Abstract
Background:
Pressure ulcers resulting from continuous EEG (cEEG) monitoring in hospitalized patients have gained attention as a preventable medical complication. We measured their incidence and risk factors.
Methods:
We performed an observational investigation of cEEG-electrode-related pressure ulcers (EERPU) among acutely ill patients over a 22-month period. Variables analyzed included age, sex, monitoring duration, hospital location, application methods, vasopressor usage, nutritional status, skin allergies, fever, and presence/severity of EERPU. We examined risk for pressure ulcers vs monitoring duration using Kaplan-Meyer survival analysis, and performed multivariate risk assessment using Cox proportional hazard model.
Results:
Among 1,519 patients, EERPU occurred in 118 (7.8%). Most (n = 109, 92.3%) consisted of hyperemia only without skin breakdown. A major predictor was monitoring duration, with 3-, 5-, and 10-day risks of 16%, 32%, and 60%, respectively. Risk factors included older age (mean age 60.65 vs 50.3, p < 0.01), care in an intensive care unit (9.37% vs 5.32%, p < 0.01), lack of a head wrap (8.31% vs 27.3%, p = 0.02), use of vasopressors (16.7% vs 9.64%, p < 0.01), enteral feeding (11.7% vs 5.45%, p = 0.04), and fever (18.4% vs 9.3%, p < 0.01). Elderly patients (71–80 years) were at higher risk (hazard ratio 6.84 [1.95–24], p < 0.01), even after accounting for monitoring time and other pertinent variables in multivariate analysis.
Conclusions:
EERPU are uncommon and generally mild. Elderly patients and those with more severe illness have higher risk of developing EERPU, and the risk increases as a function of monitoring duration.
Continuous EEG monitoring (cEEG) in acutely hospitalized patients has increased dramatically throughout the last decade.1–6 cEEG has been particularly helpful in acutely ill patients at risk for nonconvulsive seizures,4,7,8 and has been reported to contribute to reduced inpatient mortality without additional charges to hospital stay.9 In addition, cEEG is increasingly used to detect cerebral ischemia in patients at high risk,5,9–11 with ischemia monitoring protocols often involving monitoring at-risk patients for as long as 10 or more days.11
Safety concerns have recently gained national attention with the increasing demand for longer duration cEEG monitoring, with respect to the risk of electrode-induced pressure ulcers, a type of health care acquired pressure ulcers (HAPU).12,13
Electrodes can injure skin through direct pressure, chemical dermatitis, and local heating when exposed to electromagnetic fields.14 cEEG-electrode-related pressure ulcers (EERPU) are preventable lesions that increase patient discomfort and risk for infections.12,13,15,16 For administrators, pressure ulcers represent a loss of hospital revenue, as the Centers for Medicare and Medicaid Services no longer reimburses for medical care related to HAPU.17–19
Some authors have reported a 10% risk of EERPU in epilepsy monitoring units (EMU).13,20 However, EMU represent a small cohort of hospitalized patients who are generally healthier than acutely ill patients such as patients who are commonly monitored with EEG.13,20
We examined the incidence and severity of EERPU and their potential determinants among acutely hospitalized individuals monitored for more than 3 hours.
METHODS
Participants
In this prospective cohort study, we tracked all cEEG studies at a large academic hospital between March 1, 2013, and December 31, 2014. All cEEG studies were performed in the course of routine clinical care. Data regarding cEEG-electrode-related skin lesions was collected and tracked as part of a continuous quality improvement effort. Review and analysis of the clinical and cEEG data for publication was performed under a protocol approved by the local institutional review board (IRB).
Standard protocol approvals, registrations, and patient consents
This study was performed as part of quality improvement for routine clinical care practices, and per institutional policy did not require approval of the institutional review board or patient consent. No human experimentation was involved. No photographs of figures in this work contain recognizable persons. Archiving and analysis of data obtained in the course of clinical care was performed under an IRB-approved protocol.
The cohort under study consisted of inpatients at Massachusetts General Hospital (MGH). MGH contains an EMU and an independent critical care EEG monitoring (CCEM) service. The EMU serves patients electively admitted for presurgical evaluation for epilepsy surgery or for diagnostic evaluation for events concerning for seizures; these patients were excluded from the analysis. The CCEM service serves all other hospitalized patients for any pertinent indication including evaluation of acutely altered mental status.
Of 2,036 studies provided by the CCEM service during the study time frame, 517 (25.3%) studies were excluded as follows: 62 (3%) studies in which EEG were recorded with a temporary and nonstandard EEG setup used in emergency situations.18 We also excluded repeated cEEG sessions that involved the same patient more than 1 time within 24 hours after an earlier study; in these cases, we included the first but not the second cEEG monitoring session. A total of 455 (22.3%) of the original 2,036 studies were excluded for this reason. Exclusion of repeated studies allowed for more accurate estimation of the time dependence of skin ulcer risk by allowing us to treat every cEEG as starting without recent exposure to EEG electrodes.
Procedural measures
The cEEG procedure consisted of the application of electrodes using standard international 10–20 electrode placement. Studies used either plastic or metal disk electrodes. Plastic electrodes were either Ives imaging-compatible reusable or disposable electrodes (Ives EEG Solutions, Newburyport, MA). Metal disk electrodes were composed of gold, silver, or silver chloride.12
Nuprep abrasive gel (Weaver and Company, Aurora, CO) was used to clean the skin of all patients in the study. Ten20 Paste (Nihon Kohden America, Irvine, CA) or Elefix (Nihon Kohden America) were used as the conductive paste for the electrodes.12 For patients' head wraps, Conform Stretch Wrap bandaging with either 3M micropore (3M, St. Paul, MN) white or cover-roll tape was used to wrap the patients' electrodes for LTM studies, or in other cases, to affix the electrodes. Collodion was most commonly used for cases undergoing prolonged monitoring (typically 10 days) to detect delayed cerebral ischemia following subarachnoid hemorrhage. In noncollodion cases, Conform Stretch Wrap bandaging was utilized to wrap the heads. In rare cases, collodion was used with a head wrap.This study's main outcome—any EERPU—was defined as any skin lesion at or near the electrode placement site.
EEG technicians who applied the electrodes were classified as experienced when they had more than 2 years of practical experience in the field. Each EEG technician documented the procedural details every morning for each patient. In addition, they gathered the clinical variables and reported to a responsible clinician. The skin care protocols used by the nurses and technicians are summarized in the supplementary material at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0000000000000312 (Institutional skin care practices).
Clinical measures
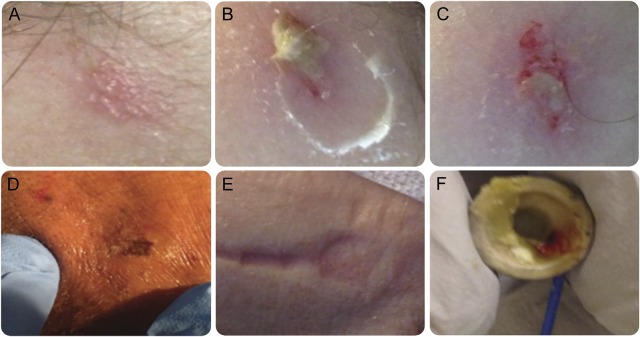
This study's main outcome—any EERPU—was defined as any skin lesion at or near the electrode placement site. The pressure ulcer had to occur after the initiation of the study and there could be no better alternative explanation for the lesion. If present, the date on which the pressure ulcer was first evident was documented and described by the technician. Lesion staging was performed by a designated nurse as described in figure 1.
Figure 1. Examples of continuous EEG (cEEG)–electrode-related pressure ulcers (EERPU).
Lesion staging was determined retrospectively by review of the EEG technician's written descriptions and by review of hospital safety reports, which are routinely filed for any lesions noted by the bedside clinician (usually the nurse) to be of severity greater than stage 1. EERPU stages were assigned following standard definitions, as follows: stage 1 for hyperemia without the presence of ulcers or blisters (A); stage 2 for shallow open ulcers or intact blisters without subcutaneous fat exposure (B–D); stage 3 when there was full-thickness tissue loss with exposure of subcutaneous fat but no visible bone, tendon, or muscles; and stage 4 for full-thickness ulcers with exposure of muscle, tendons, or bones. EEG technicians were trained to report any skin disruption to the primary care team and to the nursing staff. The decision to continue or terminate a cEEG study was made on a case-by-case basis with the involvement of the entire care team. A clinical nurse specialist was consulted by the EEG technician or the nursing staff to examine skin breakdown with attention to lesions of stage 2 or greater. The National Database of Nursing Quality Indicators was used to stage per the hospital's inpatient quality control policy. (E) Pressure implantation effect on the subjacent skin after 24 hours of lead placement. (F) An electrode with blood and paste after removal from a patient with EERPU.
Additional clinical data gathered included risk factors that we suspected a priori might confer increased risk of impaired wound healing, including a history of skin allergies, presence of fever >38.3°C, use of vasoconstrictive agents, and type of nutrition (oral vs enteral).21–24 On occasion, responsible technicians did not record all variables; in all, 973 (64%) patients had at least one variable missing. In these cases, we retrospectively cross-checked the related medical record and nursing flowsheets to fill in missing values.
Demographic data collected included age, sex, and hospital unit where the study was performed. Hospital unit was dichotomized between intensive care unit (ICU) and non-ICU. If a study was performed in an ICU during any part of cEEG monitoring, it was categorized as an ICU study.
Statistical analysis
The final sample was descriptively categorized according to the presence of EERPU. We used t tests to compare the means of patient age and study duration between the groups. We used the χ2 test of independence to test whether categorical variables (i.e., presence of head wrap, use of vasopressors, feeding modality, history of skin allergies, and presence of fever during the monitoring period) were associated with increased rates of EERPU. We expected that the incidence of EERPU might be higher in the pediatric age group due to more delicate and thinner skin. In addition, safety reports regarding skin lesions in pediatric patients tend to receive increased attention and might lead to an inflated perception of rate of EERPU to skin care in the pediatric population. Therefore, we also explicitly analyzed rates of EERPU in adults compared with rates in pediatric patients.
Time to appearance of pressure ulcers was calculated in units of days, as the time elapsed since initiation of cEEG monitoring.
To assess risk variation over time for the EEG service as a whole, and to establish a baseline complication rate, we created a proportions control chart (p-chart).25,26 We calculated and plotted the proportion of patients each month who had EERPU, the overall mean proportion 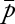 of patients with EERPU, the average number of cases undergoing cEEG monitoring
of patients with EERPU, the average number of cases undergoing cEEG monitoring 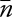 , and finally the ±3 SD control limits as
, and finally the ±3 SD control limits as 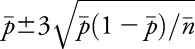 . If the lower limit given by this formula fell below zero, then we set it equal to zero.
. If the lower limit given by this formula fell below zero, then we set it equal to zero.
To assess the overall risk of EERPU in individuals as a function of monitoring duration, we used Kaplan-Meyer cumulative probability estimate curve. This analysis included all 1,519 patients.
To assess factors contributing to cumulative risk for skin ulcers in multiday cEEG monitoring sessions while accounting for different monitoring durations between patients (right censoring), we used a multivariate Cox proportional hazards (CPH) model. For this analysis, we limited the analysis to cases without any missing values for key covariates. A substantial proportion (36%, 546/1,519) of patients had complete data for the following covariates, which we included in the CPH model: sex, hospital unit, cEEG technician experience level, head wrap presence, vasopressor usage, feeding modality, skin allergies, and presence of fever. Because the risk of pressure ulcers did not follow a linear progression with age, for the CPH model we categorized age in deciles (figure e-1). This categorization ensured that the proportional hazards assumption underlying the CPH analysis was satisfied. For comparison, we also performed a logistic regression of the binary risk of developing EERPUs with duration of cEEG and age included as a continuous variable.
For all analyses, we defined the significance level as p < 0.05. We used the SAS Studio software package (SAS Institute Inc., Cary, NC), the MATLAB Statistical Toolbox (Natick, MA) and custom software written in MATLAB to perform statistical analysis.
RESULTS
Descriptive statistics
The final sample consisted of 1,519 patients. EERPU were reported in 118 (7.8%) patients. The mean monthly percentage ±3 SD range of pressure ulcers during the study period was 7.25% (0.00–16.61) (figure e-2). Among 1,519 cases of EERPU, 92.4% (109/118) occurred in adults and 7.6% (9/118) occurred in pediatric patients (<18 years old); 53.4% (63/118) of cases involved male participants and 46.6% (55/118) involved female participants. The large majority of cases consisted of stage 1 lesions (92.3%, 109/118). A small minority of lesions were stage 2 (6.7%, 8/118). A single stage 3 ulcer (0.8%, 1/118) occurred. No stage 4 pressure ulcers occurred. Most (77%, 85/109) of the stage 1 pressure ulcers and stage 2 (75%, 6/8) pressure ulcers occurred in patients older than 60 years.
Univariate risk analysis
Table 1 summarizes the overall demographic, procedural, and clinical characteristics of the study cohort. Table e-1 summarizes the characteristics of the groups with and without EERPU.
Table 1.
Cohort baseline characteristics
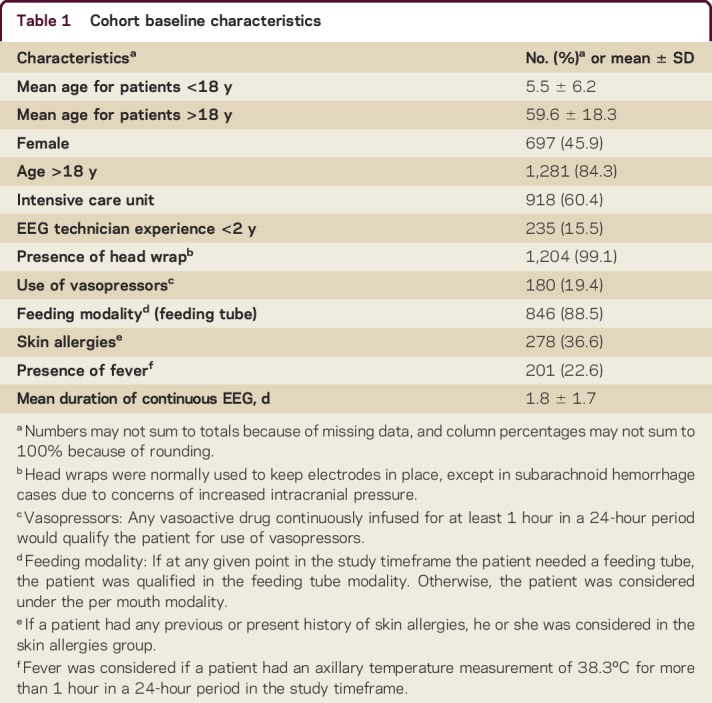
The group that developed EERPU had a higher mean age (mean age 60.65 ± 26.11 years) than the group without EERPU (50.3 ± 26.11, 95% CI [48.9–51.7], p < 0.01). Patients who received care in an ICU were more likely to develop EERPU (9.37% vs 5.32% p < 0.01). Surprisingly, patients who had cEEG monitoring performed with a head wrap had fewer EERPU than those without (8.31% vs 27.3% p = 0.03). Patients who required vasopressors (16.7% vs 9.64%, p < 0.01) or enteral feeding (11.7% vs 5.45% p = 0.04) had more EERPU than those who did not. Patients who had a fever during the monitoring period were more likely to develop EERPU (18.4% vs 9.3%, p < 0.01).
EERPU were more common in adults than in pediatric patients (8.51% vs 3.78%, p = 0.01). Figure e-1 summarizes the distribution of the patients studied by age. There were a total of 9 age categories, with each category being separated by 10 years (e.g., 0–10 years old, 11–20 years old, 21–30 years old).
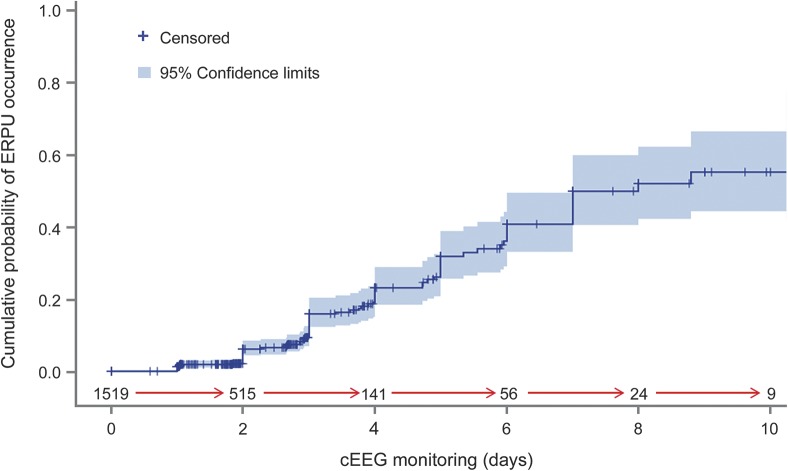
The duration of cEEG monitoring differed substantially in patients with vs without EERPU. The mean study duration was 3.4 ± 2.4 days in the EERPU group compared to 1.6 ± 1.5 days in the group without EERPU (p ≤ 0.01); see table 1. Figure e-3 details the mean duration of EERPU by stage. The risk of a EERPU at 4 days of monitoring was 20% and rose to 60% by 10 days. The Kaplan-Meier curve illustrates the overall cumulative probability of EERPU as a function of time (figure 2).
Figure 2. Cumulative probability of continuous EEG (cEEG)–electrode-related pressure ulcers (EERPU) occurrence.
Kaplan-Meier estimate of the cumulative probability of EERPU occurrence is plotted as a function of EEG study duration in days (solid line). The number of patients left at risk as time progresses is shown between the red arrows. The 95% confidence interval values are represented by the purple shaded region surrounding the darker blue line. The information for this graph was extracted from the statistical analysis of the effect of EEG duration on pressure ulcers. The risk of EERPU at 3, 5, and 10 days of monitoring was 16% (SE = 0.02), 32% (SE = 0.3), and 60% (SE = 0.7), respectively.
Multivariate analysis
Results of fitting the CPH model to this restricted subset are shown in table 2. Elderly patients (e.g., the group between 71 and 80 years old) are seen to be at higher risk for developing EERPU (hazard ratio 6.84 [95% confidence interval 1.95–24], p < 0.01), even after accounting for monitoring time and other pertinent variables. The characteristics of the cohort included in the multivariate analysis are detailed in tables e-2 to e-5.
Table 2.
Risk assessment of continuous EEG monitoring–electrode-related pressure ulcers (EERPU) using multivariable Cox proportional analysis
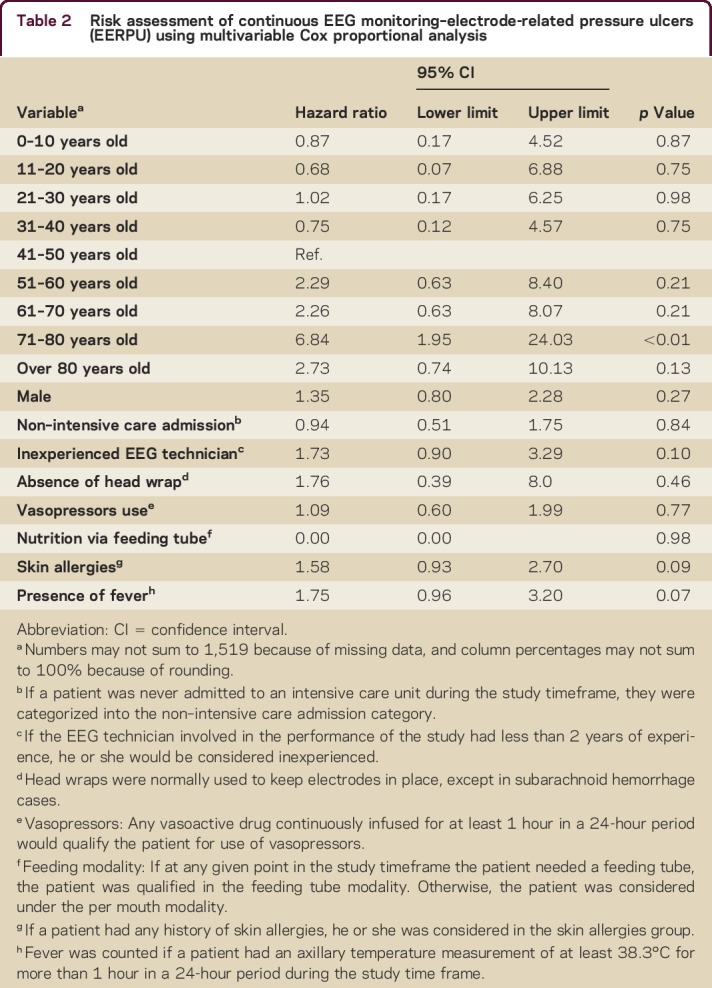
The following variables that did reach significance in univariate risk factor analysis were not found to be significant after accounting for time in the multivariable Cox proportional hazard model: presence of head wrap (p = 0.46), vasopressors usage (p = 0.77), ICU admission (p = 0.84), and presence of fever (p = 0.07).
As in the univariate analyses, the following variables did not reach significance in multivariate analysis: sex (p = 0.27), cEEG technician experience (p = 0.09), feeding modality (p = 0.98), and history of skin allergies (p = 0.09).
For comparison, we compared the CPH analysis with results of logistic regression. This analysis also suggests that duration of cEEG and age were predictive of developing EERPUs (figure e-4 and table e-6). However, logistic regression is limited by the fact that logistic regression model does not account for censored information.
DISCUSSION
This prospective study analyzes incidence, risk factors, and time dependence for EERPU from a large cohort of acutely hospitalized patients who underwent cEEG monitoring. The incidence of EERPU was infrequent (mean monthly percentage of 7.25 [0.00–16.61]) when compared to the overall incidence rate of pressure ulcers in the literature (10%–54%).22,27–29 Most EERPU that did occur consisted of hyperemia and indentation of the skin only (stage I, 7.8% of all patients). Lesions with actual breakdown were rare, and nearly all of these were shallow ulcers or blisters (stage II, 0.53% of all patients). Only a single full-thickness skin lesion (stage III, 0.07% of all patients) occurred.
In the United States, more than 2.5 million people develop HAPU yearly, and prolonged EEG studies have come under discussion as contributors to this problem.21 Thus, our finding that EERPU are uncommon and mostly mild is important. Multiple factors likely account for the relatively low rate of skin lesions in our study, ranging from population factors to institutional skin care practices (see supplemental text, Institutional skin care practices). Of note, this study was conducted in a major academic center with state-of-the-art ICUs and experienced EEG technicians and nursing staff in an environment that stresses rigorous adherence to preventive safety measures. However, further study and comparison of lesion rates between different institutions is needed to fully understand the factors governing baseline rates of EERPU.
A second major finding of this study is that the risk of EERPU increases monotonically as a function of time. Thus while the overall rate of lesions is low, one important reason for this is that most cEEG studies are relatively brief, with the majority of EEG monitoring sessions lasting less than 48 hours. This finding raises competing considerations regarding current practices surrounding EEG monitoring in the acute setting. There is strong evidence that cEEG monitoring improves diagnostic accuracy over routine (<60 minutes) EEG studies in many scenarios that occur in the acute inpatient setting, including seizure and ischemia detection.11,30 At the same time, pressure ulcers in general are considered serious preventable complications that contribute substantially to health care costs in critically ill hospitalized patients.13,21,24,28 Nevertheless, we find that EERPU are generally less severe than other commonly reported hospital-acquired pressure ulcers. Future studies should explore in depth factors related to the prevention of skin breakdown, including types of EEG leads that minimize risk, best practices for skin care, and lead placement strategies that may minimize the risk of skin breakdown.
A third important finding is that advanced age is associated with higher incidence and severity of EERPU. New-onset seizures are both common and particularly debilitating among older persons, in part because of medical conditions promoting an increasing demand for cEEG monitoring in elderly hospitalized patients.31–38 EEG evaluation strategies used in elderly populations should be reviewed and daily evaluation of need considered.
Our univariate analyses revealed that factors reflecting the severity of acute illness such as usage of vasopressors, necessity of enteral feeding, and being in an ICU were associated with higher risk for pressure ulcers. These surrogates of disease severity did not reach significance in multivariable analysis. It is possible that these variables have an important effect but that we failed to find it because of insufficient data.
Surprisingly, the absence of head wrap was associated with a lower risk for EERPU in the univariate analysis. In retrospect, we speculate 2 potential explanations for this finding. First, many of the patients who underwent EEG without a head wrap were patients with subarachnoid hemorrhage undergoing extended monitoring for detection of delayed cerebral ischemia. These patients are routinely monitored at our institutions for 10 days, generally without a head wrap, because of the a priori belief that the head wrap may tend to increase the risk of pressure ulcers by holding the EEG electrodes firmly against the scalp.9,39 Another possibility is that patients without head wraps tend to require more frequent reapplication of EEG leads leading to more frequent abrasion of the skin. These hypotheses are not mutually exclusive. Further study is required to determine whether and under what circumstances head wraps provide net benefit vs net harm during prolonged EEG monitoring.
Another limitation of the study was that the CPH model did not reliably calculate the hazard ratio for feeding modality because there were only 6 EERPU among patients fed by mouth. This is an inherent limitation of the statistical method. Sensitivity analysis performed without using feeding modality as covariate did not significantly affect the estimation for the effects of other variables.
An important limitation of our study is that we did not collect detailed data on clinical diagnoses or indications for cEEG monitoring. Nevertheless, it is possible that certain medical conditions carry more risk for skin lesions than others. Although we could not assess the direct relationship of etiology on EERPU, we used surrogates of patient clinical severity such as within-hospital location and pertinent clinical variables to address this concern. Whether specific underlying diseases confer particular risk for pressure ulcers remains to be determined in future studies.
Another limitation inherent to the observational nature of our study is that we could not control which procedures patients underwent that might have affected their risk of EERPU. Some patients had their electrodes moved from standard application sites by 0.5 centimeters as a safety measure when there was any concern that the patient was at risk for skin breakdown. Other patients intermittently needed replacement of EEG leads due to imaging studies.
The current study focused on clinical predictors for EERPU. The association between EERPU and hospital outcomes (e.g., infection rates, length of stay, scarring) was not explored.
A final limitation is that, although we examined a large sample, our study was performed at a single center. Research exploring the generalizability of our findings to other similar hospitals and to different health care settings is needed.
CONCLUSION
Pressure ulcers as a result of prolonged cEEG monitoring are an infrequent and generally mild medical complication in hospitalized patients. Disease severity, age, and monitoring duration are important risk factors that can be factored in to strategies aimed at reducing risk. This study is a part of an ongoing effort to optimize the safety of cEEG monitoring. Future studies will focus on testing interventions intended to reduce the rate of EERPU while maximizing the value of the information gained from continuous EEG monitoring in acutely hospitalized patients.
AUTHOR CONTRIBUTIONS
Lidia Moura contributed to study design, ethics institutional review board documentation, data collection, data analysis, manuscript draft and review. Thiago S. Carneiro contributed to data collection, data analysis, manuscript draft and review. David Kwasnik contributed to data collection, data analysis, manuscript draft and review. Valdery F. Moura Jr. contributed to data collection, data analysis, manuscript draft and review. Christine S. Blodgett contributed to data collection and manuscript review. Joseph Cohen contributed to data collection and manuscript review. Mary Guanci contributed to data collection and manuscript review. Daniel B. Hoch, John Hsu, and Andrew J. Cole contributed to manuscript draft and review. M. Brandon Westover contributed to study design, ethics institutional review board documentation, data collection, data analysis, manuscript draft and review.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
L.M.V.R. Moura is the recipient of a 2015–2016 Clinical Research Fellowship sponsored by the American Brain Foundation. T.S. Carneiro, D. Kwasnik, V.F. Moura Jr., C.S. Blodgett, J. Cohen, and M. Guanci report no disclosures. D. B. Hoch owns stock/stock options in Merck, Regeneron, Biogen Idec, and Medtronic. J. Hsu receives research support from NIH. A.J. Cole serves on scientific advisory boards and as a consultant for Precisis AG and Sage Therapeutics; serves as an Associate Editor for Annals Clinical and Translational Neurology; receives publishing royalties from UpToDate; receives research support from Neuropace; and has owned stock/stock options in Precisis AG and Sage Therapeutics. M. B. Westover receives publishing royalties from Pocket Neurology (Lippincott Williams & Wilkins, 2010) and Pocket Neurology, 2nd ed. (Wolters Kluwer, 2016); serves as a consultant for Roche; and receives research support from NIH/National Institute of Neurological Disorders and Stroke, The Rappaport Foundation, and the Andrew David Heitman Neuroendovascular Research Fund. Full disclosure form information provided by the authors is available with the full text of this article athttp://cp.neurology.org/lookup/doi/10.1212/CPJ.0000000000000312.
Footnotes
Supplemental data at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0000000000000312

REFERENCES
- 1.Ney JP, van der Goes DN, Nuwer MR, Nelson L, Eccher MA. Continuous and routine EEG in intensive care: utilization and outcomes, United States 2005–2009. Neurology 2013;81:2002–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vespa PM, Nuwer MR, Nenov V, et al. . Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg 1999;91:750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abend NS, Topijan AA, Gutierrez-Colina AM, Donnelly M, Clancy RR, Dlugos JD. Impact of continuous EEG monitoring on clinical management in critically ill children. Neurocrit Care 2011;15:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004;62:1743–1748. [DOI] [PubMed] [Google Scholar]
- 5.Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg 2009;109:506–523. [DOI] [PubMed] [Google Scholar]
- 6.Akman CI, Micic V, Thompson A, Riviello JJ. Seizure detection using digital trend analysis: factors affecting utility. Epilepsy Res 2011;93:66–72. [DOI] [PubMed] [Google Scholar]
- 7.Jirsch J, Hirsch LJ. Nonconvulsive seizures: developing a rational approach to the diagnosis and management in the critically ill population. Clin Neurophysiol 2007;118:1660–1670. [DOI] [PubMed] [Google Scholar]
- 8.Westover MB, Shafi MM, Bianchi MT, et al. . The probability of seizures during EEG monitoring in critically ill adults. Clin Neurophysiol 2015;126:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labar DR, Fisch BJ, Pedley TA, Fink ME, Solomon RA. Quantitative EEG monitoring for patients with subarachnoid hemorrhage. Electroencephalogr Clin Neurophysiol 1991;78:325–332. [DOI] [PubMed] [Google Scholar]
- 10.Claassen J, Vespa P. Electrophysiologic monitoring in acute brain injury. Neurocrit Care 2014;21(suppl 2):S129–S147. [DOI] [PubMed] [Google Scholar]
- 11.Claassen J, Hirsh LJ, Kreiter KT, et al. . Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol 2004;115:2699–2710. [DOI] [PubMed] [Google Scholar]
- 12.Berlin F, Carlile JA, de Burgo MI, et al. . Technical tips: electrode application and preventing skin breakdown techniques. Am J Electroneurodiagnostic Technol 2011;51:206–219. [PubMed] [Google Scholar]
- 13.Joellan M, Morton W. Preventing skin breakdown in EEG patients: best practice techniques. J Pediatr Nurs 2014;29:478–480. [DOI] [PubMed] [Google Scholar]
- 14.Netherton BL, Stecker MM, Patterson T. Mechanisms of electrode induced injury: part 3: practical concepts and avoidance. Am J Electroneurodiagnostic Technol 2007;47:257–263. [PubMed] [Google Scholar]
- 15.Litscher G, Kehl G, Schwarz G, Soyer HP. Inflammatory reactions of the skin caused by adhesive EEG electrodes. J Neurosurg Anesthesiol 1997;9:277–279. [DOI] [PubMed] [Google Scholar]
- 16.Volpi E, Jeschke MG, Herndon DN, Wolfe RR. Measurement of skin protein breakdown in a rat model. Am J Physiol Endocrinol Metab 2000;279:E900–E906. [DOI] [PubMed] [Google Scholar]
- 17.Swayne LC. Pay for performance: pay more or pay less? J Am Coll Radiol 2005;2:777–781. [DOI] [PubMed] [Google Scholar]
- 18.Committee on Quality of Health Care in America Institute of Medicine; Kohn LT, Corrigan JM, Donaldson MS, editors. To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- 19.Prous J. The changing face of healthcare. Eur Biopharm Rev 2009;35:66–67. [Google Scholar]
- 20.Stecker MM, Patterson T, Netherton BL. Mechanisms of electrode induced injury: part 1: theory. Am J Electroneurodiagnostic Technol 2006;46:315–342. [PubMed] [Google Scholar]
- 21.Russo A, Steiner C, Spector W. Hospitalizations related to pressure ulcers among adults 18 years and older, 2004. Healthc Cost Util Proj 2008;64:1–9. [PubMed] [Google Scholar]
- 22.Demarre L, Verhaeghe S, Van Hecke A, Clays E, Grypdonck M, Beeckman D. Factors predicting the development of pressure ulcers in an at-risk population who receive standardized preventive care: secondary analyses of a multicentre randomised controlled trial. J Adv Nurs 2015;71:391–403. [DOI] [PubMed] [Google Scholar]
- 23.Demarre L, Van Lancker A, Van Hecke A, et al. . The cost of prevention and treatment of pressure ulcers: a systematic review. Int J Nurs Stud 2015;52:1754–1774. [DOI] [PubMed] [Google Scholar]
- 24.Dhandapani M, Dhandapani S, Agarwal M, Mahapatra AK. Pressure ulcer in patients with severe traumatic brain injury: significant factors and association with neurological outcome. J Clin Nurs 2014;23:1114–1119. [DOI] [PubMed] [Google Scholar]
- 25.Padula WV, Mishra MK, Weaver CD, Yilmaz T, Splaine ME. Building information for systematic improvement of the prevention of hospital-acquired pressure ulcers with statistical process control charts and regression. BMJ Qual Saf 2012;21:473–480. [DOI] [PubMed] [Google Scholar]
- 26.Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Heal Care 2010;22:402–407. [DOI] [PubMed] [Google Scholar]
- 27.Little P, Dorward M, Warner G, Stephens K, Senior J, Moore M. Importance of patient pressure and perceived pressure and perceived medical need for investigations, referral, and prescribing in primary care: nested observational study. BMJ 2004;328:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flattau A, Blank AE. Risk factors for 90-day and 180-day mortality in hospitalised patients with pressure ulcers. Int Wound J 2014;11:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaitani T, Nakagami G, Iizaka S, et al. . Cost-utility analysis of an advanced pressure ulcer management protocol followed by trained wound, ostomy, and continence nurses. Wound Repair Regen 2015;23:915–921. [DOI] [PubMed] [Google Scholar]
- 30.Bourez-Swart MD, van Rooji L, Rizzo C, et al. . Detection of subclinical electroencephalographic seizure patterns with multichannel amplitude-integrated EEG in full-term neonates. Clin Neurophysiol 2009;120:1916–1922. [DOI] [PubMed] [Google Scholar]
- 31.Faught E, Richman J, Martin R, et al. . Incidence and prevalence of epilepsy among older US Medicare beneficiaries. Neurology 2012;78:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hesdorffer DC, Logroscino G, Benn EK, Katri N, Cascino G, Hauser WA. Estimating risk for developing epilepsy: a population-based study in Rochester, Minnesota. Neurology 2011;76:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leppik IE. Epilepsy in the elderly: scope of the problem. Int Rev Neurobiol 2007;81:1–14. [DOI] [PubMed] [Google Scholar]
- 34.Ramsay RE, Macias FM, Rowan AJ. Diagnosing epilepsy in the elderly. Int Rev Neurobiol 2007;81:129–151. [DOI] [PubMed] [Google Scholar]
- 35.Pugh MJV, Berlowitz DR, Kazis L. The impact of epilepsy on older veterans. Int Rev Neurobiol 2007;81:221–233. [DOI] [PubMed] [Google Scholar]
- 36.Kotsopoulos IAW, van Merode T, Kessels FGH, de Krom MCTFM, Knottnerus JA. Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia 2002;43:1402–1409. [DOI] [PubMed] [Google Scholar]
- 37.Ruggles KH, Haessly SM, Berg RL. Prospective study of seizures in the elderly in the Marshfield Epidemiologic Study Area (MESA). Epilepsia 2001;42:1594–1599. [DOI] [PubMed] [Google Scholar]
- 38.Brodie MJ, Elder AT, Kwan P. Epilepsy in later life. Lancet Neurol 2009;8:1019–1030. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt JM, Claassen J. Can quantitative EEG reliably predict deterioration from delayed cerebral ischemia secondary to vasospasm? Neurocrit Care 2011;14:149–151. [DOI] [PubMed] [Google Scholar]