Abstract
The unfolded protein response (UPR) is a highly conserved pathway that allows the cell to manage endoplasmic reticulum (ER) stress that is imposed by the secretory demands associated with environmental forces. In this role, the UPR has increasingly been shown to have crucial functions in immunity and inflammation. In this Review, we discuss the importance of the UPR in the development, differentiation, function and survival of immune cells in meeting the needs of an immune response. In addition, we review current insights into how the UPR is involved in complex chronic inflammatory diseases and, through its role in immune regulation, antitumour responses.
The endoplasmic reticulum (ER) is an extensive tubular-reticular network that is separated from the surrounding cytosol by a single lipid bilayer — the ER membrane. It is a crucial site involved in maintaining Ca2+ homeostasis and its major function is the synthesis and folding of secreted and transmembrane proteins, which constitute approximately one-third of all the proteins that are made in the cell1,2. Following translation on ER membrane-associated ribosomes, proteins enter the ER lumen where chaperone-based folding occurs, together with complex protein modifications. These include N-linked glycosylation, disulfide bond formation and proline cis–trans isomerization, which are mediated by glycosyltransferases, oxidoreductases and peptidyl-prolyl cis–trans isomerases, respectively3–6.
Adequate folding and post-translational modifications of proteins are crucial for proper function; furthermore, in a dominant manner, misfolded and aggregated proteins can cause cellular stress and cell death, as exemplified by neurodegeneration and other protein-misfolding diseases7,8. Therefore, it is of crucial importance that protein folding is subject to stringent quality control systems to allow a cell to carry out its necessary secretory functions. For example, ER-associated degradation (ERAD) ensures that misfolded and unfolded proteins are removed from the ER lumen to the cytosol for subsequent degradation by the ubiquitin-proteasome system9.
Numerous environmental conditions, both endogenous and exogenous, can disrupt the ER protein-folding environment, and when protein-folding requirements exceed the processing capacity of the ER, misfolded and unfolded proteins accumulate in the ER lumen, which triggers the unfolded protein response (UPR). The UPR is a sophisticated collection of intracellular signalling pathways that have evolved to respond to protein misfolding in the ER. In addition, it has become increasingly clear that UPR signalling has an important role in immunity and inflammation. In this Review, we discuss the role of UPR activation in the development of immune cells and discuss how the UPR is involved in mounting efficient immune responses. In addition, we highlight causes of UPR activation that are directly linked to inflammation and review current insights into the downstream pathways by which UPR activation induces inflammation. Last, we discuss how the UPR is involved in various prevalent diseases, including inflammatory bowel disease, metabolic disease and cancer. Owing to the breadth of this Review, we do not provide substantial detail for individual sections but have given an overview of the most relevant and recent findings.
The UPR pathways
In metazoans, the UPR is activated by the coordinated action of three ER transmembrane stress sensors: inositol-requiring enzyme 1α (IRE1α; also known as ERN1), PKR-like ER kinase (PERK; also known as EIF2AK3) and activating transcription factor 6α (ATF6α). Under homeostatic conditions, the luminal domains of these ER stress sensors are retained in an inactive state through association with binding immunoglobulin protein (BiP; also known as GRP78 and HSPA5). However, owing to the higher affinity of BiP for misfolded proteins, BiP dissociates from the ER stress sensors as misfolded proteins accumulate in the ER lumen, thereby releasing the stress sensors to permit downstream signalling10 (FIG. 1). In addition, it has been elegantly shown that, at least in Saccharomyces cerevisiae, basic and hydro phobic residues on unfolded proteins can directly bind to a putative peptide groove of IRE1α, and direct binding of unfolded proteins is sufficient to induce the UPR11. It is currently unclear whether direct binding of unfolded proteins as a second mechanism of UPR activation is limited to IRE1α or whether it can also be observed with PERK and ATF6α. These mechanisms require further characterization.
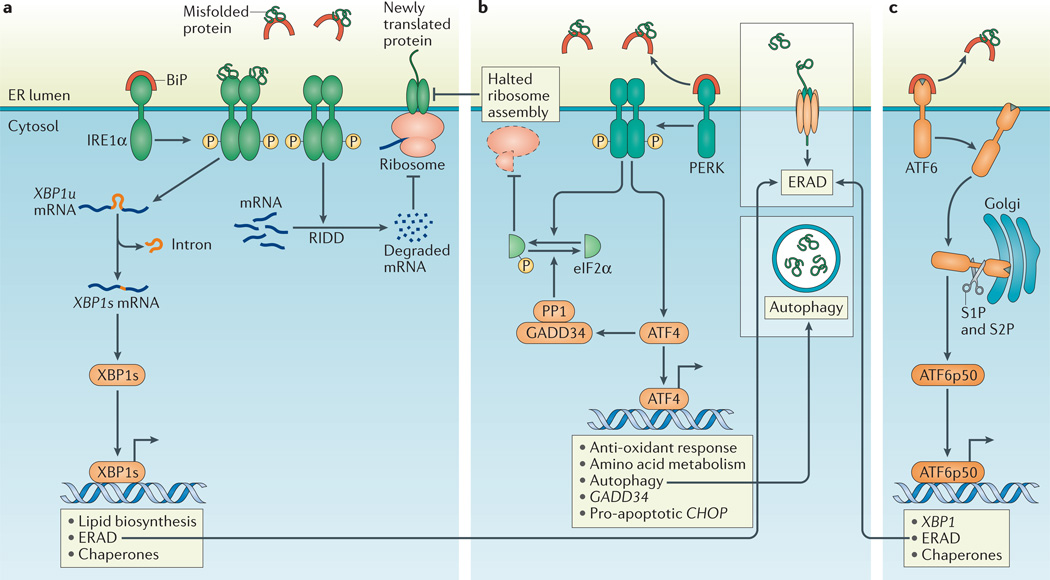
Figure 1. The accumulation of unfolded proteins in the ER lumen activates the three arms of the UPR.
a | Dissociation of binding immunoglobulin protein (BiP) from inositol-requiring enzyme 1α (IRNEa1tuα). or dirct binding of misfolded proteins to IRE1α, activates the endoribonuclease domain of IRE1α, which non-conventionally splices an intron from unspliced X-box binding protein 1 (XBP1u) mRNA to produce XBP1s mRNA that encodes a potent transcriptional activator, XBP1s. Among the target genes of XBP1s are genes encoding proteins that increase the protein-folding capacity of the endoplasmic reticulum (ER) and that assist in the degradation of misfolded proteins by ER-associated degradation (ERAD; see inset box). In addition, the entry of newly synthesized proteins into the ER is limited by the degradation of mRNA through regulated IRE1α-dependent decay of mRNA (RIDD). b | PKR-like ER kinase (PERK)-dependent phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) inhibits ribosome assembly, which causes a translational block and allows the cell to manage temporary ER stress. Activating transcription factor 4 (ATF4) escapes translation inhibition under ER stress conditions and induces the transcription of genes that promote survival, including those involved in compensatory autophagy (see inset box). Once ER stress is resolved, eIF2α is dephosphorylated by the GADD34–protein phosphatase 1 (PP1) complex to restore protein translation. However, if ER stress-induced damage is irreversible, the terminal unfolded protein response (UPR) is activated to induce apoptosis, mainly through C/EBP homologous protein (CHOP). c | Upon BiP dissociation from ATF6α during ER stress, ATF6α travels to the Golgi compartment where it is processed by the Golgi enzymes site 1 protease (S1P) and S2P to produce a cytosolic p50 fragment. ATF6p50 functions as a transcription factor that activates transcriptional programmes that increase ER capacity and protein folding, and that remove misfolded proteins from the ER for degradation (ERAD; see inset box).
The downstream transcriptional programmes of the UPR are primarily directed towards restoring proteostasis, which is achieved through at least four different strategies. First, mRNA translation is transiently attenuated through PERK-dependent phosphorylation of the α-subunit of eukaryotic translation initiation factor 2 (eIF2α), which inhibits the assembly of the eIF2-GTP–Met-tRNA ternary complex (eIF2–TC), and thereby reduces the quantity of proteins that enter the ER. Second, the entry of newly translated proteins into the ER is decreased by the degradation of ER membrane-associated mRNAs by regulated IRE1α-dependent decay (RIDD)12–14. Third, processes that eliminate unfolded proteins from the ER are induced by increasing the transcription of ERAD-related and autophagy-related proteins (BOX 1). Finally, genes are induced that increase the protein-folding capacity of the ER and mediate its expansion through increasing the biogenesis of ER and lipid components1,15.
Box 1|Autophagy as a compensation strategy for ER stress.
In response to the challenge of misfolded proteins, autophagy has a crucial function as an adaptive ‘self-eating’ process by which cellular components are encapsulated within autophagosomes and degraded. Similarly to the unfolded protein response (UPR), autophagy can result in either cell survival or cell death167,168. The mechanisms by which the UPR induces autophagy are incompletely understood, but probably involve signalling through PKR-like ER kinase (PERK)–eukaryotic translation initiation factor 2α (eIF2α) and inositol-requiring enzyme 1α (IRE1α)169.
Activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP) function both independently and together to induce a large array of autophagy genes170. In addition, eIF2α phosphorylation, in response to polyQ72 aggregate-induced endoplasmic reticulum (ER) stress, is associated with autophagosome formation and protection against neuronal cell death42. In another example, using unfolded dysferlin as a model of muscular dystrophy, spliced X-box binding protein 1 (XBP1s) was shown to be crucial for autophagy induction and protection against neurodegeneration171. Furthermore, UPR pathways can activate AMPK, which attenuates AKT–mTOR signalling to enhance autophagy172.
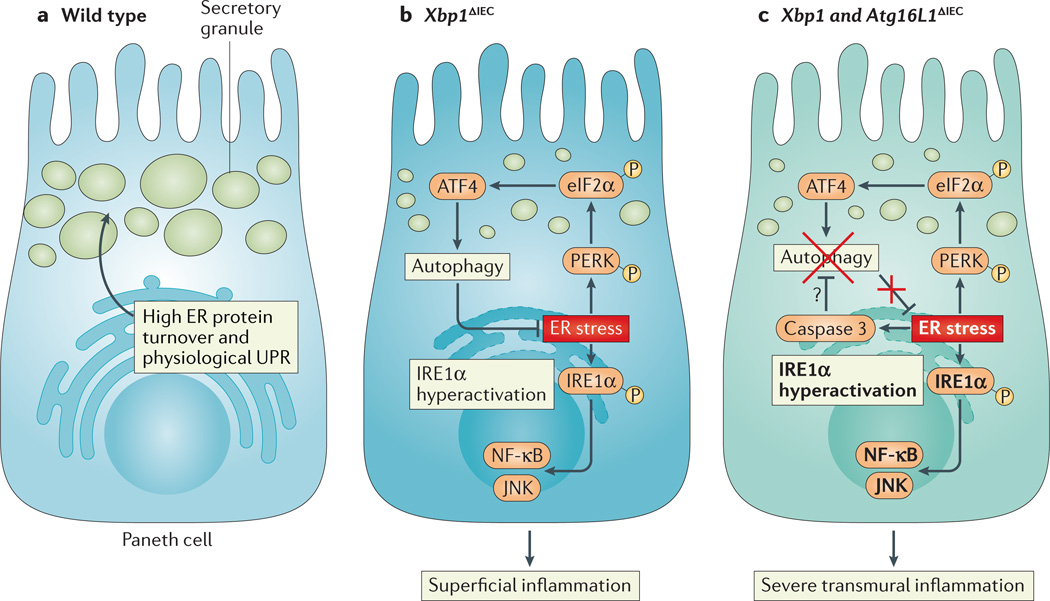
A direct demonstration that autophagy can compensate for ER stress derives from studies of ER stress-induced small intestinal inflammation77, in which concomitant deletion of epithelial XBP1 and epithelial associated autophagy related protein 7 (ATG7) or autophagy-related 16 like 1 (ATG16L1) results in increased ER stress and severe transmural Crohn’s disease-like enteritis, compared with deletion of XBP1 alone, which only induces mild superficial inflammation (FIG. 4c). In this model, the induction of autophagy depends on the phosphorylation of eIF2α78. In the case of intestinal epithelial stress that is associated with inflammatory bowel disease, autophagy probably functions to selectively remove inflammatory ER membranes (see below). In the cancer setting, ER stress induces the activation of the PERK–eIF2α –ATF4 signalling pathway to increase tumour cell survival through the induction of autophagy173,174.
The precise mechanism by which autophagy decreases ER stress remains unclear. Autophagy was previously considered to be a non-specific process (bulk macro-autophagy), but selective autophagy processes that precisely target organelles such as the mitochondria and peroxisomes into autophagosomes have now been described. Autophagy of the ER (ER-phagy) has been described in yeast175, and more recently also in mammalian cells. In mammalian cells, ER-phagy depends on the FAM134 reticulon family of proteins, which function as ER transmembrane receptors that bind LC3 proteins and GABA type A receptor-associated protein (GABARAP), thereby initiating ER degradation by autophagy176. It remains to be determined whether autophagy directly degrades stressed ER membranes that contain misfolded proteins and whether FAM134 proteins or the selective autophagy receptors NBR1, optineurin, p62 and NDP52 are involved.
However, when these attempts to restore proteostasis fail and ER stress is unabated, UPR signalling typically switches to a pro-apoptotic mode, a process that is known as the terminal UPR (reviewed in REF. 16). The terminal UPR may have evolved to eliminate, for example, excessively damaged or pathogen-infected cells.
IRE1α
The cytoplasmic tail of the type I transmembrane protein IRE1α possesses two enzymatic activities: a serine/threonine kinase domain and an endoribonuclease (RNase) domain17. On release from BiP, dimerization of IRE1α elicits RNase activity to initiate the non-conventional splicing of a single mRNA that encodes X-box binding protein 1 (XBP1). This produces a translational frameshift and creates a potent transcriptional activator known as XBP1s (‘s’ represents ‘spliced’)18,19. XBP1s translocates to the nucleus where it induces the transcription of a wide variety of ER-resident molecular chaperones and protein-folding enzymes that together increase ER size and function15,20 (FIG. 1a). In addition to the specific endoribonuclease activity that splices XBP1, IRE1α-dependent activation of RIDD degrades ER-membrane-associated mRNAs to reduce the amount of protein that enters the secretory pathway21 (FIG. 1a). In addition, emerging evidence has indicated that RIDD substrates encode proteins of a diverse nature, which modulate cellular processes that are distinct from the control of ER homeostasis, including the generation of inflammatory double-stranded RNA species through the activation of intracellular nucleic acid sensors22,23.
During prolonged ER stress, the beneficial effects of IRE1α activation through the activity of XBP1s may be temporally and quantitatively impeded, and the PERK pathway can become dominant24,25. In addition, chronic ER stress increases the oligomerization state of IRE1α to hyperactivate its cytosolic RNase domains, which leads to the cleavage of many other RNAs in addition to XBP1, including precursors of apoptosis-inhibitory micro-RNAs, which thus promotes apoptosis26–28. Therefore, prolonged ER stress may tip the properties of IRE1α from being adaptive to promoting inflammation and cell death.
PERK
Similarly to IRE1α, dissociation of BiP from the ER luminal domain of PERK permits PERK homo-dimerization and autophosphorylation to activate the cytoplasmic kinase domain. In addition, it was shown that the lipid composition of the ER membrane can activate PERK, which highlights the importance of lipid metabolism as a direct trigger of UPR activation29,30. Activated PERK phosphorylates eIF2α, which inhibits eIF2-TC formation and thereby transiently attenuates global mRNA translation, enabling the cell to manage temporary ER stress (FIG. 1b). Notably, eIF2α can be phosphorylated in mammals independently of ER stress by three additional kinases: general control nonderepressible 2 (GCN2; also known as EIF2AK4), which is induced by amino acid deprivation; the haem-regulated inhibitor kinase (HRI; also known as EIF2AK1), which is induced by oxidative stress and haem deprivation; and protein kinase R (PKR; also known as EIF2AK2), which is activated by double-stranded RNA as part of the interferon antiviral response31. Together, these additional mechanisms of eIF2α phosphorylation form the integrated stress response (ISR) system, which allows the cell to integrate multiple stress stimuli into one common node, that being the general control of protein synthesis through the phosphorylation of eIF2α31. For example, both epithelial cells and dendritic cells (DCs) activate GCN2 to phosphorylate eIF2α in response to amino acid deprivation, to induce autophagy, to reduce oxidative stress and to inhibit inflammasome activation32.
Although the translation of most mRNAs is inhibited in ER-stressed cells, the translation of some species of mRNA is favoured under ER stress conditions when eIF2α is phosphorylated and eIF2-TC availability is low (mechanisms reviewed in REF 33)34. One important example is the mRNA that encodes activating transcription factor 4 (ATF4; also known as CREB2), which is a crucial UPR mediator that transactivates genes that are involved in amino acid metabolism and oxidative stress resistance, as well as autophagy12 (FIG. 1b). As a sustained translational block is not compatible with cell survival, ATF4 also induces PPP1R15A expression, which encodes GADD34, a regulatory subunit of protein phosphatase 1 (PP1) that directs the dephosphorylation of eIF2α to restore mRNA translation185 (FIG. 1b). However, ATF4 also activates the transcription of C/EBP homologous protein (CHOP; also known as DDIT3)12,35, which is involved in ER-stress-mediated apoptosis both in vitro and in vivo36,37. To allow the cell a chance to manage the ER stress, several mechanisms suppress CHOP at early time points after ER stress, such as PERK-dependent microRNA (miRNA) miR-211 expression, which represses CHOP transcription through histone methylation38. In addition, CHOP is suppressed by Toll-like receptor (TLR) signalling during immune responses in macrophages by protein phosphatase 2A (PP2A)-mediated serine dephosphorylation of the eIF2Bε sub-unit39. Indeed, only strong and chronic activation of PERK increases steady-state levels of CHOP, owing to the short half-lives of both ATF4 and CHOP mRNAs and proteins, so that only excessive ER stress will promote the terminal UPR25. Studies of ATF4 and CHOP in cells experiencing chronic ER stress indicate that these proteins function together as a heterodimer to induce apoptosis by increasing protein synthesis, thereby enhancing protein misfolding, oxidative stress and cell death40.
ATF6
ATF6 is a type II transmembrane protein that contains a bZIP transcription factor within its cytosolic domain. Although there are two ATF6 genes in the mammalian genome (ATF6A and ATF6B), only ATF6α is required to activate UPR gene expression41. On release from BiP, ATF6α transfers to the Golgi compartment where it is processed by the Golgi enzymes site 1 protease (S1P) and S2P to produce a cytosolic p50 fragment that migrates to the nucleus. The p50 fragment activates the expression of genes encoding proteins that function to increase ER capacity and folding (including, BiP, GRP94, p58IPK (also known as DNAJC3) and XBP1), as well as the ERAD pathway19,41–43 (FIG. 1c).
The physiological UPR in immune cells
Cells that have a large secretory demand as part of their function are particularly dependent on a well-developed and large ER and, consequently, UPR signalling. Therefore, it is not surprising that highly secretory immune cells are exquisitely susceptible to environmental conditions that impose ER stress, either by directly targeting ER-folding capacity (for example, microbial toxins)44 or by markedly increasing folding demand (for example, exposure to pathogens). Thus, inflammation per se is an important factor in the induction of ER stress, and UPR activation may be a sensitive hallmark of inflammation. In addition to the pathophysiological conditions that affect specific cell types, it is now well established that activation of the UPR has a role in a wide range of physiological events that are associated with immunologically important cell types45. Furthermore, although they have not been well studied, germline polymorphisms and, potentially, somatically generated mutations may affect the ability of the host to determine whether an appropriate UPR level has been achieved based on the demand. Understanding how the UPR affects specific functions at the cellular level and the host-related factors that affect this are thus of great importance, as considered below.
B cells and plasma cells
Over the past 15 years, the role of the transcription factor XBP1 in plasma cell differentiation has become increasingly clear and has served as a paradigm for numerous secretory systems and their relationships with immune function and inflammation. Plasma cell differentiation is regulated by the transcription factors interferon-regulatory factor 4 (IRF4) and B lymphocyte-induced maturation protein 1 (BLIMP1; also known as PRDM1)46,47. In addition, the induction of Xbp1, which is downstream of BLIMP1, is required for the marked expansion of the ER and the increased protein synthesis that are necessary for high levels of antibody production and secretion during both physiological and pathological immune responses48,49 (FIG. 2a). Indeed, XBP1 deficiency in B cells leads to an absence of plasma cells and markedly reduces circulating antibody levels but has no effect on B cell maturation or isotype switching50. Although it was initially assumed that XBP1 induction was caused by increased immunoglobulin synthesis and the accumulation of unfolded immunoglobulin heavy chains51, subsequent studies have shown that ER expansion is evident in B cells before the onset of immunoglobulin synthesis52 and that Xbp1 is similarly spliced when IgM secretion is genetically abrogated53. This suggests that XBP1 induction is a differentiation-dependent event in plasma cells, rather than a response to increased immunoglobulin secretion. The factors that drive UPR activation early in plasma cell development are incompletely understood. Notably, part of the decrease in immunoglobulin production in XBP1-deficient B cells was later explained by IRE1α hyperactivation leading to increased RIDD and the subsequent degradation of immunoglobulin-µ heavy-chain mRNAs54.
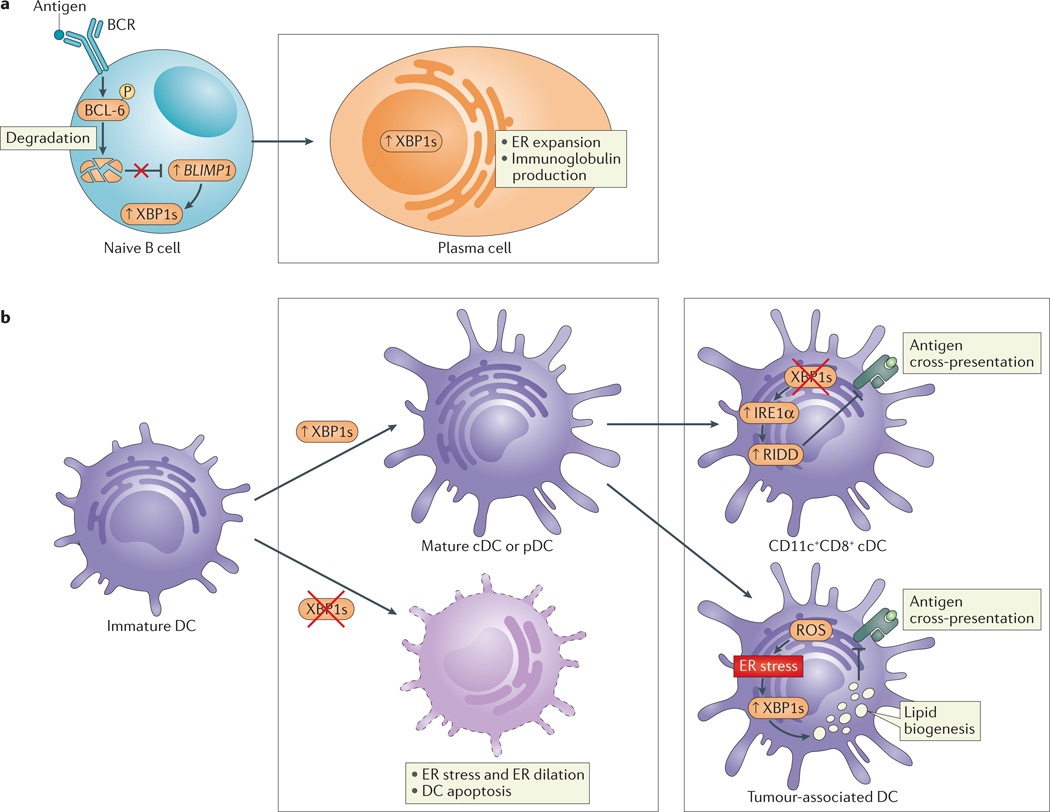
Figure 2. XBP1 has a crucial role in plasma cell differentiation and DC differentiation and function.
a | B cell receptor (BCR) ligation induces the phosphorylation of B cell lymphoma 6 (BCL-6) and its subsequent ubiquitylation and degradation. BCL-6 degradation derepresses B lymphocyte-induced maturation protein 1 (BLIMP1) in naive B cells to activate cellular programmes that are crucial for the development of plasma cells. These include activation of the unfolded protein response (UPR) and X-box binding protein 1 (XBP1) splicing, which is required for the expansion of the endoplasmic reticulum (ER) and increased protein (immunoglobulin) synthesis involved in plasma cell differentiation. b | XBP1 is crucial for the differentiation of conventional dendritic cells (cDCs) and plasmacytoid DCs (pDCs) from immature progenitors (left). Loss of XBP1 in progenitor cells abrogates maturation and decreases DC survival. XBP1 deletion in mature CD11c+ DCs results in inositol-requiring enzyme 1α (IRE1α) hyperactivation, leading to regulated IRE1α-dependent decay (RIDD)-dependent degradation of components of the MHC class I–mediated antigen cross-presentation machinery; as such, ER stress in DCs interferes with their function (right, top). By contrast, increased XBP1s in response to the production of reactive oxygen species (ROS) in tumour-associated DCs leads to augmented lipid biogenesis that is associated with the disruption of MHC class I–mediated antigen cross-presentation (right, bottom).
In addition to plasma cells, IRE1α activation is also observed in pro–B cells55. UPR activation in these early stages of B cell development might result from the increased secretory demand caused by the neo-expression of cell surface proteins that are associated with V(D)J antigen receptor rearrangements56. Indeed, a recent study has shown that IRE1α and XBP1s are important during the pre-B cell stage when immunoglobulin heavy chains are expressed for the first time and, importantly, that XBP1s in pre-B acute lymphoblastic leukaemia (ALL) cells provides a survival benefit for tumour cells57.
T cells
Studies using XBP1s–GFP reporter mice have shown that IRE1α is activated in CD4+CD8+ thymic T cells and in CD8+ splenic T cells, although the precise role of the UPR in early T cell development is not clear55. However, the activation of the IRE1α–XBP1 axis in effector CD8+ T cells was reported in response to acute infection with Listeria monocytogenes, which was associated with the expression of high levels of killer cell lectin-like receptor G1 (KLRG1). This suggests that XBP1 is important for the terminal differentiation of effector CD8+ T cells58.
DCs
Xbp1 is constitutively spliced in DCs, and loss of XBP1 leads to significantly reduced numbers of both conventional and plasmacytoid DCs59 (FIG. 2b). In addition, XBP1-deficient DCs have increased rates of apoptosis and are resistant to survival signals that are associated with TLR engagement (FIG. 2b). Interestingly, the ectopic expression of XBP1s in XBP1-deficient FLT3+ haematopoietic progenitor cells can rescue this apoptotic pheno-type59. Taken together, these results show that XBP1 is important for DC development and survival.
Consistent with the findings discussed above, constitutive activation of IRE1α is observed in DCs60 when using ER stress-activated indicator (ERAI) reporter mice61. Interestingly, although the highest levels of IRE1α activity are observed in CD8α+ DCs, the deletion of XBP1 in DCs using Cd11c–directed Cre recombinase does not result in developmental and phenotypic abnormalities of CD8α+ DCs. These different findings, compared with those from the study by Iwakoshi et al.59, may be explained by low levels of expression of CD11c, and hence Cre recombinase activity, in DC progenitor cells. Interestingly, however, XBP1-deficient CD8α+ DCs that are generated by Cd11c–Cre-mediated deletion of Xbp1 have RIDD-dependent defects in cross-presentation to OT-I T cells, owing to the degradation of components of the cross-presentation machinery, such as tapasin (also known as TAPBP)60. This study shows that the IRE1α–XBP1 arm of the UPR is crucially involved in DC function (FIG. 2b).
Consistent with a role for ER stress in the regulation of antigen presentation, several other studies have shown that ER stress affects the surface expression of MHC class I molecules by mechanisms that are not yet fully elucidated62,63. In ovarian cancer, impaired MHC class I-restricted antigen presentation to CD8+ T cells has been linked to reactive oxygen species (ROS)-induced ER stress and increased lipid metabolism in DCs induced by an unknown transmissible stress factor in the tumour microenvironment. Although the concept of lipid accumulation-dependent dysfunction of antigen presentation by DCs had been previously described64, this study links ER stress-induced Xbp1 splicing to lipid accumulation in DCs, resulting in DC antigen presentation defects65 (FIG. 2b). In accordance with this, the deletion of XBP1 in DCs abolished the accumulation of lipids in DCs and increased T cell-mediated antitumour immunity, resulting in decreased tumour burden and improved survival65.
Granulocytes
Granulocytes are represented by neutrophils, basophils and eosinophils. Eosinophils are typically associated with type 2 immune responses, allergy and parasitic infections66. Recently, it has also been shown that eosinophils might have a role in maintaining immune homeostasis in the intestine by promoting IgA class-switching in Peyer’s patches and by controlling the pool of CD103+ T cells and DCs67. Interestingly, among all types of granulocytes, eosinophils are uniquely dependent on XBP1 in that haematopoietic deletion of Xbp1 (Xbp1Vav1 mice) leads to the loss of fully mature eosinophils. In addition, the loss of XBP1 specifically in eosinophils using Epx-Cre-mediated Xbp1 deletion results in a significantly smaller pool of eosinophils in the bone marrow, which indicates that XBP1 is also needed to sustain the viability of eosinophil-committed progenitor cells68.
Macrophages
In macrophages, TLR signalling induces ER stress, and ER stress in turn amplifies the response to TLR ligation. For example, the TLR2 and TLR4 ligands Pam3CSK4 and lipopolysaccharide (LPS), respectively, induce IRE1α activation in mouse J774 macrophages. Furthermore, treatment with TLR agonists increases Xbp1 splicing in these macrophages, which is dependent on TNF receptor-associated factor 6 (TRAF6) recruitment to IREα and which requires the NAPDH oxidase NOX2 (REF. 69). Importantly, IRE1α-induced Xbp1 splicing in response to TLR ligation has been shown to be crucial for cytokine production, as macrophage-specific deficiency of XBP1 impairs the production of interleukin-6 (IL-6), tumour necrosis factor (TNF) and interferon-β (IFNβ)69 (FIG. 3a).
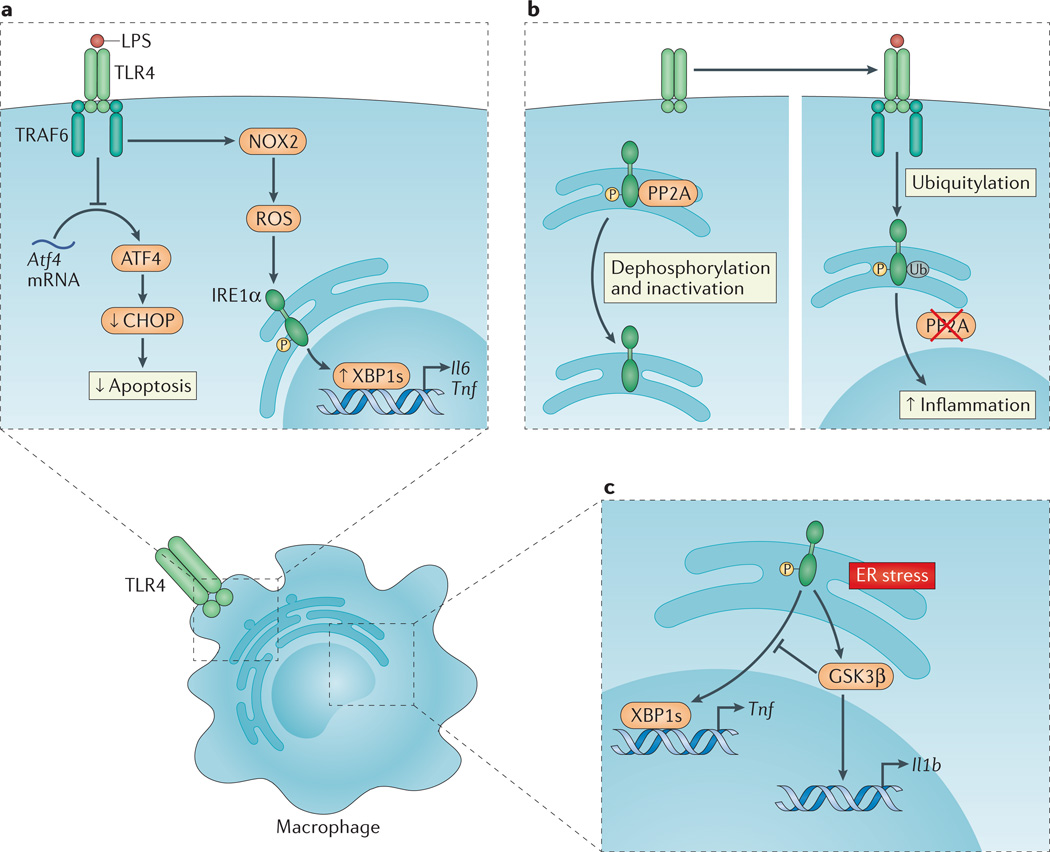
Figure 3. TLR signalling and the UPR coordinate immune responses in macrophages.
a | Upon Toll-like receptor (TLR) ligation, the inositol-requiring enzyme 1α (IRE1α) arm of the unfolded protein response (UPR) is activated through a mechanism that requires TNF receptor-associated factor 6 (TRAF6) recruitment to the TLR and reactive oxygen species (ROS) production by the NADPH oxidase NOX2. Spliced X-box binding protein 1 (XBP1s) functions as a transcription factor for the pro-inflammatory cytokines interleukin-6 (Il6) and tumour necrosis factor (Tnf). In a separate pathway, translation of activating transcription factor 4 (Atf4) mRNA is inhibited by TLR4 ligation, which decreases C/EBP homologous protein (CHOP) levels and apoptosis in activated macrophages. This favours macrophage survival and as such facilitates the immune response. b | In the absence of TLR signalling, phosphorylated IRE1α is subject to protein phosphatase 2A (PP2A)-mediated dephosphorylation and inactivation (left panel). Upon TLR ligation, however, TRAF6 interacts with and catalyses the ubiquitylation of IRE1α, which prevents PP2A–mediated dephosphorylation and inactivation of IRE1α, thereby amplifying inflammation (right panel). c | Endoplasmic reticulum (ER) stress-induced activation of IRE1α can induce Il1b transcription through glycogen synthase kinase 3β (GSK3β), which simultaneously inhibits Xbp1 splicing and thereby the transcription of XBP1s target genes, including Tnf.
TLR signalling also amplifies IRE1α signalling by modulating the phosphorylation status of this protein. In the absence of TLR signalling, phosphorylated IRE1α is dephosphorylated and thus inactivated by PP2A. Upon TLR ligation, however, TRAF6 interaction with IRE1α catalyses the ubiquitylation of IRE1α, which prevents its interaction with PP2A — through its adaptor, receptor for activated C kinase 1 (RACK1) — thus avoiding IRE1α dephosphorylation and inactivation70,71 (FIG. 3b). By contrast, the induction of ER stress in bone marrow-derived macrophages strongly potentiates LPS-induced pro-inflammatory signalling, including the induction of genes encoding CXC-chemokine ligand 1 (CXCL1), CXCL2, TNF, IL-1α and IL-6, in a pathway that depends on receptor-interacting serine/threonine-protein kinase 1 (RIPK1)72.
The IRE1α–XBP1 pathway has also been linked to increased IL-1β production through the IRE1α-dependent activation of glycogen synthase kinase 3β (GSK3β). In addition, GSK3β inhibits further Xbp1 slicing and thereby Tnf transcription, which modulates the inflammatory response to ER stress and, potentially, TLR signalling73 (FIG. 3c).
The PERK pathway has been shown to induce inflammation through the direct binding of ATF4 to the Il6 promoter74. In macrophages, however, TLR signalling inhibits translation of Atf4 mRNA and thereby its downstream target CHOP75. Similarly, CHOP is further suppressed by TLR–TRIF signalling in macrophages through PP2A–mediated serine dephosphorylation of the eIF2Bε subunit39. These mechanisms of CHOP suppression are required for macrophage survival during an immune response. This demonstrates that specific control of the different arms of the UPR is crucial for innate immune function69 (FIG. 3a).
Paneth cells
The conditional deletion of Xbp1 in intestinal epithelial cells (IECs) has shown that Paneth cells (which are an important source of anti-microbial peptides and stem cell survival signals)76 and, to a lesser extent, goblet cells (which are dedicated to mucus production) are highly dependent on this UPR transcription factor77. Paneth cells in mice with specific deletion of XBP1 in IECs are severely hypomorphic. The resultant decreased antimicrobial function has been shown to be associated with attenuated killing of L. monocytogenes in the intestine and increased bacterial translocation to the liver. In addition, IRE1α hyperactivation that leads to the phosphorylation of JUN N-terminal kinase (JNK) is also observed in XBP1-deficient intestinal epithelium, resulting in spontaneous superficial small intestinal enteritis77 (FIG. 4). Moreover, deletion of Xbp1 specifically in Paneth cells is sufficient to induce small intestinal enteritis78. As the spontaneous inflammation that is observed in the setting of IEC-specific deletion of Xbp1 is reversed under germ-free conditions, these studies indicate that the UPR in IECs and, particularly, the UPR in Paneth cells is crucial to the maintenance of intestinal homeostasis in the presence of microbial commensalism. Whether XBP1 has a crucial role in the development of Paneth cells, as it does in highly secretory haematopoietic cells, is not fully clear.
Figure 4. UPR signalling in intestinal epithelial cells.
a | Intestinal epithelial cells, particularly Paneth cells, have a well-developed endoplasmic reticulum (ER) to manage their high secretory demands, including the production of antimicrobial proteins such as lysozyme and defensins. b | Deletion of X-box binding protein 1 (XBP1) in the small intestinal epithelium (Xbp1ΔIEC mice) induces ER stress, resulting in hypomorphic Paneth cells that have signs of inositol-requiring enzyme 1α (IRE1α) hyperactivation and downstream activation of nuclear factor-κB (NF-κB) and JUN N-terminal kinase (JNK). The induction of autophagy through the PKR-like ER kinase (PERK)-eukaryotic translation initiation factor 2α (eIF2α)-activating transcription factor 4 (ATF4) axis of the unfolded protein response (UPR) alleviates ER stress. c | Simultaneous deletion of XBP1 and autophagy-related 16 like 1 (ATG16L1) in the small intestinal epithelium thus further increases ER stress and ER stress-induced inflammation. Hypothetically, ER stress, which induces caspase 3, could be an important pathway responsible for the degradation of ATG16L1 in patients carrying the Crohn’s disease risk allele ATG16L1T300A, which is prone to caspase 3-mediated cleavage.
Pathological causes of UPR activation
It is clear that the UPR has a crucial function under physiological conditions, particularly during periods of high secretory demand, as shown by the loss of numerous haematopoietic cells (such as DCs, eosinophils and plasma cells) and parenchymal cells (such as hepatocytes, pancreatic β-cells, acinar cells of the salivary glands and Paneth cells) when important elements of the UPR are absent79. In addition to this physiological UPR, ER stress can further result from various intrinsic and environmental factors, such as inadequate energy supply (as observed during hypoxia and nutrient deprivation) and increased secretory demand during exposure to pathogens and inflammatory stimuli80. These environmental challenges induce UPR activation and thereby contribute to inflammation through various mechanisms.
Hypoxia
Hypoxia inhibits oxygen-dependent protein-folding processes, which directly affects disulfide bond formation during post-translational folding of proteins and cis–trans prolyl isomerization in the ER81, leading to ER stress. Hypoxia can occur in adipose tissue in the setting of obesity, probably as a result of the diffusion limit of oxygen imposed by the hypertrophic adipocytes. Adipocyte hypoxia has been shown to be associated with the induction of ER stress and the increased expression of CHOP, which is at least partly responsible for the reduced expression of adipocytokines that provide important anti-inflammatory functions82. This raises the possibility that hypoxia-induced ER stress may be linked to obesity-related metabolic diseases.
Hypoxia, owing to inefficient vascularization, is also a hallmark of the tumour microenvironment and is one of the factors that accounts for UPR activation in solid tumours. UPR activation in tumours triggers protective responses that enable the tumour to cope with conditions of low oxygen and nutrient supply83. Splicing of XBP1 mRNA was recently shown to provide a survival benefit to highly aggressive triple-negative breast cancer cells. The inhibition of XBP1 in these tumour cells using RNA interference decreased tumour growth and particularly affected angiogenesis, which links XBP1 to tumour hypoxia. Moreover, it was shown that XBP1 directly interacts with the central mediator of the cellular response to hypoxia, hypoxia-inducing factor 1α (HIF1α), and assists in regulating the expression of HIF1α target genes84. As HIF1α also has a prominent role in the antitumour immune response by inhibiting the effector functions of tumour-infiltrating lymphocytes85, targeting the UPR and HIF1α as connected pathways in solid tumours might provide a therapeutic benefit by boosting antitumour immune responses.
ROS
The oxidizing environment of the ER is optimized for disulfide bond formation, and the redox status of the ER is tightly regulated in mammalian cells86. This environment is primarily derived from ROS that are generated by endoplasmic reticulum oxireductin 1 (ERO1), with protein disulfide isomerase being responsible for disulfide bond formation. However, cellular ROS production increases considerably and to supraphysiological levels under ER stress and inflammatory conditions. These conditions are closely connected in a reciprocal manner such that they induce self-reinforcing pathways that may further promote inflammation4,87. ROS can be induced by a wide variety of immunological signals. These include, for example, TLR and TNF receptor signalling, which further trigger inflammatory responses by enhancing the phosphorylation of IκB to induce nuclear factor-κB (NF-κB) signalling88. Conversely, it has been shown that extracellular sources of ROS can induce ER stress, possibly by disrupting Ca2+ retention in the ER89. Once they are produced, ROS can also promote NLRP3 (NLR family, pyrin domain containing 3) inflammasome activation90 and can regulate lymphocyte function88 and, as discussed above, NOX2-derived ROS in macrophages are required for XBP1-dependent cytokine production following TLR2 and TLR4 ligation.
In mouse models of diabetes, ER stress and ROS production have recently been linked to inflammasome activation and pancreatic β-cell death in a pathway that involves the induction of thioredoxin-interacting protein (TXNIP). This protein reduces the anti oxidant effect of thioredoxin. Two recent studies have shown that ER stress-induced inflammasome activation and pancreatic β-cell apoptosis were preceded by increased Txnip mRNA expression through a pathway that involves IRE1α27 and/or PERK91. As a consequence, the genetic deletion of TXNIP partly protects against diabetes progression27.
Pathogens
Many pathogens interfere with the function of the host ER as part of their infectious life cycle, and can therefore activate distinct arms of the UPR. Pathogen-induced UPR activation can be beneficial to the host by enabling an innate immune response directed against the invading pathogens. However, invading pathogens can selectively modulate UPR pathways to promote their own survival92.
Viral replication requires the host ER for the production of viral structural and non-structural proteins, and as such viral replication interferes with the host protein synthesis machinery93. Not surprisingly, viral infection can trigger the UPR (reviewed in REF. 94). The IRE1α-mediated arm of the UPR can block viral replication in the case of respiratory syncytial virus (RSV), probably through RIDD-dependent viral RNA degradation, as XBP1 is not required for defence against RSV95. However, some viruses, such as Japanese encephalitis virus, use RIDD to their advantage to degrade host RNAs without affecting viral RNA96. Similarly, many viruses have evolved mechanisms to circumvent the disadvantageous consequences of the UPR; the selective activation of the ATF6α- and IRE1α-mediated arms of the UPR sustains viral replication by increasing the production of ER chaperones, and viruses simultaneously block PERK-mediated UPR activation to circumvent a translational block and/or ER stress-induced apoptosis. For example, herpes simplex virus type 1 selectively blocks the activation of the PERK-mediated arm of the UPR through the association of viral glycoprotein gB with the luminal domain of PERK97. Also, whereas hepatitis C virus (HCV) induces IRE1α and ATF6α activation, it suppresses the downstream effects of XBP1s to prevent the activation of ERAD, which enables HCV replication and contributes to the persistence of the virus in infected hepatocytes98,99.
An important example of how the UPR protects the host from bacterial infection has come from studies using Caenorhabditis elegans. In this nematode, infection with Pseudomonas aeruginosa induces a p38 MAPK-driven innate immune response, which in turn triggers the IRE1α–XBP1 pathway of the UPR. XBP1 activation is protective for the host, as xbp-1-mutant larvae exhibit ER disruption and increased lethality in response to infection. Interestingly, lethality could also be prevented by the loss of p38 MAPK, which shows that it is not the infection itself but rather the innate immune response that is lethal in the absence of a simultaneous protective XBP1-dependent UPR response100.
A recent study in primary bronchial epithelial cells has further supported the link between an innate immune response and the UPR by showing that virulence factors that are derived from P. aeruginosa strongly induce UPR activation in a p38 MAPK-dependent manner, as evidenced by Xbp1 splicing and the induction of BiP and CHOP. However, induction of GADD34 occurred through the activation of the ISR, which was associated with the improved survival of host cells101.
L. monocytogenes has a well-documented ability to induce the UPR, which was recently shown to occur without the active infection of cells by secretion of the cytolysin listeriolysin O. This toxin induces all branches of the UPR, possibly by altering intracellular Ca2+ homeostasis, which ultimately leads to apoptosis102. Similarly, the AB5 subtilase cytotoxin from Shiga-toxigenic Escherichia coli cleaves BiP, thus inducing all three arms of the UPR and resulting in ER stress-induced cell death103. Pathogen-associated molecular patterns may also activate specific arms of the UPR. For example, Streptomyces spp. produce a toxin (trierixin) that directly inhibits Xbp1 splicing44. By contrast, TLR2 and TLR4 ligands trigger the IRE1α– XBP1 arm of the UPR in macrophages while suppressing ATF4–CHOP signalling to promote macrophage survival during infections69,75. Thus, pathogens can either subvert or induce the UPR during their life cycles, and UPR induction may alert the immune system to the presence of these pathogens.
Cell damage and immunogenic cell death
Dying cells can elicit inflammatory responses both through the induction of cell surface receptors that are recognized by immune cells, such as the ER-resident protein calreticulin (CRT; also known as CALR), and through secreted factors, including high mobility group box 1 (HMGB1) and ATP104,105. HMGB1 activates all arms of the UPR in endothelial cells, resulting in increased expression of intercellular adhesion molecule 1 (ICAM1) and P-selectin, which probably function to attract immune cells to dying cells106. In splenic DCs, HMGB1 exposure induces UPR activation with increased levels of BiP and Xbp1 splicing; this pathway seems to be important for mounting an immune response, as silencing of Xbp1 is associated with the downregulation of the costimulatory cell surface receptors CD80 and CD86, decreased MHC class II expression and, subsequently, impaired stimulation of T cells in co-culture systems107.
Even before cells die and intracellular components such as HMGB1 are released to elicit inflammation, stressed cells induce immune responses; for example, the cell surface expression of CRT is a recognition signal for engulfment by antigen-presenting cells. Human bladder carcinoma T24 cells that are exposed to photo-dynamic therapy, which causes the ROS-mediated induction of ER stress, increase their cell surface expression of CRT and secretion of ATP through pathways involving PERK108. Interestingly, this was associated with the increased engulfment of cancer cells by DCs and increased DC expression of CD80, CD86 and MHC class II, as well as increased IL-1β and nitric oxide production, all of which are important for an effective antitumour CD8+ T cell response108. Increased cell surface expression of CRT also occurs in a PERK-dependent manner in cancer cells that have non-physiological increases in chromosome content (this is known as hyperploidy and occurs in the early stages of various cancers) and this is an important immunosurveillance system against hyperploidy in cancers109.
Whether UPR activation can drive the specific upregulation of ligands that are recognized by innate immune cells, including natural killer cells, requires further research. Interestingly, CHOP has been linked to cell death in human colon cancer HCT116 cells through the upregulation of death receptor 5 (DR5; also known as TNFRSF10B), which is a receptor for TNF-related apoptosis-inducing ligand (TRAIL; also known as TNFSF10). However, in this setting, DR5 drives apoptosis in a ligand-independent manner through the activation of caspase 8 (REF. 110). In addition, it is important to recognize that, although UPR activation in tumour cells can sometimes increase their immunogenicity and is therefore beneficial to the host, some tumours can also take advantage of the UPR to prevent antitumour immune responses (BOX 2). Elucidating the crosstalk between UPR activation in tumour cells, the release of damage-associated molecules, cell surface receptor expression and immune activation is of great importance to improve antitumour immune responses.
Box 2|Transmission of ER stress.
Can immune cells sense endoplasmic reticulum (ER) stress in other cells? Intriguing studies have shown that the activation of the unfolded protein response (UPR) in tumour cells can, through an unknown transmissible factor, induce ER stress in macrophages involving the upregulation of mRNAs encoding binding immunoglobulin protein (BiP), C/EBP homologous protein (CHOP) and GADD34 and increased splicing of the mRNA encoding X-box binding protein 1 (XBP1). This occurs in a Toll-like receptor 4 (TLR4)-dependent manner, which suggests that a TLR4 ligand is the transmissible factor. Importantly, ER stress-induced cell death was not responsible for the observed transmissible ER stress, which indicates that there is active secretion of the transmissible factor rather than a passive leakage of damage-associated molecules from dying cells165. Macrophages that were stimulated by neighbouring ER-stressed tumour cells had increased inflammatory responses, which is in keeping with previous observations that TLR4 ligation amplifies pro-inflammatory cytokine signalling and may be the responsible signalling moiety69. In addition to macrophages, dendritic cells (DCs) are also susceptible to an ER stress-transmissible factor that is secreted by tumour cells and that interferes with DC-mediated antigen cross-presentation to CD8+ T cells and results in a less effective antitumour immune response166. It was recently shown that XBP1s–induced increases in lipid metabolism in DCs could be the underlying mechanism of impaired MHC class I-mediated antigen presentation to cytotoxic T cells65.
It is not clear whether the unidentified transmissible factor is a byproduct of ER stress in tumour cells or is actively secreted by these cells to modulate the antitumour response. Identification of the ER stress-transmissible factor would greatly enhance our understanding of how tissue-specific ER stress can become systemic in nature. In addition, this could provide possible new therapeutic targets to improve the antitumour immune response.
The UPR as an inflammatory nidus
ER stress is implicated in various chronic pathological conditions that involve inflammation (such as metabolic diseases, inflammatory bowel diseases, atherosclerosis and neurodegenerative diseases, among others). The investigation of the pathogenic mechanisms involved has revealed a reciprocal regulation between ER stress and inflammation — whereby ER stress can directly initiate inflammatory pathways, and, in turn, pro-inflammatory stimuli such as ROS, TLR ligands and cytokines can trigger ER stress — such that the resulting UPR activation can further amplify inflammatory responses111.
ER stress-induced inflammatory signalling
NF-κB, a master transcriptional regulator of pro-inflammatory pathways, is activated by the interaction of IRE1α with TRAF2 in response to ER stress, which leads to the recruitment of IκB kinase (IKK) and the phosphorylation and subsequent degradation of IκB. This releases NF-κB for translocation to the nucleus112 (FIG. 5). The PERK– eIF2α-mediated and ATF6-mediated branches of the UPR activate NF-κB through different mechanisms from IRE1α. Engaging the PERK–eIF2α signalling pathway in response to ER stress halts overall protein synthesis and increases the ratio of NF-κB to IκB, owing to the shorter half-life of IκB, thereby favouring NF-κB-dependent transcription113,114 (FIG. 5). ATF6α activation through exposure to the bacterial subtilase cytotoxin can induce the phosphorylation of AKT to activate NF-κB115,116 (FIG. 5).
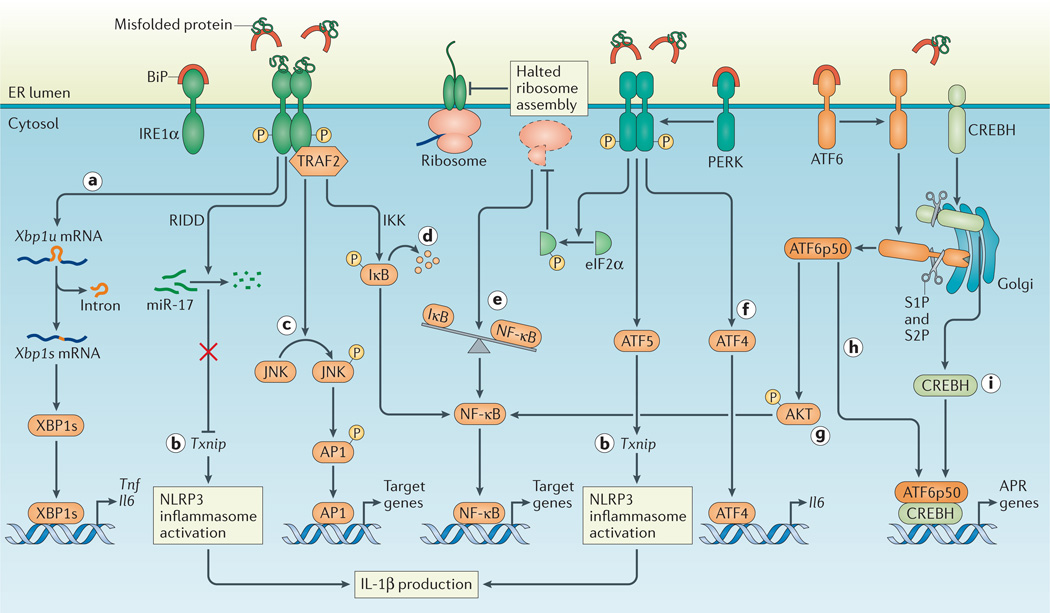
Figure 5. The UPR as an inflammatory nidus.
a | Inositol-requiring enzyme 1α (IRE1α) activation and subsequent splicing of X-box binding protein 1 (Xbp1) produces the transcription factor XBP1s that directly binds the promoters of tumour necrosis factor (Tnf) and interleukin-6 (Il6). b | Regulated IRE1α-dependent decay (RIDD)-dependent degradation of miR-17, which in unstressed conditions represses thioredoxin-interacting protein (Txnip), allows for increased TXNIP levels and NLRP3 inflammasome activation with upregulation of IL-1β. In addition, Txnip can be induced through the PKR-like ER kinase (PERK)-activating transcription factor 5 (ATF5) pathway to induce inflammasome activation. c | Activated IRE1α forms a complex with TNF receptor-associated factor 2 (TRAF2) to induce phosphorylation of Jun N-terminal kinase (JNK) and upregulation of pro-inflammatory genes through activated activator protein 1 (AP1). d | In addition, the IRE1α –TRAF2 complex recruits IκB kinase (IKK), and subsequent phosphorylation of IκB leads to its degradation, which releases nuclear factor-κB (NF-κB) for nuclear translocation. e | Translation attenuation by PERK-dependent phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) results in decreased translation of both IκB and NF-κB. However, owing to the shorter half-life of IκB, the net result is an increased NF-κB to IκB ratio, which promotes inflammation. f | ATF4 directly binds the Il6 promoter. g | ATF6α induces NF-κB signalling via AKT phosphorylation. h,i | Site 1 protease (S1P)- and S2P–mediated cleavage of ATF6α and CREBH allows their cleavage fragments to translocate to the nucleus and induce genes of the acute phase response (APR). BiP, binding immunoglobulin protein.
JNK, together with p38 and ERK, is one of the stress-inducible MAPKs that mediate a wide variety of responses, including but not limited to proliferation, autophagy and inflammation. The IRE1α–TRAF2 complex can, in addition to the activation of NF-κB, recruit apoptosis signal-regulating kinase 1 (ASK1; also known as MAP3K5) and can subsequently activate JNK, leading to the increased expression of pro-inflammatory genes through enhanced activator protein 1 (AP1) activity117,118 (FIG. 5). Loss of XBP1 in IECs of the small intestine results in the hyperactivation of IRE1α, which drives inflammatory responses, including cytokine production, that are partly mediated by the phosphorylation of JNK77. ER stress in human cancer cell lines has also been shown to trigger ERK activation through PI3K, which was associated with increased resistance to ER stress-induced cell death119.
UPR activation in immune cells and various stromal cells leads to the induction and secretion of cytokines, such as IL-6 and TNF120,121. In macrophages, XBP1s directly binds the Tnf and Il6 promoters to regulate their expression69, and ATF4 — downstream of PERK activation — functions as a transcription factor for Il6 (REF. 74) (FIG. 5). ER stress in pancreatic β-cells can trigger the activation of the NLRP3 inflammasome and IL-1β secretion through IRE1α-mediated and PERK-mediated induction of TXNIP27 (FIG. 5).
Conversely, cytokines themselves can directly regulate the UPR. IL-10, for example, can inhibit inflammation-induced ER stress by blocking the shuttling of ATF6p50 to the nucleus in a pathway that involves p38 MAPK122. However, circulating pro-inflammatory cytokines including IL-1, IL-6, CXCL8 (also known as IL-8) and TNF, can trigger UPR activation in the liver and can thereby stimulate the release of products of the acute phase response (APR), which amplify inflammatory responses to eliminate infection and to restore tissue homeostasis. Upon ER stress in hepatocytes, ATF6α and CREBH traffic to the Golgi to undergo S1P- and S2P–mediated cleavage; the cytosolic active transcription factors that are released induce the expression of APR genes, including those encoding C-reactive protein and serum amyloid P component111 (FIG. 5).
The pathological UPR in inflammatory disease
UPR-associated inflammatory pathways are increasingly being recognized to be involved in various complex inflammatory diseases. We discuss below several examples of autoimmune, metabolic and neoplastic conditions in which the UPR may have an important role.
Inflammatory bowel disease
Over the past decade, genome-wide association studies (GWAS) have identified various susceptibility loci for Crohn’s disease and ulcerative colitis, which are chronic inflammatory diseases of the gastrointestinal tract, collectively known as inflammatory bowel disease (IBD)123,124. Many of these risk genes encode proteins that have an important role in proteostasis. Orosomucoid-like 3 (ORMDL3), which is a risk locus for both Crohn’s disease125 and ulcerative colitis126 (as well as, interestingly, asthma127), encodes an ER transmembrane protein that activates ATF6α in lung epithelial cells and induces the expression of SERCA2B (also known as ATP2A2), which might be associated with airway remodelling127. In addition, ORMDL3 represses serine palmitoyltransferase activity and thereby decreases ceramide levels, which might protect against apoptosis128. However, the mechanism by which ORMDL3 is involved in IBD pathogenesis is unstudied. Candidate gene approaches have also identified anterior gradient 2 (AGR2)129 and XBP1 as risk loci for Crohn’s disease and ulcerative colitis. AGR2 is a member of the protein disulfide isomerase family and is particularly highly expressed by the secretory cells of the intestinal tract. In line with this, Agr2−/− mice have decreased expression of mucin 2 (MUC2) in goblet cells and abnormal localization of Paneth cells in the small intestine, which coincides with UPR activation and the development of spontaneous ileocolitis130.
The conditional deletion of Xbp1 in IECs causes ER stress and results in spontaneous inflammation of the small intestine and increased susceptibility to dextran sodium sulfate (DSS)-induced colitis77. As for AGR2 deficiency, the deletion of XBP1 in IECs particularly affects secretory cells, as Xbp1ΔIEC mice have decreased numbers of goblet cells and severely hypomorphic Paneth cells with decreased antimicrobial peptide production, which is associated with an increased susceptibility to infection with L. monocytogenes77. It is not completely clear how UPR activation in AGR2-deficient and Xbp1ΔIEC mice eventually causes intestinal inflammation. Xbp1ΔIEC mice show signs of IRE1α-dependent JNK phosphorylation and NF-κB activation; blockade of NF-κB activation, genetic deletion of IRE1α in IECs, or the systemic deletion of TNF receptor 1 protects Xbp1ΔIEC mice from spontaneous enteritis78,123. Therefore, IRE1α– NF-κB signalling, in a pathway that is driven by TNF, has a crucial role in the development of inflammation upon deletion of XBP1 in IECs.
As autophagy compensates for ER stress (BOX 1), the genetic deficiency of autophagy in Xbp1ΔIEC mice is associated with even more severe spontaneous enteritis that extends transmurally78. Indeed, signs of ER stress have been detected in Paneth cells in patients with Crohn’s disease who carry the risk allele of autophagy-related 16-like 1 (ATG16L1T300A)131. Moreover, as the ATG16L1T300A risk allele encodes a protein that is otherwise functionally intact but that has increased sensitivity to caspase 3-mediated cleavage132,133, and as XBP1 deletion in IECs leads to caspase 3 activation77, it is possible that in humans environmentally and/or genetically determined ER stress can reveal the phenotypic manifestations of this common risk variant134. The epithelium of the small intestine may be particularly sensitive to ER stress-induced caspase 3 activation and ATG16L1T300A cleavage as there is evidence for ER stress even under baseline, non-inflammatory conditions135.
In addition to IRE1α, the intestinal and lung epithelia express IRE1β (encoded by ERN2), particularly in goblet cells. Ern2−/− mice have increased basal levels of BiP in the intestinal epithelium, aberrant MUC2 accumulation in the ER of goblet cells136 and increased sensitivity to DSS-induced colitis137.
Various other perturbations in UPR signalling have been associated with intestinal inflammation. Mice that lack CREB3L1 (also known as OASIS)138,139, ATF6α, S1P or p58IPK are more susceptible to DSS-induced colitis137,186. Furthermore, nonphosphorylatable Ser51Ala eIF2α-mutant mice have defective UPR signalling in IECs, leading to secretory dysfunction of Paneth cells and increased sensitivity to oral Salmonella enterica subsp. enterica serovar Typhimurium infection and DSS-induced colitis140.
Forward genetic approaches using N-ethyl-N-nitrosourea mutagenesis have yielded mouse strains (known as Winnie and Eeyore) that carry single missense mutations in Muc2. These mutations cause misfolding of MUC2, which results in strong UPR activation and spontaneous ulcerative colitis-like colitis, characterized by inflammatory responses that involve both innate and adaptive immunity (including the IL-23–T helper 17 cell inflammatory axis)141,142.
Together, these studies show that the UPR is important in maintaining intestinal epithelial homeostasis in a highly complex intestinal luminal environment challenged by microorganisms and other external factors. However, intestinal inflammation can also be promoted by ER stress in the haematopoietic system of mucosal tissues, as shown by studies using HLA-B27 transgenic rats143, and the contribution of UPR activation in distinct cell types to intestinal inflammation requires further research.
Diabetes mellitus
Pancreatic β-cells rapidly increase protein synthesis during acute and chronic stimulation and are therefore dependent on a well-developed ER and on protein-quality control mechanisms. β-cell-specific deletion of IRE1α or XBP1 results in impaired β-cell proliferation, defective proinsulin synthesis and processing, and decreased insulin secretion144. Similarly, genetic deletion of PERK leads to ER stress-induced loss of pancreatic β-cells and a progressive decline in endocrine (but also exocrine) pancreatic function, with hyperglycaemia developing within 4 weeks145, which further emphasizes the central role of the UPR in pancreatic β-cell survival and diabetes. In Akita mice, the oxidative folding of proinsulin by ER-resident oxireductases, which is required to obtain the native shape of proinsulin and to allow its trafficking from the ER further down the secretory pathway, is impeded by the expression of a mutant form of proinsulin (Ins2 C96Y). This results in the accumulation of misfolded proinsulin proteins in the ER, which triggers UPR activation, β-cell inflammation and eventually β-cell death. This leads to diabetes in the mice as early as 4 weeks of age146,147, which is similar to PERK-deficient mice148. Importantly, IRE1α oligomer formation, which is indicative of high levels of chronic ER stress, is a crucial propagator of the phenotype of Akita mice, as pharmacological inhibition of IRE1α kinase activity alleviates the disease phenotype26.
ER stress-induced JNK activation occurs in the adipose and liver tissue of obese mice, whether obesity is induced by a high-fat diet or genetically through leptin deficiency (ob/ob mice). Obese mice develop insulin resistance through ER stress-mediated JNK-induced phosphorylation of insulin receptor substrate 1 (IRS1), which impairs insulin action and causes insulin resistance149. Importantly, lower levels of XBP1s in the livers of obese mice were recently shown to result from inducible nitric oxide synthase (iNOS; also known as NOS2)-dependent — and thus inflammation-dependent — S-nitrosylation of the RNase domain of IRE1α, although the kinase domain was unaffected. This resulted in decreased levels of protective XBP1s but maintained the levels of pro-inflammatory phosphorylated JNK. This study thereby demonstrates how obesity-induced inflammation can alter UPR signalling and provides a new link between inflammation, UPR activation and metabolic dysfunction150.
ROS production that is secondary to ER stress can be another trigger for inflammation as ROS production activates the NLRP3 inflammasome and IL-1β secretion in β-cells through increased levels of TXNIP, either as a result of RIDD-dependent decay of the Txnip-suppressing miR-17 or through the PERK pathway, ultimately leading to β-cell apoptosis and diabetes27,91. Indeed, the suppression of JNK activity protects β-cells from oxidative stress and ameliorates glucose tolerance151. Although this is a field of extensive research, much still needs to be learned about the interplay between ER stress, inflammation and the development of metabolic diseases such as diabetes, as the UPR could be an attractive signalling pathway for therapeutic intervention.
Non-alcoholic steatohepatitis
The crucial role of the UPR in the liver was first discovered in 2000, when it was shown that XBP1 was required for liver development152. In the liver, the ER and UPR are highly important for lipid synthesis and metabolism, in addition to their well-known role in protein quality control153. UPR activation in the liver has been extensively studied in light of the increasing prevalence of non-alcoholic fatty liver disease (NAFLD), which is the leading cause of non-alcoholic and non-viral liver-associated illness and death in the United States154. Hepatic steatosis, which is the mildest form of NAFLD, can progress to non-alcoholic steatohepatitis (NASH) through a process that involves free fatty acid and lipid accumulation in hepatocytes followed by a series of innate immune responses in leukocytes, including in liver-resident macrophages that are known as Kupffer cells155. It has been shown that ER stress can induce liver steatosis through effects on lipid synthesis and inflammation, but high-fat feeding-induced steatosis itself can also trigger ER stress, thereby providing a positive feedback loop that amplifies liver inflammation and injury156. In turn, UPR activation can modulate inflammatory signalling to induce liver inflammation, partly through JNK activation, and κB phosphorylation leading to NF-κB activation112,113, which is an important mediator of methionine- and choline-deficient (MCD) diet-dependent development of NASH157. In addition, the accumulation of lipids in hepatocytes can lead to mitochondrial dysfunction with increased ROS levels, which further triggers downstream inflammatory responses. The importance of ROS in the development of NASH has specifically been shown in mice deficient in NRF2 (also known as NFE2L2), a protein that is crucially involved in the antioxidant response and that can be induced by PERK; Nrf2−/− mice develop NASH more rapidly than wild-type mice when fed high-fat, high-cholesterol or MCD diets158.
Cancer
The activation of the UPR in cancers can initiate transcriptional programmes that allow tumour cells to combat harsh environmental conditions such as hypoxia, oxidative stress and low nutrient availability. In addition, these transcriptional programmes can actively shape a tumorigenic proinflammatory milieu. For example, UPR activation in prostate cancer cells results in the transcriptional upregulation of IL6 and TNF59, the promoters of which contain functional binding sites for XBP1s69. These proinflammatory mediators may promote inflammation-induced malignancies160,161. Moreover, both the PERK-mediated162 and IRE1α-mediated163 arms of the UPR in tumour cells can increase the transcription of genes that encode pro-angiogenic mediators such as vascular endothelial growth factor A (VEGFA), fibroblast growth factor 2 (FGF2) and IL-6 (REFS 162–164).
Intriguingly, UPR activation in cancer cells can also affect the antitumour immune response. Soluble factors that are secreted by ER-stressed tumour cells, but not by non-stressed tumour cells, can upregulate proinflammatory cytokine expression in macrophages, including the tumorigenic cytokines IL-6, IL-23p19 and TNF165. These soluble factors are also involved in dampening the antitumour immune response by inhibiting antigen presentation by antigen-presenting cells to cytotoxic CD8+ T cells65,166. The mechanism by which this occurs is only partly elucidated but may include ROS-induced ER stress in DCs, transmitted through an as yet undefined factor, which increases lipid synthesis through XBP1 activation and thereby disrupts antigen presentation65.
Conclusions
Much has been learned about the functions of the UPR beyond being simply a means to manage ER stress. The UPR has also now been recognized for its role in immune cell differentiation and function, and in regulating immune and inflammatory responses, including those associated with infections, tumours and autoimmune responses. It is clear that the UPR, and thus a certain level of ER stress, is crucial for cellular and, consequently, tissue homeostasis (the ‘eustress’ response). Therefore, understanding how the balance tips towards a pathophysiological UPR (the ‘distress’ response) that is associated with disease, and how this balance can be manipulated to enable appropriate immune responses and to restore homeostasis, are important directions for future research.
With the development of therapeutic agents that enhance proteostasis or that interfere with specific components of the UPR (BOX 3), used either alone or together, the hope is that an improved understanding of the UPR in immunity and inflammation will eventually lead to the development of novel therapeutic strategies for chronic immune-mediated diseases.
Box 3|Therapeutic opportunities.
Tauro-ursodeoxycholic acid (TUDCA) and 4-phenyl butyrate (PBA) are small-molecule chaperones that contribute to proper protein folding in the endoplasmic reticulum (ER); they have proved successful in alleviating ER stress-induced hyperglycaemia, restoring insulin sensitivity and ameliorating fatty liver disease in obese mice177. TUDCA and PBA have also been shown to reduce ER stress in the intestinal epithelium and thereby decrease the severity of dextran sodium sulfate (DSS)-induced colitis178. In addition, lipopolysaccharide (LPS)-induced lung inflammation was reduced by PBA, through decreasing ER stress and modulating the nuclear factor-κB (NF-κB) and hypoxia-inducing factor 1 α (HIF1α) signalling pathways179.
In haematological malignancies that produce large amounts of protein, such as multiple myeloma, blocking the 26S proteasome with bortezomib induces the activation of the PKR-like ER kinase (PERK)-mediated unfolded protein response (UPR) pathway, which increases activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP) activity and sensitizes multiple myeloma cells to apoptosis180. In addition, blockade of inositol-requiring enzyme 1 α (IRE1α) endonuclease activity with the small-molecule inhibitor MKC-3946 increases the death of multiple myeloma cells in response to the proteasome inhibitor bortezomib181. In line with this, CD138+ plasma cells from patients with multiple myeloma are highly prone to cell death after treatment with STF-083010, another specific IRE1α RNase domain inhibitor, which further illustrates the importance of X-box binding protein 1 (XBP1) in plasma cells. In addition, STF-083010 has potent cytotoxic effects in multiple myeloma cell lines and xenograft models182.
Sunitinib is a receptor tyrosine kinase inhibitor that targets the receptors for platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) and thereby affects tumour angiogenesis and tumour cell proliferation. It has been approved by the United States Food and Drug Administration for the treatment of renal cell carcinoma and gastrointestinal stromal tumours. However, sunitinib also seems to influence the kinase activity of IRE1α183 and protein kinase R (PKR)-dependent phosphorylation of eukaryotic translation initiation factor 2α (eIF2α)184. Therefore, sunitinib is potentially beneficial for the treatment of UPR-prone cancers but it has also been shown to have negative effects on the anti-viral immune response, as measured by decreased levels of interferon-β (IFNβ) in response to infection with encephalomyocarditis virus, which was dependent on decreased RNase L activity184. This study exemplifies that broadly targeting the UPR might have important side effects that must be considered.
Finally, given the recent reports showing that UPR activation in cancers can modulate the tumour immune microenvironment, antigen presentation by dendritic cells and thereby antitumour immune responses, targeting the UPR in cancers may improve tumour recognition by the immune system or, synergistically, may improve the efficacy of immunotherapy in cancer.
Acknowledgments
The authors thank M. Wang for assistance with preparation of this Review and apologize to those whose work was not included owing to size limitations. This work was supported by the Netherlands Organization for Scientific Research Rubicon grant 825.13.012 (J.G.); US National Institutes of Health (NIH) grants DK044319, DK051362, DK053056 and DK088199, and the Harvard Digestive Diseases Center (HDDC) grant DK034854 (R.S.B.); NIH grants DK042394, DK088227, DK103183 and CA128814 (R.J.K.); and European Research Council (ERC) Starting Grant 260961, ERC Consolidator Grant 648889, and the Wellcome Trust Investigator award 106260/Z/14/Z (A.K.).
Glossary
- ER-associated degradation (ERAD)
A pathway that removes terminally misfolded proteins from the endoplasmic reticulum (ER) through their retrotranslocation to the cytosol, which targets them for degradation by the ubiquitin-proteasome system.
- Unfolded protein response (UPR)
A highly conserved pathway that regulates the balance between the folding capacity of the endoplasmic reticulum (ER) and protein synthesis.
- Integrated stress response (ISR)
An ancient stress response that modulates protein biosynthesis by integrating various types of stress signals, including endoplasmic reticulum (ER) stress, amino acid deprivation, virus infection and oxidative stress.
- Paneth cells
Highly specialized small-intestinal epithelial cells that shape the composition of the microbiota through the secretion of antimicrobial proteins and that sustain and modulate epithelial stem cells by the secretion of niche factors.
- Acute phase response (APR)
A group of systemic and innate physiological processes in the early response to infection or injury.
- Crohn’s disease
An inflammatory disease of the small and large intestines that is thought to arise from an inappropriate immune response towards the intestinal microbiota in a genetically susceptible host.
- Ulcerative colitis
A chronic disease of the colon with unknown aetiology, characterized by inflammation and ulceration in the colon.
- Non-alcoholic fatty liver disease (NAFLD)
Liver disease characterized by the accumulation of fat (steatosis) in the liver which is often associated with obesity. Although NAFLD is benign it can progress towards steatohepatitis and even cirrhosis.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer. 2014;14:581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 3.Sevier CS, Kaiser CA. Formation and transfer of disulphide bonds in living cells. Nat. Rev. Mol. Cell Biol. 2002;3:836–847. doi: 10.1038/nrm954. [DOI] [PubMed] [Google Scholar]
- 4.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 2013;14:630–642. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 7.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 9.Smith MH, Ploegh HL, Weissman JS. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science. 2011;334:1086–1090. doi: 10.1126/science.1209235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pincus D, et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8:e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–1894. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding HP, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 13.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 14.Scheuner D, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 15.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 17.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korennykh AV, et al. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457:687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 20.Hassler JR, et al. The IRE1α/XBP1s pathway is essential for the glucose response and protection of β cells. PLoS Biol. 2015;13:e1002277. doi: 10.1371/journal.pbio.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 22.Maurel M, Chevet E, Tavernier J, Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 2014;39:245–254. doi: 10.1016/j.tibs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Eckard SC, et al. The SKIV2L RNA exosome limits activation of the RIG-I-like receptors. Nat. Immunol. 2014;15:839–845. doi: 10.1038/ni.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin JH, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutkowski DT, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghosh R, et al. Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell. 2014;158:534–548. doi: 10.1016/j.cell.2014.07.002.. This study shows that the oligomerization state of the cytosolic domains of IRE1α, which increases under chronic ER stress, crucially determines the balance between the adaptive UPR and the terminal UPR.
- 27.Lerner AG, et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell. Metab. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Upton JP, et al. IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic caspase-2. Science. 2012;338:818–822. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu S, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volmer R, van der Ploeg K, Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc. Natl Acad. Sci. USA. 2013;110:4628–4633. doi: 10.1073/pnas.1217611110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Claudio N, Dalet A, Gatti E, Pierre P. Mapping the crossroads of immune activation and cellular stress response pathways. EMBO J. 2013;32:1214–1224. doi: 10.1038/emboj.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravindran R, et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature. 2016;531:523–527. doi: 10.1038/nature17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl Acad. Sci. USA. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J. Biol. Chem. 2011;286:10939–10949. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marciniak SJ, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chitnis NS, et al. miR-211 is a prosurvival microRNA that regulates chop expression in a PERK-dependent manner. Mol. Cell. 2012;48:353–364. doi: 10.1016/j.molcel.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woo CW, Kutzler L, Kimball SR, Tabas I. Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat. Cell Biol. 2012;14:192–200. doi: 10.1038/ncb2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, et al. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Kouroku Y, et al. ER stress (PERK/eIF2α phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 43.Talloczy Z, et al. Regulation of starvation- and virus-induced autophagy by the eIF2α kinase signaling pathway. Proc. Natl Acad. Sci. USA. 2002;99:190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tashiro E, et al. Trierixin a novel Inhibitor of ER stress-induced XBP1 activation from Streptomyces sp. 1. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. (Tokyo) 2007;60:547–553. doi: 10.1038/ja.2007.69. [DOI] [PubMed] [Google Scholar]
- 45.Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J. Cell Biol. 2010;189:783–794. doi: 10.1083/jcb.201003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner CA, Jr, Mack DH, Davis MM. Blimp-1, anovel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 47.Sciammas R, et al. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–236. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Shaffer AL, et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21:81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Reimold AM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 50.Todd DJ, et al. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J. Exp. Med. 2009;206:2151–2159. doi: 10.1084/jem.20090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwakoshi NN, et al. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nature Immunol. 2003;4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- 52.van Anken E, et al. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity. 2003;18:243–253. doi: 10.1016/s1074-7613(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 53.Hu CC, Dougan SK, McGehee AM, Love JC, Ploegh HL. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO J. 2009;28:1624–1636. doi: 10.1038/emboj.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benhamron S, et al. Regulated IRE1-dependent decay participates in curtailing immunoglobulin secretion from plasma cells. Eur. J. Immunol. 2014;44:867–876. doi: 10.1002/eji.201343953. [DOI] [PubMed] [Google Scholar]
- 55.Brunsing R, et al. B-and T-cell development both involve activity of the unfolded protein response pathway. J. Biol. Chem. 2008;283:17954–17961. doi: 10.1074/jbc.M801395200. [DOI] [PubMed] [Google Scholar]
- 56.Zhang K, et al. The unfolded protein response sensor IRE1α is required at 2 distinct steps in B cell lymphopoiesis. J. Clin. Invest. 2005;115:268–281. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kharabi Masouleh B, et al. Mechanistic rationale for targeting the unfolded protein response in pre-B acute lymphoblastic leukemia. Proc. Natl Acad. Sci. USA. 2014;111:E2219–E2228. doi: 10.1073/pnas.1400958111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamimura D, Bevan MJ. Endoplasmic reticulum stress regulator XBP-1 contributes to effector CD8+ T cell differentiation during acute infection. J. Immunol. 2008;181:5433–5441. doi: 10.4049/jimmunol.181.8.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J. Exp. Med. 2007;204:2267–2275. doi: 10.1084/jem.20070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Osorio F, et al. The unfolded-protein-response sensor IRE-1α regulates the function of CD8α + dendritic cells. Nat. Immunol. 2014;15:248–257. doi: 10.1038/ni.2808.. This study shows how XBP1 deletion in mature conventional DCs hyperactivates IRE1α and RIDD, thereby degrading mRNAs that encode proteins that are important for cross-presentation, and thus interfering with DC function.
- 61.Iwawaki T, Akai R, Kohno K, Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 2004;10:98–102. doi: 10.1038/nm970. [DOI] [PubMed] [Google Scholar]
- 62.Granados DP, et al. ER stress affects processing of MHC class I-associated peptides. BMC Immunol. 2009;10:10. doi: 10.1186/1471-2172-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qian SB, et al. Tight linkage between translation and MHC class I peptide ligand generation implies specialized antigen processing for defective ribosomal products. J. Immunol. 2006;177:227–233. doi: 10.4049/jimmunol.177.1.227. [DOI] [PubMed] [Google Scholar]
- 64.Herber DL, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cubillos-Ruiz JR, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161:1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Furuta GT, Atkins FD, Lee NA, Lee JJ. Changing roles of eosinophils in health and disease. Ann. Allergy Asthma Immunol. 2014;113:3–8. doi: 10.1016/j.anai.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chu VT, et al. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40:582–593. doi: 10.1016/j.immuni.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 68.Bettigole SE, et al. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat. Immunol. 2015;16:829–837. doi: 10.1038/ni.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 2010;11:411–418. doi: 10.1038/ni.1857.. This study shows that the IRE1α-XBP1 arm of the UPR is crucially involved in macrophage cytokine responses to TLR ligation in a pathway that involves TRAF6 and the NAPDH oxidase NOX2.
- 70.Qiu Y, et al. A crucial role for RACK1 in the regulation of glucose-stimulated IRE1α activation in pancreatic β cells. Sci. Signal. 2010;3:ra7. doi: 10.1126/scisignal.2000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiu Q, et al. Toll-like receptor-mediated IRE1α activation as a therapeutic target for inflammatory arthritis. EMBO J. 2013;32:2477–2490. doi: 10.1038/emboj.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao C, et al. Cellular stress amplifies TLR3/4-induced CXCL1/2 gene transcription in mononuclear phagocytes via RIPK1. J. Immunol. 2014;193:879–888. doi: 10.4049/jimmunol.1303396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim S, et al. Endoplasmic reticulum stress-induced IRE1α activation mediates cross-talk of GSK-3β and XBP-1 to regulate inflammatory cytokine production. J. Immunol. 2015;194:4498–4506. doi: 10.4049/jimmunol.1401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iwasaki Y, et al. Activating transcription factor 4 links metabolic stress to interleukin-6 expression in macrophages. Diabetes. 2014;63:152–161. doi: 10.2337/db13-0757. [DOI] [PubMed] [Google Scholar]
- 75. Woo CW, et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by Toll-like receptor signalling. Nat. Cell Biol. 2009;11:1473–1480. doi: 10.1038/ncb1996.. This study shows that TLR-TRIF signalling in macrophages suppresses CHOP to inhibit ER stress-induced apoptosis during the host response to pathogen invasion.
- 76.Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu. Rev. Physiol. 2013;75:289–311. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- 77.Kaser A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Adolph TE, et al. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272–276. doi: 10.1038/nature12599.. This study shows that XBP1 deletion in epithelial cells, or specifically in Paneth cells, induces ileitis. In addition, further loss of compensatory autophagy, resulting from concomitant deletion of ATG16L1 in XBP1-deficient epithelial cells, induces transmural Crohn’s disease-like ileitis.
- 79.Bettigole SE, Glimcher LH. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 2015;33:107–138. doi: 10.1146/annurev-immunol-032414-112116. [DOI] [PubMed] [Google Scholar]
- 80.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat. Rev. Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 81.Koritzinsky M, et al. Two phases of disulfide bond formation have differing requirements for oxygen. J. Cell Biol. 2013;203:615–627. doi: 10.1083/jcb.201307185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hosogai N, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 83.Wang G, Yang ZQ, Zhang K. Endoplasmic reticulum stress response in cancer: molecular mechanism and therapeutic potential. Am. J. Transl Res. 2010;2:65–74. [PMC free article] [PubMed] [Google Scholar]
- 84.Chen X, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature. 2014;508:103–107. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143:512–519. doi: 10.1111/imm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsunoda S, et al. Intact protein folding in the glutathione-depleted endoplasmic reticulum implicates alternative protein thiol reductants. eLife. 2014;3:e03421. doi: 10.7554/eLife.03421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malhotra JD, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl Acad. Sci. USA. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 90.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 91.Oslowski CM, et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell. Metab. 2012;16:265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Celli J, Tsolis RM. Bacteria, the endoplasmic reticulum and the unfolded protein response: friends or foes? Nat. Rev. Microbiol. 2015;13:71–82. doi: 10.1038/nrmicro3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walsh D, Mohr I. Viral subversion of the host protein synthesis machinery. Nat. Rev. Microbiol. 2011;9:860–875. doi: 10.1038/nrmicro2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li S, Kong L, Yu X. The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit. Rev. Microbiol. 2015;41:150–164. doi: 10.3109/1040841X.2013.813899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hassan I, et al. Inositol-requiring enzyme 1 inhibits respiratory syncytial virus replication. J. Biol. Chem. 2014;289:7537–7546. doi: 10.1074/jbc.M113.510594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhattacharyya S, Sen U, Vrati S. Regulated IRE1-dependent decay pathway is activated during Japanese encephalitis virus-induced unfolded protein response and benefits viral replication. J. Gen. Virol. 2014;95:71–79. doi: 10.1099/vir.0.057265-0. [DOI] [PubMed] [Google Scholar]
- 97.Mulvey M, Arias C, Mohr I. Maintenance of endoplasmic reticulum (ER) homeostasis in herpes simplex virus type 1-infected cells through the association of a viral glycoprotein with PERK, a cellular ER stress sensor. J. Virol. 2007;81:3377–3390. doi: 10.1128/JVI.02191-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tardif KD, Mori K, Kaufman RJ, Siddiqui A. Hepatitis C virus suppresses the IRE1-XBP1 pathway of the unfolded protein response. J. Biol. Chem. 2004;279:17158–17164. doi: 10.1074/jbc.M312144200. [DOI] [PubMed] [Google Scholar]
- 99.Zheng Y, et al. Hepatitis C virus non-structural protein NS4B can modulate an unfolded protein response. J. Microbiol. 2005;43:529–536. [PubMed] [Google Scholar]
- 100. Richardson CE, Kooistra T, Kim DH. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature. 2010;463:1092–1095. doi: 10.1038/nature08762.. This study shows that XBP1 induction is crucial for the ability of C. elegans to survive the consequences of mounting an immune response to microorganisms.
- 101.van ‘t Wout EF, et al. Virulence factors of Pseudomonas aeruginosa induce both the unfolded protein and integrated stress responses in airway epithelial cells. PLoS Pathog. 2015;11:e1004946. doi: 10.1371/journal.ppat.1004946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pillich H, Loose M, Zimmer KP, Chakraborty T. Activation of the unfolded protein response by Listeria monocytogenes. Cell. Microbiol. 2012;14:949–964. doi: 10.1111/j.1462-5822.2012.01769.x. [DOI] [PubMed] [Google Scholar]
- 103.Paton AW, et al. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443:548–552. doi: 10.1038/nature05124. [DOI] [PubMed] [Google Scholar]
- 104.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 106.Luo Y, Li SJ, Yang J, Qiu YZ, Chen FP. HMGB1 induces an inflammatory response in endothelial cells via the RAGE-dependent endoplasmic reticulum stress pathway. Biochem. Biophys. Res. Commun. 2013;438:732–738. doi: 10.1016/j.bbrc.2013.07.098. [DOI] [PubMed] [Google Scholar]
- 107.Zhu XM, et al. Endoplasmic reticulum stress and its regulator XBP-1 contributes to dendritic cell maturation and activation induced by high mobility group box-1 protein. Int. J. Biochem. Cell Biol. 2012;44:1097–1105. doi: 10.1016/j.biocel.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 108.Garg AD, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Senovilla L, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337:1678–1684. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- 110.Lu M, et al. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345:98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhang K, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040.. This study shows that inflammatory signals in the liver activate the UPR, resulting in S1P- and/or S2P–mediated cleavage of CREBH to free its nuclear fraction and to allow the transcription of acute-phase response genes.
- 112.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factorα links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1 α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deng J, et al. Translational repression mediates activation of nuclear factor κB by phosphorylated translation initiation factor 2. Mol. Cell. Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tam AB, Mercado EL, Hoffmann A, Niwa M. ER stress activates NF-κB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS ONE. 2012;7:e45078. doi: 10.1371/journal.pone.0045078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakajima S, et al. Selective abrogation of BiP/GRP78 blunts activation of NF-κB through the ATF6 branch of the UPR: involvement of C/EBPβ and mTOR-dependent dephosphorylation of Akt. Mol. Cell. Biol. 2011;31:1710–1718. doi: 10.1128/MCB.00939-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamazaki H, et al. Activation of the Akt-NF-κB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J. Immunol. 2009;183:1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eferl R, Wagner E. F AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 118. Urano F, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664.. This study demonstrates that ER stress-induced IRE1α activation results in the recruitment of TRAF2 to activate JNK and downstream inflammatory signalling.
- 119.Hu P, Han Z, Couvillon AD, Exton JH. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J. Biol. Chem. 2004;279:49420–49429. doi: 10.1074/jbc.M407700200. [DOI] [PubMed] [Google Scholar]
- 120.Li Y, et al. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-α and interleukin-6: model of NF-κB- and map kinase-dependent inflammation in advanced atherosclerosis. J. Biol. Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 121.Meares GP, et al. PERK-dependent activation of JAK1 and STAT3 contributes to endoplasmic reticulum stress-induced inflammation. Mol. Cell. Biol. 2014;34:3911–3925. doi: 10.1128/MCB.00980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shkoda A, et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 123.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu. Rev. Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Van Limbergen J, Wilson DC, Satsangi J. The genetics of Crohn’s disease. Annu. Rev. Genomics Hum. Genet. 2009;10:89–116. doi: 10.1146/annurev-genom-082908-150013. [DOI] [PubMed] [Google Scholar]
- 125.Barrett JC, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat. Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McGovern DP, et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat. Genet. 2010;42:332–337. doi: 10.1038/ng.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moffatt MF, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 128.Kiefer K, et al. Coordinated regulation of the orosomucoid-like gene family expression controls de novo ceramide synthesis in mammalian cells. J. Biol. Chem. 2015;290:2822–2830. doi: 10.1074/jbc.M114.595116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zheng W, et al. Evaluation of AGR2 and AGR3 as candidate genes for inflammatory bowel disease. Genes Immun. 2006;7:11–18. doi: 10.1038/sj.gene.6364263. [DOI] [PubMed] [Google Scholar]
- 130.Zhao F, et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev. Biol. 2010;338:270–279. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Deuring JJ, et al. Genomic ATG16L1 risk allele-restricted Paneth cell ER stress in quiescent Crohn’s disease. Gut. 2013;63:1081–1091. doi: 10.1136/gutjnl-2012-303527. [DOI] [PubMed] [Google Scholar]
- 132.Lassen KG, et al. Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc. Natl Acad. Sci. USA. 2014;111:7741–7746. doi: 10.1073/pnas.1407001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Murthy A, et al. A Crohn’s disease variant in Atg16l1 enhances its degradation by caspase 3. Nature. 2014;506:456–462. doi: 10.1038/nature13044. [DOI] [PubMed] [Google Scholar]
- 134.Hampe J, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 135.Bogaert S, et al. Involvement of endoplasmic reticulum stress in inflammatory bowel disease: a different implication for colonic and ileal disease? PLoS ONE. 2011;6:e25589. doi: 10.1371/journal.pone.0025589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsuru A, et al. Negative feedback by IRE1β optimizes mucin production in goblet cells. Proc. Natl Acad. Sci. USA. 2013;110:2864–2869. doi: 10.1073/pnas.1212484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bertolotti a, et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1β-deficient mice. J. Clin. Invest. 2001;107:585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Asada R, et al. The endoplasmic reticulum stress transducer OASIS is involved in the terminal differentiation of goblet cells in the large intestine. J. Biol. Chem. 2008;287:8144–8153. doi: 10.1074/jbc.M111.332593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hino K, Saito A, Asada R, Kanemoto S, Imaizumi K. Increased susceptibility to dextran sulfate sodium-induced colitis in the endoplasmic reticulum stress transducer OASIS deficient mice. PLoS ONE. 2014;9:e88048. doi: 10.1371/journal.pone.0088048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cao SS, et al. Phosphorylation of eIF2α is dispensable for differentiation but required at a posttranscriptional level for paneth cell function and intestinal homeostasis in mice. Inflamm. Bowel Dis. 2014;20:712–722. doi: 10.1097/MIB.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 141.Heazlewood CK, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eri RD, et al. An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol. 2011;4:354–364. doi: 10.1038/mi.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human β2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 144.Lee AH, Heidtman K, Hotamisligil GS, Glimcher LH. Dual and opposing roles of the unfolded protein response regulated by IRE1α and XBP1 in proinsulin processing and insulin secretion. Proc. Natl Acad. Sci. USA. 2011;108:8885–8890. doi: 10.1073/pnas.1105564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harding HP, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 146.Ron D. Proteotoxicity in the endoplasmic reticulum: lessons from the Akita diabetic mouse. J. Clin. Invest. 2002;109:443–445. doi: 10.1172/JCI15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang J, et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. J. Clin. Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Oyadomari S, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 2002;109:525–532. doi: 10.1172/JCI14550.. This study reveals that inflammation decreases levels of protective XBP1s in the liver through S-nitrosylation of the RNase domain of IRE1α, thereby negatively affecting glucose homeostasis.
- 149.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 150.Yang L, et al. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science. 2015;349:500–506. doi: 10.1126/science.aaa0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kaneto H, et al. Oxidative stress and the JNK pathway in diabetes. Curr. Diabetes Rev. 2005;1:65–72. doi: 10.2174/1573399052952613. [DOI] [PubMed] [Google Scholar]
- 152.Reimold AM, et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000;14:152–157. [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang K, et al. The unfolded protein response transducer IRE1α prevents ER stress-induced hepatic steatosis. EMBO J. 2011;30:1357–1375. doi: 10.1038/emboj.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Milic S, Stimac D. Nonalcoholic fatty liver disease/steatohepatitis: epidemiology, pathogenesis, clinical presentation and treatment. Dig. Dis. 2012;30:158–162. doi: 10.1159/000336669. [DOI] [PubMed] [Google Scholar]
- 155.Wenfeng Z, et al. Kupffer cells: increasingly significant role in nonalcoholic fatty liver disease. Ann. Hepatol. 2014;13:489–495. [PubMed] [Google Scholar]
- 156.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dela Pena A, et al. NF-κB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129:1663–1674. doi: 10.1053/j.gastro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 158.Gupte AA, Lyon CJ, Hsueh WA. Nuclear factor (erythroid-derived 2)-like-2 factor (Nrf2), a key regulator of the antioxidant response to protect against atherosclerosis and nonalcoholic steatohepatitis. Curr. Diab.Rep. 2013;13:362–371. doi: 10.1007/s11892-013-0372-1. [DOI] [PubMed] [Google Scholar]
- 159.Mahadevan NR, Fernandez A, Rodvold JJ, Almanza G, Zanetti M. Prostate cancer cells undergoing ER stress in vitro and in vivo activate transcription of pro-inflammatory cytokines. J. Inflamm. Res. 2010;3:99–103. doi: 10.2147/JIR.S11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pikarsky E, et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 161.Clarke HJ, Chambers JE, Liniker E, Marciniak SJ. Endoplasmic reticulum stress in malignancy. Cancer Cell. 2014;25:563–573. doi: 10.1016/j.ccr.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 162.Blais JD, et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol. Cell. Biol. 2006;26:9517–9532. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Auf G, et al. Inositol-requiring enzyme 1α is a key regulator of angiogenesis and invasion in malignant glioma. Proc. Natl Acad. Sci. USA. 2010;107:15553–15558. doi: 10.1073/pnas.0914072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang Y, et al. The unfolded protein response induces the angiogenic switch in human tumor cells through the PERK/ATF4 pathway. Cancer Res. 2012;72:5396–5406. doi: 10.1158/0008-5472.CAN-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mahadevan NR, et al. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc. Natl Acad. Sci. USA. 2011;108:6561–6566. doi: 10.1073/pnas.1008942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mahadevan NR, et al. Cell-extrinsic effects of tumor ER stress imprint myeloid dendritic cells and impair CD8+ T cell priming. PLoS ONE. 2012;7:e51845. doi: 10.1371/journal.pone.0051845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Suh DH, Kim MK, Kim HS, Chung HH, Song YS. Unfolded protein response to autophagy as a promising druggable target for anticancer therapy. Ann. NY Acad. Sci. 2012;1271:20–32. doi: 10.1111/j.1749-6632.2012.06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.B’Chir W, et al. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41:7683–7699. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hetz C, et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Qin L, Wang Z, Tao L, Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010;6:239–247. doi: 10.4161/auto.6.2.11062. [DOI] [PubMed] [Google Scholar]
- 173.Hart LS, et al. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J. Clin. Invest. 2012;122:4621–4634. doi: 10.1172/JCI62973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rouschop KM, et al. The unfolded protein response protects human tumor cells during hypoxiathrough regulation of the autophagy genes MAP1LC3B and ATG 5 . J. Clin. Invest. 2010;120:127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bernales S, Schuck S, Walter P. ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy. 2007;3:285–287. doi: 10.4161/auto.3930. [DOI] [PubMed] [Google Scholar]
- 176.Khaminets A, et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522:354–358. doi: 10.1038/nature14498. [DOI] [PubMed] [Google Scholar]
- 177.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cao SS, et al. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology. 2013;144:989–1000. doi: 10.1053/j.gastro.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim HJ, et al. Inhibition of endoplasmic reticulum stress alleviates lipopolysaccharide-induced lung inflammation through modulation of NF-κB/HIF-1α signaling pathway. Sci. Rep. 2013;3:1142. doi: 10.1038/srep01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mujtaba T, Dou QP. Advances in the understanding of mechanisms and therapeutic use of bortezomib. Discov. Med. 2011;12:471–480. [PMC free article] [PubMed] [Google Scholar]
- 181.Mimura N, et al. Blockade of XBP1 splicing by inhibition of IRE1α is a promising therapeutic option in multiple myeloma. Blood. 2012;119:5772–5781. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Papandreou I, et al. Identification of an Ire1α endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ali MM, et al. Structure of the Ire1 autophosphorylation complex and implications for the unfolded protein response. EMBO J. 2011;30:894–905. doi: 10.1038/emboj.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jha BK, et al. Inhibition of RNase L and RNA-dependent protein kinase (PKR) by sunitinib impairs antiviral innate immunity. J. Biol. Chem. 2011;286:26319–26326. doi: 10.1074/jbc.M111.253443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Connor JH, et al. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol. Cell Biol. 2001;21:6841–6850. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brandl K, et al. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proc. Natl Acad. Sci. USA. 2009;106:3300–3305. doi: 10.1073/pnas.0813036106. [DOI] [PMC free article] [PubMed] [Google Scholar]