of once-daily versus three-times-daily dosing during long-term studies of pramipexole in early and advanced Parkinson's disease. Eur J Neurol 2013;20:50–56. [DOI] [PubMed] [Google Scholar]
- 24.Baruzzi A, Contin M, Riva R, et al. Influence of meal ingestion time on pharmacokinetics of orally administered levodopa in parkinsonian patients. Clin Neuropharmacol 1987;10:527–537. [DOI] [PubMed] [Google Scholar]



