Abstract
The role of specific subsets of peripheral nerves in pain related behavior remains unclear. To better understand the contribution of differential activation of fast-conducting, high-threshold mechanoreceptor (AHTMR) input, we hypothesized that neuronal activation would be distinct with nerve injury, and that nociceptive input would predictt behavior in the freely exploring animal. A series of surfaces was used to deliver mechanical input to the hindpaws of rats upon voluntary movement and exploration. Neuronal activation increased as apex surface decreased (0.2, 0.6, 1.0 and 1.5 mm) using in vivo recording in L4 DRG neurons, and this relationship was enhanced following partial ligation of L5 (pSNL). In behaving animals, apex size was correlated to time spent on each surface following pSNL, but not with sham. Morphine normalized the discriminatory behavior following pSNL. These data indicate that noxious mechanical activation of AHTMR upon normal movement predicts behavior using paradigms that do not rely on reflexive withdrawal responses suggesting that AHTMR activation and central nervous system input contribute to higher order pain behavior after nerve injury beyond the immediate early pain input long attributed to these neurons.
Keywords: mechanical, hyperalgesia, nociceptive, noxious, open field, pain, aversion, allodynia
Graphical abstract
Introduction
The withdrawal response elicited by a threshold stimulus is the most common endpoint in evaluation of pain in the laboratory [1]. Withdrawal is divided into response to thermal or mechanical force and is based on the evoked response component of pain [2,3]. Withdrawal to mechanical force is based on the development of hyperalgesia and allodynia from tissue and nerve injury [4–7]. This is an evoked response that is fundamentally reflexive and is largely a function of spinal cord circuitry based on motor responses to nociceptive input. Ascending inputs seem to be required and the response is influenced by higher order central nervous system (CNS) inputs through descending modulation to spinal cord circuitry, but conscious decision-making about pain or discomfort as part of its character or occurrence is likely absent [8–10]. Furthermore, in neuropathic pain patients, hyperalgesia alone may not tell the whole story and the predominant functional symptom of pain is likely the quality and character, which may be more related to the spontaneous and persistent component of pain [11–13].
Spontaneous, or non-reflexive, measures of pain likely provide different information about the nociceptive input and its impact to the whole animal, more along the lines of the subjective description of actual pain [14,15]. However, some types of non-reflexive or spontaneous pain behaviors in the animal may still rely predominantly on a reflexive pathway in the spinal cord. Decision-making can be a component of escape and avoidance or place preference and may include attention-related responses [16–19]. These behaviors involve higher levels in CNS and reflect the influence and impact globally to the animal. This emerging consideration of higher level CNS modulation has resulted in greater use of novel approaches to measure the extent to which nociceptive information changes non-elicited behavioral outputs in freely behaving animals [16–18,20–23].
A geometric surface based on single cell fast-conducting, high-threshold mechanoreceptor (AHTMR) threshold to mechanical force after nerve or tissue injury was used to activate peripheral neuronal input [24,25]. We hypothesized that increasing calibrated force, related to reducing the size of the surface activated by a given pressure, would activate AHTMR differently due to nerve injury induced hypersensitivity. Furthermore, we hypothesized that the surface induced mechano-activation of AHTMR in the face of nerve injury would induce injury related nociceptive peripheral input to higher order CNS structures in the brain that rely on decision making and this would result in pain related altered place avoidance in a freely behaving animal.
Methods
Surgical Procedures
A total of 48 male Sprague-Dawley rats (Postnatal day 45) were used in the study. A power analysis per se was not performed. However, based on previous studies in our laboratory, a minimum of 12 animals are required in each group to detect a meaningful difference using open field (OF). Three groups of 12 animals in each group were used for OF testing (nerve injury, sham, and nerve injury with morphine) and 2 groups of 11 animals in each group were used for electrophysiology (sham and nerve injury) (also based on previous studies of electrophysiologically determined responses of a single neuron). Animals were randomly assigned to receive surgery or sham and morphine with nerve injury or nerve injury only. OF and electrophysiology groups were done at separate times while animal groups within these experiments were all done at the same time. The use and handling of animals were in accordance with guidelines provided by the National Institutes of Health and received approval from the Institutional Animal Care and Use Committee of the Wake Forest University Health Sciences.
Partial Spinal Nerve Ligation (pSNL)
Animals underwent right L5 pSNL and recovery as previously described [25]. In a sham control group, the surgical procedure was identical to that described except that the left L5 spinal nerve was not injured.
Behavioral Testing
Individuals blinded to treatment determined mechanical withdraw threshold (MWT) by application of calibrated von Frey hairs to the plantar surface of the paw as previously described [25]. MWTs were determined before and 1 week after pSNL (postoperative day 7 [POD7]). All animals were included in the data analysis.
Electrophysiology
A week after either pSNL or Sham surgery, cellular recordings were made under anesthesia, after a dorsal midline incision was made in trunk skin and the L4 dorsal root ganglion (DRG) and adjacent spinal cord were exposed by laminectomy as previously described (illustrated in Figure 1A) [26]. The tissue was continuously superfused with oxygenated artificial cerebrospinal fluid as described [26].
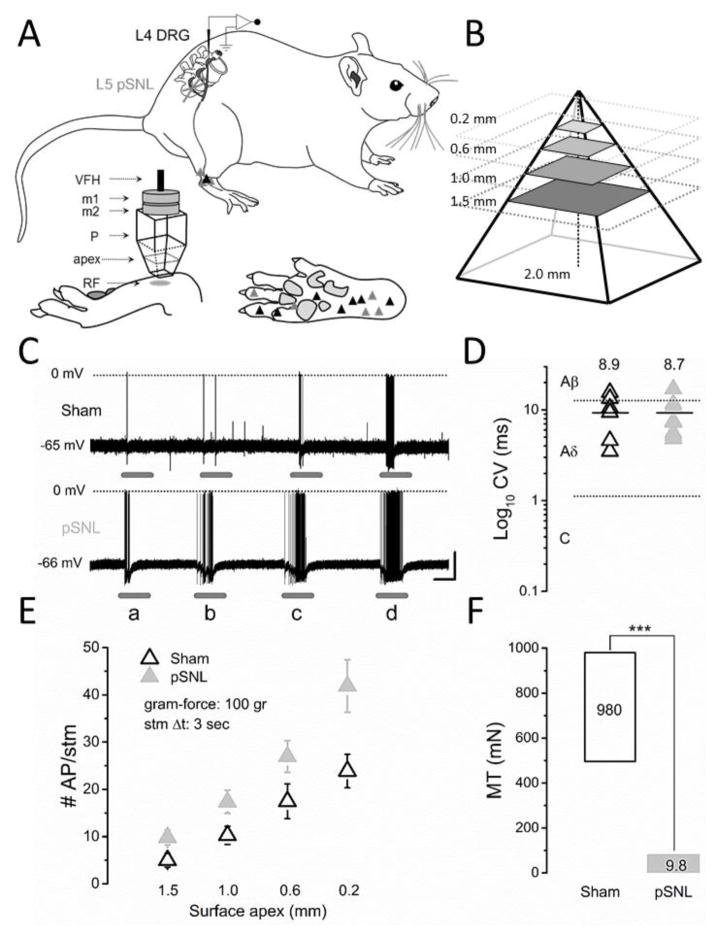
Figure 1. Single Neuron Electrophysiology.
A) Schematic diagram of the in vivo L4 preparation with partial spinal nerve ligation (pSNL) in L5. Single cell fast-conduction high-threshold mechanoreceptor (AHTMR) neuronal receptor fields (RF) are illustrated in sham (n=8) (black) and pSNL (n=8) (gray) animals. The pyramid apex was used for stimulation (RF: receptor field; P: pyramid; m1 and m2: magnets; VFH: von Frey hair (100 gr). B) Apexes for open field or RF testing were generated using a pyramidal base with size apexes as shown (0.2, 0.6, 1, and 1.5 mm). C) Representative effect of apex mechanical stimulation (a: 1.5; b: 1.0; c: 0.6; d: 0.2 mm, respectively) on the responses of AHTMRs (Sham and pSNL). Scale bar: 2 sec, 20 mV. D) Conduction Velocity (CV) is presented in log10 scale with mean (bar and value). E) Effect of apex on action potentials (AP) per stimuli (#AP/stm). There is an apex size-dependent response with increased responses with decreasing apex size (P<0.05) and more APs per given apex size stimulus with pSNL versus sham (P<0.05). Data are means (± standard error). F) Afferents mechanical thresholds (MT) in Sham (black) and pSNL (gray) animals presented with the median values. p<0.001 (***).
The electrophysiological recordings from L4 DRG neurons were limited to of 71 min. DRG soma were impaled with borosilicate microelectrodes (80–250 MΩ) containing 1 M potassium acetate. Intracellular penetrations with a resting membrane potential of ≤−40 mV were characterized further as previously described [26–28]. Only cells capable of generating a somatic action potential (AP) (by current somatic injection, 25 and 500 ms pulses) and with impalements stable long enough to adequately explore the full extent of the skin at the L4 dermatome (>2 min) were included. In the electrophysiology studies the investigator could not be blinded to treatment since the change in neural thresholds of different nerve populations made it clear which animals had nerve injury.
Cellular Classification Protocol
To identify the receptor field (RF), the skin was searched and a cellular classification process was performed to determine afferent identity as previously described [26–28]. The results of this procedure were combined with specific cellular properties (action potential [AP] shape and somatic passive characteristics) to assign every cell into one of three simplified categories: Low threshold MR (LTMR), AHTMR, c-nociceptors (CHTMR), based on the strongest defining characteristics [27]. For the purpose of this study, only cells classified as AHTMR were studied further. In all cases, RFs were characterized (Fig. 1A).
Somatic Electrical Properties
Active and passive membrane properties of AHTMR neurons were analyzed as previously described [27]. All included cells satisfied the following requirements: resting membrane potential more negative than −40 mV, AP amplitude ≥30 mV and the presence of AHP. Passive membrane properties indicative of poor (extremely low Ri and/or Tau) impalement were also reasons for exclusion.
Conduction Velocity (CV) and Receptive Field (RF)
Spike latency was obtained by stimulating the RF at the skin surface using a bipolar electrode following all natural stimulation to prevent potential alterations in RF properties by electrical stimulation as previously described [26–28]. After establishing the afferent identity as AHTMR subtype, the RF was carefully searched with suprathreshold mechanical stimuli and threshold obtained using Von Frey filaments as previously described [25].
Surface Apex Size Neuronal Mechano-Stimulation
Intracellular recordings of 16 fast-conducting mechanical nociceptive afferents (one from each animal; 8 pSNL and 8 Sham animals) were obtained from neurons innervating skin within the L4 dermatome as previously described [24,25] from 22 animals (in 6 animals recordings of the AHTMR could not be obtained). These animals were also part of another study to determine changes in AHTMR after injury. Following characterization of peripheral RF, CV, and membrane electrical properties (passive and active), stimulation with individual pyramids (single apex of the surface) was implemented. The RF of selected cells was stimulated by the use of 4 individual pyramids with different apexes (1.5, 1.0, 0.6, 0.2 mm; weight: 1.8 gr) (Fig. 1A and Fig. 1B). For the stimulation, each pyramid was attached to a 100 gr von Frey filament to maintain the same force. This force was the minimum needed to elicit any response in the sham so as to be able to compare responses after nerve injury. Pyramids were positioned perpendicular (90°) to the maximal sensitivity point within the RF and the mechanical stimulation (3 sec) with each pyramid was applied with increasing intensity (decreasing apex area 1.5 to 0.2 mm; interval: ±10 sec). Response characteristics were analyzed including maximal instantaneous frequency (MIF, Hz) and discharge rate (# AP per stimuli) (Fig. 1C and 1E).
Open Field Discrimination (OFD) Zone Preference with Different Apex Surfaces Stimulation
OFD exploratory behavior was assessed on POD7 after pSNL or sham using commercially available equipment and software (Med Associates Inc., St. Albans, VT) as previously described [18]. Morphine 1 mg/kg or saline was administered subcutaneously 30 minutes prior to OFD in pSNL animals. Three groups of 12 animals in each group were used for OFD (pSNL, sham, and pSNL with morphine). Nerve injured animals were randomized to receive morphine or nothing. Briefly, animals were placed in activity chambers divided into 4 equal quadrants and 0.5 cm thick stainless steel plates with surfaces of equally spaced apices of 0.2, 0.6, 1, and 1.5 mm in quadrants/zones (Fig. 1F) as floor to analyze zone preference in free exploration. The distance between each apex was 0.5 cm. Plates were contiguous with no gaps or dividers. Data were collected in 6-min bins for 1 h. The primary outcome measure was total time within an apex zone/quadrant. Secondary outcome measures included total distance traveled in the X-Y plane, zone entries, and resting time, repeated beam breaks within 3 cm of the animal in the absence of locomotion (stereotypy), and total beam breaks in the upper Z direction (rearing) all within an apex zone/quadrant.
Statistical Analysis
MWT data were analyzed using the paired t-test within group and the unpaired t-test between groups. Data from the OFD testing was analyzed using non-parametric testing since the apex sizes are not continuously related. Friedman repeated measure one-way analysis of variance (ANOVA) with apex size was used to test for overall effects of the different apex size surfaces. The Mann-Whitney U was used to test for between group effects (pSNL or sham and drug treatment or saline). Post-hoc pairwise analyses were performed using the Tukey’s test. Correlation testing was planned prior to the start of the experiments based on previous data using OFD and graded responses in quadrants. Correlation was determined using the Spearman rank order correlation and rho (ρ), P-values, and linear regression lines were determined by Sigmaplot (Systat Software Inc., San Jose, CA). Appropriate corrections for multiple comparisons were used where appropriate. Data are means (± standard error). Where P-values are reported, these are uncorrected P-values. P≤0.05 was considered statistically significant.
Results
Fast-conducting Mechanoreceptor Responses to Different Apex Size Activation
Sixteen neurons (n=8 sham and n=8 pSNL) from 22 different animals were identified and met criteria to be classified as AHTMR consistent with our previous reports [24,25,29]. In these neurons the center of the RF was used for response testing to the apex of the pyramids within the RF (Fig. 1A). Decreasing the apex size with a standardized force resulted in an apex-dependent increase in AP/stimulus that was greater in the neurons from the pSNL animals compared to the sham animals (P<0.05) (Fig. 1C and E). CV was no different between pSNL and Sham while RF thresholds were lower in the pSNL group (Fig. 1D and F, respectively).
Open Field Discrimination (OFD) Zone Preference
The mean weight of the animals in OFD was 204±3 g and not different between groups (sham (n=12) and pSNL (n=12) groups). There was no difference between groups in total distance travelled (5922±1005 cm and 6669±1069 cm, respectively), total zone entries (403±65 cm and 493±61, respectively), and distance travelled or zone entries in a given apex zone (Fig. 2B and 2F). The total time spent in each zone was dependent on the apex size on the surface for the pSNL, but not for the sham groups (P<0.05) (Representative figure of OFD between pSNL and sham Fig. 2A).
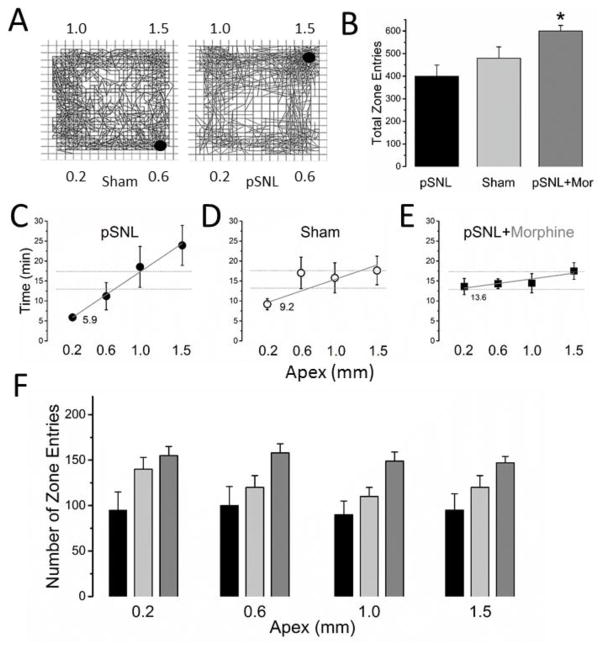
Figure 2. Open Field Discrimination (OFD).
Total time spent in each quadrant was measured for OFD 7 days after partial spinal nerve injury (pSNL) or sham. A) Representative OFD session tracings of sham and pSNL animals. The discrimination for the pSNL animal is seen. The quadrants are denoted by apexes in mm. The red circle is represents the animal at the time of capture. B) Total zone entries were not different between the sham and pSNL. However, total zone entries were greater in the pSNL-morphine group compared to the pSNL, but not the sham group (5A) (*P<0.05). C,D,E) Time in apex zones (pSNL C, sham D, and pSNL-morphine E). A moderate correlation (rho (ρ), =0.47) was found with an apex dependent increase in time in quadrants for the pSNL group only (the least time in the 0.2 apex surfaces zone and increasing time to the most time in the 1.5 apex surface zone (P=0.0008)). Apex size total zone time relationship was different between the pSNL and the other groups (P<0.05). The 0.2 apex was different from the 1.0 and the 1.5 mm apexes for the pSNL only (P<0.05). A representative regression line (red line pSNL, black line sham) is shown along with a horizontal line (thin black) (theoretical equal distribution). F) No difference in zone entries was found between the different apex size zones for any group. All data are means (± standard error).
In the pSNL group, there was a positive correlation with increasing time spent in the zones with the larger apex (least time spent in the 0.2 apex surface zone and the most time spent in the 1.5 apex surface zone (ρ=0.47 and P=0.0008) (Fig. 2C). However, no significant correlation was found for the surface apex size for the sham group (ρ of 0.21, NS) (Fig. 2D). Pairwise comparison of the pSNL group only revealed that the 0.2 apex zone time was different from both the 1.0 and the 1.5 (P<0.05), but not the 0.6 apex zone. Additionally, morphine in pSNL animals eliminated the zone discrimination effect (Fig. 2E). Total distance travelled distance travelled between the apex zones in the pSNL-morphine group (7338±664 cm) was not different from the saline pSNL or Sham groups. However, the total number of zone entries was greater in the pSNL-morphine group (608±38) than in the pSNL (P<0.05) and no different from sham (Fig. 2B). Similar to the pSNL and the sham groups, no difference was found in zone entries in a given apex size zone for the pSNL-morphine group (Fig 2F). Of the time in each zone, approximately 67% was comprised of resting time (no difference between groups or different apexes). Zone resting time showed the same correlation to apex size for the pSNL group and no difference for the sham group or pSNL-morphine group. No differences in ambulatory counts, vertical counts, and stereotypy were found between the groups or surface quadrants.
Mechanical Withdrawal Threshold
MWT was measured at baseline and POD7 after sham or pSNL. MWT was also measured 30 minutes after either saline in the sham or morphine in the pSNL groups. The mean baseline MWT was 24±3 g and there was no difference at baseline between the two groups. However, POD7 after pSNL, MWT was significantly lower (9±4 g) compared to baseline and to the sham group at POD7 (37.1±6.1 g) (P<0.05). After morphine the MWT increased to 32±3 g and was no different than the sham.
Discussion
Mechanical activation of AHTMR neurons generates graded input that corresponds to behavior of the freely moving animal. The discriminatory nature of the behavior to the quadrants that produce the greatest activation of AHTMR input to the spinal cord permits the evaluation of spontaneous behavior independent of evoked withdraw responses. This moves mechanical stimulation in the periphery from primarily motor response with descending modulation to a decision making response suggesting that the input is indeed inducing some type of pain to the animal. The normalization of the behavior in the OFD after pSNL with morphine suggests that the preference is nociceptive in nature as it is reversed by this standard analgesic.
Mechanical stimulation in the periphery activates both fast-conducting (A) and slower conducting (C) type neurons. A-type neurons primarily carry information of light touch and nociceptive mechanical force. The nociceptive A-type neurons have long been thought to subserve the “first pain” response, but recently have been shown to play a role in nociceptive input related to nerve injury and tissue damage well beyond the initial injury [24,25,28]. AHTMRs contribute to mechanical hyperalgesia related to nerve injury suggesting a contribution of these neurons to pain related nociceptive input in the more chronic neuropathic condition [25]. The data presented in this paper further corroborate the notion that AHTMR are involved in nerve injury related nociceptive input beyond the initial “acute” phase since their activation produces a significant behavioral effect in the freely behaving animal 7 days after initial injury.
From the mechanical thresholds in normal AHTMR neurons, the pyramids would only generate AP activity in injured and hypersensitive AHTMR neurons at any force below 15 g (the lower limit of the normal threshold for AHTMR tested). AP generation is likely to occur in a limited way, if at all, in any AHTMR in a normal animal from any apex used during the course of exploring in the OFD test. However, in nerve injured animals, the threshold of AHTMR is such that the body weight of the animal is sufficient force on any apex in the floor of the OFD test to generate AP’s and nociceptive input and an unpleasant association with that region. Since these are mechanically activated nociceptors, no activation would be expected in the normal animal in the absence of injury and therefore no “pain” input would be generated in the brain. This is consistent with the results in the OFD test.
LTMR are certainly activated by the surfaces as the animal ambulates and may play a role in allodynia. Previous studies have not been able to demonstrate that LTMR activation produces pain as measured by place aversion and this may be because the hyperalgesia is related to the higher threshold mechanical nociceptive units and their activation of second order neurons in nociceptive lamina [9,29–31]. Nevertheless, activation of LTMR tactile units may have an impact as surfaces produce a unique and different sensation. Akin to a possible strange sensation from the different quadrants in the normal animal, an altered sensory input may have occurred in the pSNL animals from activation of other mechanoreceptors. If this were the case, one would have expected possibly less overall distance travelled in the pSNL group related to less exploration and anxiety to the different and unique sensations from the quadrants. However, the light touch sensation in injured animals is slightly reduced, with increasing forces needed to generate an AP in the LTMR neurons at least in the L4 dermatome [24,28]. This behavior may incorporate allodynia in the pSNL animals from LTMR, not just the hyperalgesia from the AHTMR. However, the very low thresholds of the AHTMR after injury suggest that they may be activated at levels of mechanical sensibility below LTMR or A-beta neurons and therefore, may be a component of allodynia. The contributions of these other neurons to the surface discrimination are unclear from this study. Altered light touch input may generate a place preference if one sensation is more pleasant, but this would be less likely to occur rapidly and may require conditioning over a longer period of time. Further studies of the activation of other subsets of neurons by the apexes may be valuable for further understanding behavioral responses and the implications.
No special place association stimuli were used in the OFD test. Animals were exposed to the OFD one time and therefore no prior conditioning occurred. The OFD was generated through experiential movement through the zones and onto the surfaces and a decision to avoid further time in certain quadrants. This is not the first study to use OF activity after injury. OF has been used to examine the effect of injury on the development of anxiety [18]. The altered preferences in the current study may be partly driven by anxiety induced in the different quadrants, but the contribution of anxiety and the exact brain circuitry responsible for this are unclear. Aversion to mechanical stimulation has been shown previously [17]. In this paradigm the aversion develops over time as a response to evoked mechanical stimulation as opposed to aversion developing spontaneously in a freely exploring animal. This is more of a conditioned response to aversion similar to the conditioned place preference that has also been used as a novel way to examine the effect of nociceptive input on higher order behaviors [21]. Furthermore, our study is the only study to directly correlate the behavior with activation of mechanical nociceptive input from any neuron suggesting that the aversion is likely related to nociceptive input and the representation of pain in the animal. This, however, does not eliminate a contribution of other nerve subtypes in the preference and likely the AHTMR are not the only nerve fiber involved [25].
Conclusions
The impact of differential activation of AHTMR on behaviors other than withdrawal is important in understanding the implications of these specific peripheral neurons in generating the “pain” signal input to the brain. The use of this type of non-reflexive behavior will be valuable to verify drug effects on pain related behaviors in animals beyond evoked withdrawal or hypersensitivity. Ultimately, mechanical and thermal activation may be combined to understand complex and related effects of more than one modality and how this affects nerve fiber activity-specific behavior, pharmacologic efficacy, and nerve injury related nociceptive input.
Highlights.
Graded fast conducting high threshold mechanoreceptor activation can be elicited
Induced neuronal activity in this subset is further increased after nerve injury
Activity of these neurons predicts pain related place aversion
This nerve subset may contribute to pain beyond acute pain signaling
Consideration of these neurons in chronic pain may further knowledge and treatment
Acknowledgments
We thank Renee Parker, Susy Kim, Tracy Strassburg, and Addie Larimore for technical assistance and Jame Eisenach, MD for support and discussions. We also wish to thank Wes Lampkin and Pao Ly of Control Technologies LLC (Hickory, NC) for manufacture of surfaces and pyramids.
Funding
This work was supported by the Department of Anesthesiology and the National Institutes of Health (Grant Numbers GM104249 [DGR] and GM113852 [TJM]). Neither funding sponsor had any role in the design, conduct, analysis, and interpretation of the study, nor were they involved in the writing or decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest
The authors have no conflict of interests to declare.
Contributions
M. Danilo Boada: Conception and design of research, performed experiments, analyzed data, interpreted results, prepared figures, drafted, edited, revised, and approved final manuscript.
Thomas J. Martin: Conception and design of research, interpreted results, edited, revised, and approved final manuscript.
Douglas G. Ririe: Conception and design of research, performed experiments, analyzed data, interpreted results, prepared figures, drafted, edited, revised, and approved final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barrot M. Tests and models of nociception and pain in rodents. Neuroscience. 2012;211:39–50. doi: 10.1016/j.neuroscience.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 2.Backonja MM, Galer BS. Pain assessment and evaluation of patients who have neuropathic pain. Neurol Clin. 1998;16:775–790. doi: 10.1016/s0733-8619(05)70097-9. [DOI] [PubMed] [Google Scholar]
- 3.Bouhassira D. Neuropathic pain: the clinical syndrome revisited. Acta Neurol Belg. 2001;101:47–52. [PubMed] [Google Scholar]
- 4.Backonja MM. Defining neuropathic pain. Anesth Analg. 2003;97:785–790. doi: 10.1213/01.ANE.0000062826.70846.8D. [DOI] [PubMed] [Google Scholar]
- 5.Jensen TS. An improved understanding of neuropathic pain. Eur J Pain. 2002;6(Suppl B):3–11. doi: 10.1016/s1090-3801(02)90002-9. [DOI] [PubMed] [Google Scholar]
- 6.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 7.Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 8.King T, Porreca F. Preclinical assessment of pain: improving models in discovery research. Curr Top Behav Neurosci. 2014;20:101–120. doi: 10.1007/7854_2014_330. [DOI] [PubMed] [Google Scholar]
- 9.Ruscheweyh R, Forsthuber L, Schoffnegger D, Sandkühler J. Modification of classical neurochemical markers in identified primary afferent neurons with Abeta-, Adelta-, and C-fibers after chronic constriction injury in mice. J Comp Neurol. 2007;502:325–336. doi: 10.1002/cne.21311. [DOI] [PubMed] [Google Scholar]
- 10.Woolf CJ. Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain. 1984;18:325–343. doi: 10.1016/0304-3959(84)90045-9. [DOI] [PubMed] [Google Scholar]
- 11.Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- 13.Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Mogil JS, Stöhr T Preclinical Pain Consortium. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139:243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 14.IASP Taxonomy Work Group: Part III. [accessed 10.12.15];Pain Terms: A Current List with Definitions and Notes on Usage. Available at: http://www.iasp-pain.org/files/Content/ContentFolders/Publications2/ClassificationofChronicPain/Part_III-PainTerms.pdf.
- 15.Tappe-Theodor A, Kuner R. Studying ongoing and spontaneous pain in rodents--challenges and opportunities. Eur J Neurosci. 20140;39:1881–1890. doi: 10.1111/ejn.12643. [DOI] [PubMed] [Google Scholar]
- 16.De Felice M, Eyde N, Dodick D, Dussor GO, Ossipov MH, Fields HL, Porreca F. Capturing the aversive state of cephalic pain preclinically. Ann Neurol. 2013;74:257–265. doi: 10.1002/ana.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol. 2000;163:490–494. doi: 10.1006/exnr.2000.7395. [DOI] [PubMed] [Google Scholar]
- 18.Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Martin TJ, Grigg A, Kim SA, Ririe DG, Eisenach JC. Assessment of attention threshold in rats by titration of visual cue duration during the five choice serial reaction time task. J Neurosci Methods. 2015;241:37–43. doi: 10.1016/j.jneumeth.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 23.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151:12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Boada MD, Gutierrez S, Aschenbrenner CA, Houle TT, Hayashida K, Ririe DG, Eisenach JC. Nerve injury induces a new profile of tactile and mechanical nociceptor input from undamaged peripheral afferents. J Neurophysiol. 2015;113:100–109. doi: 10.1152/jn.00506.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boada MD, Martin TJ, Peters CM, Hayashida K, Harris MH, Houle TT, Boyden ES, Eisenach JC, Ririe DG. Fast-conducting mechanoreceptors contribute to withdrawal behavior in normal and nerve injured rats. Pain. 2014;155:2646–2655. doi: 10.1016/j.pain.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boada MD, Houle TT, Eisenach JC, Ririe DG. Differing neurophysiologic mechanosensory input from glabrous and hairy skin in juvenile rats. J Neurophysiol. 2010;104:3568–3575. doi: 10.1152/jn.00415.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boada MD, Gutierrez S, Houle T, Eisenach JC, Ririe DG. Developmental differences in peripheral glabrous skin mechanosensory nerve receptive field and intracellular electrophysiologic properties: phenotypic characterization in infant and juvenile rats. Int J Dev Neurosci. 2011;29:847–854. doi: 10.1016/j.ijdevneu.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boada MD, Gutierrez S, Giffear K, Eisenach JC, Ririe DG. Skin incision-induced receptive field responses of mechanosensitive peripheral neurons are developmentally regulated in the rat. J Neurophysiol. 2012;108:1122–1129. doi: 10.1152/jn.00399.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baseer N, Al-Baloushi AS, Watanabe M, Shehab SA, Todd AJ. Selective innervation of NK1 receptor-lacking lamina I spinoparabrachial neurons by presumed nonpeptidergic Aδ nociceptors in the rat. Pain. 2014;155:2291–2300. doi: 10.1016/j.pain.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King T, Qu C, Okun A, Mercado R, Ren J, Brion T, Lai J, Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011;152:1997–2005. doi: 10.1016/j.pain.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitcher GM, Henry JL. Nociceptive response to innocuous mechanical stimulation is mediated via myelinated afferents and NK-1 receptor activation in a rat model of neuropathic pain. Exp Neurol. 2004;186:173–197. doi: 10.1016/j.expneurol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Allchorne AJ, Broom DC, Woolf CJ. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol Pain. 2005;1:365. doi: 10.1186/1744-8069-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]