Abstract
Patient: Male, 73
Final Diagnosis: Henoch-Schönlein purpura (HSP)
Symptoms: Abdominal pain • bloating • blood in stool • nausea • vomiting
Medication: —
Clinical Procedure: EGD • colonoscopy • kidney biopsy • skin biopsy • arthrocentesis
Specialty: Rheumatology
Objective:
Unknown ethiology
Background:
Henoch-Schönlein purpura (HSP), a small vessel vasculitis mediated by deposition of immune-complexes containing IgA in the skin, gut, and glomeruli, often presents with abdominal pain, purpuric rash in the lower extremities and buttocks, joint pain, and hematuria. The disease most commonly targets children but can affect adults who tend to have a worse prognosis.
Case Report:
We discuss a case of HSP in an elderly Chinese male who presented with severe proximal bowel inflammation, vasculitic rash, and proteinuria; he was found to have positive stool rotavirus and giardia. He improved significantly with high dose steroids. We believe rotavirus may have been a triggering event in this patient. A brief review of the literature is also presented.
Conclusions:
This is the first case report describing a classic presentation of HSP in an adult following a rotavirus infection. HSP can cause significant morbidity and mortality in adult patients predominantly from progressive renal failure; therefore careful management and monitoring is important. GI infections seem to be a common trigger for HSP and this case report suggests that rotavirus may be part of the spectrum.
MeSH Keywords: Autoimmune Diseases; Gastrointestinal Tract; Purpura, Schoenlein-Henoch; Rotavirus Infections; Rotavirus Vaccines; Vasculitis
Background
Henoch-Schönlein purpura (HSP) is a small vessel vasculitis mediated by deposition of immune-complexes containing IgA in the skin, gut, and glomeruli [1,2]. The disease constellation was first described in the 1800s in young boys with abdominal pain, vomiting, melena, joint pain, a purpuric rash, and hematuria [1,2]. William Osler believed the disease was an anaphylaxis reaction [1,2]. We now know that HSP is primarily a vasculitis involving deposition of IgA immune complexes [3]. However, the etiology of HSP is still uncertain; factors such as infection and hypersensitivity triggering the disease have been reported [3]. In adults, malignancy and drug exposure have been associated with HSP, which differs from drug-induced hypersensitivity vasculitis as the latter lacks IgA deposition [4–6]. Gastrointestinal (GI) infections with campylobacter, helicobacter pylori, and other organisms have been reported to trigger HSP [7]. Our case suggests that rotavirus infection also may trigger the disease in adults.
Case Report
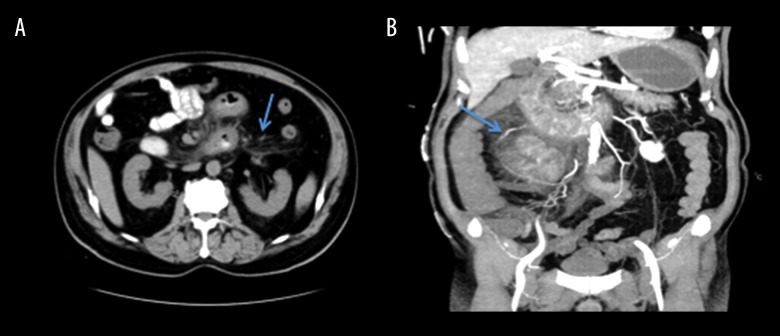
A 73-year-old Chinese man who was in his usual state of health presented with 2–3 days of progressively worsening lower abdominal pain, dark stools, nausea, vomiting, and bloating. Prior to this, he had two weeks of constipation, which evolved into loose, dark stool one week prior to presentation. He travels frequently between China and the United States, but denied recent illness, tick or animal bites, or the addition of new medicines. His past medical history included chronic obstructive pulmonary disease, coronary artery disease, type II diabetes mellitus, and resection of a right parasagittal meningioma. On examination, he had a mildly distended abdomen with tenderness in the lower abdomen. Fecal occult blood test was positive, white blood cell count (WBC) was 14 k/mcL, and lactate was normal. Computerized tomography (CT) of the abdomen and pelvis showed markedly abnormal loops of proximal small bowel beginning in the distal duodenum and extending into the proximal jejunum manifested by extensive stranding and marked wall thickening (Figure 1A). He was managed conservatively for suspected non-specific small bowel inflammation with improvement in leukocytosis and abdominal pain, and was discharged on empiric antibiotics, including levofloxacin and metronidazole for seven days and pantoprazole 40 mg daily.
Figure 1.
Computerized tomography (CT). (A) Slice of CT of the abdomen and pelvis with contrast showing stranding around proximal small bowel (arrow) consistent with small bowel inflammation. (B) Coronal slice of a CT angiography (CTA) of the abdomen and pelvis showing proximal small bowel inflammation demonstrated by significant stranding around loops of bowel (arrow) and normal blood vessels with IV contrast.
He returned three days later with worsening and more diffuse abdominal pain, recurrent hematochezia, nausea and vomiting. CT angiography of the abdomen and pelvis showed an increase in the extent of small bowel inflammation proximally, involving more of the duodenum and jejunum without mesenteric ischemia (Figure 1B). A wireless capsule study was attempted, but the capsule was retained in the stomach. Upon retrieval of the capsule with enteroscopy and esophagogastroduodenoscopy (EGD), he was found to have ulcers at the gastroesophageal (GE) junction, erythematous mucosa throughout the stomach, and extensive ulcerations in the second portion of the duodenum without evidence of Helicobacter pylori (H. pylori) or other signs of infection. Biopsies of the lesions revealed severe active enteritis with ulceration and lamina propria hemorrhage, without H. pylori, cytomegalovirus (CMV) or herpes simplex viral inclusions. He developed ileus and was managed with nasogastric tube (NGT) suction with high gastric outputs, and supportive total parenteral nutrition (TPN). Testing for tuberculosis, Yersinia, CMV, human immunodeficiency virus (HIV), Epstein-bar virus (EBV), hepatitis B virus (HBV), hepatitis C virus (HCV), H. pylori, salmonella, campylobacter, toxoplasma, shigella, ova and parasites, and clostridium difficile were all negative. Stool studies for giardia and rotavirus that were collected from the first admission were found to be positive during his second admission. He was treated with nitazoxonide. Hematology was consulted for hypogammaglobulinemia (IgG and IgM were low, IgA normal) and to rule out lymphoma, myeloproliferative disorders or solid tumors. Blood smear, bone marrow biopsy, flow cytometry, and various mutation testing were all unremarkable. Repeat EGD with colonoscopy showed slightly improved duodenal ulcers, and a tubular adenoma and hyperplastic polyps, respectively.
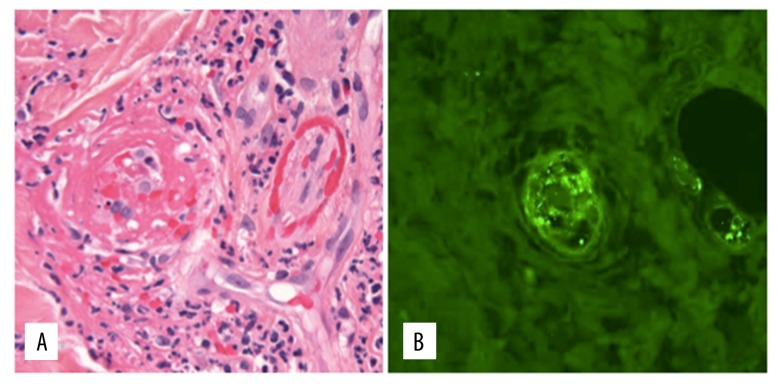
Three days after his second admission, he developed left knee pain and a purpuric rash in his palms, soles, wrists, and ankles. Skin biopsy disclosed a leukocytoclastic vasculitis more consistent with a primary vasculitis rather than a drug rash. Immunofluorescence performed after antigen retrieval of formalin fixed paraffin embedded tissue sections disclosed granular IgA deposition in the dermal vessels (Figure 2). He was started on 40 mg daily IV Solu-Medrol with gradual improvement of his symptoms, including abdominal pain, rash, and hematochezia. Left knee joint arthrocentesis showed clear fluid with 85 WBCs, no crystals or bacteria, consistent with non-inflammatory arthritis. Laboratory studies revealed ANA 1: 40, ANCA negative, C3 66 mg/dL (reference range: 88–252 mg/dL), C4 13 mg/dL (reference range: 12–72 mg/dL), erythrocyte sedimentation rate (ESR) 55 mm/hour and C-reactive protein (CRP), 72.2 mg/dL, with normalization of inflammatory markers with steroid treatment.
Figure 2.
Skin biopsy. (A) Dermal small vessel vasculitis showing fibrin, neutrophils, and karyorrhectic debris within and surrounding vessel walls with extravasated erythrocytes (H & E ×400). (B) Granular deposition of IgA within dermal vessels (following antigen retrieval on formalin fixed paraffin embedded tissue, ×400).
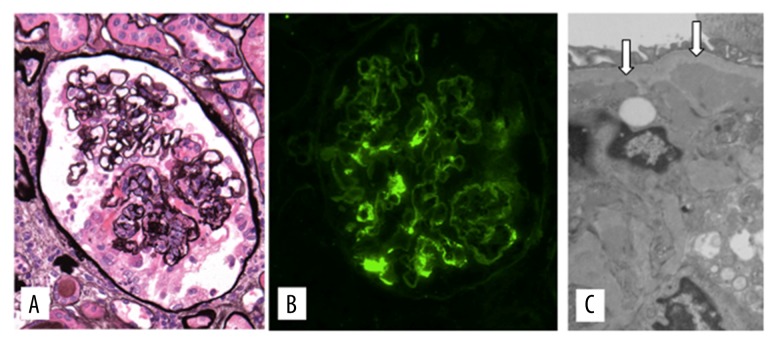
His urine analysis (UA) showed 2+ protein with a protein creatinine ratio (UPC) of 1.7 g/g, 15 red blood cell (RBC) and 18 WBC with normal serum creatinine of 0.7 mg/dL. UA done several months prior to this event was normal. He was diagnosed with HSP given the abdominal, renal, skin, and joint findings. Despite being treated with steroids, he developed facial and lower extremity edema with a rising creatinine to 1.2 mg/dL and worsening proteinuria, now 2.9g/24 hours, prompting renal biopsy (Figure 3).
Figure 3.
Kidney biopsy. (A) Glomerulus showing a segmental necrotizing and crescentic lesion, and mild mesangial widening but without mesangial hypercellularity (Jones methenamine silver ×240). (B) Glomerulus staining for IgA in mesangial regions (×240). (C) Electron micrograph with mesangial electron dense deposits (arrows) often beneath the paramesangial basement membrane (original magnification ×10,000).
The renal biopsy contained a total of 60 glomeruli of which 11 were globally sclerotic. And 20% of the glomeruli had active necrotizing and crescentic lesions and 10% had endocapillary hyper-cellularity without mesangial hypercellularity. Immunofluorescence disclosed mesangial staining for IgA with lesser staining for IgM, C3, fibrinogen, and both light chains, and mesangial deposits were identified ultrastructurally consistent with HSP.
He was managed with higher doses of IV steroids and angiotensin converting enzyme inhibitor (ACEI) for three weeks, with stabilization of renal function, and he was discharged with a prolonged oral steroid taper. One month later, he had improved but with persistent hematuria (115 to 37 RBC) and proteinuria (UPC 3+ to 2+). Screening colonoscopy with biopsy revealed rare CMV inclusions, which were treated with two weeks of IV ganciclovir. He completed the steroid taper after two months and has continued to do well.
Discussion
Henoch-Schönlein’s purpura (HSP) is a small vessel vasculitis mediated by deposition of immune-complexes containing IgA in the skin, gut, and glomeruli [1,2]. Histologically, patients affected with HSP have polymorphonuclear leukocyte infiltration of small vessel walls with leukocytoclasia and immunofluorescence showing IgA deposits [1–3]. The clinical features of HSP include palpable purpura, arthritis, gastrointestinal (GI) involvement, and glomerulonephritis [1–3]. GI involvement is very common affecting 50%–80% of patients and can often precede purpura [8]. Nephritis is a serious complication of HSP, which infrequently may result in end stage renal failure [8–11].
HSP mainly affects children with an annual incidence estimated to be 15 cases/100,000 per year compared to 1.3 cases/100,000 per year in adults [4]. Although the incidence of HSP is lower in adults, studies suggest adults have more renal disease compared to children [5,6,9–11]. Uppal et al. compared adults and children with HSP and found that renal involvement was more frequent and severe in adults (90% in adults versus 52.4% in children). Adults had more diarrhea and leukocytosis, required more aggressive therapy and had longer hospital stays [9–11]. Outcome was also slightly worse in adults with complete recovery in 90% compared to 98.8% in children [9–11]. Therefore, age at onset is considered an important factor for disease manifestation, severity, and outcome [9–11]. Risk factors for progression to end-stage renal disease (ESRD) following HSP in children include older age, abdominal symptoms, low factor XIII activity, and persisting purpura [9–11]. However, prognostic factors in adults are less clear and there are no convincing and carefully done studies examining risk factors for developing ESRD following HSP in adults.
There are different criteria for HSP developed by the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR); but both require palpable purpura, abdominal pain and evidence of small vessel vasculitis on biopsy [12,13]. The diagnostic criteria do not include serologic findings but IgA level is often elevated [14,15]. The serum IgA was normal in our patient, which is unusual. However, Fahima et al. reported a case of HSP in a middle-aged man who presented with enteritis and had normal IgA levels [16]. Yang et al. found IgA anti-endothelial cell antibodies (AECA) positively correlated with HSP and could stimulate IL-8 production in endothelial cells [14,15]. It is possible that IgA levels could be normal in HSP but undergo alteration of the IgA profile. Furthermore, our patient had low IgG and IgM but normal IgA levels, suggesting a relatively increased IgA level in the setting of low baseline total immunoglobulin levels.
Complement levels are usually normal in HSP, C3 was low and C4 was borderline normal in our patient. However, there are reports of post-infectious development of HSP associated with hypocomplementemia [18]. Lin et al. identified 53 children with HSP and usually transiently decreased C3 and/or C4 levels, and found this did not impact the extent of nephritis or outcome; however, this cohort had elevated ASO titers suggesting a prior triggering infection [19]. Yan-xiang et al. suggested that complement activation by immunoglobulin in patients with HSP plays an important role in renal injury [17]. Future studies are needed to investigate whether C3 and C4 levels have any prognostic value in terms of renal involvement in adults with HSP [17–19].
Adults are also more likely than children to have secondary causes of HSP [6]. HSP has been associated with hypersensitivity to drugs [20–27]. Cancer, blunt trauma, monoclonal IgA gammopathy, Wiskott-Aldrich syndrome carrier status, and chronic alcoholic liver disease also have been reported to cause HSP [28–32]. Lahita described a 22 year old female with recurrent episodes of HSP since puberty occurring monthly with menstruation, who achieved remission during pregnancy, and in a dose responsive manner when prescribed birth control pills containing progesterone and estradiol, suggesting a pathologic role of male sex hormones [33].
Rotavirus is a non-enveloped virus first described in 1973 by Bishop and colleagues and is responsible for half of all cases of diarrhea requiring hospital admission amongst children younger than 2 years [34,35]. In contrast, most non-bacterial causes of adult gastroenteritis are related to Norwalk-like viruses [34]. Rotavirus outbreaks typically occur in the winter/spring seasons [35]. Our patient presented in the spring, which is consistent with epidemiological data of peak rotavirus infections. There are seven distinct groups of rotavirus with groups A, B, and C reported as human pathogens, with group A, the main detected pathogen worldwide [34]. Adults tend to shed less virus, which may be undetectable by most routine assays [34]. The mechanism by which rotavirus induces diarrhea is still poorly understood and most infections are self-limited. The presentation of adult acute rotavirus infection is highly variable and includes diarrhea, fever, headache, malaise or cramping [34]. Interestingly, asymptomatic infections may occur in up to 40% of infected adults [34]. Past infections with rotavirus do not prevent reinfections, as the initial antibody response to infection is serotype specific [34]. However, rotavirus reinfection is associated with fewer symptoms and an antibody response [34]. Our patient presented in the spring, the peak time for rotavirus infection, and was asymptomatic as many adults are. It also is possible that our patient was a carrier for rotavirus without having rotaviral disease. Champsaur et al. in 1984 studied 179 children and discovered that viral carriage, without infection or serologic response, was as high as 27% [36]. Since GI symptoms often precede the development of purpura and nephritis, it is reasonable to suspect that HSP in children may be related to asymptomatic rotavirus infection. Although rotavirus infection is a relatively rare in adults, it also may be a contributor to HSP in this population.
Giardia also has been reported to be associated with HSP [7]. It was unlikely the sole cause of HSP in our patient given lack of diarrhea or history to suggest chronic giardiasis. However, it is possible that giardia may have been a predisposing factor with concomitant or superimposed rotavirus infection triggering active HSP. Additionally, our patient was found to have cytomegalovirus (CMV) on follow-up, which may be reactivation or de novo infection. Ninomiya et al. described a 61year-old male treated for HSP who was later found to have CMV as a complication of the patient’s immunosuppressed state [37]. Murakami et al. described a 14-year-old boy who suffered from reactivation of latent CMV after diagnosis and treatment for HSP [38]. These are similar to our case, in which CMV was identified following immunosuppression with high dose steroids. D’Alessandro et al. suggested that CMV vasculitides may mimic HSP, rather than being sequelae of steroid treatment or temporary immunosuppression [39]. It is unlikely that CMV vasculitis was the cause of our patient’s presentation as multiple endoscopic exams, GI biopsies, and serologic tests were negative for CMV at presentation, and rare CMV inclusions were found only after steroid treatment. Nonetheless, viral vasculitides should remain in the differential while evaluating a patient with HSP.
Herein we describe a 73-year-old Asian gentleman who presented with GI symptoms and was found to have rotavirus and giardia infections, who later developed a cutaneous vasculitis and glomerulonephritis diagnostic for HSP. While rotavirus is a common cause of diarrhea in children, infection in adults is rare in the developed world [35]. Various pathogens have been associated with HSP, including bacteria, viruses, and protozoa; to date, there have been no reported cases or studies associating rotavirus infections with HSP [7,40,41]. However, a link between rotavirus and HSP may be inferred from reports of disease following rotavirus vaccination. Felicetti et al. found that 15.5% of adults and up to 34.5% of children developed a vasculitis following vaccination, with HSP the most frequent lesion, most often associated with meningococcal, measles, and HPV vaccines [42]. Currently, universal rotavirus vaccination is considered safe and effective, and is recommended in infants [43]. The seroresponse rate to rotavirus vaccine is most importantly determined by the anti-rotavirus IgA seroconversion rate, emphasizing the importance of IgA production in the immunologic response to rotavirus infection [44,45]. Vaccine side effects include intussusception, gastroenteritis and vasculitis, with vasculitides associated with rotavirus vaccines almost entirely reported in infants less than one year of age, with 91.5% of these the Kawasaki disease type [46]. It is possible that this rise in IgA titer is associated with post-vaccination HSP, likely in predisposed patients.
In addition to infection, certain genetic variations have been associated with susceptibility to and pathogenesis of HSP. A role for genetic predisposition to HSP was suggested by epidemiologic differences across different ethnic groups, and familial aggregation of HSP [47]. Major histocompatibility complex (MHC) and non-MHC genes, proinflammatory cytokines genes, endothelial nitric oxide synthase (eNOS), matrix metalloproteinase (MMPs), MEFV gene, and many others have been implicated as possible predisposing or protective factors in the pathogenesis of HSP [48–62]; however, most of these studies were limited to the pediatric population and focused on specific ethnic groups. An et al. studied the association between C1GALT1 polymorphisms, a gene coding an enzyme important for kidney homeostasis, and the risk of HSP in a Chinese population [49]. They found that certain single nucleotide polymorphisms (SNPs) and genotypes of C1GALT1 were associated with significantly higher risk of developing HSP [49]. Lopez-Majias et al. found that certain HLA alleles were associated with susceptibility for HSP while others have a protective effect [50,51]. Ding et al. recently studied polymorphisms in heat-shock protein 70-2 (HSP70-2) and tumor necrosis factor alpha (TNF-α) and found the HSP70-2 (+1267A/G) and TNF-α (+308G/A) gene polymorphisms were associated with HSP in a Chinese cohort of children [53]. HSP70-2 polymorphisms previously were associated with certain rheumatic diseases such as ankylosing spondylitis (AS) and systemic lupus erythematosis (SLE) [55,56]. Chen et al. showed that certain genotypes of PAX2, an essential nuclear transcription factor in the development of human embryonic kidney, may be associated with higher risk of renal involvement in children with HSP [60]. Mahsa et al. found association between carriage of IL-1 receptor antagonist allele 2 and allele A of the IL-8 gene and increased susceptibility to renal involvement [61,62]. It is therefore likely that under certain environmental influences or following an infection, individuals with a particular geno-type would be predisposed to developing HSP.
Conclusions
In conclusion, this patient was found to have giardia and rotavirus infections with diarrhea in association with active HSP. This is the first case report describing a classic presentation of HSP in an adult following a rotavirus infection. HSP can cause significant morbidity and mortality in adult patients predominantly from progressive renal failure, therefore careful management and monitoring is important. GI infections appear to be a common trigger for HSP and this case report suggests that rotavirus may be part of this spectrum.
References:
- 1.Chen JY, Mao JH. Henoch-Schönlein purpura nephritis in children: Incidence, pathogenesis and management. World J Pediatr. 2015;11(1):29–34. doi: 10.1007/s12519-014-0534-5. [DOI] [PubMed] [Google Scholar]
- 2.Davin J-C, Berge IJT, Weening JJ. What is the difference between IgA nephropathy an Henoch-Schonlein purpura nephritis? Kidney Int. 2001;59:823–34. doi: 10.1046/j.1523-1755.2001.059003823.x. [DOI] [PubMed] [Google Scholar]
- 3.Rostoker G. Schönlein-henoch purpura in children and adults: Diagnosis, pathophysiology and management. BioDrugs. 2001;15(2):99–138. doi: 10.2165/00063030-200115020-00004. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Porrua C, Gonzalez-Gay MA. Comparative clinical epidemiological study of hypersensitivity vasculitis versus Henoch-Schonlein purpura in adults. Semin Arthritis Rheum. 1999;28(6):404–12. doi: 10.1016/s0049-0172(99)80006-7. [DOI] [PubMed] [Google Scholar]
- 5.Kang Y, Park J, Ha Y, et al. Differences in clinical manifestations and outcomes between adult and child patients with Henoch-Schonlein Purpura. J Korean Med Sci. 2014;29:198–203. doi: 10.3346/jkms.2014.29.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uppal SS, Hussain MAS, Al-Raqum HA, et al. Henoch-Schonlein’s purpura in adults versus children/adolescents: A comparative study. Clin Exp Rheumatol. 2006;24(Suppl. 41):S26–30. [PubMed] [Google Scholar]
- 7.Rigane D, Catellazzi L, Bosco A, Esposito S. Is there a crossroad between infections, genetics, and Henoch-Schonlein purpura? Autoimmun Rev. 2013;12(10):1016–21. doi: 10.1016/j.autrev.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Chen SY, Kong MS. Gastrointestinal manifestations and complications of Henoch-Schonlein purpura. Chang Gung Med. 2004;27:175–81. [PubMed] [Google Scholar]
- 9.Chang WL, Yang YH, Wang LC, et al. Renal manifestations in Henoch-Schonlein purpura: A 10-year clinical study. Pediatr Nephrol. 2005;20:1269–72. doi: 10.1007/s00467-005-1903-z. [DOI] [PubMed] [Google Scholar]
- 10.Almeida JLJ, Campos LMA, Paim LB. Renal involvement in Henoch-Schönlein purpura: A multivariate analysis of initial prognostic factors. Pediatr (Rio J) 2007;83(3):259–66. doi: 10.2223/JPED.1638. [DOI] [PubMed] [Google Scholar]
- 11.Mohey H, Laurent B, Mariat C, Berthoux F. Validation of the absolute renal risk of dialysis/death in adults with IgA nephropathy secondary to Henoch-Schonlein purpura: A monocentric cohort study. BMC Nephrology. 2013;14:169. doi: 10.1186/1471-2369-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69:798–806. doi: 10.1136/ard.2009.116657. [DOI] [PubMed] [Google Scholar]
- 13.Mills JA, Michel BA, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of Henoch-Schonlein purpura. Arthritis Rheum. 1990;33(8):1114–21. doi: 10.1002/art.1780330809. [DOI] [PubMed] [Google Scholar]
- 14.Yang YH, Wang SJ, Chuang YH, et al. The level of IgA antibodies to human umbilical vein endothelial cells can be enhanced by TNF-α treatment in children with Henoch-Schonlein purpura. Clin Exp Immunol. 2002;130:352–57. doi: 10.1046/j.1365-2249.2002.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawakami T, Watabe H, Mizoguchi M, Soma Y. Elevated serum IgA anticardiolipin antibody levels in adult Henoch-Schonlein purpura. British J Derm. 2006;154:983–87. doi: 10.1111/j.1365-2133.2006.07457.x. [DOI] [PubMed] [Google Scholar]
- 16.Fahima DK, Atluri P, Wasser K, Baig N. Atypical presentation of IgA vasculitis in an adult. Med Sci Case Rep. 2016;3:32–36. [Google Scholar]
- 17.Pan Yx, Ye Q, Shao Wx, et al. Relationship between immune parameters and organ involvement in children with Henoch-Schonlein purpura. PLoS One. 2014;9(12):e115261. doi: 10.1371/journal.pone.0115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera F, Anaya S, Perez-Alvarez J, et al. Henoch-Schonlein nephritis associated with streptococcal infection and persistent hypocomplementemia: A case report. J Med Case Rep. 2010;4:50. doi: 10.1186/1752-1947-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Q, Min Y, Li Y, et al. Henoch-Schonlein purpura with hypocomplementemia. Pediatr Nephrol. 2012;27(5):801–6. doi: 10.1007/s00467-011-2070-z. [DOI] [PubMed] [Google Scholar]
- 20.Moors RJ, Keeling PJ, Morgan SH. Adult Schonlein-Henoch purpura after enalapril. Lancet. 1992;340:304–5. doi: 10.1016/0140-6736(92)92391-r. [DOI] [PubMed] [Google Scholar]
- 21.Drago F, Arditi MR, Rebora R. Henoch-Schonlein induced by luoroquinolones. Br J Dermatol. 1994;131:448. doi: 10.1111/j.1365-2133.1994.tb08538.x. [DOI] [PubMed] [Google Scholar]
- 22.Prajapati C, Casson IF. Henoch-Schonlein purpura associated with ranitidine. Int J Clin Pract. 1997;51:251. [PubMed] [Google Scholar]
- 23.Chevalier X, Rostoker G, Larget-Piet B, et al. Schonlein-Henoch purpura with necrotizing vasculitis after cocaine snorting. Clin Nephrol. 1995;43:348–49. [PubMed] [Google Scholar]
- 24.Kaneko K, Igarashi J, Suzuki Y, et al. Carbamazepine-induced thrombocytopenia and leucopenia complicated by Henoch-Scholein purpura symptoms. Eur J Pediatr. 1993;152:769–70. doi: 10.1007/BF01953999. [DOI] [PubMed] [Google Scholar]
- 25.Niedermaier G, Briner V. Henoch-Schonlein syndrome induced by carbidopa/levodopa. Lancet. 1997;349:1071–72. doi: 10.1016/S0140-6736(05)62294-5. [DOI] [PubMed] [Google Scholar]
- 26.Michail S, Vaiopoulos G, Nakopoulou L, et al. Henoch-Schonlein purpura and acute interstitial nephritis after intravenous vancomycin administration in a patient with a staphylococcal infection. Scand J Rheumatol. 1998;27:233–35. doi: 10.1080/030097498440886. [DOI] [PubMed] [Google Scholar]
- 27.Sola Alberich R, Jammoul A, Masana L. Henoch-Schonlein purpura associated with acetyl salicylic acid. Clin Exp Immunol. 1995;100:470–74. [Google Scholar]
- 28.Cairns SA, Mallick NP, Lawler W, et al. Squamous cell carcinoma of bronchus presenting with Henoch-Schonlein purpura. Br Med J. 1978;2:474–75. doi: 10.1136/bmj.2.6135.474-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talbot D, Craig R, Falconer S, et al. Henoch-Schonlein purpura secondary to trauma. Arch Dis Child. 1988;63:1114–15. doi: 10.1136/adc.63.9.1114-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dosa S, Cairns SA, Mallick MP, et al. Relapsing Henoch-Schonlein syndrome with renal involvement in a patient with an IgA monoclonal gammopathy. Nephron. 1980;26:145–48. doi: 10.1159/000181970. [DOI] [PubMed] [Google Scholar]
- 31.Lasseur K, Allen AC, Deminie’re C, et al. Henoch-Schonlein purpura with IgA nephropathy and abnormalities of IgA in a Wiskott-Aldrich syndrome carrier. Am J Kidney Dis. 1997;2:285–87. doi: 10.1016/s0272-6386(97)90043-3. [DOI] [PubMed] [Google Scholar]
- 32.Feriozzi S, Onetti Muda A, Faraggiana T, et al. Henoch-Schonlein disease with IgA nephropathy associated with chronic alcoholic liver disease. Nephrol Dial Transplant. 1995;10:1231–33. [PubMed] [Google Scholar]
- 33.Lahita MJ. Henoch-schonlein purpura remitting in pregnancy and during sex steroid therapy. Br J Rheumatol. 1994;33:586–88. doi: 10.1093/rheumatology/33.6.586. [DOI] [PubMed] [Google Scholar]
- 34.Anderson EJ, Weber SG. Rotavirus infection in adults. Lancet Infect Dis. 2004;4:91–99. doi: 10.1016/S1473-3099(04)00928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardy DB. Epidemiology of rotaviral infection in adults. Rev Infect Dis. 1987;9(3):461–69. doi: 10.1093/clinids/9.3.461. [DOI] [PubMed] [Google Scholar]
- 36.Champsaur H, Henry-Amar M, Goldszmidt D, et al. Rotavirus carriage, asymptomatic infection, and disease in the first two years of life. II. Serological Response. J Infect Dis. 1984;149(5):675–82. doi: 10.1093/infdis/149.5.675. [DOI] [PubMed] [Google Scholar]
- 37.Ninomiya R, Omi T, Kato T, et al. IgA vasculitis complicated by cytomegalo-virus enteritis: A case report. J Nippon Med Sch. 2014;81(1):48–52. doi: 10.1272/jnms.81.48. [DOI] [PubMed] [Google Scholar]
- 38.Murakami H, Takahashi S, Kawakubo Y, et al. Adolescent with Henoch-Schonlein purpura glomerulonephritis and intracranial hemorrhage possibly secondary to reactivation of latent CMV. Pediatr Int. 2008;50:112–15. doi: 10.1111/j.1442-200X.2007.02531.x. [DOI] [PubMed] [Google Scholar]
- 39.D’Alessandro M, Buoncompagni A, Minoia F, et al. Cytomegalovirus-related necrotizing vasculitis mimicking Henoch-Schonlein syndrome. Clin Exp Rheumatol. 2014;32(3 Suppl. 82):S73–75. [PubMed] [Google Scholar]
- 40.Mroczkowska EK, Pańczyk-Tomaszewska M, Szmigielska A, et al. Mycoplasma pneumoniae as a trigger for Henoch-Schönlein purpura in children. Cent Eur J Immunol. 2015;40(4):489–92. doi: 10.5114/ceji.2015.56976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong LJ, Mao M. Current views of the relationship between Helicobacter pylori and Henoch-Schonlein purpura in children. World J Clin Pediatr. 2016;5(1):82–88. doi: 10.5409/wjcp.v5.i1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felicetti P, Trotta F, Bonetto C, et al. Spontaneous reports of vasculitis as an adverse event following immunization: A descriptive analysis across three international databases. Vaccine. 2016;34(51):6634–40. doi: 10.1016/j.vaccine.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 43.Cortese MM, Parashar UD, Centers for Disease Control and Prevention (CDC) Prevention of rotavirus gastroenteritis among infants and children recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep. 2009;58(RR-2):1–25. [PubMed] [Google Scholar]
- 44.Ramani S, Mamani N, Villena R, et al. Rotavirus serum IgA immune response in children receiving Rotarix® Co-administered with bOPV or IPV. Pediatr Infect Dis J. 2016;35(10):1137–39. doi: 10.1097/INF.0000000000001253. [DOI] [PubMed] [Google Scholar]
- 45.Lambertu LM, Ashraf S, Walker CF, Black RE. A systematic review of the effect of rotavirus vaccination on diarrhea outcomes among children younger than 5 years. Pediatr Infect Dis J. 2016;35(9):992–98. doi: 10.1097/INF.0000000000001232. [DOI] [PubMed] [Google Scholar]
- 46.Prelog M, Gorth P, Zwazl I, et al. Universal mass vaccination against Rotavirus: Indirect effects on Rotavirus infections in neonates and non-vaccinated small infants not eligible for vaccination. J Infect Dis. 2016;214(4):546–55. doi: 10.1093/infdis/jiw186. [DOI] [PubMed] [Google Scholar]
- 47.He X, Yu C, Zhao P, et al. The genetics of Henoch-Schonlein purpura: A systematic review and meta-analysis. Rheum Int. 2013;33:1387–95. doi: 10.1007/s00296-012-2661-4. [DOI] [PubMed] [Google Scholar]
- 48.Altug U, Ensari G, Sayin DB, Ensari A. MEFV gene mutations in Henoch-Schonlein purpura. Int J Rheum Dis. 2013;16:347–51. doi: 10.1111/1756-185X.12072. [DOI] [PubMed] [Google Scholar]
- 49.An J, Lu Q, Zhao H, et al. A study on the association between C1GALT1 polymorphisms and the risk of Henoch-Schonlein purpura in a Chinese population. Rheumatol Int. 2013;33:2539–42. doi: 10.1007/s00296-013-2761-9. [DOI] [PubMed] [Google Scholar]
- 50.López-Mejías R, Genre F, Pérez BS, et al. Association of HLA-DRB1*01 with IgA vasculitits (Henoch-Schonlein) Arthritis & Rheumatology. 2015;67:823–27. doi: 10.1002/art.38979. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Mejias, et al. Association of HLA-B*41: 02 with Henoch-Schonlein Purpura (IgA vasculitis) in Spanish individuals irrespective of the HLA-DRB1 status. Arthritis Res Ther. 2015;17:102. doi: 10.1186/s13075-015-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu H, Lu P, Yang Y, et al. Chemokine MCP1/CCL2 and RNATES/CCL5 gene polymorphisms influence Henoch-Schonlein Purpura susceptibility and severity. J Formos Med Assoc. 2015;114(4):347–52. doi: 10.1016/j.jfma.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Ding G, Wang C, Che R, et al. Heat shock protein 70-2 and tumor necrosis factor alpha gene polymorphisms in Chinese children with Henoch Schonlein Purpura. World J Pediatr. 2016;12(1):49–54. doi: 10.1007/s12519-015-0048-9. [DOI] [PubMed] [Google Scholar]
- 54.Xu ED, Xiao YF, Wang YY, Dong L. Association study between matrix metalloproteinase-9 gene (MMP9) polymorphisms and the risk of Henoch-Schonlein purpura in children. Genet Mol Res. 2015;15(2) doi: 10.4238/gmr.15028095. [DOI] [PubMed] [Google Scholar]
- 55.Vargas-Alarcon G, Londono JD, Hernandez-Pacheco G, et al. Heat shock protein 70 gene polymorphisms in Mexican patients with spondyloarthropathies. Ann Rheum Dis. 2002;61:48–51. doi: 10.1136/ard.61.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pablos JL, Carreira PE, Martin-Villa JM, et al. Polymorphism of the heat-shock protein gene HSP70-2 in systemic lupus erythematosus. Br J Rheumatol. 1995;34:721–23. doi: 10.1093/rheumatology/34.8.721. [DOI] [PubMed] [Google Scholar]
- 57.López-Mejías R, Genre F, Remuzgo-Martínez S, et al. Interleukin 1 beta (IL1β) rs16944 genetic variant as a genetic marker of severe renal manifestations and renal sequelae in Henoch-Schönlein purpura. Clin Exp Rheumatol. 2016;34(3 Suppl. 97):S84–88. [PubMed] [Google Scholar]
- 58.Zhong W, Zhou TB, Jiang Z. Association of endothelial nitric oxide synthase gene polymorphism with the risk of Henoch-Schönlein purpura/Henoch-Schönlein purpura nephritis. Ren Fail. 2015;37(3):372–76. doi: 10.3109/0886022X.2014.1000802. [DOI] [PubMed] [Google Scholar]
- 59.Mao S, Huang S. Association of AGT M235T gene polymorphism with HSP/HSPN risk. Ren Fail. 2015;37(1):16–21. doi: 10.3109/0886022X.2014.977142. [DOI] [PubMed] [Google Scholar]
- 60.Chen J, Fang X, Dang X, et al. Association of then paired box 2 gene polymorphism with the susceptibility and pathogenesis of Henoch-Schonlein purpura in children. Mol Med Rep. 2015;11:1997–2003. doi: 10.3892/mmr.2014.2908. [DOI] [PubMed] [Google Scholar]
- 61.Amoli MM, Thomson W, Hajeer AH, et al. Interleukin 8 gene polymorphism is associated with increased risk of nephritis in cutaneous vasculitis. J Rheumatol. 2002;29:2367–70. [PubMed] [Google Scholar]
- 62.Amoli MM, Thomson W, Hajeer AH, et al. Interleukin 1 receptor antagonist gene polymorphism is associated with severe renal involvement and renal sequelae in Henoch-Schonlein purpura. J Rheumatol. 2002;29:1404–7. [PubMed] [Google Scholar]