Abstract
Background
The effects of low serum C3 levels and the activation of the complement system on the development and the prognosis of IgAN are unclear. The present study aimed to determine whether decreased levels of complement C3 influence the prognosis of IgAN patients with chronic kidney disease.
Material/Methods
We enrolled a total of 1564 patients with primary IgAN diagnosed by renal biopsy at the Chinese PLA General Hospital from January 2011 to March 2015. The endpoint was end-stage renal disease (ESRD) or a doubling of the baseline serum creatinine (D-SCr) level. All patients were using 1: 1 propensity score matching (PSM), and the baseline values were not significantly different between these 2 groups (P>0.05).
Results
During a follow-up period, 14 patients in the group with decreased C3 levels reached the endpoint, with 12 patients with normal C3 levels. There was no significant difference between the 2 groups in achieving D-SCr or ESRD (P=0.676). In multivariate Cox analysis, adjusted for demographic and laboratory examination, the risk of reaching the endpoint was comparable in the 2 groups (HR, 0.70; 95% CI, 0.27–1.78; P=0.449;). Furthermore, the risk of reaching ESRD (HR, 0.83; 95% CI, 0.25–2.75; P=0.757) and D-SCr (HR, 1.45; 95% CI, 0.20–10.60; P=0.718) did not differ between the 2 groups.
Conclusions
Decreased serum C3 levels in IgA nephropathy with chronic kidney disease did not play a decisive role in renal progression.
MeSH Keywords: Complement Activation; Glomerulonephritis, IGA; Prognosis; Renal Insufficiency, Chronic
Background
IgA nephropathy (IgAN) is the most common primary glomerulonephritis in the Asia-Pacific region [1] and accounts for 20–45% of glomerular diseases [2–4]. The clinical and pathological manifestations of IgAN vary, and approximately one-third of patients advance to end-stage renal disease (ESRD) within 20 to 25 years [5]. The clinicopathological characteristics associated with poor prognosis determined by previous studies include massive proteinuria, loss of renal function, hypertension, renal interstitial fibrosis, and glomerulosclerosis [2–4]. However, the correlation between complement level changes and prognosis remains uncertain. IgA-based deposition in the mesangial area has been used as the diagnostic criteria for IgAN, while accompanying C3 deposition is also very common in IgAN patients; however, the effect of complement activation on the progression and prognosis of the disease remains unclear. Activation of the alternative complement pathway and the lectin pathway have been reported to be involved in the pathogenesis of IgAN [6–10]. In a Korean study, decreased circulating C3 level was positively correlated with C3 deposition strength in the glomerular mesangial area and was a risk factor for progression into end-stage renal disease (ESRD) [11]. In several studies in Asia, a high serum IgA: C3 ratio was associated with disease progression of IgAN [12,13].
The present study aimed to follow up the renal function changes of IgAN patients with chronic kidney disease and decreased levels of complement C3 to determine whether low levels of complement C3 influence the renal prognosis of IgAN with chronic kidney disease.
Material and Methods
Patients
The study subjects were inpatients at the Department of Nephrology who were diagnosed with IgAN by renal biopsy between January 2011 and March 2015. Among the 1564 primary IgAN patients aged between 18 and 70 years, patients were excluded if their complement level was not detected during renal biopsy; if they had liver cirrhosis, malignant tumors, or acute renal injury (AKI); or if they had entered ESRD. A total of 496 patients were included. A C3 level <90 mg/dL was considered as a decreased C3 level. A 1: 1 propensity score matching method was used, and a total of 107 patients with decreased serum C3 levels and 107 patients with normal serum C3 levels were included (Table 1). All renal biopsy patients had signed the research protocol of the Renal Clinical Database Establishment when hospitalized, allowing their data to be used for clinical research, and the research was approved by the Ethics Committee of the General Hospital of the Chinese People’s Liberation Army.
Table 1.
Demographic, clinical and biochemical characteristics.
| Variables | Unmatched cohort | Matched cohort | ||||
|---|---|---|---|---|---|---|
| C3 <90mg/dl (n=109) | C3 ≥90mg/dl (n=387) | P-value | C3 <90mg/dl (n=107) | C3 ≥90mg/dl (n=107) | P-value | |
| Age (years) | 40.1±11.5 | 37.6±11.7 | 0.04 | 37.9±11.7 | 38.9±11.4 | 0.50 |
| Male gender, n (%) | 52 (47.7) | 273 (70.4) | <0.001 | 52 (48.6) | 61 (57) | 0.27 |
| Hypertension, n (%) | 29 (26.7) | 76 (19.6) | 0.87 | 28 | 25 | 0.95 |
| DM, n (%) | 3 (2.8) | 8 (2.1) | 0.52 | 3 | 5 | 0.67 |
| SBP (mm Hg) | 140 (130, 172) | 140 (120, 168) | 0.96 | 132.9±17.1 | 133.6±23.8 | 0.97 |
| DBP (mm Hg) | 84 (60, 120) | 80 (50, 130) | 0.69 | 84.6±12.5 | 85.3±13.4 | 0.68 |
| MAP (mm Hg) | 100 (57.7, 160) | 100 (73.3, 140) | 0.73 | 100.7±13.2 | 101.4±15.2 | 0.73 |
| BMI | 22.7±3.7 | 25.5±3.7 | <0.001 | 22.7±3.7 | 23.2±3.9 | 0.53 |
| Laboratory measurements | ||||||
| Hemoglobin (g/L) | 119.6±20.6 | 131.6±20.9 | <0.001 | 119.5±20.7 | 128.4±20.3 | 0.02 |
| Alanine transaminase (U/L) | 16.5 (5.7, 116.7) | 15.7 (4.4, 383.2) | 0.06 | 11.9 (4.5, 113) | 16.6 (4.4, 72.9) | 0.06 |
| Aspartate transaminas (U/L) | 15.6 (9.6, 66.7) | 15.8 (5.5, 379.3) | 0.79 | 15.3 (5.5, 66.5) | 16 (9.1, 41.2) | 0.18 |
| Total protein (g/L) | 66.1±7.8 | 62.7±9.4 | <0.001 | 60.1±8.2 | 62.6±7.7 | 0.10 |
| Serum albumin (g/L) | 40.1±5.3 | 36.8±6.4 | <0.001 | 36±6.5 | 38.1±5.8 | 0.05 |
| Urea nitrogen (mmol/L) | 5.8±1.3 | 8.7±3.5 | <0.001 | 8.2±3.5 | 8.2±3.3 | 0.83 |
| Serum creatinine (mg/dl) | 107.5±22.9 | 151.6±62.2 | <0.001 | 151.8±68.1 | 154.1±64.7 | 0.57 |
| Serum uric acid (umol/L) | 392.0±96.1 | 431.1±103.7 | <0.001 | 399.3±94.3 | 435.6±107.3 | 0.01 |
| eGFR (ml/min per 1.73 m2) | 51.8±20.5 | 57.8±19.6 | 0.01 | 51.9±20.3 | 52.3±20 | 0.88 |
| Cholesterol (mmol/L) | 6.5±1.8 | 5.1±1.5 | 0.14 | 6.5±1.6 | 5.1±1.4 | 0.20 |
| Triglyceride (mmol/L) | 1.7±1.2 | 2.2±1.4 | <0.001 | 1.9±1.2 | 2.3±1.7 | 0.002 |
| 24-h protein excretion (g/day) | 1.7 (0.2, 10.3) | 1.8 (0, 17.7) | 0.76 | 1.7 (0.18, 10.3) | 1.9 (0.03, 12.1) | 0.68 |
| IgA (mg/dl) | 324.9±137.3 | 315.3±123.9 | 0.68 | 323.7±137.1 | 328.9±138.3 | 0.78 |
| IgG (mg/dl) | 940.2±317.8 | 1012.7±333.7 | 0.04 | 939.5±320.7 | 1026.3±298.9 | 0.52 |
| IgM (mg/dl) | 119.1±74 | 102.4±52.4 | 0.03 | 119.8±74.5 | 101.9±53.9 | 0.45 |
| C4 (mg/dl) | 21.2±5.6 | 28.2±7.6 | <0.001 | 21.3±5.8 | 27.8±6.5 | <0.001 |
| Medications | ||||||
| ACE inhibitors or ARBs | 74 | 225 | 0.08 | 72 | 65 | 0.39 |
| Other antihypertensive | 42 | 183 | 0.08 | 42 | 46 | 0.68 |
| Corticosteroid | 3638 | 87 | 0.01 | 32 | 29 | 0.76 |
| Immunosuppressive agents | 21 | 79 | 0.89 | 20 | 16 | 0.58 |
| FU duration (month) | 34.4±13.5 | 32.8±18.7 | 0.37 | 34.5±13.3 | 32.9±17.5 | 0.45 |
Data are expressed as mean ±standard deviation (SD) or median (interquatile range) as appropriate; SBP – systolic pressure; DBP – diastolic pressure; MAP – mean arterial pressure; eGFR – estimated glomerular filtration rate.
Data collection
The following parameters were collected in a clinical database: age, height, weight, body mass index (BMI), systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure, where hypertension was defined as a systolic blood pressure >140 mmHg or a diastolic blood pressure >90 mmHg with the need for antihypertensive drugs. An automatic biochemical analyzer was used to detect levels of hemoglobin, total protein, serum albumin, serum creatinine, uric acid, triglycerides, total cholesterol, and other indicators. The colorimetric method was used to quantify 24-h urinary protein. Patients were classified using the chronic kidney disease (CKD) diagnostic criteria in the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines of 2006. The estimated glomerular filtration rate (eGFR) was calculated using the CKD-epidemiology collaboration (CKD-EPI) formula: eGFR=141× (serum creatinine (mg/dl)/0.9)−1.209×(0.993)age, [14[, and the conversion between the units of serum creatinine was as follows: 1 mg/dL=88.4 μmol/L. Complement C3, C4, and immunoglobulin were measured, a serum C3 level <90 mg/dl was considered a decreased serum C3 level, and the normal value of C3 was 90–180 mg/dL.
Follow-up
The study subjects were followed up. The average follow-up time for the group with decreased C3 levels prior to matching was 34.4 months. The follow-up time for the group with normal C3 levels was 32.8 months, and no significant difference in the follow-up time was observed between the 2 groups (P=0.365). The average follow-up time of the group with decreased C3 levels after matching was 34.5 months, and the follow-up time for the group with normal C3 levels was 32.9 months. No significant difference in the follow-up time after matching was observed between the 2 groups (P=0.451). The endpoint of this study was the doubling of the baseline serum creatinine and developing into ESRD. ESRD was eGFR <15 ml/min/1.73 m2, peritoneal dialysis, permanent hemodialysis, or renal transplantation, which also was initiation of renal replacement therapy.
Statistical analysis
All patients were assigned a 1: 1 propensity score match by using the PSM extension program in SPSS software (Chicago, IL) with the occurrence of serum C3 as the dependent variable. We derived age, sex, diabetes, hypertension, or the existence of two factors from a multiple logistic regression model with the following variables: serum albumin, serum creatinine, eGFR (CKD-EPI), total cholesterol concentration, and 24-h urinary protein excretion levels, which were used as covariates. Otherwise, the quality of the matching results in the process was assured by defining the caliper values. The standard differences of the covariates between 2 groups were set to 0.2. After performing all propensity score matching, we used the Wilcoxon signed-rank test to analyze continuous variables and the McNemar test to analyze categorical variables to evaluate the balance of baseline covariates between the 2 groups.
SPSS version 19.0 was used for the statistical analysis. Continuous variables are expressed χ̄±s, while categorical variables are expressed as numbers (percentages). Comparison between 2 groups using the t test. Normality of distribution of parameters was analyzed by the Kolmogorov-Smirnov test. Nonparametric variables are described as medians and quartile ranges, and the comparisons between groups were made using the Mann-Whitney test or the Kruskal-Wallis test. The Kaplan-Meier curves method was used to show the probability of kidney survival and the log-rank test was used to compare survival between the 2 groups. The independent prognostic value of the clinical parameters of the study results was analyzed by multiple Cox regression analysis. Risk ratios (HR) and 95% confidence intervals (CI) were calculated using estimated regression coefficients and standard errors in Cox regression analysis. P<0.05 was considered a significantly significant difference.
Results
Baseline variables
Figure 1 is a flow diagram depicting patient samples and exclusions. Baseline characteristics of the patients who met the inclusion criteria are described in Table 1. All 496 patients were enrolled in the unpaired cohort study, and 109 (22%) exhibited varying decreases in serum C3 levels. There were no differences in diabetes, hypertension, MAP, and laboratory measurements including serum albumin, total cholesterol, IgA, IgG, and IgM. The ages of patients with decreased C3 levels were higher than those of patients with normal complement levels, and the difference was statistically significant (40.1 vs. 11.5 years of age, P=0.036). It is noteworthy that the BMI of patients with decreased C3 levels was significantly lower than that of the normal group (22.7 vs. 25.5, P<0.001). Baseline 24-h urinary protein excretion, serum albumin, creatinine, and eGFR were comparable between the 2 groups.
Figure 1.
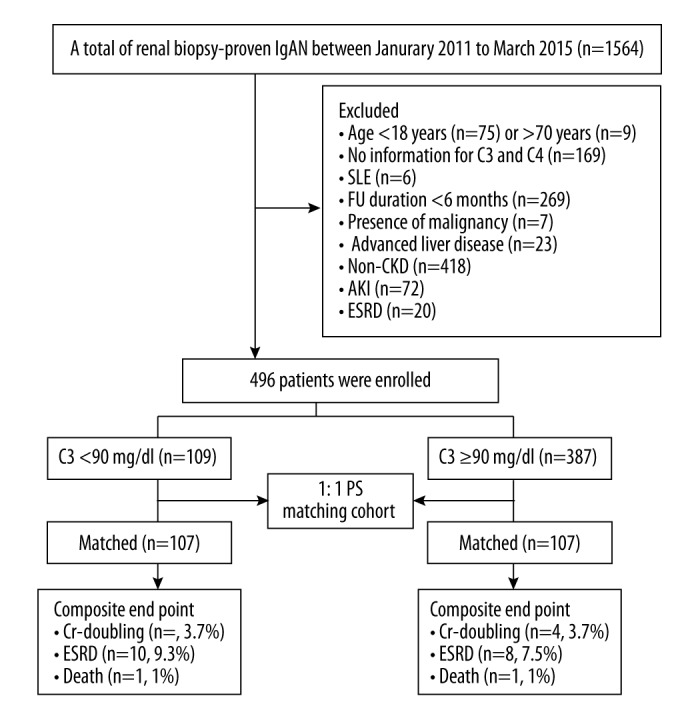
Flow diagram of patient progress and outcomes. From January 2011 to March 2015, we enrolled 109 patients with decreased serum C3 level and 387 patients with normal serum C3 level. After 1: 1 propensity score (PSM) matching, 107 patients with decreased serum C3 level and 107 patients with normal serum C3 level were ultimately analyzed. Cr – creatinine; ESRD – end-stage renal disease; FU – follow-up.
PS matching was used to control the unbalanced conditions at baseline between the 2 groups. After 1: 1 PS matching, we matched pairs of 107 patients with decreased serum C3 level and 107 patients with normal serum C3 level, resulting in no differences in demographic and laboratory measurements, including hypertension, mean arterial pressure, serum albumin, creatinine and eGFR, 24-h urinary protein excretion, and FU duration (Table 1).
Patient progression for the unmatched cohort
In addition, according to its clinical characteristics, kidney prognosis can be classified as remission (without renal function impairment, hematuria, and urinary protein), persistent proteinuria and/or hematuria, D-Scr, and ESRD. We defined persistent proteinuria as 24-h protein excretion >0.5 g/day and hematuria as >3 red blood cells per high-power field. In the unpaired cohort study, a total of 15 (13.8%) patients with decreased C3 levels reached the endpoint event, and a total of 28 patients in group with normal C3 levels reached the endpoint event; the difference between the 2 groups was statistically significant (P=0.032). In the patient described above, 5 patients with decreased C3 levels (4.6%) developed D-SCr compared with 7 patients (1.8%) with normal serum C3 level, but the difference was not statistically significant (P=0.095; Table 2). Moreover, ESRD occurred in 10 patients (9.2%) with decreased serum C3 level and 21 patients (5.4%) with normal serum C3 level (P=0.153). One patient in each group died during follow-up. Kaplan-Meier survival analysis (Figure 2A–2C) was performed on patients in the 2 groups, which suggested that the renal survival rate of the endpoint event was significantly different between the 2 groups. The renal survival rate of the group with decreased C3 levels was lower than that of the group with normal C3 levels. When analyzing a single endpoint, we found that the renal survival rate of ESRD was not significantly different between the 2 groups; however, the number of patients who achieved D-SCr in the group with decreased C3 levels was lower than that of the group with normal C3 levels.
Table 2.
Renal outcomes before and after propensity score 1: 1 matching.
| Renal outcomes, n (%) | Unmatched cohort | Matched cohort | ||||
|---|---|---|---|---|---|---|
| C3 <90mg/dl (n=109) | C3 ≥90mg/dl (n=387) | P-value | C3 <90mg/dl (n=107) | C3 ≥90mg/dl (n=107) | P-value | |
| Remission | 5 (4.6) | 25 (6.5) | 0.47 | 5 (4.7) | 2 (1.9) | 0.44 |
| Persistent proteinuria and/or hematuria | 88 (80.7) | 333 (86.1) | 0.17 | 87 (81.3) | 92 (85.9) | 0.27 |
| SCr doubling | 5 (4.6) | 7 (1.8) | 0.10 | 4 (3.7) | 4 (3.7) | 0.73 |
| ESRD | 10 (9.2) | 21 (5.4) | 0.15 | 10 (9.3) | 8 (7.5) | 0.62 |
| Death | 1 (0.9) | 1 (0.2) | 0.39 | 1 (1) | 1 (1) | 1 |
Data are expressed as mean ± standard deviation (SD) or median (interquatile range) as appropriate.
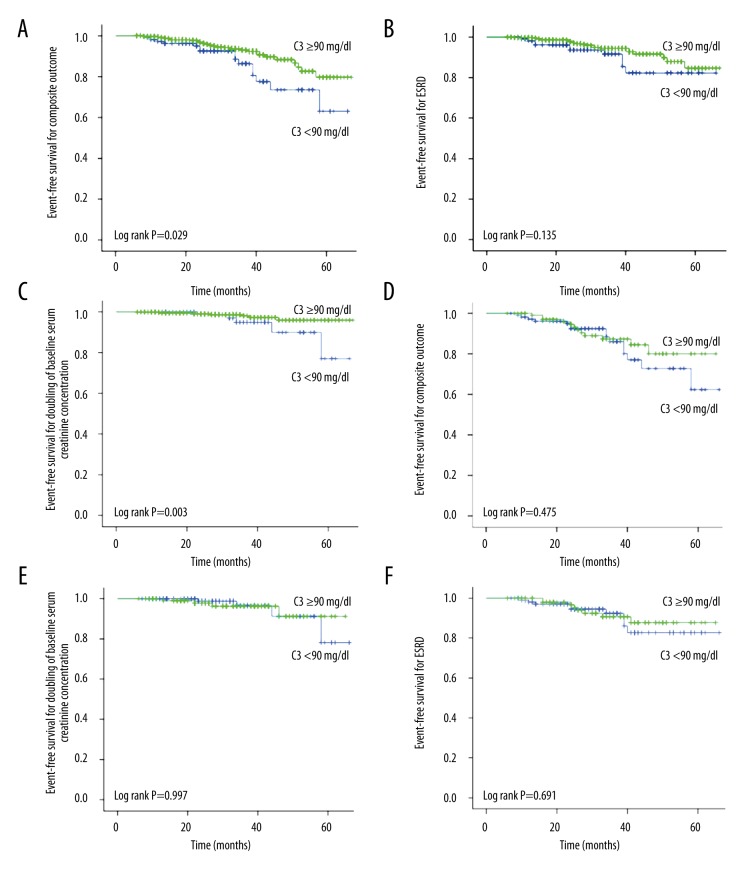
Figure 2.
Clinical outcomes between decreased serum C3 level and normal serum C3 level. (A–C) Kaplan-Meier plots for outcomes in the unmatched and (D–F) the matched cohort. In the unmatched cohort, the event-free survival for composite outcome (P=0.047) (A) and doubling of serum creatinine (P=0.03) (C) was significantly lower in patients with decreased serum C3 level than in patients with normal serum C3 level. There was no significant difference in the ESRD between the 2 groups (B) (P=0.135). In the matched cohort, the event-free survival for composite outcome (D), doubling of the baseline serum creatinine (E), and ESRD (F) did not differ between the 2 groups.
Patient progression for the matched cohort
Before performing 1: 1 propensity score matching, 14 patients (13.1%) with decreased serum C3 level developed the study outcome compared with 12 patients (11.3%) with normal serum C3 level (P=0.676). There were no significant differences between groups in the individual kidney outcome of D-SCr (4.7% vs. 4.7%, P=1) or ESRD. In a multivariate Cox analysis adjusted for unbalanced factors, the risk of reaching renal outcome was comparable in 2 groups (HR, 0.70; 95% CI, 0.27–1.78; P=0.449; Table 3). Furthermore, the risk of developing D-SCr (HR, 1.45; 95% CI, 0.20–10.60; P=0.718) and ESRD (HR, 0.83; 95% CI, 0.25–2.75; P=0.757) were not different between the 2 groups (Table 4). Kaplan-Meier survival analysis (Figure 2D–2F) was performed on the patients of the 2 groups after matching, which showed no significant difference in the overall endpoint events (P>0.05), suggesting that decreased serum C3 levels in IgA nephropathy patients with chronic kidney disease was not an independent risk factor for progression.
Table 3.
Multivariate Cox proportional analyses for SCr doubling and ESRD.
| Variables | Unmatched cohort | Matched cohort | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | 1.01 (0.97–1.04) | 0.79 | 1.02 (0.97–1.08) | 0.36 |
| Male (vs. Female) | 0.98 (0.44–2.19) | 0.87 | 0.72 (0.25–2.06) | 0.54 |
| MAP (mm Hg) | 1.01 (0.98–1.03) | 0.14 | 0.98 (0.95–1.02) | 0.37 |
| Total protein (g/L) | 0.99 (0.94–1.05) | 0.08 | 1.00 (0.94–1.08) | 0.91 |
| Serum albumin (g/L) | 0.98 (0.89–1.08) | 0.03 | 0.98 (0.88–1.10) | 0.75 |
| Urea nitrogen (mmol/L) | 1.04 (0.94–1.16) | <0.001 | 0.92 (0.74–1.14) | 0.43 |
| Serum creatinine (mg/dl) | 1.00 (0.99–1.02) | <0.001 | 1.00 (1.00–1.01) | 0.63 |
| Serum uric acid (umol/L) | 1.00 (1.00–1.01) | 0.04 | 1.00 (1.00–1.01) | 0.11 |
| eGFR (ml/min per 1.73 m2) | 0.98 (0.95–1.02) | <0.001 | 0.92 (0.85–0.99) | 0.17 |
| Cholesterol (mmol/L) | 0.77 (0.59–1.01) | 0.35 | 1.01 (0.91–1.12) | 0.89 |
| Triglyceride (mmol/L) | 1.01 (0.78–1.31) | 0.91 | 0.82 (0.54–1.24) | 0.34 |
| 24-h protein excretion (g/day) | 1.04 (0.88–1.22) | 0.01 | 1.16 (0.86–1.55) | 0.33 |
| Patients with decreased C3 level (vs. no) | 0.54 (0.26–1.12) | 0.03 | 0.70 (0.27–1.78) | 0.45 |
95% CI – 95% confidence interval; MAP – mean arterial pressure.
Table 4.
Multivariate Cox proportional analyses for SCr doubling and ESRD.
| Unmatched cohort | Matched cohort | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| SCr×2 | 0.04 (0.01–0.31) | 0.002 | 1.45 (0.20–10.60) | 0.72 |
| ESRD | 0.87 (0.36–2.11) | 0.14 | 0.83 (0.25–2.75) | 0.76 |
95% CI – 95% confidence interval.
Discussion
IgAN was first defined by Jean Berger in 1968, and it is the most common primary glomerular disease worldwide. Its pathological feature is IgA or IgA-based deposition in the glomerular mesangial area or mesangium and the capillary wall, which is commonly accompanied by the deposition of complement C3 and the immunoglobulins G and M. IgAN is a progressive disease that is characterized by a highly changeable clinical course and outcome, and approximately one-third of patients progress to ESRD within 20 to 25 years [5]. Previous studies have shown that the alternative complement pathway and the lectin pathway are involved in IgAN [6–10]. Treatment targeting complement activation (such as anti-C5 and anti-C5aR antibodies) may be effective for a subset of IgAN patients. Studies by Seung Jun Kim et al. confirmed that C3 hypocomplementemia and C3 deposition in the glomerular mesangial area are independent risk factors of the prognosis of IgAN [11].
In the present study, we included inpatients in our hospital who had undergone a renal biopsy between January 2011 and March 2015 and were pathologically confirmed to have primary IgAN. Patients were classified into a decreased serum complement C3 group and a group with normal C3 levels according to serum C3 levels. The patients in the 2 groups were followed up before matching (34.4±13.5 vs. 32.8±18.7, P=0.365). The risk of the kidney outcome was statistically comparable. The event-free survival for composite outcome and D-Scr was significantly lower in patients with decreased serum C3 level than in patients with normal serum C3 level. In the unpaired cohort study, a total of 15 (13.8%) patients with decreased C3 levels reached the endpoint event, and a total of 28 patients in the group with normal C3 levels reached the endpoint event; the difference between the 2 groups was statistically significant (P=0.032). Kaplan-Meier survival analysis (Figure 2A–2C) was performed on the patients in the 2 groups, which suggested that the renal survival rate until the endpoint event was significantly different between the 2 groups. The renal survival rate of the group with decreased C3 levels was lower than that of the group with normal C3 levels.
After performing 1: 1 propensity score matching, a total of 107 patients with decreased serum C3 levels and 107 patients with normal serum C3 levels were enrolled. The follow-up time was 34.5±13.3 vs. 32.9±17.5 months (P=0.451), which suggested no significant difference between the prognosis and C3 hypocomplementemia for IgAN patients with accompanying chronic kidney disease. Furthermore, Cox analysis data were also consistent with the above results, suggesting that it was not an independent risk factor for progression.
The reasons for the inconsistency of the conclusions obtained from the improved statistical methods are as follows. The use of the propensity score matching method makes this study different from previous studies. The propensity score matching method has been regarded as an effective method for clinical research in recent years. Oh et al. used this method to match primary IgAN patients with purpuric nephritis patients and found no difference in prognosis, whereas the data before the matching showed a significant difference in their prognosis [18]. A study by Valiga used the propensity score matching method to match a group treated with hormones and renin-angiotensin system (RAS) blockers to a group treated with RAS blockers alone, and found that glucocorticoids can effectively reduce urinary protein, slow the rate of renal function decline, and improve the kidney survival rate [19].
To exclude the effect of confounding factors, we used the propensity score matching method so that the clinical baseline indicator was not different between the 2 groups of patients. Before matching, the 2 groups had significant differences in age, sex, BMI, albumin, serum creatinine, eGFR, triglycerides, and other baseline indicators.
The age of the patients in the decreased complement C3 level group was older than that of the group with normal C3 levels at the time of renal biopsy (P=0.036), and age was an independent risk factor for the prognosis of IgA patients.
Before matching, the group with normal C3 levels had higher BMI and triglyceride levels. Most of the previous studies have shown that an increase in complement level is related to obesity and insulin resistance, while some studies have shown that complement activation often occurs in patients with an extremely low BMI, such as those with anorexia nervosa. Low levels of complement C3 may reflect the severity of poor health in patients, such as those with malnutrition (malnutrition will accelerate the progress of chronic kidney disease) [20].
Recently, Yan Ouyang et al. demonstrated that low BMI was an independent risk factor for deterioration of renal function in IgAN [21] and metabolic disorders in CKD patients, which results in protein degradation rather than protein synthesis [22]. In particular, additional protein loss as well as long-term moderate and severe proteinuria will accelerate this deterioration, and it is not recommended that patients with CKD lose excessive weight. In contrast, another report found that the risk of death of hemodialysis patients with a low BMI was increased [23,24]. During renal biopsy, a higher level of SCr constitutes an independent risk factor for long-term renal survival [25–30]. We confirmed that baseline SCr and eGFR values were associated with kidney progression in IgAN patients. Therefore, we used the propensity score matching method to balance these non-IgAN factors, resulting in a more reliable conclusion.
Because the previous study did not strictly match the baseline indicators, the difference in baseline indicators may have influenced the clinical prognosis. The present study used the propensity score matching method. After matching, we found no significant difference in the renal prognosis between the 2 groups. Our study has certain limitations. First, because this was a retrospective study, selection bias may have been present, although the propensity score matching method was used to correct this bias. Second, this was a single-center study with short follow-up times. Third, because this was a retrospective study, data collection was not complete, and we did not monitor the effect of different drug treatments on the progression of IgAN. Fourth, we only included the complement level of patients at the time of renal biopsy, and we could not detect a correlation between changes in complement levels and the clinical indicators. Fifth, we divided the patients into a group with decreased C3 levels and a group with normal C3 levels, but we did not examine the effect of the degree of complement decline on the progression of the disease. Sixth, we did not include IgAN patients younger than 18 or older than 70 years. Subsequent studies should expand the age range and increase the sample size, and we plan to conduct a long-term follow-up study to verify the conclusions of the present study.
Conclusions
In conclusion, our study showed that decreased serum C3 levels in IgA nephropathy with chronic kidney disease does not play a decisive role in renal progression.
Footnotes
Source of support: This work was supported by the National Sciences Foundation of China [grant numbers 81273968, 81471027, and 81072914]; Ministerial Projects of the National Working Commission on Aging [grant number QLB2014W002]; and The Four Hundred Project of 301 [YS201408]
References
- 1.D’Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987;64:709–27. [PubMed] [Google Scholar]
- 2.Glassock, Richard J. The pathogenesis of IgA nephropathy. Curt Opin Nephrol Hypertens. 2011;20(2):153–60. doi: 10.1097/MNH.0b013e3283436f5c. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Tang Z, Zeng C, et al. Pathological demography of native patients in a nephrology center in China. Chin Med J(Engl) 2003;116(9):1377–81. [PubMed] [Google Scholar]
- 4.Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13, 519 renal biopsies. Kidney Int. 2004;66(3):920–23. doi: 10.1111/j.1523-1755.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 5.Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010;5(3):425–30. doi: 10.2215/CJN.06530909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roos A, Rastaldi MP, Calvaresi N, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17(6):1724–34. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]
- 7.Endo M, Ohi H, Ohsawa I, et al. Glomerular deposition of mannose-binding lectin (MBL) indicates a novel mechanism of complement activation in IgA nephropathy. Nephrol Dial Transplant. 1998;13(8):1984–90. doi: 10.1093/ndt/13.8.1984. [DOI] [PubMed] [Google Scholar]
- 8.Stad RK, Bruijn JA, van Gijlswijk-Janssen DJ, et al. An acute model for IgA-mediated glomerular inflammation in rats induced by monoclonal polymeric rat IgA antibodies. Clin Exp Immunol. 1993;92(3):514–21. doi: 10.1111/j.1365-2249.1993.tb03430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinosa M, Ortega R, Gomez-Carrasco JM, et al. Mesangial C4d deposition: A new prognostic factor in IgA nephropathy. Nephrol Dial Transplant. 2009;24(3):886–91. doi: 10.1093/ndt/gfn563. [DOI] [PubMed] [Google Scholar]
- 10.Oortwijn BD, Eijgenraam JW, Rastaldi MP, et al. The role of secretory IgA and complement in IgA nephropathy. Semin Nephrol. 2008;28(1):58–65. doi: 10.1016/j.semnephrol.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Koo HM, Lim BJ, et al. Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS One. 2012;7(7):e40495. doi: 10.1371/journal.pone.0040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komatsu H, Fujimoto S, Hara S, et al. Relationship between serum IgA/C3 ratio and progression of IgA nephropathy. Intern Med. 2004;43(11):1023–28. doi: 10.2169/internalmedicine.43.1023. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Wang C, Tang Y, et al. Serum immunoglobulin A/C3 ratio predicts progression of immunoglobulin A nephropathy. Nephrology (Carlton) 2013;18(2):125–31. doi: 10.1111/nep.12010. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347(10):738–48. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 16.Yu HH, Chiang BL. Diagnosis and classification of IgA nephropathy. Autoimmun Rev. 2014;13(4–5):556–59. doi: 10.1016/j.autrev.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Donadio JV, Bergstralh EJ, Grande JP, Rademcher DM. Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol Dial Transplant. 2002;17(7):1197–203. doi: 10.1093/ndt/17.7.1197. [DOI] [PubMed] [Google Scholar]
- 18.Oh HJ, Ahn SV, Yoo DE, et al. Clinical outcomes, when matched at presentation, do not vary between adult-onset Henoch-Schonlein purpura nephritis and IgA nephropathy. Kidney Int. 2012;82(12):1304–12. doi: 10.1038/ki.2012.302. [DOI] [PubMed] [Google Scholar]
- 19.Tesar V, Troyanov S, Bellur S, et al. Corticosteroids in IgA Nephropathy: A retrospective analysis from the VALIGA study. J Am Soc Nephrol. 2015;26(9):2248–58. doi: 10.1681/ASN.2014070697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartley GH. Nutritional status, delaying progression and risks associated with protein restriction. EDTNA ERCA J. 2001;27(2):101–4. doi: 10.1111/j.1755-6686.2001.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang Y, Xie J, Yang M, et al. Underweight is an independent risk factor for renal function deterioration in patients with IgA nephropathy. PLoS One. 2016;11(9):e0162044. doi: 10.1371/journal.pone.0162044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3(1):73–95. doi: 10.1002/j.1550-8528.1995.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 23.Leavey SF, Mccullough K, Hecking E, et al. Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: Results from the dialysis outcomes and practice patterns study (DOPPS) Nephrol Dial Transplant. 2001;16(12):2386–94. doi: 10.1093/ndt/16.12.2386. [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005;6(3):489–500. doi: 10.1053/j.ajkd.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Le W, Liang S, Hu Y, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: Results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27(4):1479–85. doi: 10.1093/ndt/gfr527. [DOI] [PubMed] [Google Scholar]
- 26.D’Amico G. Influence of clinical and histological features on actuarial renal survival in adult patients with idiopathic IgA nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis: Survey of the recent literature. Am J Kidney Dis. 1992;20(4):315–23. doi: 10.1016/s0272-6386(12)70293-7. [DOI] [PubMed] [Google Scholar]
- 27.Koyama A, Igarashi M, Kobayashi M. Natural history and risk factors for immunoglobulin A nephropathy in Japan. Research Group on Progressive Renal Diseases. Am J Kidney Dis. 1997;29(4):526–32. doi: 10.1016/s0272-6386(97)90333-4. [DOI] [PubMed] [Google Scholar]
- 28.Berthoux F, Mohey H, Laurent B, et al. Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol. 2011;22(4):752–61. doi: 10.1681/ASN.2010040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie J, Kiryluk K, Wang W, et al. Predicting progression of IgA nephropathy: New clinical progression risk score. PLoS One. 2012;7(6):e38904. doi: 10.1371/journal.pone.0038904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauta V, Gronhagen-Riska C. IgA nephropathy: From predicting progression to treatment. Duodecim. 2006;122(2):215–22. [PubMed] [Google Scholar]