Abstract
Mitochondrial metabolism plays an integral role in glucose-stimulated insulin secretion (GSIS) in β-cells. In addition, the diabetogenic role of glucagon released from α-cells plays a major role in the etiology of both type 1 and type 2 diabetes because unopposed hyperglucagonemia is a pertinent contributor to diabetic hyperglycemia. Titrating expression levels of the mitochondrial protein mitoNEET is a powerful approach to fine-tune mitochondrial capacity of cells. Mechanistically, β-cell–specific mitoNEET induction causes hyperglycemia and glucose intolerance due to activation of a Parkin-dependent mitophagic pathway, leading to the formation of vacuoles and uniquely structured mitophagosomes. Induction of mitoNEET in α-cells leads to fasting-induced hypoglycemia and hypersecretion of insulin during GSIS. MitoNEET-challenged α-cells exert potent antiapoptotic effects on β-cells and prevent cellular dysfunction associated with mitoNEET overexpression in β-cells. These observations identify that reduced mitochondrial function in α-cells exerts potently protective effects on β-cells, preserving β-cell viability and mass.
Introduction
The critical roles mitochondria play in numerous aspects of metabolic regulation position them center stage in the regulation of energy homeostasis. Pancreatic β-cells are glucose sensors that adjust insulin release to blood glucose levels to sustain euglycemia, a process where mitochondria are integral to coupling glucose metabolism with insulin exocytosis (1). The pertinent role of ATP production in β-cells is reflected by the blockade of glucose-stimulated insulin secretion (GSIS) with inhibition of mitochondrial electron transport chain complexes (2,3).
Obesity-associated type 2 diabetes mellitus (T2DM) is characterized by insulin resistance such that β-cells are unable to appropriately compensate with elevated insulin secretion (4). Reduced β-cell volume, caused by β-cell death from glucolipotoxicity, results in low GSIS by residual β-cells in patients with diabetes (5,6). Mitochondrial dysfunction in β-cells plays a pivotal role in the anomalies of obesity-related T2DM (7,8). Impaired β-cell function is associated with mitochondrial DNA mutations in humans and is induced in rodents by using β-cell–specific deletions of targeted mitochondrial genes; in both cases, low oxidative capacity and β-cell dysfunction ensue (6,9). Mitochondria in β-cells in patients with diabetes also exhibit morphologic and functional abnormalities, concurrent with compromised function (5,6). The precise mechanisms that impede mitochondrial function and the key pathways that activate β-cell failure and loss of β-cell mass remain unknown (4). Delineating mechanisms that perturb mitochondrial β-cell function should help to define the pathophysiology of β-cell dysfunction in T2DM and identify novel avenues that preserve β-cell mass.
MitoNEET has been identified as a dimeric mitochondrial membrane protein (10,11). Located on the outer mitochondrial membrane (OMM), mitoNEET was named according to its COOH-terminal amino acid sequence Asn-Glu-Glu-Thr (NEET) (10). Oriented toward the cytoplasm, mitoNEET binds redox-active 2Fe-2S clusters (12–14). We previously determined that in white adipose tissue (WAT), mitoNEET lowers mitochondrial oxidative capacity; this triggers a profound compensatory response in the mature adipocyte such that peroxisome proliferator–activated receptor-γ (PPARγ) and adiponectin levels increase to activate massive WAT expansion (15). MitoNEET achieves these remarkable metabolic effects by acting as a powerful regulator of iron content in the mitochondrial matrix (15).
Glucagon secretion from α-cells sustains glucose levels during fasting by stimulating hepatic glucose production (16). When glucose demand is increased, insulin secretion falls, thus stimulating glucagon production and removing the inhibitory action of insulin on the liver while augmenting the stimulatory effect of glucagon on gluconeogenesis (17). Conversely, under nutrient excess, the reverse occurs. The hepatic effects of insulin and glucagon are in diametric opposition, with both regulating glucose metabolism to preserve normoglycemia. This finely tuned balance is perturbed in patients with diabetes (18). Fasting and postprandial hyperglucagonemia exist alongside insulin insufficiency and enhanced hepatic glucose output, both contributors to hyperglycemia (19,20). Preclinical studies also demonstrate that postabsorptive hyperglucagonemia accounts for ∼50% of the pathological increment in glucose excursions (21). Therefore, targeting α-cell–derived glucagon excess to eliminate diabetic glycemic volatility is appealing for the treatment of T2DM such that novel avenues aimed to suppress glucagon hypersecretion or signaling should prove beneficial (20). Although how mitochondria affect glucagon secretion from α-cells is unknown, identifying strategies that target α-cell mitochondrial function, with the aim of curbing glucagon secretion during T2DM, is a widely unexplored area. Preserving insulin secretion and preventing loss of β-cell mass while suppressing local glucagon production during insulin resistance are attractive therapeutic avenues. We used mitoNEET as a unique modulator of mitochondrial activity to influence whole-islet physiology by inducing the protein either in β-cells, α-cells, or both cell types simultaneously. The hope was to unravel the critical mechanisms by which compromised mitochondrial function alters β-cell insulin secretion and α-cell glucagon production with the goal of preserving insulin sensitivity under metabolic challenge.
Research Design and Methods
Animals
All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of The University of Texas Southwestern (UTSW) Medical Center (Dallas, TX). Tet-responsive element (TRE)-mitoNEET mice were generated as previously described (15). Mouse insulin promoter (MIP)-rtTA mice and preproglucagon (PPG)-rtTA mice were generated by subcloning the rtTA into a plasmid containing the 8.3-kilobase (kb) MIP or the 1.7-kb PPG promoter, respectively. After linearization, the construct was injected into C57/BL6-derived blastocysts. TRE-LacZ and Parkin knockout (Park-KO) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Transgene-positive offspring were genotyped with PCR by using the following primer sets: TRE-mitoNEET, 5′-AGAGAATCGCACCAAAGCTATG and 5′-CAAACTCACCCTGAAGTTCTCAG; TRE-LacZ, 5′-ATCCTCTGCATGGTCAGGTC and 5′-CGTGGCCTGATTCATTCC; TRE-green fluorescent protein (GFP), 5′- GTAAACGGCCACAAGTTCAGC and 5′-GATGCCGTTCTTCTGCTTGTC; MIP-rtTA, 5′-CACCTGGAGACCTTAATGGGCCAAAC and 5′-CGATTGGCAGGGCATCGAGC; PPG-rtTA, 5′-TTTGAACAGAAGGGGCTTTTT and 5′-GAGCGAGTTTCCTTGTCGTC; Park-KO mutant, 5′-ATGTTGCCGTCCTCCTTGAAGTCG and Parkin wild-type (WT), 5′-GCAGAATTACAGCAGTTACCTGG and 5′-CCTACACAGAACTGTGACCTGG (all three primers mixed in one reaction). All experiments were performed in a pure C57/BL6 background and conducted using male and female littermate control mice. All doxycycline (Dox)-chow diet (25, 50, 100, or 600 mg/kg Dox) or Dox high-fat diet (HFD) (600 mg/kg Dox) experiments were initiated at ∼6–12 weeks of age. Mice were fed a pioglitazone (Pio)-containing (40 mg/kg/day; Watson Pharmaceuticals) Dox chow (600 mg/kg) diet.
Systemic Tests
For the oral glucose tolerance test (OGTT), mice were fasted for 3 h before administration of glucose 2.5 g/kg body weight (BW) by gastric gavage. For the arginine tolerance test, mice were fasted overnight (12–16 h) before intraperitoneal injection of l-arginine 1 mg/g BW (Sigma-Aldrich). At the indicated time points, venous blood samples from the tail vein were collected in heparin-coated capillary tubes. Glucose levels were determined by using an oxidase-peroxidase assay (Sigma-Aldrich, St. Louis, MO). Insulin levels were measured with commercially available ELISA kits (Millipore LINCO Research, St. Charles, MO). Pancreatic insulin content was measured as previously described (22). Glucagon levels were measured by using an ELISA kit (Mercodia Inc., Winston-Salem, NC).
GSIS on Perfused Pancreata
Mouse pancreata were perfused with buffers containing either 2.7 mmol/L or 16.7 mmol/L glucose (Sigma-Aldrich). All buffers before reaching the celiac artery were maintained at 37°C. Perfusates were then collected at 1-min intervals for 25 min. Insulin levels were measured in perfusates with an insulin assay kit (Cisbio).
Isolation of Islets
Mice were sacrificed by cervical dislocation after isoflurane anesthesia, then pancreatic islets were isolated as previously detailed (23). Briefly, pancreata were inflated using Liberase TL 0.1 g/L (#05401020001; Roche) containing DNase I 0.1 g/L (#10104159001; Roche) and then digested by incubation at 37°C for 25 min. Islets were hand selected under a dissection microscope, washed in sterile 1× PBS, and immediately snap frozen in liquid nitrogen followed by storage at −80°C until analysis.
Isolation of GFP-Labeled α-Cells by FACS Sorting
After islet isolation, every 70–80 islets (mixed sizes) were handpicked and added to a tube containing 500 μL enzyme-free cell dissociation solution (Cat. S-004-B; Millipore). Islets were allowed to dissociate in a 37°C water bath for 5–7 min before gently pipetting up and down until no visual clumps were observed. This procedure was repeated once. A small aliquot of islet suspension was examined under a light microscope to confirm the presence of a dispersed cell suspension. Cells were immediately centrifuged (4°C at 500g for 5 min) to remove the supernatant and then resuspended in 500 μL cold sample application buffer (114 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L KH2PO4, 1.16 mmol/L MgSO4, 20 mmol/L HEPES, and 2.5 mmol/L CaCl2; pH 7.3) containing 5 mmol/L glucose and 1% FBS. This procedure was repeated once. All dispersed cells from the same group were pooled together and washed again. The final cell pellets were diluted to 0.5–1 × 106 cells/mL for FACS sorting. Cells were sorted by a BD FACSAria with an argon laser beam tuned to 488 nm at 50-mW output with a 530 ± 10 nm emission filter. C57/BL6 cells were used as the control and to set the sorting parameters. Cells were sorted at a rate of 1,000 events/s by using normal recovery mode with the temperature setting at 4°C.
Quantitative Real-Time PCR
Pancreatic tissues were excised from mice and snap frozen in liquid nitrogen. Total RNA was isolated after tissue homogenization in TRIzol (Invitrogen, Carlsbad, CA) using a TissueLyser (MagNA Lyser; Roche) and then isolated using an RNeasy RNA extraction kit (QIAGEN). The quality and quantity of RNA was determined by absorbance at 260/280 nm. cDNA was prepared by reverse transcribing 1 μg of RNA with an iScript cDNA Synthesis Kit (Bio-Rad, Hercules CA). Supplementary Table 2 details the primer sequences used for quantitative real-time PCR. Results were calculated by threshold cycle method (24), with β-actin used for normalization.
Histology, Immunofluorescence, and Immunohistochemistry
Pancreata were dissected and fixed in 10% PBS-formalin for 24 h. After paraffin embedding and sectioning (5 μm), tissues were stained with hematoxylin-eosin (H-E). For immunofluorescence (IF) and immunohistochemistry (IHC), paraffin-embedded sections were incubated with primary antibodies for 24 h at 4°C and subsequently decorated with secondary antibodies for 1 h at room temperature (RT). Antibodies used were a polyclonal anti-mitoNEET antibody (1:250; Santa Cruz Biotechnology, Dallas, TX), a guinea pig anti-swine insulin antibody (1:500; Dako, Carpinteria, CA), a rabbit antiglucagon antibody (1:250; Zymed, Grand Island, NY), and a polyclonal goat beclin-1 antibody (1:250; Santa Cruz Biotechnology). For IHC, sections were incubated with biotinylated secondary antibodies (anti-guinea pig antibody [1:500], anti-rabbit antibody [1:200], or anti-goat antibody [1:500]; Dako) for 1 h at RT, then reactions were developed using DAB (3,3'-diaminobenzidine) chromogen and DAB substrate buffer (Dako). All images were acquired with an Olympus FSZ100 light microscope or a Zeiss AxioObserver epifluorescence microscope. For microtubule-associated protein light chain 3 (LC3-II) IF staining, isolated islets were treated for 30 min at 37°C with a mixture containing CYTO-ID Green Detection Reagent Hoechst 33342 Nuclear Stain per manufacturer protocol (CYTO-ID Autophagy Detection Kit; Enzo Life Sciences, Inc., Farmingdale, NY). As a positive control, WT islets were pretreated with rapamycin (500 nmol/L). Islets were then washed twice with 1× assay buffer and transferred to a glass microscope slide. Autophagic signal was imaged by using confocal microscopy in the fluorescein isothiocyanate 488-nm excitable green fluorescent detection range (Leica TCS SP5 confocal microscope).
β-Galactosidase Staining of Tissues
Mice at age 10–12 weeks were anesthetized and perfused with 0.2% glutaraldehyde in 1× PBS. Pancreatic, gastric fundal, small intestinal, eye, and brain tissues were excised and placed into a 6-cm cell culture dish containing 0.2% glutaraldehyde and cut into small slices. Tissues were washed with rinse buffer (100 mmol/L sodium phosphate, 2 mmol/L MgCl2, 0.01% sodium deoxycholate, and 0.02% NP-40) three times and then soaked in β-galactosidase (β-gal) staining buffer (5 mmol/L potassium ferricyanide, 5 mmol/L potassium ferrocyanide, and 1 mg/mL β-gal substrate in rinse buffer) for 48 h at RT with mild shaking. Tissues were transferred to 10% formalin overnight for postfixation, and postfixed tissues were processed with a standard paraffin tissue–embedding protocol. After paraffin embedding and sectioning, tissues were counterstained with nuclear fast red. Stained sections were imaged with an Olympus FSX100 light microscope.
Transmission Electron Microscopy
Fresh pancreatic tissues were fixed by perfusion with 4% paraformaldehyde and 1% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer. Fixed tissues were transferred to 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer, postfixed in buffered 1% osmium tetroxide, en bloc stained in 4% uranyl acetate in 50% ethanol, dehydrated with a graded series of ethanol, then embedded in EMbed 812 resin. Thin sections were cut on a Leica Ultracut UCT ultramicrotome and poststained with 2% uranyl acetate and lead citrate. Images were acquired on an FEI Tecnai G2 transmission electron microscope equipped with a LaB6 source and operating at 120 kV.
Immunoblotting
Frozen islets were homogenized in TNET buffer (50 mmol/L Tris-HCl [pH 7.6], 150 mmol/L NaCl, 5 mmol/L EDTA, phosphatase inhibitors [Sigma-Aldrich], protease inhibitors [Roche], and 1% Triton X-100) and then centrifuged at 13,000 rpm for 5 min. Protein concentrations were determined by using a bicinchoninic acid assay kit (Pierce). Proteins were resolved on 10–20% Tricine gels (Invitrogen) and then transferred to nitrocellulose membranes (Protran; Whatman GmbH, Dassel, Germany). Affinity-purified polyclonal anti-mitoNEET antibodies (1:1,000), monoclonal anti-Parkin antibodies (1:1,000; Cell Signaling Technology, Boston, MA), or monoclonal anti-β-actin antibodies (1:1,000) were used. Primary antibodies were detected with secondary IgG labeled with infrared dyes emitting at 700 and 800 nm (LI-COR Biosciences, Lincoln, NE) and then visualized on a LI-COR Odyssey infrared scanner. The scanned data were analyzed using Odyssey version 2.1 software (LI-COR Biosciences).
Mass Spectroscopy for Lipid Species Quantification
Lipid species were quantified, as previously detailed, by liquid chromatography and tandem mass spectrometry using a TSQ Quantum Ultra Triple Quadrupole Mass Spectrometer (Thermo Fisher Scientific) equipped with an electrospray ionization probe and interfaced with an Agilent 1100 series high-performance liquid chromatography system (Agilent Technologies) (25).
INS-1 Cell Culture and Plasmid Transfection
INS-1 cells were seeded on a 35-mm dish for 16–18 h before plasmid transfection at ∼25% confluence. For each dish, 2.5 μg plasmid DNA (a cytomegalovirus [CMV]-mitoNEET-internal ribosome entry site [IRES]-GFP plasmid or a control CMV-GFP plasmid) was transfected into cells by using Lipofectamine 3000 (L3000-008; Invitrogen). After transfection (20–24 h), cells were imaged using a Zeiss LSM 780 confocal microscope. The GFP imaging was set at excitation 488 nm and emission 500–530-nm band path. The tetramethylrhodamine, methyl ester (TMRM) imaging was set at excitation 543 nm and emission 565–615-nm band path.
TMRM Staining and Carbonyl Cyanide-4-Phenylhydrazone Treatment
After plasmid transfection (20–24 h), INS-1 cells were washed three times with 1× PBS and then incubated with TMRM 20 nmol/L for 30 min at RT. The cells were washed three times with 1× PBS before imaging. As a control, carbonyl cyanide-4-phenylhydrazone 1 μmol/L was used to depolarize the mitochondria membrane potential (ΔΨm).
Results
Generation of an Inducible β-Cell or α-Cell Labeling System
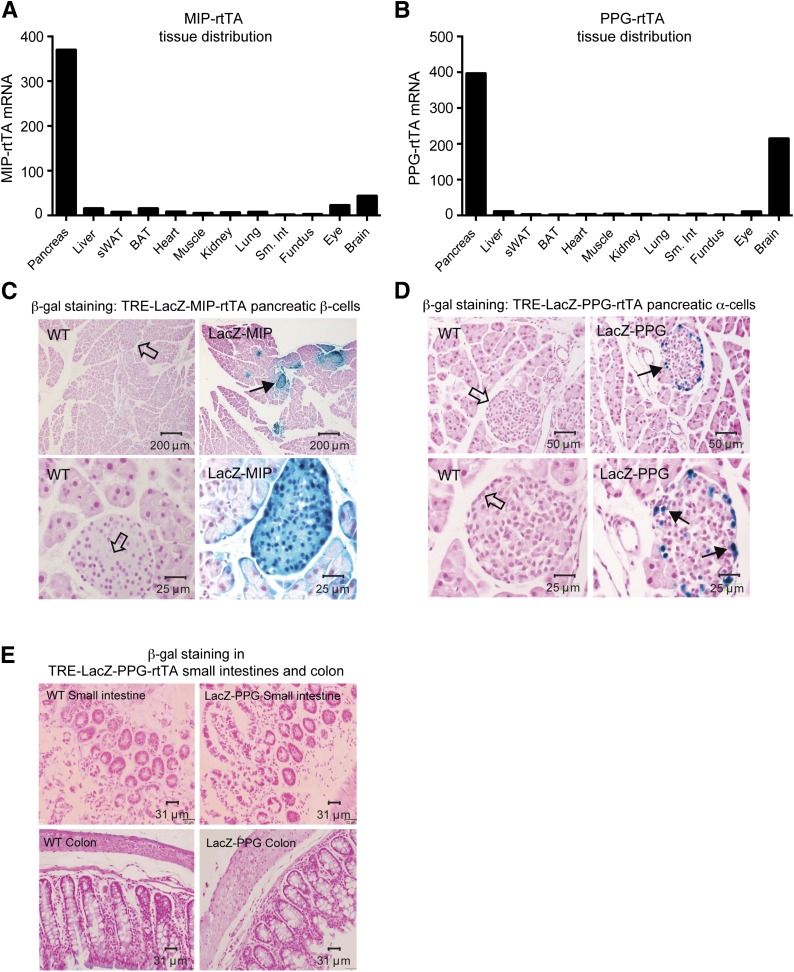
To create a system that allows us to induce any given protein specifically in β-cells, we generated a novel insulin promoter–driven reverse tetracycline-on controlled transactivator (MIP-rtTA) mouse. We also generated a novel PPG promoter–driven rtTA mouse (PPG-rtTA) that allows induction specifically in α-cells. MIP-rtTA and PPG-rtTA mRNA levels are highest and primarily restricted to the pancreas (Fig. 1A and 1B). To define the specificity, we used an inducible β-cell or α-cell labeling system. We crossed MIP-rtTA or PPG-rtTA mice with Tet-responsive LacZ (TRE-LacZ) mice. Upon Dox-chow feeding, β-cells or α-cells expressing rtTA activate the TRE promoter, and LacZ expression is induced only within these cell types. Tissues are then stained blue with β-gal substrate. Upon Dox-chow feeding, β-cells or α-cells of TRE-LacZ-MIP-rtTA or TRE-LacZ-PPG-rtTA mice were uniformly labeled blue (Fig. 1C and 1D), reflecting the presence of β-gal. Of note, β-cells within TRE-LacZ-PPG-rtTA islets were not labeled (Fig. 1D).
Figure 1.
Generation and validation of MIP-rtTA and PPG-rtTA mice by using an inducible β-gal system. A and B: Representative tissue distributions of MIP-rtTA (A) and PPG-rtTA mRNA expression (B) in the pancreas, liver, subcutaneous (sWAT), brown adipose tissue (BAT), heart, skeletal muscle, kidney, lung, small intestine (Sm. Int), stomach fundus, eye, and whole-brain tissues derived from a male C57/BL6 MIP-rtTA mouse or a PPG-rtTA mouse after 1 week of Dox-chow (600 mg/kg) feeding. C and D: β-gal staining of pancreata derived from male C57/BL6 WT TRE-LacZ-MIP-rtTA (LacZ-MIP) (C) or TRE-LacZ-PPG-rtTA (LacZ-PPG) (D) mice after Dox-chow (600 mg/kg) feeding. Representative blue LacZ-positive β-gal staining is highlighted by solid arrows in centrally located β-cells within islets of LacZ-MIP pancreata (C) in addition to peripherally located α-cells in LacZ-PPG pancreata (D). Open arrows represent LacZ-negative β-cells and α-cells. E: A β-gal stain of L cells in small intestines and the colon of WT and LacZ-PPG mice after Dox-chow feeding.
The rat insulin promoter cassette conveys expression in the hypothalamus (26). To confirm specificity of our inducible MIP–driven system, we stained brain slices from Dox-chow–fed TRE-LacZ-MIP-rtTA mice and observed no positive β-gal signal in the hypothalamus, cerebellum, or cerebrum (Supplementary Fig. 1A). Although we observed low PPG-rtTA gene expression in the whole brain (Fig. 1B), staining of hypothalamic, cerebellar, and cerebral tissues from TRE-LacZ-PPG-rtTA mice revealed no positive β-gal signal (Supplementary Fig. 1B), highlighting stringent pancreatic specificity of our PPG-rtTA mice. The PPG gene is also expressed in the intestinal endocrinocytes (L cells), albeit the tissue distribution diversifies during posttranslational processing (27). No positive β-gal signal was observed in small intestinal or colonic tissues from Dox-chow–fed TRE-LacZ-PPG-rtTA mice (Fig. 1E). The gastric fundus also is a source of glucagon in insulin-deprived depancreatized alloxan-diabetic dogs (28), suggesting that extrapancreatic glucagon contributes to hyperglucagonemia upon pancreatic injury. In animals with noncompromised pancreata, the current analyses revealed no β-gal signal in the gastric fundus (Supplementary Fig. 1C).
Induction of MitoNEET in β-Cells Causes Hyperglycemia, Glucose Intolerance, Cellular Dysfunction, and Loss of β-Cell Mass: A Novel and Highly Specific β-Cell Mouse Model of Titratable Diabetes
MitoNEET exerts a profound effect on whole-body energy utilization by lowering adipocyte mitochondrial iron content, which hinders mitochondrial function (15). The adipocyte compensates by upregulating PPARγ and adiponectin, which results in massive WAT expansion (15). We subsequently sought to explore whether mitoNEET influences pancreatic function and whether the β-cell activates compensatory mechanisms under mitochondrial dysfunction. We used the TRE-mitoNEET (TRE-MitoN) mouse (15) in which the expression of mitoNEET is driven by a tetracycline-inducible promoter element (or TRE). For the promoter element to be operational, the presence of the tetracycline-on transcription factor rtTA is necessary. We provide the rtTA factor in a β-cell– or α-cell–specific manner through mice harboring rtTA under the control of the MIP (Supplementary Fig. 2A) or the PPG promoter (Supplementary Fig. 2B), respectively. After Dox feeding, we achieved β-cell– or α-cell–specific induction of the mitoNEET transgene when TRE-MitoN mice were crossed with either MIP-rtTA mice or PPG-rtTA mice, respectively (Supplementary Fig. 2A and 2B). MitoNEET overexpression was strictly confined to the pancreas in TRE-MitoN-MIP-rtTA (MIP-MitoN) mice and TRE-MitoN-PPG-rtTA (PPG-MitoN) mice (Supplementary Fig. 2C) because endogenous mitoNEET levels in WT mice did not follow a comparable expression pattern (Supplementary Fig. 2D). IF staining revealed mitoNEET induction specifically in centrally located β-cells in MIP-MitoN islets (Supplementary Fig. 2E). In some β-cells, we saw a single prominent structure stained, whereas in other β-cells, we observed a more diffuse staining pattern. MitoNEET overexpression was also evident in peripherally located α-cells in PPG-MitoN islets (Supplementary Fig. 2F).
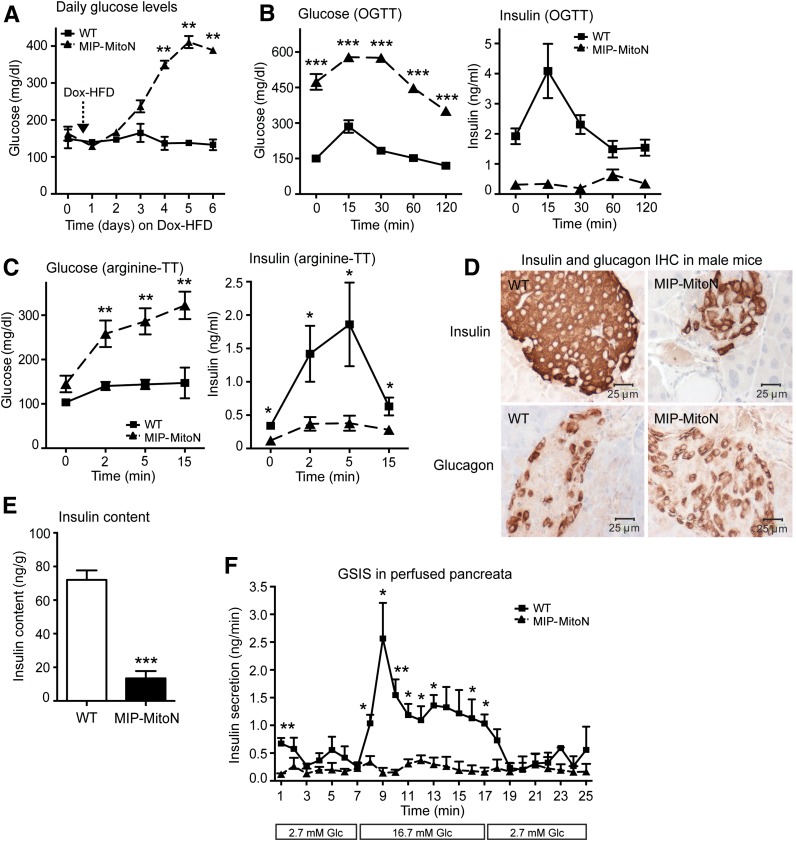
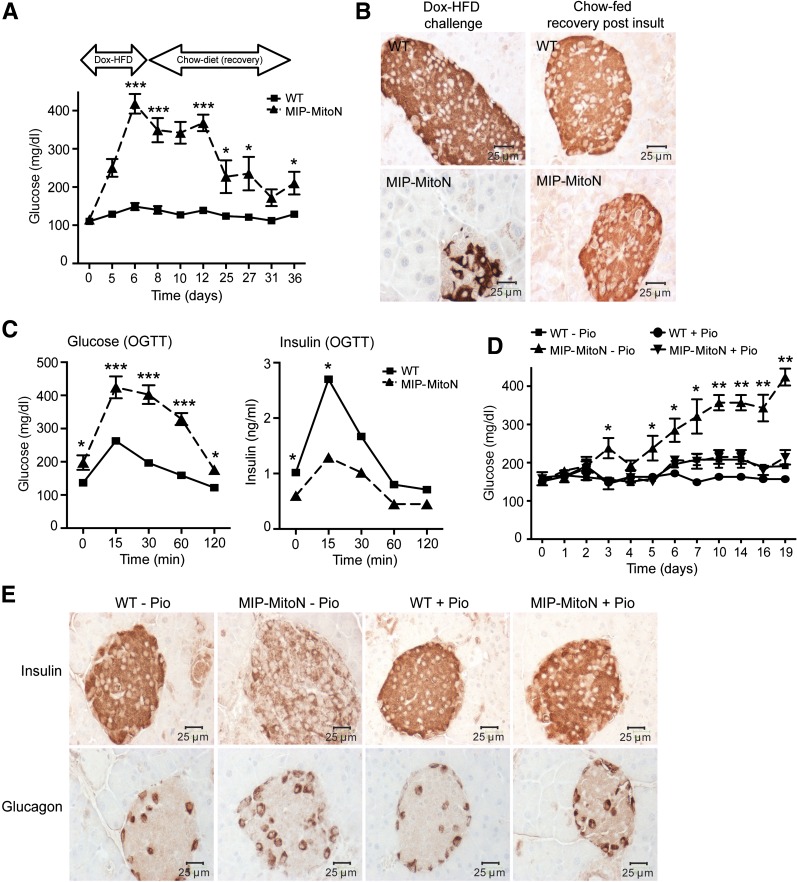
Initial observations in MIP-MitoN mice were striking. After only 5 days of Dox-HFD feeding (600 mg/kg Dox), mice exhibited chronic hyperglycemia (Fig. 2A). MIP-MitoN mice were profoundly glucose intolerant, as demonstrated by diminished insulin secretion capacity during OGTT (Fig. 2B). An l-arginine tolerance test (an amino acid that stimulates the acutely releasable pool of insulin from β-cells) revealed that MIP-MitoN mice exhibited significantly impaired insulin release (Fig. 2C). IHC showed smaller-sized islets and less insulin-positive staining in β-cells, whereas α-cell glucagon signal remained intact (Fig. 2D). The system not only affects islet size but also islet numbers because quantitative analysis revealed an ∼50% reduction of islets in MIP-MitoN pancreata (WT mice 24 islets per tissue section, MIP-MitoN mice 14 islets). This was corroborated by significantly lower total pancreatic insulin content in MIP-MitoN mice, integrating the reduction of both size and number of islets per pancreas (Fig. 2E). Perfusing isolated pancreata with low followed by high concentrations of glucose to examine GSIS confirmed β-cell dysfunction in MIP-MitoN mice (Fig. 2F). Although WT mice responded with a classical glucose-induced biphasic insulin response consisting of a transient first phase with a longer second phase (29), MIP-MitoN mice completely lacked a response (Fig. 2F).
Figure 2.
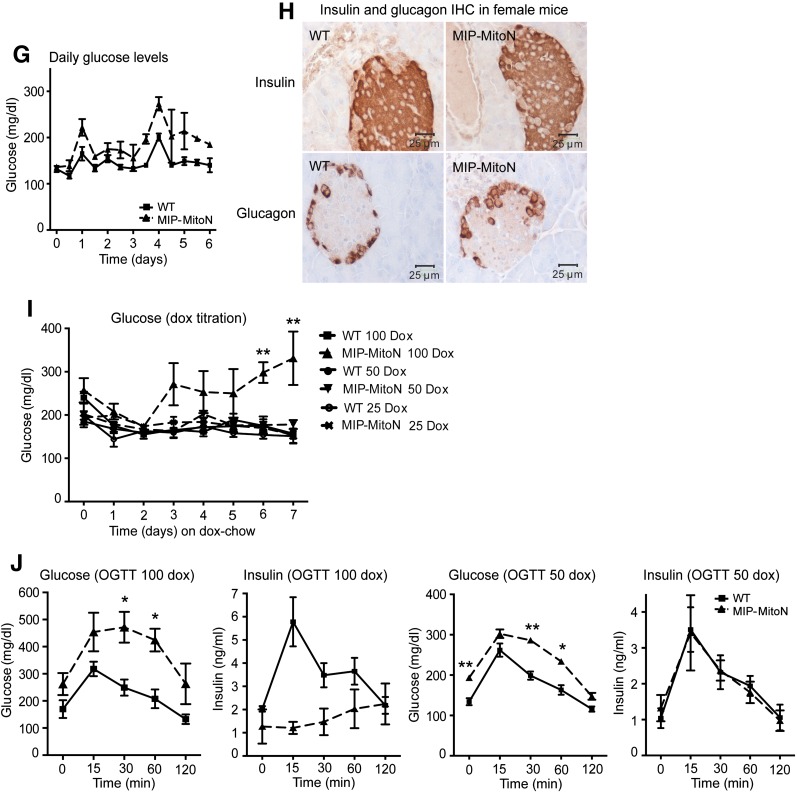
β-cell–specific MIP-MitoN mice exhibit hyperglycemia, glucose intolerance, β-cell dysfunction, and loss of β-cell mass. A: Daily ad libitum glucose levels in male C57/BL6 WT vs. MIP-MitoN mice during 6 days of Dox-HFD (600 mg/kg Dox) feeding (n = 6). Data are mean ± SEM. **P < 0.01. B: Glucose levels (left) and insulin levels (right) during an OGTT (2.5 g/kg BW by gastric gavage following a 3-h fast) on male WT vs. MIP-MitoN mice (n = 5). ***P < 0.001. C: Glucose levels (left) and insulin levels (right) during an arginine tolerance test (TT) on male WT vs. MIP-MitoN mice fed Dox-HFD for 6 days. Mice were fasted (14–16 h) before intraperitoneal injection of l-arginine (1 mg/g BW) (n = 5). *P < 0.05, **P < 0.01. D: Insulin (top) and glucagon (bottom) IHC staining on sectioned pancreata obtained from male WT vs. MIP-MitoN mice. E: Total pancreatic insulin content (ng/g) in WT vs. MIP-MitoN mice after Dox-HFD feeding (n = 8). ***P < 0.001. F: Insulin secretion rates (ng/min) during perfusion of pancreata in 600 mg/kg Dox-chow fed WT vs. MIP-MitoN mice. Pancreata were perfused with a low glucose concentration (2.7 mmol/L) for up to 7 min followed by a high glucose concentration (16.7 mmol/L) for up to 10 min and then again with a low glucose dose for up to 8 min (n = 3). *P < 0.05, **P < 0.01. G: Daily ad libitum glucose levels during 6 days of Dox-HFD (600 mg/kg) feeding of female WT vs. MIP-MitoN mice (n = 3). H: Insulin (top) and glucagon (bottom) IHC of female WT vs. MIP-MitoN pancreata on day 7 of Dox-HFD feeding. I: Daily ad libitum glucose levels during a Dox titration time course in male WT vs. MIP-MitoN mice during 7 days of Dox-chow (25, 50, or 100 mg/kg Dox) feeding (n = 6). **P < 0.01. J: Glucose and insulin levels in male WT vs. MIP-MitoN mice during an OGTT (2.5 g/kg BW) following 7 days of 100 mg/kg Dox-chow (left) or 50 mg/kg Dox-chow (right) feeding (n = 6). *P < 0.05, **P < 0.01. Glc, glucose.
An interesting sexual dimorphism was noted in the present MIP-MitoN mice. Profound hyperglycemia was apparent in male MIP-MitoN mice during Dox-HFD feeding (Fig. 2A). Conversely, female MIP-MitoN mice are resistant to mitoNEET-driven hyperglycemia (Fig. 2G) and retain full insulin-positive β-cell mass (Fig. 2H) and islet numbers (WT females 25 islets, MIP-MitoN females 19 islets). This could be due to an inherently higher resistance to the negative consequences of compromised mitochondrial function. Although female MIP-MitoN mice induced mitoNEET ∼2.5-fold in β-cells, overexpression was not as pronounced in male mice (Supplementary Fig. 3A). Nevertheless, a comparable fold induction in male MIP-MitoN mice still very effectively caused significant β-cell dysfunction, hyperglycemia, and glucose intolerance (Fig. 2J), suggesting that female mice are inherently more resistant to the effects of mitoNEET. Female mice also have higher circulating levels of adiponectin compared with male mice (30), and adiponectin-overexpressing mice display protection from β-cell apoptosis and diabetes (31). Female mice also display potent protection from caspase-8–mediated apoptosis (R.Y., P.E.S., unpublished observations). Future studies investigating how female MIP-MitoN mice cope with compromised mitochondrial function with ease and manage to sustain normoglycemia during a mitoNEET-driven β-cell insult should prove illuminating.
To examine the effects of mitoNEET on β-cell function without concomitant cellular death, we titrated the dose of Dox. Figure 2I shows that from a range of Dox concentrations (25, 50, or 100 mg/kg), the 100 mg/kg dose resulted in significantly higher glucose levels in MIP-MitoN mice. By using lower doses of Dox (50 or 100 mg/kg) in MIP-MitoN mice, glucose tolerance was impaired without associated hyperglycemia (Fig. 2J). This reflects the high level of reproducibility in the present mouse model, with a precisely titratable degree of β-cell dysfunction linked to an inducible diabetic readout. It further highlights the extremely high degree of sensitivity of the β-cell to an even slight reduction in mitochondrial perturbation.
MitoNEET Induction Forms Large Autophagic Vacuoles and Mitophagosomes in β-Cells Through Activation of Parkin-Dependent Mitophagy
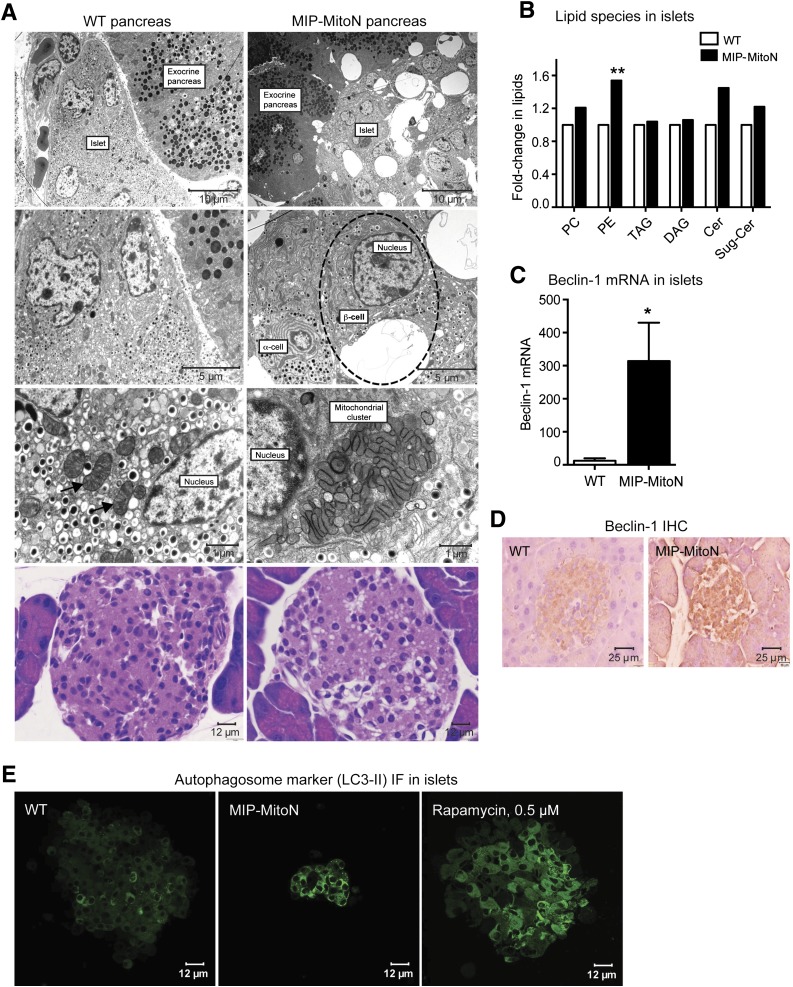
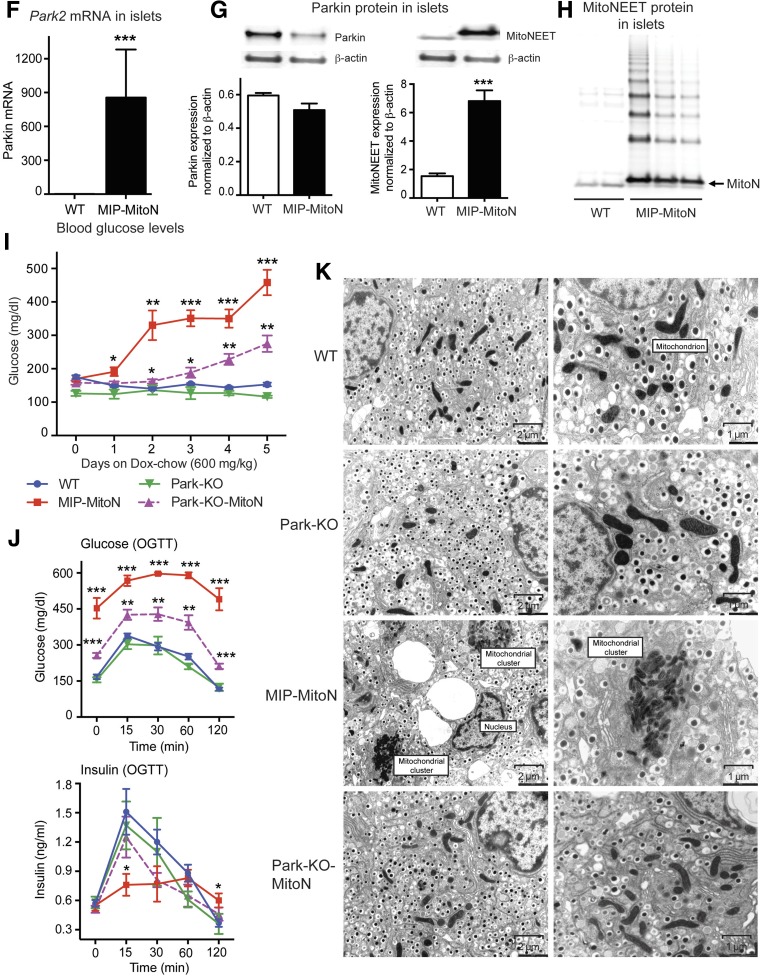
Pancreatic β-cells harbor an interconnected filamentous network of mitochondria. However, when mitochondria become fused or fragmented, GSIS is impaired (6,32). By using electron microscopy (EM), we previously identified that WAT-specific induction of mitoNEET causes elongated, fused, and dysfunctional mitochondria (15). However, we did not observe any apoptotic adipocytes or aggregates. Here, EM of pancreata from 100 mg/kg Dox-chow fed mice was striking. MIP-MitoN islets displayed enlarged vacuoles that were restricted to the islet and not present in the exocrine pancreas or WT islets (Fig. 3A, first panel). Higher magnification revealed each MIP-MitoN β-cell to contain a single large vacuole, which was not evident in MIP-MitoN α-cells (Fig. 3A, second panel), highlighting a mitoNEET-driven β-cell–autonomous phenomenon. Vacuoles were not present in MIP-MitoN mice fed 600 mg/kg Dox-chow (data not shown), suggesting a dose-dependent effect of mitoNEET. Fewer insulin granules were evident in MIP-MitoN β-cells, whereas the numbers of glucagon granules appeared similar (Fig. 3A, second panel).
Figure 3.
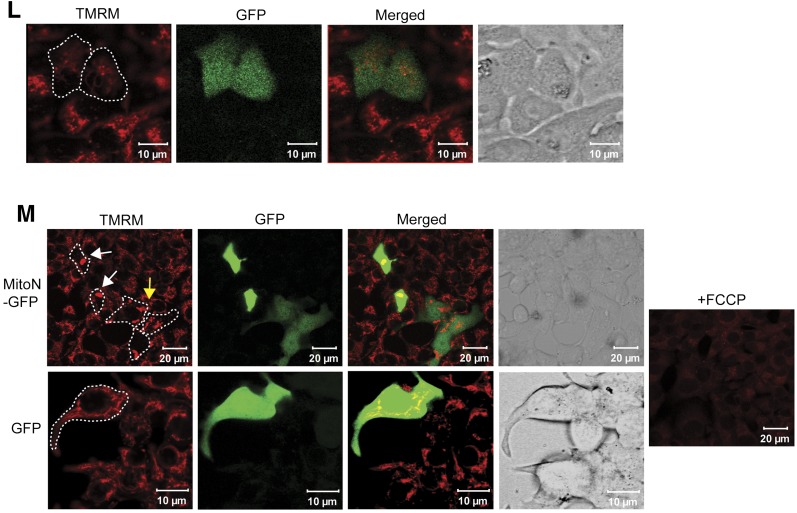
MitoNEET causes the formation of autophagic vacuoles and mitophagosomes in β-cells. A: Representative EM images of WT (left) and MIP-MitoN (right) pancreata from mice fed Dox-chow (100 mg/kg) for 2 weeks. The dashed outline highlights an MIP-MitoN β-cell containing an autophagic vacuole. The arrows point to WT β-cell mitochondria. The bottom panels show representative H-E staining of pancreata from WT and MIP-MitoN mice that underwent Dox-chow (100 mg/kg) feeding. B: Fold changes in lipid species content (phosphatidylcholine [PC], PE, triacylglycerol [TAG], diacylglycerol [DAG], ceramide [Cer], and sugar-ceramide [Sug-Cer] levels) in islets isolated from WT and MIP-MitoN mice after 2 weeks of Dox-chow (100 mg/kg) feeding (n = 3). Data are mean ± SEM. **P < 0.01. C: Beclin-1 gene expression levels in islets isolated from WT and MIP-MitoN mice after 2 weeks of Dox-chow (100 mg/kg) feeding (n = 5). *P < 0.05. D: Beclin-1 IHC in pancreata from WT and MIP-MitoN mice after 2 weeks of Dox-chow (100 mg/kg) feeding. E: Representative confocal images of IF staining by using the autophagosome marker LC3-II in islets isolated from WT and MIP-MitoN mice following 2 weeks of Dox-chow (100 mg/kg) feeding. F: Park2 gene expression levels in islets isolated from WT and MIP-MitoN mice after 2 weeks of Dox-chow (100 mg/kg) feeding (n = 5). ***P < 0.001. G: Representative Western blots showing Parkin and mitoNEET protein expression levels in islets isolated from WT and MIP-MitoN mice after 2 weeks of Dox-chow (100 mg/kg) feeding. Bar graphs show Parkin and mitoNEET levels normalized to β-actin. Of note, the ectopically expressed mitoNEET is carboxy-terminally FLAG-tagged, giving rise to a slightly slower migrating band by Western blotting, with the faster migrating band corresponding to endogenous mitoNEET present in both WT and transgenic cells. H: Western blot showing mitoNEET protein expression levels in islets isolated from WT and MIP-MitoN mice after 2 weeks of Dox-chow (100 mg/kg) feeding. I: Daily ad libitum glucose levels in WT, MIP-MitoN, Park-KO, and Park-KO-MIP-MitoNEET (Park-KO-MitoN) mice during Dox-chow (600 mg/kg) feeding for 7 days (n = 7). *P < 0.05, **P < 0.01, ***P < 0.001. J: Glucose and insulin levels in WT, MIP-MitoN, Park-KO, and Park-KO-MitoN mice during an OGTT performed after 1 week of Dox-chow (600 mg/kg) feeding (n = 6). K: Representative EM images of WT, Park-KO, MIP-MitoN, and Park-KO-MitoN pancreata from mice fed Dox-chow (100 mg/kg) for 2 weeks. L: Representative confocal microscopy images of INS-1 β-cells transfected with CMV-mitoNEET-IRES-GFP plasmid and stained with the ΔΨm dye TMRM. The dashed outlines highlight the cells of interest that overexpress mitoNEET. M: Representative confocal microscopy images of INS-1 β-cells transfected with CMV-mitoNEET-IRES-GFP plasmid (top) or CMV-GFP plasmid (bottom) stained with TMRM. The dashed outlines highlight the cells of interest that overexpress mitoNEET. The yellow arrow indicates the midstages of small mitophagosome formation with moderate mitoNEET induction. The white arrows highlight late-stage large mitophagosome formation with chronic mitoNEET induction. FCCP, carbonyl cyanide-4-phenylhydrazone.
To assess whether the vacuoles in MIP-MitoN β-cells are lipid droplets, we quantified lipid species in islets from WT and MIP-MitoN mice fed 100 mg/kg Dox-chow. Although no marked differences in triacylglyceride or diacylglyceride levels were apparent, a significant increase in phosphatidylethanolamine (PE) levels, with a trend toward elevated levels of other membrane-associated lipid species (phosphatidylcholine, ceramides, and glycoceramides) was observed (Fig. 3B). However, the overall increase in lipid species did not account for the massive structure observed, suggesting that this may be a vacuolar organelle. Consistent with this, we were unable to stain islets with the neutral lipid stain oil red O (data not shown). A key reaction in autophagy entails the conjugation of the ubiquitin-like protein Atg8 to the lipid PE in autophagic membranes (33); levels of Atg8-PE conjugate correlate with autophagosomal size (34). We therefore suggest that the structures present in MIP-MitoN β-cells are autophagic vacuoles targeted for lysosomal degradation. Although WT islet mitochondria exhibit a classical mitochondrial shape with clearly visible cristae (35), conversely, MIP-MitoN islets harbor numerous abnormally shaped mitochondrial clusters (comparable in size to the diameter of the nucleus) that exhibit undefined cristae (Fig. 3A, third panel). Mitochondrial cluster formation (although not to the same extent) has been reported in β-cells in which autophagy is activated by exposure of the cells to palmitate (36). In the current study, mitoNEET may have promoted mitophagy and/or mitophagosomal accumulation. H-E staining confirmed the presence of the large vacuolar structures in MIP-MitoN islets that were apparent through EM (Fig. 3A, fourth panel).
Autophagy is a lysosomal degradation pathway essential for cell survival that is a critical compensatory response to numerous insults (37). In the current study, beclin-1 levels, an established marker of autophagy (38), were significantly upregulated in MIP-MitoN islets (Fig. 3C and 3D). Similarly, LC3-II, a marker of autophagosomes (39), revealed higher LC3-II–positive autophagic activity in MIP-MitoN islets on par with positive-control rapamycin-treated islets (Fig. 3E). Low-dose mitoNEET induction in β-cells, therefore, activates a critical autophagic pathway that forms unique mitophagosomes in β-cells hitherto never observed to this extent in the pancreas.
Parkin is an E3 ubiquitin-protein ligase that together with PTEN-induced putative kinase 1 (PINK1) orchestrates a mitochondrial quality control system to promote cellular survival through autophagy of damaged mitochondria, a process termed mitophagy (40,41). A single nucleotide polymorphism highlighted the Park2 gene as a candidate susceptibility gene for diabetes that plays a role in β-cell insulin secretion (42). Several studies corroborate a Parkin-mediated mitophagy pathway in β-cells (43), and in HeLa cells, Parkin polyubiquitinates mitoNEET on the OMM (44). Here, a massive transcriptional upregulation of Parkin was evident in MIP-MitoN islets (Fig. 3F). Conversely, we were surprised to observe a moderate reduction in Parkin protein expression in transgenic islets (Fig. 3G). Such disconnect between transcriptional and posttranslational mechanisms that mediate Parkin expression in MIP-MitoN islets suggests either destabilization of the mitochondrial architecture and/or self-ubiquitinated proteasomal-mediated degradation of Parkin on the OMM, the latter a phenomenon that regulates Parkin degradation rates (45). Given the reduction in protein levels, Parkin may undergo rapid proteolysis postrecruitment to the OMM; the system may compensate through transcriptional upregulation in PARK2 message. MitoNEET may serve as one of the substrates for Parkin-mediated ubiquitination. Western blotting for mitoNEET in transgenic islets implicated extensive ladder formation, consistent with the addition of a number of 8.5-kDa ubiquitin chains (Fig. 3H). Of note, mitoNEET contains 13 lysine residues, and blotting revealed a minimum of nine distinct mitoNEET bands with the potential to have additional, unresolved populations at higher molecular weights.
To test our hypothesis that mitoNEET functions through a Parkin-dependent mechanism, we crossed MIP-MitoN mice with Park-KO mice. Although MIP-MitoN mice exhibited chronic hyperglycemia within only a few days of Dox-chow feeding (600 mg/kg), MIP-MitoN mice lacking Parkin (Park-KO-MitoN mice) displayed significantly lower glucose levels (Fig. 3I) and were at least partially protected from glucose intolerance (Fig. 3J). Of note, mitoNEET transgene levels upregulated regardless of a Parkin knockout (Supplementary Fig. 3C), indicating no inhibition of transgene expression in the absence of Parkin. We also confirmed that no defects in insulin secretion are evident in Park-KO mice (Fig. 3J). Although mitophagosomes and enlarged vacuoles were present in MIP-MitoN pancreata, no such structures were evident in Park-KO-MitoN pancreata (Fig. 3K). After transfection of INS-1 β-cells with either a CMV-mitoNEET-IRES-GFP plasmid or a control CMV-GFP plasmid, we observed a mitoNEET-driven lowering of ΔΨm in the early stages of mitoNEET induction (Fig. 3L), with large punctate mitophagosomal aggregates forming in the later stages of mitoNEET overexpression (Fig. 3M). Of note, no mitophagosomes were evident in cells transfected with CMV-GFP alone, highlighting a mitoNEET-specific effect (Fig. 3M).
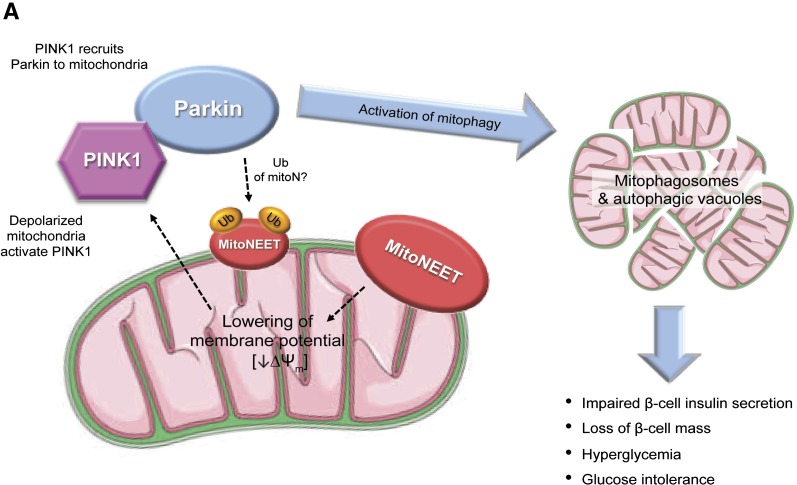
Collectively, these observations point to a mechanism whereby Parkin-dependent mitophagy in β-cells causes β-cell dysfunction in MIP-MitoN mice. Therefore, the current model is similar to our previous observations in the adipocyte (15): In β-cells, mitoNEET lowers ΔΨm. A reduction in ΔΨm recruits the PINK1-Parkin complex to mitochondria to activate mitophagy (46) (Fig. 7A). However, although Parkin ubiquitinates mitoNEET (47), and likely numerous other proteins, we do not know what the functional implications are for this ubiquitination reaction and whether this further stimulates mitophagy. Future studies examining the consequences of this ubiquitination should therefore prove illuminating.
Figure 7.
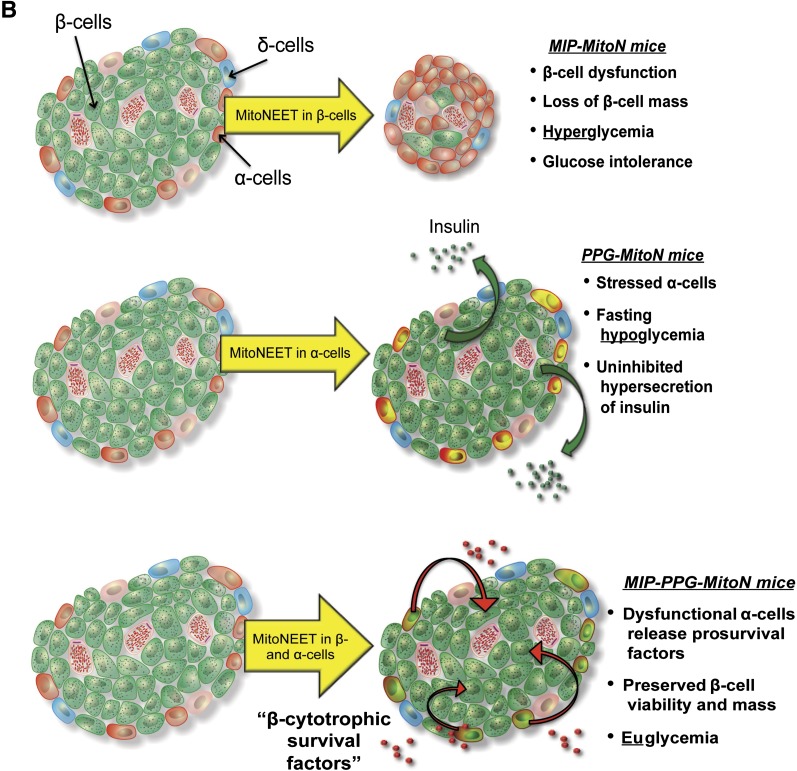
A: The mechanistic action of mitoNEET in MIP-MitoN β-cells. MitoNEET lowers ΔΨm. This depolarization of ΔΨm activates PINK1 and recruitment of Parkin to the OMM to trigger mitophagy, as observed by the formation of mitophagosomes and autophagic vacuoles. Such effects impair the ability of the MIP-MitoN β-cell to secrete insulin and, under increasing concentrations of Dox, promote β-cell death. At the whole-body level, MIP-MitoN mice exhibit hyperglycemia and severe glucose intolerance. Parkin is also known to polyubiquitinate (Ub) mitoNEET; however, the functional implications of this modification are currently unknown. B: Proposed model of mitoNEET-driven cellular and whole-body action in MIP-MitoN mice (β-cell induction of mitoNEET), PPG-MitoN mice (α-cell induction), and MIP-PPG-MitoN mice (β- and α-cell induction).
Modes of Regenerating or Preserving β-Cell Mass and Function: The Impact of Thiazolidinediones
Inducing mitoNEET in β-cells causes cellular dysfunction and loss of β-cell mass. We evaluated whether β-cells can regenerate and/or exhibit a full functional recovery after a mitoNEET-driven insult. If so, would an improvement in whole-body glucose tolerance ensue? MIP-MitoN mice were fed high-dose Dox-HFD (600 mg/kg) for 1 week. To lower mitoNEET levels and minimize HFD-induced insulin resistance, mice were switched to a non–Dox-containing chow diet to allow recovery and/or potential regeneration of β-cells postinjury. Upon reverting back to chow diet, MIP-MitoN mice achieved almost normoglycemia within 2–3 weeks after mitoNEET insult (Fig. 4A). The restoration of islet numbers (WT 33 islets; MIP-MitoN 33 islets) and insulin-positive signal (Fig. 4B) suggests recovery of β-cell mass. However, β-cell functionality was not entirely restored. Despite near-euglycemic glucose levels and the ability to mount an insulin response to glucose challenge, persistent glucose intolerance in MIP-MitoN mice was evident (Fig. 4C).
Figure 4.
Regeneration of β-cell mass in MIP-MitoN mice after mitoNEET-induced insult but minimal recovery of β-cell functionality. A: Glucose levels in WT vs. MIP-MitoN mice during Dox-HFD (600 mg/kg) feeding for 1 week then postrecovery (standard chow diet feeding) for 4 weeks (n = 5). Data are mean ± SEM. *P < 0.05, ***P < 0.001. B: Insulin IHC in WT vs. MIP-MitoN pancreata after Dox-HFD challenge (600 mg/kg) and after chow feeding (the recovery stage). C: Glucose and insulin levels in WT vs. MIP-MitoN mice during an OGTT during the recovery chow diet feeding stage (3 weeks after the initial Dox-HFD mitoNEET-induced insult) (n = 5). *P < 0.05, ***P < 0.001. D: Daily ad libitum glucose levels in WT vs. MIP-MitoN mice during feeding of Dox-chow (600 mg/kg) either with the TZD Pio (40 mg/kg/day) or without Pio (n = 5). *P < 0.05, **P < 0.01. E: Insulin and glucagon IHC staining of pancreata from WT vs. MIP-MitoN mice fed either Dox-chow (600 mg/kg) alone (−Pio), or Pio-containing Dox-chow (+Pio).
Thiazolidinediones (TZDs) are agonists for nuclear PPARγ that enhance insulin sensitivity in patients with diabetes (48). In β-cells, TZDs exert β-cytoprotective effects by preserving β-cell mass and islet architecture in a diabetic setting (49–51). We previously reported that treatment of PANIC-ATTAC mice (an inducible mouse model of β-cell loss and hyperglycemia) with a PPARγ agonist restores normoglycemia, improves glucose tolerance, and leads to the rapid regain of β-cell mass (51). Given that PANIC-ATTAC mice and MIP-MitoN mice share similarities in terms of β-cell dysfunction, β-cell loss, and subsequent recovery of β-cell mass, we hypothesized that TZDs exert β-cell protection in a similar manner as in MIP-MitoN mice to PANIC-ATTAC mice. WT and MIP-MitoN mice were therefore fed Dox-chow (600 mg/kg Dox) or Pio-containing Dox-chow (40 mg/kg day Pio and 600 mg/kg Dox). MIP-MitoN mice fed Dox-chow alone exhibited the expected gradual hyperglycemia; however, MIP-MitoN mice fed Pio-containing Dox-chow were completely protected from mitoNEET-induced hyperglycemia (Fig. 4D). IHC for insulin and glucagon revealed that Pio preserves islet morphology and β-cell mass, as demonstrated by dense insulin staining in MIP-MitoN Pio-treated islets (Fig. 4E). Supplementary Fig. 3B shows that mitoNEET transgene levels are significantly upregulated in MIP-MitoN pancreata both in the absence and in the presence of Pio, confirming that the ability of Pio to maintain normoglycemia in MIP-MitoN mice is not a consequence of transgene suppression. TZDs, therefore, provide potent β-cytoprotective effects from mitoNEET-induced mitochondrial dysfunction and the ensuing β-cell loss.
An α-Cell–Specific Induction of MitoNEET Perturbs Local Glucagon Homeostasis and Causes Fasting-Induced Hypoglycemia and Hypersecretion of Insulin
Targeting the diabetogenic role of α-cells is becoming increasingly important in diabetes research (20). Suppression of glucagon may offer valuable therapeutic avenues. Manipulating mitochondrial function to curb α-cell–derived hyperglucagonemia in an obese and/or diabetic setting is a novel and hitherto unexplored area for the modification of α-cell physiology. The precise mechanisms by which mitochondria influence α-cell glucagon secretion remain to be established.
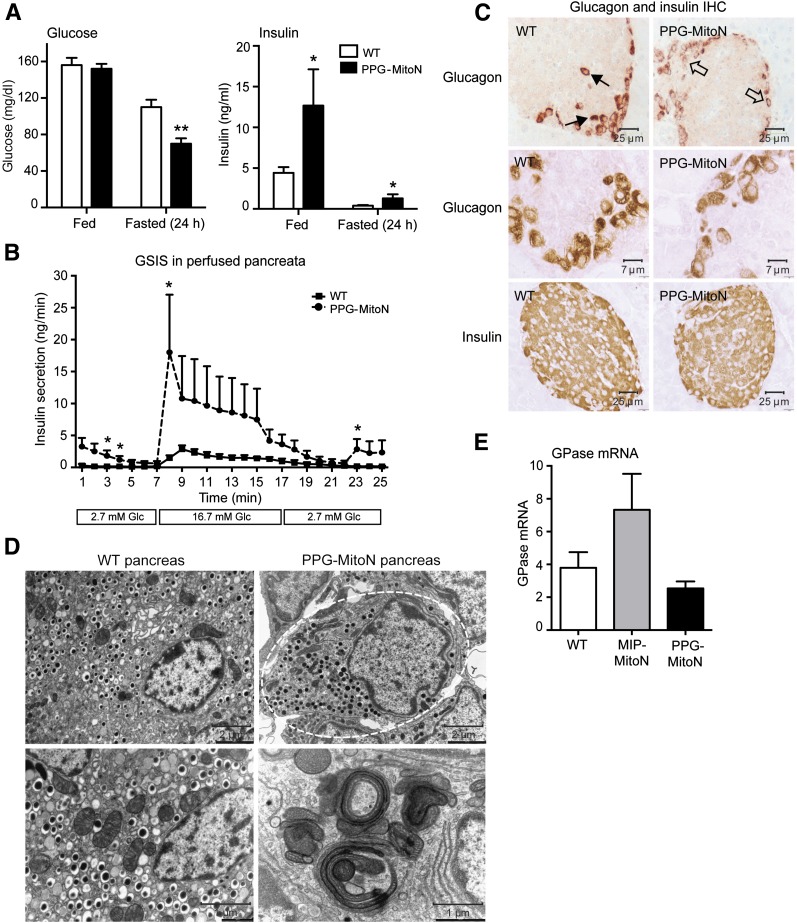
To induce mitoNEET in α-cells, TRE-MitoN mice were crossed with PPG-rtTA mice (Supplementary Fig. 2B). No marked differences in glucose tolerance were observed after 3 weeks of Dox-HFD (600 mg/kg) feeding (data not shown). However, PPG-MitoN mice exhibited fasting-induced hypoglycemia complemented by significantly higher insulin levels (Fig. 5A). Perfusing pancreata with low and then high concentrations of glucose to assess GSIS revealed hypersecretion of insulin from PPG-MitoN islets, with a profound insulin spike evident in the transient first phase of insulin release followed by significantly higher insulin levels in the prolonged second phase (Fig. 5B). IHC showed some loss of glucagon-positive signal in α-cells of PPG-MitoN islets (Fig. 5C, top and middle panels), with no difference in insulin staining apparent between WT and PPG-MitoN pancreata (Fig. 5C, bottom panel). Although EM revealed a relatively normal cellular architecture in PPG-MitoN α-cells (Fig. 5D, top panel), mitophagosomes were evident (Fig. 5D, bottom panel) similar to those in MIP-MitoN β-cells (Fig. 3A). This implies that mitoNEET-induced mitochondrial dysfunction activates mitophagy in the α-cell, forming large mitophagosomes similar to that in the β-cell. Consistent with local dysregulation in glucagon homeostasis, we observed a trend toward downregulation in downstream hepatic glucagon signaling targets of gluconeogenesis in PPG-MitoN mice. Glucose-6-phosphatase gene expression levels were moderately lower in PPG-MitoN livers after a 24-h fast; however, the differences did not reach statistical significance (Fig. 5E). PPG-MitoN mice, therefore, exhibit perturbed local glucagon signaling, with a disruption in islet homeostasis, as evidenced by dysinhibited secretion of insulin during GSIS.
Figure 5.
Enrichment of mitoNEET in α-cells results in fasting-induced hypoglycemia, hypersecretion of insulin during GSIS, and low glucagon–positive signal in islets. A: Ad libitum and fasted (24 h) glucose and insulin levels in male C57/BL6 WT vs. PPG-MitoN mice after 2 weeks of Dox-HFD (600 mg/kg) feeding (n = 4). Data are mean ± SEM. *P < 0.05, **P < 0.01. B: Insulin secretion rates (ng/min) during perfusion of pancreata of Dox-HFD (600 mg/kg)–fed WT and PPG-MitoN mice. Pancreata were perfused with a low glucose dose (2.7 mmol/L glucose) for up to 7 min followed by a high glucose dose (16.7 mmol/L glucose) for up to 10 min and then again with a low glucose dose for up to 8 min (n = 3). *P < 0.05. C: IHC staining of glucagon (top and middle panels, with the middle panel showing a higher resolution image) in addition to insulin IHC (bottom panel) in pancreata derived from WT vs. PPG-MitoN mice fed Dox-HFD (600 mg/kg) for 3 weeks. Solid arrows point to normal glucagon expression in α-cells in WT pancreata, whereas open arrows indicate loss of glucagon-positive staining in PPG-MitoN α-cells. D: Representative EM images of WT and PPG-MitoN pancreata from mice fed Dox-HFD (600 mg/kg) for 3 weeks. The dashed outline highlights glucagon granules within one α-cell. Scale bars indicate the resolution at which each image was taken. E: Hepatic glucose-6-phosphatase (GPase) gene expression levels after a 24-h fast in WT, MIP-MitoN, and PPG-MitoN mice after 3 weeks of Dox-HFD (600 mg/kg) feeding (n = 4). Glc, glucose.
Simultaneous Induction of MitoNEET in Both β-Cells and α-Cells Preserves β-Cell Viability Through Upregulation of Antiapoptotic Factors
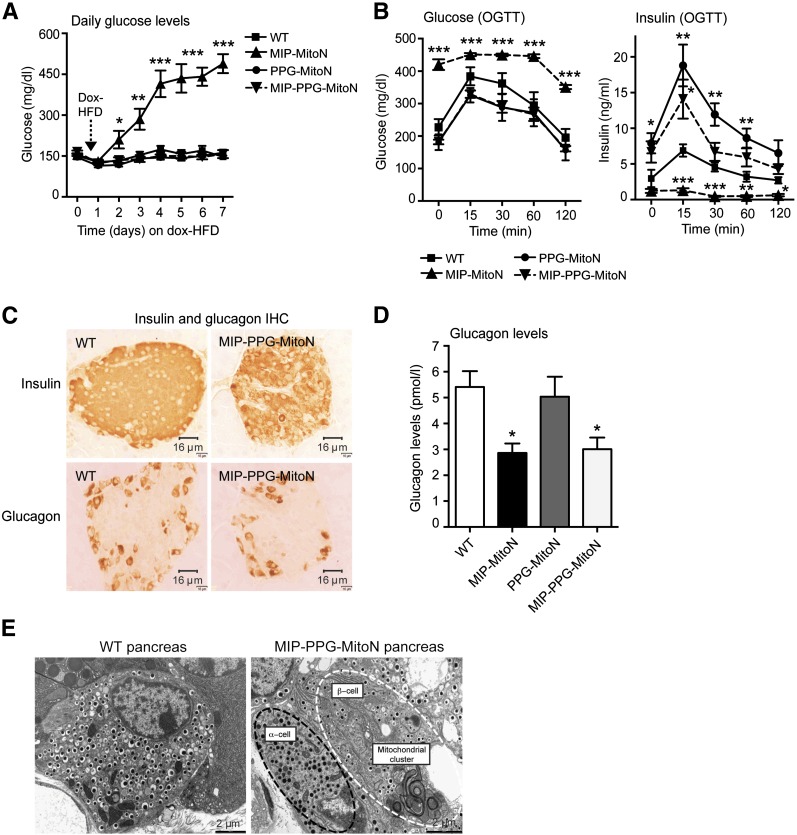
MitoNEET promotes β-cell dysfunction and lowers α-cell glucagon production when overexpressed in each cell type individually. We therefore asked whether simultaneous mitoNEET-driven perturbation of both β-cells and α-cells would disrupt whole-islet homeostasis. To achieve this, MIP-MitoN mice were crossed with PPG-MitoN mice. Supplementary Fig. 2G confirms that we achieved sufficient induction of mitoNEET in MIP-PPG-MitoN pancreata after Dox-HFD feeding. Although MIP-MitoN mice (β-cells only) exhibited chronic hyperglycemia after only 5 days of Dox-HFD (Fig. 6A), MIP-PPG-MitoN littermates (both β-cell and α-cell induction of mitoNEET) retained normoglycemia (Fig. 6A). This finding suggests that dual overexpression of mitoNEET in both β-cells and α-cells is protective against the mitoNEET-driven β-cell dysfunction typically observed when mitoNEET is induced in β-cells alone. Consistent with this, 24-h fasting glucose levels confirmed hyperglycemia in β-cell–specific MIP-MitoN mice, significant hypoglycemia in α-cell–specific PPG-MitoN mice, but normoglycemia in α- and β-cell–specific MIP-PPG-MitoN mice (Supplementary Table 1). Although MIP-MitoN mice exhibited markedly lower insulin levels (Supplementary Table 1) due to loss of β-cell mass (Fig. 2E and 2F), their PPG-MitoN and MIP-PPG-MitoN littermates displayed significantly higher levels of insulin (Supplementary Table 1), again suggesting uninhibited insulin secretion due to dysregulated glucagon homeostasis. In line with a diabetic phenotype, MIP-MitoN mice harbor elevated circulating levels of neutral lipids (Supplementary Table 1), whereas no differences were apparent in PPG-MitoN mice or MIP-PPG-MitoN mice. Although MIP-MitoN mice displayed the expected impairment in glucose tolerance, no differences were observed in MIP-PPG-MitoN and PPG-MitoN mice (Fig. 6B). MIP-PPG-MitoN and PPG-MitoN mice, however, exhibited higher levels of insulin in response to glucose load (Fig. 6B) consistent with an enhanced insulin secretion capacity. IHC showed comparable staining of insulin and glucagon between WT and MIP-PPG-MitoN pancreata (Fig. 6C), suggesting that the α-cell copes with mitochondrial dysfunction more effectively when the β-cell is challenged under similar conditions, and vice versa. Although fasting (24 h) glucagon levels were comparable between WT and PPG-MitoN mice after 1 week of Dox-HFD feeding, MIP-MitoN and MIP-PPG-MitoN mice displayed significantly lower glucagon levels (Fig. 6D). In MIP-PPG-MitoN pancreata, EM revealed the presence of mitophagosomes in β-cells but not in α-cells (Fig. 6E), indicating that under these conditions, the α-cell overcomes the challenge of mitochondrial perturbation, leading to the formation of mitophagic structures. Under the same conditions, the β-cells formed mitophagosomes, suggesting that the formation of the structure per se does not lead to cellular dysfunction and may in fact preserve it. Consistent with that, we observed at least in INS-1 cells that these mitophagosomes preserve ΔΨm (Fig. 3M). Both PPG-MitoN mice (α-cells) and MIP-PPG-MitoN mice (β- and α-cells) exhibited compromised α-cell function, yet the net result prevented the loss of β-cell mass typically seen in MIP-MitoN mice, suggesting that α-cell perturbation may emanate a potent protective signal toward β-cells to preserve their viability. We therefore hypothesized that compromised α-cells release an antiapoptotic β-cytotrophic factor that acts locally to target juxtaposed stressed β-cells. To explore this hypothesis, we sought to identify a potential α-cell–derived β-cytotrophic candidate originating from mitoNEET-enriched α-cells. We crossed TRE-GFP mice with PPG-MitoN mice to achieve GFP-specific labeling of mitoNEET-abundant α-cells. By using FACS, we isolated GFP-labeled α-cells from GFP-PPG-MitoN islets versus control GFP-PPG islets and then performed microarray methodology to screen for potential α-cell–derived survival candidates. The most prominent genes upregulated by mitoNEET in α-cells were nerve growth factor α (Ngfa), protein disulfide isomerase (pancreatic) (Pdip), and trefoil factor 2 (Tff2) (Table 1). Ngfa and Tff2 are critical trophic factors that help to maintain the survival of β-cells through antiapoptotic properties (52–54). The profound upregulation of these survival factors in metabolically challenged PPG-MitoN α-cells suggests their importance in sustaining β-cell viability in this mouse model. Future studies delineating the precise mechanisms of how this mitoNEET-driven upregulation in Ngfa and Tff2 promotes β-cell survival in a mouse model with perturbed α-cell homeostasis (the PPG-MitoN mouse) should prove interesting.
Figure 6.
Dual induction of mitoNEET in both β-cells and α-cells protects mice from mitoNEET-driven hyperglycemia typically observed in β-cell–alone overexpressors. A: Daily ad libitum glucose levels in male C57/BL6 WT, MIP-MitoN, PPG-MitoN, and MIP-PPG-MitoN mice during 7 days of Dox-HFD (600 mg/kg) feeding (n = 6). B: Glucose and insulin levels during an OGTT in male WT, MIP-MitoN, PPG-MitoN, and MIP-PPG-MitoN mice (n = 6). Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. C: IHC staining of insulin and glucagon in pancreata from WT vs. MIP-PPG-MitoN mice fed Dox-HFD (600 mg/kg). D: Circulating glucagon levels after a 24-h fast in WT, MIP-MitoN, PPG-MitoN, and MIP-PPG-MitoN mice after 1 week of Dox-HFD (600 mg/kg) feeding (n = 4). E: Representative EM images of WT and MIP-PPG-MitoN pancreata from mice fed Dox-HFD (600 mg/kg). The white outline highlights a β-cell, and the black outline highlights an α-cell.
Table 1.
Top upregulated genes in α-cells isolated from GFP-PPG-MitoN pancreata
| Gene | Gene definition | Fold-up | Function |
|---|---|---|---|
| Ngfa | Nerve growth factor, α | 11.5 | Ngfa is a critical trophic factor in maintaining the survival of β-cells; it exerts profound antiapoptotic and survival features. Pancreatic β-cells synthesize and secrete Ngfa to increase insulin production. Increased pancreatic Ngfa is an acute response of the pancreas to damage. Ngfa reduction activates β-cell apoptosis. |
| Pdip | Protein disulfide isomerase, pancreatic | 7.1 | A major intracellular storage protein that promotes the accumulation of pancreatic estrogen. Reported to provide strong protection to the cell against heat shock or oxidative stress–induced death. Estrogen is further known to protect β-cells from apoptosis and oxidative injury in addition to preventing insulin-deficient diabetes in mice. |
| Tff2 | Trefoil factor 2 | 5.6 | Antiapoptotic. Identified as a novel target for inducing β-cell proliferation. Promotes cell proliferation in β-cells through CXCR-4–mediated ERK1/2 phosphorylation. |
Top genes identified by Illumina microarray that display the highest-fold upregulation in α-cells isolated from TRE-GFP-PPG-rtTA (GFP-PPG) pancreata (n = 4) vs. TRE-MitoN-TRE-GFP-PPG-rtTA (GFP-PPG-MitoN) pancreata (n = 7) from mice that underwent 2–3 weeks of Dox-HFD (600 mg/kg) feeding. Inclusion criteria were genes that are significantly altered more than fivefold in GFP-PPG α-cells vs. GFP-PPG-MitoN α-cells and exhibit an absolute value of >500 in abundance. Gene abbreviations, definitions, and fold alterations between GFP-PPG α-cells vs. GFP-PPG-MitoN α-cells are indicated.
MitoNEET Promotes Transcriptional Profile Reprogramming of an α-Cell Into a β-Cell–Like Status
Upon further analyses of the PPG-MitoN α-cell microarray, we observed a striking switch in transcriptional patterning of the transgenic α-cells. PPG-MitoN α-cells exhibited a marked downregulation in several α-cell–predominant genes (Gcg, Ttr, Nkx2.2, and Isl1) (Table 2). At the same time, a profound upregulation in numerous β-cell–specific genes (Ins1, Glut2, Nkx6.1, Pdx1, Iapp, ZnT8, Chga, Ngn3, and Ptfa1) was evident in the transgenic α-cells (Table 2), suggesting that a mitoNEET-induced perturbation in α-cell mitochondrial function not only preserves β-cell viability through the production of a β-cytotrophic factor, but also simultaneously promotes transcriptional conversion from an α-cell state into a more β-cell–like profile. However, complete rescue observed at the level of β-cell mass seems unlikely to be exclusively due to a conversion of α-cells. This can be inferred from the fact that many more β-cells than original α-cells remained present and that there did not appear to be a net depletion of α-cells converting to β-cells.
Table 2.
MitoNEET promotes transcriptional reprogramming to drive an α-cell profile to a more β-cell conversion
| Cell type–specific* gene | Gene definition | Fold change | Alteration |
|---|---|---|---|
| α-cell specific | |||
| Gcg | Glucagon | 1.8 | ↓ |
| Ttr | Transthyretin | 1.6 | ↓ |
| Nkx2.2 | NK2 homeobox 2 | 1.3 | ↓ |
| Isl1 | LIM homeodomain | 1.3 | ↓ |
| β-cell specific | |||
| Ins1 | Insulin I | 2.2 | ↑ |
| Ins2 | Insulin II | 1.0 | — |
| Glut2 | Facilitated glucose transporter, 2 | 4.0 | ↑ |
| Nkx6.1 | NK6 homeobox 1 | 2.2 | ↑ |
| Pdx1 | Pancreatic and duodenal homeobox 1 | 1.6 | ↑ |
| Iapp | Islet amyloid polypeptide | 2.1 | ↑ |
| ZnT8 | Solute carrier family 30 (Zn transporter), 8 | 1.3 | ↑ |
| Chga | Chromagranin A | 1.2 | ↑ |
| Ngn3 | Neurogenin 3 | 1.4 | ↑ |
| Ptfa1 | Pancreas-specific transcription factor 1a | 5.0 | ↑ |
| δ-cell specific | |||
| Sst | Somatostatin | 1.1 | ↓ |
Fold alterations in cell type–specific pancreatic genes identified by Illumina microarray analyses in α-cells isolated from TRE-GFP-PPG-rtTA (GFP-PPG) pancreata (n = 4) vs. TRE-MitoN-TRE-GFP-PPG-rtTA (GFP-PPG-MitoN) pancreata (n = 7) from mice that underwent 2–3 weeks of Dox-HFD (600 mg/kg) feeding. Gene abbreviations, definitions, and fold alterations between GFP-PPG α-cells and GFP-PPG-MitoN α-cells are indicated.
*Known to be highly expressed in α-cells, β-cells, or δ-cells.
Discussion
We generated and validated a novel mouse model that allows inducible cell-specific overexpression of any gene in pancreatic β-cells (the MIP-rtTA mouse). In conjunction, we also generated and characterized a novel α-cell–specific mouse model (the PPG-rtTA mouse). We confirmed cell specificity in both mouse models by using an inducible TRE-LacZ labeling system in which positive β-gal signal is strictly confined to α-cells and β-cells, respectively. By utilizing the β-cell–specific MIP-rtTA mouse and exploiting the unique properties of the OMM protein mitoNEET to compromise mitochondrial function, we created a novel, inducible, and highly titratable mouse model of overt diabetes (the MIP-MitoN mouse). We demonstrate that male MIP-MitoN mice are profoundly hyperglycemic, are glucose and arginine intolerant, and exhibit low pancreatic insulin content due to widespread loss of β-cell mass. The severity of the β-cell insult can be tightly and effectively fine-tuned by careful titration of Dox administration, which at lower levels prompts β-cell dysfunction without resulting in β-cell loss. In vivo findings are corroborated by pancreatic perfusion experiments showing impaired GSIS in MIP-MitoN mice, with a complete lack in a first-phase insulin spike in response to glucose infusion. Mechanistically, low-dose mitoNEET induction activates Parkin-mediated mitophagy in β-cells, which leads to the formation of large vacuoles and mitophagosomes localized exclusively and specifically to each β-cell enriched with mitoNEET. Of note, this mechanism is reinforced by using MIP-MitoN mice null for Parkin lacking any mitophagosomes in β-cells and exhibiting normoglycemia at the whole-body level. The TZD Pio, on the other hand, is protective against mitoNEET-driven hyperglycemia and prevents loss of β-cell mass. By removing Dox and thus restoring the physiological levels of mitoNEET, β-cells in MIP-MitoN mice regenerate to full mass. By using the PPG-rtTA mouse, we achieved induction of mitoNEET specifically in pancreatic α-cells. PPG-MitoN mice exhibited fasting-induced hypoglycemia as a result of an overproduction of insulin; this was substantiated by marked hypersecretion of insulin during glucose tolerance and GSIS. Such findings implicate mitoNEET-mediated dysregulation in local α-cell homeostasis, which likely unleashes uninhibited insulin secretion from the adjacent β-cell. By inducing mitoNEET in both β-cells and α-cells simultaneously, we rescued the β-cell dysfunction typically seen when mitoNEET is enriched in β-cells alone (i.e., double transgenic β-cell-α-cell MIP-PPG-MitoN mice retain normoglycemia despite their single transgenic β-cell–targeted MIP-MitoN littermates exhibiting severe hyperglycemia). This finding indicates that reduced mitochondrial function in α-cells is potently protective in terms of sustaining β-cell viability.
Efficient mitochondrial function is essential for the β-cell to exquisitely sense and translate available nutrient metabolites into signals to stimulate insulin secretion (55). Mitochondrial dysfunction, therefore, is a fundamental contributor to β-cell failure in diabetes (56), with defective mitochondria tightly coupled with blunted GSIS (57). In the current study, we present a unique MIP-MitoN mouse model of titratable mitochondrial impairment that can be used to examine the critical step-by-step stages from normal β-cell function to progressively impaired GSIS as a function of mitochondrial activity. The current mouse model also allows assessment of how β-cells protect themselves by unleashing compensatory mechanisms in response to incremental degrees of compromised mitochondrial function. Other means of compromising mitochondria in β-cells, such as hypoxia or chemical inhibition of the mitochondrial electron transport chain, severely impair GSIS or result in total ablation of β-cells (9,58,59), thus preventing the analyses of intermediate compensatory mechanisms. The titratable nature of the MIP-MitoN mouse provides a snapshot of the apparent transitional mechanisms by perturbing β-cell mitochondrial function, which may not be fully defined through more severe models of overt diabetes.
Mitophagy, the selective autophagy of mitochondria, is associated with T2DM (60). Two proteins mediate the selective removal of defective mitochondria: the E3 ubiquitin-protein ligase Parkin acts in concert with the serine/threonine-protein kinase PINK1 (61). Both PARK2 and PINK1 are strong candidates as susceptibility genes associated with T2DM (62,63). In response to mitochondrial membrane depolarization, PINK1 recruits cytosolic Parkin to ubiquitinate proteins on the OMM (61,64); this induces LC3-II to form autophagosomes (65) and to trigger a mitophagic clearance system. In the current study, low-dose mitoNEET induction in β-cells caused large autophagic vacuoles to form within each MIP-MitoN β-cell; this is supported by an upregulation in beclin-1 levels and enhanced LC3-II activity in MIP-MitoN islets. MitoNEET also promotes the highly interconnected mitochondrial network within the β-cell body to collapse and form mitophagosomes. Collectively, the formation of autophagic vacuoles and mitophagosomes in MIP-MitoN β-cells is a direct consequence of mitoNEET activating an autophagic degradation pathway through a PINK1/Parkin- and beclin-1–dependent mechanism. Vives-Bauza et al. (64) reported that co-overexpression of PINK1 and Parkin in HEK293T cells causes overt alteration of the mitochondrial network by forming fragmented mitochondrial aggregates that undergo mitophagy, the latter confirmed by LC3-positive autophagic vacuoles. Of note, we previously identified that mitoNEET lowers ΔΨm in adipocytes (15) and have confirmed this effect in β-cells in the present study. Furthermore, lowering ΔΨm is perceived by the cell as mitochondrial damage (64). Given that loss of ΔΨm activates PINK1 to relocalize Parkin to the OMM and trigger mitophagy (64), mechanistically, we propose that mitoNEET impairs mitochondrial function to lower the ΔΨm and that this initiates a compensatory response by activating PINK1/Parkin-mediated mitophagy in an attempt to protect mitochondrial integrity (Fig. 7A). This mechanism was strengthened by data showing that MIP-MitoN mice that lack Parkin fail to form mitophagosomes and thus sustain normoglycemia. Finally, a Parkin-mitoNEET negative feedback loop may prime mitoNEET for degradation, as Lazarou et al. (47) identified mitoNEET as a direct substrate for polyubiquitination by Parkin on the OMM (Fig. 7A). This may explain the markedly lower mitoNEET expression levels observed in islets from leptin-deficient ob/ob mice (data not shown).
The intricate topographical architecture of α- and β-cells within the islet indicates coordinated reciprocal paracrine communication between the two cell types to achieve global glycemic stability. In the fed state, insulin suppresses glucagon to lower hepatic gluconeogenesis (66); vice versa during fasting, low insulin levels prompt a reciprocal increase in glucagon to stimulate hepatic glucose production. Coordination of these two hormones that diametrically oppose each other’s action on hepatic fuel metabolism provides glycemic homeostasis. Glucagon, however, is a potent diabetogenic hormone such that a lack in local insulin availability to suppress glucagon release causes uncontrolled hyperglucagonemia, a major contributor to T2DM (66). Perturbing glucagon homeostasis could harbor therapeutic potential. The induction of mitoNEET in mature adipocytes effectively compromises mitochondrial function to alter cellular signaling (15). In the current study, we used mitoNEET as a genetic tool to impair α-cell mitochondrial function in the hope of disrupting glucagon homeostasis. PPG-MitoN mice exhibited fasting-induced hypoglycemia, a synergistic overproduction in insulin, and hypersecretion of insulin during GSIS. Indeed, impaired glucagon signaling unleashes uninhibited insulin secretion from β-cells (66).
Mice lacking proper glucagon signaling post streptozotocin-induced loss of β-cell mass do not develop diabetes (67,68). How can impaired glucagon signaling with complete β-cell loss equate to glycemic stability given that whole-islet signaling is compromised? To mimic this, we used mitoNEET to perturb α-cells and β-cells simultaneously to dysregulate the entire islet. Although MIP-MitoN mice (β-cell alone) exhibited hyperglycemia, MIP-PPG-MitoN mice (both β-cell and α-cell) retained normoglycemia. Furthermore, although MIP-MitoN mice are glucose intolerant and lack insulin responses, MIP-PPG-MitoN mice hypersecrete insulin, indicating that dual induction of mitoNEET in both cell types (thus simultaneous cell impairment) is protective against mitoNEET-driven hyperglycemia and β-cell loss.
Given that both PPG-MitoN mice (α-cells) and MIP-PPG-MitoN mice (β- and α-cells) prevent the loss of β-cell mass typically seen in MIP-MitoN mice (β-cells), we transcriptionally screened for potential survival factors emanating from the perturbed α-cell that could protect the viability of the adjacent β-cell. Three genes profoundly upregulated in PPG-MitoN α-cells stood out as potential β-cytotrophic candidates: Ngfa, Pdip, and Tff2 (Table 1). Ngfa is a critical neurotrophic factor that enhances GSIS to improve viability and survival of islets (52). Conversely, withdrawal or neutralization of Ngfa induces β-cell apoptosis (53) and lowers insulin levels (69). Future studies exploring the mechanisms of how a mitoNEET-mediated induction of Ngfa exerts autocrine antiapoptotic properties to maintain β-cell survival in a mouse model with dysregulated α-cell homeostasis (the PPG-MitoN mouse) should prove beneficial. Finally, further transcriptional screening of PPG-MitoN α-cells revealed a surprising switch in transcriptional patterning. Perturbed transgenic α-cells transcriptionally converted from an α-cell program to a more β-cell–like profile (Table 2). Although conversion of α-cells to β-cells has been reported after extreme β-cell loss (70), the complete rescue of β-cell mass in PPG-MitoN mice is unlikely to be exclusively due to a conversion of α-cells. This could be a result of more β-cells remaining than original α-cells present; additionally, there does not appear to be a net depletion of α-cells seemingly converting to β-cells.
In summary, we identify a novel inducible mitoNEET-driven mouse model of diabetes. Mechanistically, we show that mitoNEET enhances Parkin-mediated mitophagy, resulting in the formation of unique mitophagosomes in β-cells. We further characterize a novel α-cell–specific model of mitoNEET induction that causes a dysregulation in local glucagon homeostasis to unleash hypersecretion of insulin. Finally, we used mitoNEET to simultaneously disrupt α- and β-cell homeostasis, which exerts a protective effect by preserving β-cell viability. Figure 7B summarizes our three novel mouse models of β-cell and/or α-cell impairment, which highlight the importance of proper mitochondrial function in the maintenance of pancreatic function and, secondary to that, whole-body glucose homeostasis. We used these systems to define potent β-cytoprotective factors derived from α-cells with compromised mitochondrial function.
Supplementary Material
Article Information
Acknowledgments. The authors thank J. Song and S. Connell (UTSW Medical Center, Dallas, TX) for technical assistance as well as the rest of the Scherer laboratory for helpful discussions. They also thank Pierre Cosson and Lelio Orci (Department of Cell Physiology and Metabolism, University of Geneva, Geneva, Switzerland) for valuable comments, Bob Hammer and the UTSW Transgenic Core Facility for the generation of mouse models, and the UTSW Metabolic Core Facility (Xioli Lin for perfusions for EM experiments) and EM Core Facility, particularly Rebecca Jackson, Robyn Leidel, Phoebe Doss, and Anza Darehshouri. Finally, the authors thank George Adams and Shengxi Liu (Nancy) at the Illumina Microarray Core of UTSW and Young Lee (UTSW Medical Center) for providing INS-1 cells.
Funding. C.M.K. was supported by a fellowship from JDRF International (3-2008-130). C.M.K. and P.E.Sc. were supported by a JDRF/Eli Lilly Company Program Award (2-SRA-2016-149-Q-R; PI: William Holland). W.-h.L. was supported by National Institutes of Health grant R01-GM 077593. The authors were also supported by National Institutes of Health grants R01-DK-55758, R01-DK-099110, and P01-DK-088761 (to P.E.Sc.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.M.K. designed and performed all experiments, analyzed data, and contributed to the writing of the manuscript. S.C. and W.-h.L. performed the GSIS perfused pancreata experiments, isolated α-cells from PPG-MitoN mice for microarray analysis, and performed the ΔΨm experiments. R.Y. contributed to the injections during the isolation of islets and performed the β-gal staining on TRE-LacZ-MIP-rtTA brain tissues. K.S. generated the TRE-MitoN mouse. Q.A.W. generated the PPG-rtTA mouse and contributed to the β-gal staining of PPG-rtTA tissues. S.B.S. generated the MIP-rtTA mouse. P.E.Sa. and J.T.B. contributed to the use of mass spectrometry to measure the various lipid species in islets isolated from the MIP-MitoN mice. W.J.G. was involved in key discussions that were critical to the mechanistic insights of the manuscript. R.H.U. provided expertise in islet biology. P.E.Sc. contributed to the experimental design, performance of experiments, data analysis and interpretation, and the writing of the manuscript. P.E.Sc. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1323/-/DC1.
See accompanying article, p. 1484.
References
- 1.Maechler P, Carobbio S, Rubi B. In beta-cells, mitochondria integrate and generate metabolic signals controlling insulin secretion. Int J Biochem Cell Biol 2006;38:696–709 [DOI] [PubMed] [Google Scholar]
- 2.Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic β cells. Trends Endocrinol Metab 2012;23:477–487 [DOI] [PubMed] [Google Scholar]
- 3.Maechler P, de Andrade PB. Mitochondrial damages and the regulation of insulin secretion. Biochem Soc Trans 2006;34:824–827 [DOI] [PubMed] [Google Scholar]
- 4.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest 2006;116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchetti P, Lupi R, Del Guerra S, Bugliani M, Marselli L, Boggi U. The beta-cell in human type 2 diabetes. Adv Exp Med Biol 2010;654:501–514 [DOI] [PubMed] [Google Scholar]
- 6.Anello M, Lupi R, Spampinato D, et al. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia 2005;48:282–289 [DOI] [PubMed] [Google Scholar]
- 7.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem 2006;75:367–401 [DOI] [PubMed] [Google Scholar]
- 8.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 2005;307:384–387 [DOI] [PubMed] [Google Scholar]
- 9.Silva JP, Köhler M, Graff C, et al. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat Genet 2000;26:336–340 [DOI] [PubMed] [Google Scholar]
- 10.Colca JR, McDonald WG, Waldon DJ, et al. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol Endocrinol Metab 2004;286:E252–E260 [DOI] [PubMed] [Google Scholar]
- 11.Paddock ML, Wiley SE, Axelrod HL, et al. MitoNEET is a uniquely folded 2Fe 2S outer mitochondrial membrane protein stabilized by pioglitazone. Proc Natl Acad Sci U S A 2007;104:14342–14347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiley SE, Paddock ML, Abresch EC, et al. The outer mitochondrial membrane protein mitoNEET contains a novel redox-active 2Fe-2S cluster. J Biol Chem 2007;282:23745–23749 [DOI] [PubMed] [Google Scholar]
- 13.Wiley SE, Murphy AN, Ross SA, van der Geer P, Dixon JE. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc Natl Acad Sci U S A 2007;104:5318–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin J, Zhou T, Ye K, Wang J. Crystal structure of human mitoNEET reveals distinct groups of iron sulfur proteins. Proc Natl Acad Sci U S A 2007;104:14640–14645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusminski CM, Holland WL, Sun K, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med 2012;18:1539–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamatani K, Saito K, Ikezawa Y, et al. Relative contribution of Ca2+-dependent mechanism in glucagon-induced glucose output from the liver. Arch Biochem Biophys 1998;355:175–180 [DOI] [PubMed] [Google Scholar]
- 17.Cherrington A, Vranic M. Role of glucagon and insulin in control of glucose turnover. Metabolism 1971;20:625–628 [DOI] [PubMed] [Google Scholar]
- 18.Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007;28:253–283 [DOI] [PubMed] [Google Scholar]
- 19.Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1987;64:106–110 [DOI] [PubMed] [Google Scholar]
- 20.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012;122:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 2000;85:4053–4059 [DOI] [PubMed] [Google Scholar]
- 22.Mu J, Woods J, Zhou YP, et al. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes 2006;55:1695–1704 [DOI] [PubMed] [Google Scholar]
- 23.Ye R, Holland WL, Gordillo R, et al. Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes β-cell regeneration. eLife 2014;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Li Y, Chakraborty M, et al. Liver-specific deficiency of serine palmitoyltransferase subunit 2 decreases plasma sphingomyelin and increases apolipoprotein E levels. J Biol Chem 2009;284:27010–27019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gannon M, Shiota C, Postic C, Wright CV, Magnuson M. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis 2000;26:139–142 [DOI] [PubMed] [Google Scholar]
- 27.Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem 1986;261:11880–11889 [PubMed] [Google Scholar]
- 28.Blazquez E, Muñoz-Barragan L, Patton GS, Orci L, Dobbs RE, Unger RH. Gastric A-cell function in insulin-deprived depancreatized dogs. Endocrinology 1976;99:1182–1188 [DOI] [PubMed] [Google Scholar]
- 29.Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia 2009;52:739–751 [DOI] [PubMed] [Google Scholar]
- 30.Combs TP, Berg AH, Rajala MW, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 2003;52:268–276 [DOI] [PubMed] [Google Scholar]
- 31.Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 2011;17:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu H, Koshkin V, Allister EM, Gyulkhandanyan AV, Wheeler MB. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes 2010;59:448–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichimura Y, Kirisako T, Takao T, et al. A ubiquitin-like system mediates protein lipidation. Nature 2000;408:488–492 [DOI] [PubMed] [Google Scholar]
- 34.Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol Biol Cell 2008;19:3290–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature 2001;414:807–812 [DOI] [PubMed] [Google Scholar]
- 36.Martino L, Masini M, Novelli M, et al. Palmitate activates autophagy in INS-1E β-cells and in isolated rat and human pancreatic islets. PLoS One 2012;7:e36188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aita VM, Liang XH, Murty VV, et al. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics 1999;59:59–65 [DOI] [PubMed] [Google Scholar]
- 38.Liang XH, Jackson S, Seaman M, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999;402:672–676 [DOI] [PubMed] [Google Scholar]
- 39.Wang RC, Wei Y, An Z, et al. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 2012;338:956–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sauvé V, Gehring K. Phosphorylated ubiquitin: a new shade of PINK1 in Parkin activation. Cell Res 2014;24:1025–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trempe JF, Sauvé V, Grenier K, et al. Structure of Parkin reveals mechanisms for ubiquitin ligase activation. Science 2013;340:1451–1455 [DOI] [PubMed] [Google Scholar]
- 42.Jin HS, Kim J, Lee SJ, et al. The PARK2 gene is involved in the maintenance of pancreatic β-cell functions related to insulin production and secretion. Mol Cell Endocrinol 2014;382:178–189 [DOI] [PubMed] [Google Scholar]
- 43.Hoshino A, Ariyoshi M, Okawa Y, et al. Inhibition of p53 preserves Parkin-mediated mitophagy and pancreatic β-cell function in diabetes. Proc Natl Acad Sci U S A 2014;111:3116–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazarou M, Jin SM, Kane LA, Youle RJ. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell 2012;22:320–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finney N, Walther F, Mantel PY, Stauffer D, Rovelli G, Dev KK. The cellular protein level of Parkin is regulated by its ubiquitin-like domain. J Biol Chem 2003;278:16054–16058 [DOI] [PubMed] [Google Scholar]
- 46.Okatsu K, Kimura M, Oka T, Tanaka K, Matsuda N. Unconventional PINK1 localization to the outer membrane of depolarized mitochondria drives Parkin recruitment. J Cell Sci 2015;128:964–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazarou M, Narendra DP, Jin SM, Tekle E, Banerjee S, Youle RJ. PINK1 drives Parkin self-association and HECT-like E3 activity upstream of mitochondrial binding. J Cell Biol 2013;200:163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes 1996;45:1661–1669 [DOI] [PubMed] [Google Scholar]
- 49.Kawashima S, Matsuoka TA, Kaneto H, et al. Effect of alogliptin, pioglitazone and glargine on pancreatic β-cells in diabetic db/db mice. Biochem Biophys Res Commun 2011;404:534–540 [DOI] [PubMed] [Google Scholar]
- 50.Ishida H, Takizawa M, Ozawa S, et al. Pioglitazone improves insulin secretory capacity and prevents the loss of beta-cell mass in obese diabetic db/db mice: possible protection of beta cells from oxidative stress. Metabolism 2004;53:488–494 [DOI] [PubMed] [Google Scholar]
- 51.Wang ZV, Mu J, Schraw TD, et al. PANIC-ATTAC: a mouse model for inducible and reversible beta-cell ablation. Diabetes 2008;57:2137–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miao G, Mace J, Kirby M, et al. In vitro and in vivo improvement of islet survival following treatment with nerve growth factor. Transplantation 2006;81:519–524 [DOI] [PubMed] [Google Scholar]
- 53.Pierucci D, Cicconi S, Bonini P, et al. NGF-withdrawal induces apoptosis in pancreatic beta cells in vitro. Diabetologia 2001;44:1281–1295 [DOI] [PubMed] [Google Scholar]
- 54.Orime K, Shirakawa J, Togashi Y, et al. Trefoil factor 2 promotes cell proliferation in pancreatic β-cells through CXCR-4-mediated ERK1/2 phosphorylation. Endocrinology 2013;154:54–64 [DOI] [PubMed] [Google Scholar]
- 55.Wiederkehr A, Wollheim CB. Mitochondrial signals drive insulin secretion in the pancreatic β-cell. Mol Cell Endocrinol 2012;353:128–137 [DOI] [PubMed] [Google Scholar]
- 56.Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev 2010;31:364–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia 2010;53:1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohta M, Nelson D, Nelson J, Meglasson MD, Erecińska M. Oxygen and temperature dependence of stimulated insulin secretion in isolated rat islets of Langerhans. J Biol Chem 1990;265:17525–17532 [PubMed] [Google Scholar]
- 59.Kennedy ED, Maechler P, Wollheim CB. Effects of depletion of mitochondrial DNA in metabolism secretion coupling in INS-1 cells. Diabetes 1998;47:374–380 [DOI] [PubMed] [Google Scholar]
- 60.Lee MS. Role of islet β cell autophagy in the pathogenesis of diabetes. Trends Endocrinol Metab 2014;25:620–627 [DOI] [PubMed] [Google Scholar]
- 61.Geisler S, Holmström KM, Skujat D, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 2010;12:119–131 [DOI] [PubMed] [Google Scholar]
- 62.Wongseree W, Assawamakin A, Piroonratana T, Sinsomros S, Limwongse C, Chaiyaratana N. Detecting purely epistatic multi-locus interactions by an omnibus permutation test on ensembles of two-locus analyses. BMC Bioinformatics 2009;10:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leak TS, Mychaleckyj JC, Smith SG, et al. Evaluation of a SNP map of 6q24-27 confirms diabetic nephropathy loci and identifies novel associations in type 2 diabetes patients with nephropathy from an African-American population. Hum Genet 2008;124:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vives-Bauza C, Zhou C, Huang Y, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A 2010;107:378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wild P, Dikic I. Mitochondria get a Parkin’ ticket. Nat Cell Biol 2010;12:104–106 [DOI] [PubMed] [Google Scholar]
- 66.Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci U S A 2010;107:16009–16012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hancock AS, Du A, Liu J, Miller M, May CL. Glucagon deficiency reduces hepatic glucose production and improves glucose tolerance in adult mice. Mol Endocrinol 2010;24:1605–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 2011;60:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gezginci-Oktayoglu S, Karatug A, Bolkent S. The relation among NGF, EGF and insulin is important for triggering pancreatic β cell apoptosis. Diabetes Metab Res Rev 2012;28:654–662 [DOI] [PubMed] [Google Scholar]
- 70.Thorel F, Népote V, Avril I, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010;464:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.