This study is the first to test the effect of an arithmetic task on the time course of balance readjustment following visual withdrawal or addition. Performing such a cognitive task increases the time delay following addition of vision but has no effect on withdrawal dynamics. This suggests that sensorimotor integration following addition of a stabilizing signal is performed at a cortical level, whereas the response to its withdrawal is “automatic” and accomplished at a subcortical level.
Keywords: processing time, sensorimotor integration, sensory reweighting, balance, vision, cognitive task
Abstract
The aim of this study was to test the effects of a concurrent cognitive task on the promptness of the sensorimotor integration and reweighting processes following addition and withdrawal of vision. Fourteen subjects stood in tandem while vision was passively added and removed. Subjects performed a cognitive task, consisting of counting backward in steps of three, or were “mentally idle.” We estimated the time intervals following addition and withdrawal of vision at which body sway began to change. We also estimated the time constant of the exponential change in body oscillation until the new level of sway was reached, consistent with the current visual state. Under the mentally idle condition, mean latency was 0.67 and 0.46 s and the mean time constant was 1.27 and 0.59 s for vision addition and withdrawal, respectively. Following addition of vision, counting backward delayed the latency by about 300 ms, without affecting the time constant. Following withdrawal, counting backward had no significant effect on either latency or time constant. The extension by counting backward of the time interval to stabilization onset on addition of vision suggests a competition for allocation of cortical resources. Conversely, the absence of cognitive task effect on the rapid onset of destabilization on vision withdrawal, and on the relevant reweighting time course, advocates the intervention of a subcortical process. Diverting attention from a challenging standing task discloses a cortical supervision on the process of sensorimotor integration of new balance-stabilizing information. A subcortical process would instead organize the response to removal of the stabilizing sensory input.
NEW & NOTEWORTHY This study is the first to test the effect of an arithmetic task on the time course of balance readjustment following visual withdrawal or addition. Performing such a cognitive task increases the time delay following addition of vision but has no effect on withdrawal dynamics. This suggests that sensorimotor integration following addition of a stabilizing signal is performed at a cortical level, whereas the response to its withdrawal is “automatic” and accomplished at a subcortical level.
healthy adults find it seemingly easy to maintain balance in the different situations experienced in everyday life. However, achieving successful balance in a varying environment requires the combination of complex and often redundant vestibular, somatosensory, and visual inputs (Assländer and Peterka 2014; Logan et al. 2014; Mergner et al. 2002; Peterka 2002; Polastri et al. 2012). Appropriate sensorimotor integration allows for the proper orchestration of postural muscle activity and regulation of body sway (Kelly et al. 2012; Saffer et al. 2008; Sozzi et al. 2013).
Cortical processing is involved in balance control. There is increasing evidence that postural responses to balance perturbations are not mediated only through subcortical circuits but also through the motor cortex (Bolton 2015; Jacobs and Horak 2007; Saradjian et al. 2013; Schieppati et al. 1995; Varghese et al. 2015). The motor cortex might play a role in the modulation of the late components of the postural responses to perturbations (Nardone and Schieppati 2008) and might have a permissive role in the improvement of stance stability observed during balance training (Taube et al. 2007). Studies of correlations of balance performance with imaging data and studies using transcranial direct current stimulation have also pointed to a critical role of other regions of the cortex in the integration of relevant sensory information (Ishigaki et al. 2016; Van Impe et al. 2012; Zwergal et al. 2012).
It is known that performing certain cognitive or attention-demanding tasks during quiet standing can modify body sway (Andersson et al. 2002; Kerr et al. 1985; Lajoie et al. 1993; Maki and McIlroy 1996; Mitra and Frazier 2004; Nafati and Vuillerme 2011; Rougier and Bonnet 2016). Interaction between cognitive processes and postural control has been repeatedly shown for all subject ages (Schmid et al. 2007; see Lacour et al. 2008 for a critical review). Research in the elderly has shown that increasing the complexity of the postural task degrades either the postural or the concurrent cognitive task performance, or both (Boisgontier et al. 2013; Stelmach et al. 1990). Attention influences the integration of sensory inputs for balance as well as sensory selection by enhancing specific sensory channels (Maylor et al. 2001; Redfern et al. 2001, 2002). Notably, Mahboobin et al. (2007) have posited that integration of sensory inputs for balance requires a nonnegligible delay and that this time interval can be impacted by dual-task interference.
We have previously shown that following modification in sensory state (vision, haptic) while standing, a nonnegligible time interval, henceforth termed latency, elapses before changes in body sway are detectable. These latencies were found to range between 0.6 and 1.2 s, on average, following passive withdrawal or addition of vision, respectively (Assländer and Peterka 2016; Honeine and Schieppati 2014; Sozzi et al. 2011, 2012). This period likely accommodates for the neural computation connected with the sensorimotor integration process. Subsequent to this time interval, the level of body oscillation increases or decreases exponentially until a new state of dynamic stability is reached, depending on whether vision is removed or provided. The new state of dynamic stability is reached within a period ranging from 2 to 5 s (Assländer and Peterka 2016; Honeine et al. 2015; Sozzi et al. 2011, 2012). It has been argued that during the exponential transition to a new sway level, the central nervous system is gradually reweighting the adaptive gain given to each sensory modality involved in maintaining balance (see Carver et al. 2006; Hwang et al. 2014; Peterka 2002).
Interestingly, latencies and time constants following the change in sensory state are in general shorter following withdrawal than following addition of vision (Assländer and Peterka 2016; Honeine et al. 2015; Sozzi et al. 2011, 2012). Sozzi et al. (2011) and Honeine and Schieppati (2014) advocated that the shorter time interval for adaptation following withdrawal is due to switching to a new, “default” postural set. This would be rapidly implemented to counteract the increased risk of falling caused by the removal of the stabilizing input. One possibility is that integration of vision requires at least a coarse analysis of the visual scene and the intervention of the cortex (Brandt et al. 1998; Cutfield et al. 2014). On the other hand, sudden withdrawal of the visual input would be processed at the subcortical level (Cullen and Roy 2004; Elias et al. 2014; Murnaghan et al. 2014), where vestibular and proprioceptive inputs are combined to encode body-in-space motion.
The aim of the present study was to investigate the effects of performing a cognitive task on the sensorimotor integration time following addition and withdrawal of vision. We hypothesized that performing an attention-diverting task would have an effect on the timing of the sensorimotor integration process following addition of visual information and on the later transient recovery of body sway to a new dynamic state. We also assumed that balance control following vision withdrawal involves a default postural set. Therefore, we expected the latencies following the loss of visual input and the time required to reweight the remaining inputs to be unaffected by the cognitive task.
To test our hypothesis, we measured the latencies in the level of oscillation of the center of feet pressure (CoP) during tandem stance following withdrawal and following addition of visual input while subjects performed a cognitive task and compared the results with those obtained when the subjects were “mentally idle.”
METHODS
Participants.
Fourteen healthy young subjects (8 women and 6 men) participated in the study. Their mean age, mass, and height were 25.1 yr (range 21–41 yr), 64.2 kg (range 48–91 kg), and 1.73 m (range 1.60–1.91 m), respectively. All subjects provided written informed consent to the experiment as conformed to the Declaration of Helsinki. The local review board approved the research protocol.
Postural tasks and procedures.
Subjects stood on a force platform (Kistler 9286BA) with feet in tandem position (Fig. 1A). Subjects completed two experimental conditions. In the condition termed “counting backward,” subjects performed a cognitive task while standing. In the control condition, subjects stood mentally idle, again standing in the same tandem position.
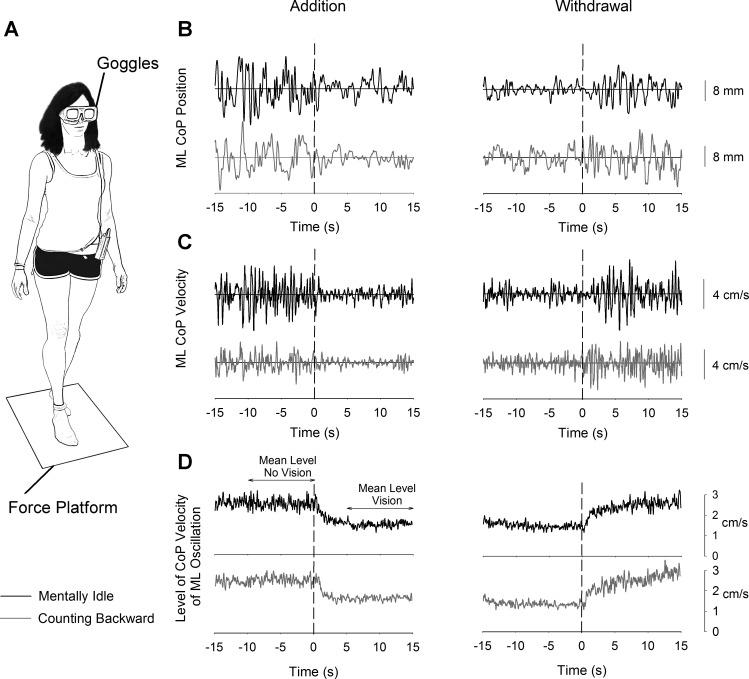
Fig. 1.
Experimental setup and raw traces. A shows a subject standing on the force platform in the tandem posture. Electronic goggles worn by the subjects allowed control of vision inflow via a TTL signal. B shows the raw traces of ML CoP position (1 trial) in the mentally idle (black traces) and counting-backward (gray traces) conditions during addition (left) and withdrawal (right) of vision. C shows the ML CoP velocity traces of the same trials. D shows the level of ML CoP velocity of oscillation (average of 92 rectified ML CoP velocity traces). The horizontal segments with arrowheads over the level of ML CoP oscillation trace indicate the time windows in which the mean level of oscillation was calculated in the presence and in the absence of vision.
For each subject, 80–100 standing trials were performed in each of the 2 conditions. The trials were allocated into 3 days to avoid fatigue. The contour of the feet was drawn on the platform so that the same feet positions were reproduced in subsequent days. Each day, subjects performed 2 sets of conditions (control and cognitive) of about 30 trials per set. The order of the conditions was randomized. A trial lasted around 40 s. In each trial, a shift in the visual state occurred randomly during a time period ranging from 17 to 22 s with respect to the beginning of the trial acquisition. Randomization of the time at which the change in visual state occurred was adopted to minimize the risk of subjects anticipating the sensory-state shift (Cenciarini and Peterka 2006). Usually, subjects rested following a block of 5 consecutive trials, i.e., 200 s, for as long as they required (normally less than a couple of minutes).
The shift in visual state was achieved by means of electronically controlled shutter goggles (PLATO visual occlusion spectacles; Translucent Technologies, Toronto, ON, Canada; Fig. 1A). The goggles allowed subjects to wear corrective eyeglasses, if required. A TTL signal was used to control the opacity of the lenses. The response time to the voltage transient is ∼1 ms to open and 3–5 ms to close (manufacturer's specification). When vision was available, subjects could see the patterned wall of the laboratory at about 6 m distance. The laboratory was well illuminated and luminosity levels were comparable between sessions.
Cognitive task.
During the mentally idle condition, the subjects were simply asked to stand in the tandem position and were not given any other instruction, such as to concentrate on the task (Mitra and Fraizer 2004; Redfern et al. 2004; for a discussion of this issue see Bonnet 2016 and Zok et al. 2008 with the companion commentaries) or to make deliberate efforts to reduce body sway (Dreher and Teasdale 2016; Reynolds 2010; Sciadas et al. 2016).
The cognitive task consisted of counting backward in steps of three, mentally and not aloud (Ceyte et al. 2014; Chong et al. 2010; Grangeon et al. 2011; Pellecchia 2003; Rankin et al. 2000; Woollacott and Shumway-Cook 2002). The subjects were instructed to perform the task as best as they could. They were also asked to continue counting backward regardless of having committed an error or not. The starting number was randomly assigned before each acquisition block. At the end of each block, subjects were asked for the resulting number.
Calculation of mean level of CoP velocity of oscillation.
The ground reaction force was measured by the force platform at a sampling frequency of 560 Hz. The position of the CoP was computed offline. Since tandem stance increases instability mainly in the frontal plane (Jonsson et al. 2005; Sozzi et al. 2013), only mediolateral (ML) CoP oscillation are presented in this study. The instantaneous ML CoP position curves of each trial were reduced to 30-s-long matrices comprising 15 s before the instant of the shift in visual state and 15 s afterward. The instant of the shift corresponded to t = 0 s, and the changes in CoP oscillation on changing visual condition were referenced to the same time instant. The CoP position signals were bandpass filtered between 0.1 and 5 Hz (Sozzi et al. 2011; see Yamamoto et al. 2015) with a no-lag second-order Butterworth filter (Fig. 1B). The velocity of ML oscillation was then obtained by calculating the time derivative of the filtered CoP position traces of each trial (Fig. 1C) by using the simple finite differencing method. The velocity traces were not filtered. The mean level of velocity of ML CoP oscillation was then calculated by rectifying the velocity curves of each trial and then averaging them (∼80–100) for each condition (Fig. 1D). The mean level of velocity of oscillation before the shift in sensory state was measured by averaging the traces in the period spanning from t = −10 s to t = 0 s. The mean level following the shift in visual state was the average velocity of oscillation in the period spanning from t = 5 s to t = 15 s (see arrows in Fig. 1D).
Estimation of the latency at which sway begins to change following the shift in visual state and of the time constant to reach a new dynamic state.
To estimate the latency and the following time constant, we used the same custom-made fitting algorithm as Honeine et al. (2015). This algorithm, which is based on the work of Assländer and Peterka (2014, 2016), fits a time-shifting function composed of a flat line (constant value over time) followed by an exponential function (see Eqs. 1 and 2) to the level of CoP velocity of ML oscillation traces. The advantage of fitting the velocity traces instead of the level of oscillation traces is the reduction in the drift of the position signal (see Assländer and Peterka 2016).
The variables a and b in the equation determine the level before and after the change in dynamic state. Δt is the time delay or latency, and tau (τ) is the time constant. The fitting process employed the least-squares minimization procedure based on the “trust-region-reflective” algorithm provided by MATLAB. The model was allowed to fit a, b, Δt, and τ. The initial values of a and b (i.e., those used in the first iteration) were the mean levels of oscillation before and after visual change, respectively. After the fitting procedure, the output for variables a and b deviated slightly from their initial values and always remained within 0.2 standard deviations (SD) of the mean values of velocity level of oscillation; the resulting time shift (Δt in the equations) was considered to be the duration of the integration time period, or latency, whereas the subsequent τ was the time employed during the reweighting period to reach 63% or to fall to 37% of maximum after withdrawal or addition of stabilizing information, respectively.
Statistics.
A 2 (mentally idle/counting backward) × 2 (vision/no vision) repeated-measures ANOVA was used to test the effect of the counting-backward task on the mean level of velocity of ML CoP oscillation during vision with respect to no vision. To test the effects of the cognitive task on the latencies following addition or withdrawal of vision, a 2 (mentally idle/counting backward) × 2 (addition/withdrawal) repeated-measures ANOVA was used. To test the effects of the cognitive task on the time constant following addition or withdrawal of vision, a 2 (mentally idle/counting backward) × 2 (addition/withdrawal) repeated-measures ANOVA was used. Post hoc analysis was made using the Fischer's least significant difference test. The level of significance in all tests was set to P < 0.05. The software Statistica (StatSoft, Tulsa, OK) was used.
RESULTS
Effect of vision on the mean level of CoP velocity of oscillations.
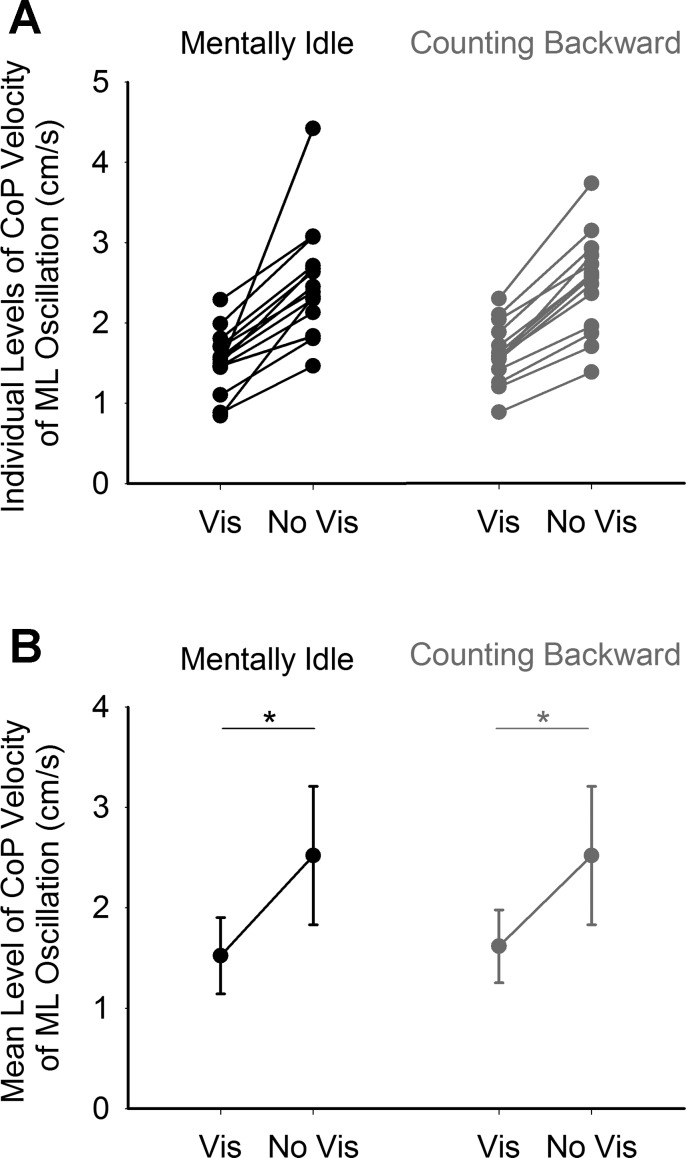
The level of velocity of ML CoP oscillations was lower in the presence of vision, under both mentally idle and counting-backward conditions. The levels of velocity of ML CoP oscillation of each subject in the presence and absence of vision are shown in Fig. 2A for both conditions. The grand means and SD of the levels of velocity of oscillation are shown in Fig. 2B. ANOVA revealed a significant lower value in oscillation level when vision was present with respect to absent [F(1,13) = 63.9, P < 0.0001]. Performing the counting-backward task had no significant effect on the mean level of velocity of CoP oscillation [F(1,13) = 0.19, P = 0.67]. There was no interaction between cognitive task and vision [F(1,13) = 1.1, P < 0.32].
Fig. 2.
Mean level of velocity of ML CoP oscillation. A shows the levels of oscillation velocity of each subject during the presence (Vis) and absence of vision (No Vis) under the mentally idle (black) and counting-backward (gray) conditions. B shows the grand mean and SD of the levels of velocity of oscillation of all the subjects. *P < 0.05 indicates significant difference.
Latencies of the changes in CoP oscillation in response to the visual shift.
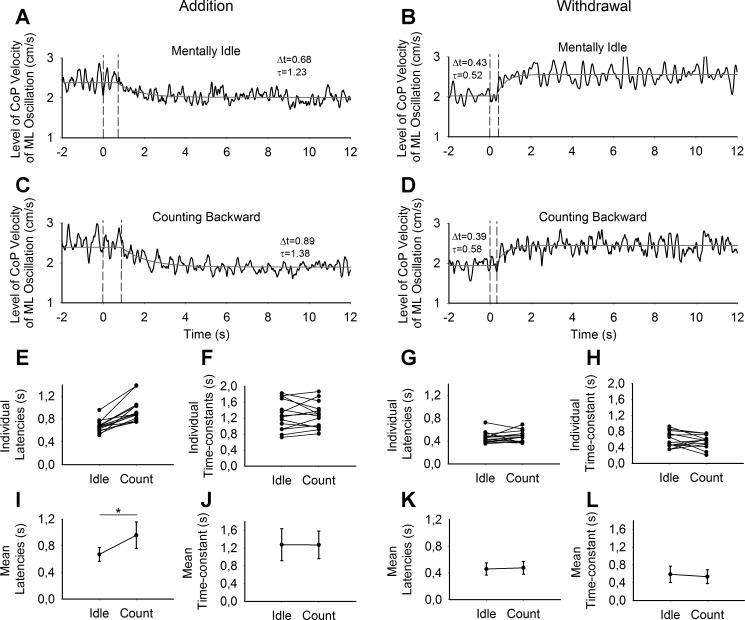
Figure 3, A–D, shows the traces of the velocity of ML CoP oscillation in a single subject following addition (A and C) and withdrawal (B and D) of vision in the two task conditions (mentally idle, A and B; counting backward, C and D). The data show that under both conditions, a short time interval (latency) elapsed following the visual shift (t = 0 s). Subsequently, an exponential decrease or increase ensued depending on whether vision was added or withdrawn, respectively.
Fig. 3.
Latencies and time constants following the shift in sensory state. A–D show the time course of the level of velocity of ML CoP oscillation traces (black) and the fitted exponential curve (gray lines) in 4 conditions: addition (A and C) and withdrawal (B and D) when the subject was mentally idle (A and B) and when the subject was counting backward (C and D). These curves were obtained from 1 representative subject. E–H show the individual latencies (E and G) and time constants (F and H) of all the subjects following addition (E and F) and withdrawal (G and H) of vision under the mentally idle (Idle) and counting backward (Count) conditions. I–L show the grand mean and SD of latencies (I and K) and time constants (J and L) following addition (I and J) and withdrawal (K and L) of vision under the mentally idle (Idle) and counting backward (Count) conditions. *P < 0.05 indicates significant difference.
The individual latencies and time constants following addition and withdrawal of vision under the mentally idle and counting-backward conditions are shown in Fig. 3, E–H. Means and SD of the latencies and time constants are reported in Fig. 3, I–L. The latencies and time constants have been calculated by fitting a time-delayed exponential curve to the data of velocity of CoP oscillation. ANOVA showed a significant effect of counting backward on the latencies of the oscillation change following the shift in visual state [F(1,13) = 31.4, P < 0.0001]. ANOVA also showed a difference between the latency after withdrawal and the latency after the addition of vision [F(1,13) = 52.3, P < 0.0001]. There was a significant interaction between mentally idle/counting-backward task and withdrawal/addition [F(1,13) = 34.7, P < 0.0001]. Post hoc tests were used to compare the effect of the counting-backward condition on the identified latencies. For the addition of visual information, a significant increase in latency was found in the counting-backward condition compared with the mentally idle condition (P < 0.0001). For withdrawal of visual information, no significant changes in latency were found between cognitive conditions (P = 0.60).
Exponential recovery to a new level of CoP oscillation.
As expected, the time constants were longer following the addition than following withdrawal of vision [F(1,13) = 52.8, P < 0.0001; Fig. 3, F, J, H, L]. Performing the counting-backward task had no significant effect on the time constants [F(1,13) = 0.5, P = 0.49] following either sensory shift. There was no interaction between cognitive conditions and withdrawal/addition [F(1,13) = 0.37, P = 0.55].
DISCUSSION
Cortical processing is involved in the control of sway during standing (Kerr et al. 1985; Lajoie et al. 1993; Maki and McIlroy 1996; Soto et al. 2006; Stins et al. 2009; Taube et al. 2006, 2007; Valls-Solé et al. 1994). It is also admitted that the complex multisensory integration process that follows the shift in the sensory state takes place, at least in part, at the supraspinal level (Bolton 2015; Bronstein et al. 1990; Honeine and Schieppati 2014; Maki and McIlroy 2007; Teasdale and Simoneau 2001). During stance, the cortico-striato-pallido-thalamo-cortical loop (Jahn et al. 2004) and the cerebellum (Inukai et al. 2016) seem to represent the main sensorimotor interface. Other studies conducted with functional MRI have emphasized the role of the premotor cortex and the parietal cortex in sensorimotor integration (Fattori et al. 2009; Macaluso and Maravita 2010; Sepulcre 2014). Under critical balance conditions, as in our tandem-stance task, in which controlled processing of standing is associated with major adjustments in lower limb muscle activity (Sozzi et al. 2013), the motor cortex would play a prominent role in addition to the parietal cortex (Boisgontier et al. 2013; Mihara et al. 2008; Ouchi et al. 1999; Papegaaij et al. 2016; Slobounov et al. 2005). The tandem posture would be more susceptible than quiet stance to cognitive demands (Dault et al. 2001).
In this study, subjects performed a cognitive task consisting of counting backward in steps of three while vision was withdrawn or added. The time course following a change in visual state under this condition was compared with that obtained when subjects stood mentally idle. Number subtraction involves enhanced activity of several cortical regions such as left and right cingulate/medial frontal, left inferior frontal, left precentral/middle frontal, left and right intraparietal sulci, left occipital, left and right insula, and left thalamus areas (Fehr et al. 2007; Kong et al. 2005; Menon et al. 2000; Rickard et al. 2000). The subtraction task also calls into action the basic anatomical substrate of working memory and numerical knowledge that includes the parietal cortex and the prefrontal cortex (Burton et al. 2008; Fehr et al. 2007; Mirelman et al. 2014; Sveljo et al. 2014). Since the arithmetic task seems to share cortical areas involved in sensorimotor integration, and since effects of counting on the reaction time following a perturbation of balance are documented (Ceyte et al. 2014; Chong et al. 2010; Pellecchia 2003; Rankin et al. 2000; Siu and Woollacott 2007; Woollacott and Shumway-Cook 2002), we investigated whether counting backward while standing in tandem would have an effect on the time to integrate the sudden addition or loss of vision. We also examined whether the subtraction task would influence the duration of the ensuing reweighting process that is observable as an exponential change in CoP level of oscillation.
Cognitive effect on the level of CoP velocity of oscillation.
Previous studies have not always agreed on the effect of performing a cognitive task on the level of sway, with authors reporting an increase (Mitra et al. 2013; Pellecchia 2003; Shumway-Cook and Woollacott 2000) or a decrease (Andersson et al. 2002; Negahban et al. 2015; Swan et al. 2004; Vuillerme et al. 2000) depending on the nature of the cognitive task and the difficulty of the balancing task (Woollacott and Shumway-Cook 2002). Pellecchia (2003) found that counting backward in steps of three increased the sway path during standing on a foam pad with eyes open. On the other hand, undertaking the Brooks spatial memory task while maintaining balance in tandem stance had an effect on the mean number of errors in the cognitive task but had no effect on postural sway (Kerr et al. 1985). The possible complications inherent in these studies are mainly due to the differences in the standing postures and the cognitive tasks (see Mitra and Frazier 2004 and Lacour et al. 2008 for an extensive discussion).
A negligible increase in body sway was found in our study during counting backward compared with the mentally idle condition during eyes open. The mean level of oscillation was also comparable between the counting-backward and mentally idle conditions when vision was absent. Overall, it is safe to assume that, under a tandem-stance condition, performance of the counting-backward task does not modify the level of oscillation. Perhaps, under tandem stance, there is sort of a ceiling effect, since sway is already much larger (even with eyes open) under tandem stance than with feet parallel, and subjects would not tolerate any further increase of body sway with the cognitive task, amplifying the risk of falling due to the small support base. From an analytical point of view, the negligible effect of the counting-backward task per se on the sway level simplifies the interpretation of the changes in latency in response to the visual shifts (see below), since the starting sway conditions (mentally idle/counting backward) are equivalent.
When our subjects were asked at the end of the experiment which of the two conditions seemed to be the hardest to endure, all agreed that it was the mentally idle condition. Surprisingly, there was unanimous agreement on the fact that the counting-backward condition seemed to be easier because it took attention away from the balancing task itself. This would imply that attention to the critical standing condition and related oscillations would favor a costly moment-to-moment correction strategy (Tokuno et al. 2009). If this assumption is tenable, a smaller sway may not necessarily correspond to a smaller overall neural and muscle effort (Kiemel et al. 2011). It might be speculated that the arithmetic task promotes intermittent rather than continuous activation of feedback control (Asai et al. 2009).
Cognitive effect on the latency following addition of vision.
Our hypothesis that the events occurring after addition of vision, i.e., the integration of a newly entering stabilizing information, would involve higher order processing has been confirmed by our results. The latency of the onset of the changes in CoP oscillation increased during the cognitive task in all subjects. When the visual information is added, this input about the environment likely has to first be recognized, before the brain gives it appropriate weight (see for a review Ernst and Bülthoff 2004). This time period would allow for the computation necessary for the integration of the new input critically relevant for balance control and for the organization of the appropriate postural set. This process would occur under both static (Amblard and Carblanc 1980; Pinar et al. 2010) and dynamic conditions (De Nunzio and Schieppati 2007; see Sozzi et al. 2016).
The counting-backward task increased the latencies to the onset of reduction in body oscillation by about 300 ms, on average, following the sudden addition of vision. This is in keeping with the hypothesis that the sensorimotor integration process following addition of vision (or, in general, of a stabilizing sensory input) implies resource competition at high-level cortical processing. Previously, Teasdale et al. (1993) and Lajoie et al. (1993) showed that the reaction times to an acoustic stimulus increased during standing (feet together) with respect to sitting (eyes open). Teasdale and Simoneau (2001) also found that the reaction times to an acoustic signal during quiet standing slightly increased when subjects had to reintegrate the proprioceptive input while eyes were closed. Mcllroy et al. (1999) and Norrie et al. (2002) found that when balance was perturbed during tracking of a moving target on a computer screen with a potentiometer, a delay between the onset of balance reaction and a pause in the tracking of 235 ms occurred. Rankin et al. (2000) reported an effect of counting backward in steps of three on the EMG response of tibialis anterior and gastrocnemius muscles to a perturbation of balance. The cognitive task caused a reduction in the long-loop EMG activity of those leg muscles appearing from 200 to 500 ms following the perturbation. Our results strengthen the contention that sensorimotor integration of a newly entering balance-stabilizing input is performed at a cortical level, sharing neural circuits that play a role in performing an arithmetic task.
Cognitive effect on the latency following withdrawal of vision.
In line with previous research, the latency of the changes in sway following withdrawal of vision was always shorter than following addition, under both the mentally idle and the counting-backward conditions (Assländer and Peterka 2016; Honeine and Schieppati 2014; Honeine et al. 2015; Sozzi et al. 2011, 2012). In particular, the counting-backward task has no significant effect on that latency with respect to the mentally idle condition. This result is in keeping with the supposition that the sensory integration process that occurs following withdrawal of a sensory input relies on a rapid, subcortically expressed default postural set, which relies on proprioception and vestibular input. This is reasonable in the sense that when an afferent input is suddenly withdrawn, the body has to respond quickly and swiftly reweight its reliance on the remaining sensory information (here proprioceptive and vestibular; Cullen 2016) to minimize the risk of falling. The information to be “reweighted” is already present in the period preceding vision withdrawal and is therefore known to the brain and ready to be upgraded.
Cognitive effect on the time constant following addition or withdrawal of vision.
After the integration process, be it withdrawal or addition of a stabilizing input, the level of body oscillation changes exponentially as the central nervous system gradually reweights the adaptive gain specified by each available sensory modality involved in maintaining balance (Carver et al. 2006; Hwang et al. 2014; Peterka 2002). In line with previous research, the duration of the reweighting period is longer following addition than following withdrawal (Assländer and Peterka 2014, 2016; Honeine et al. 2015; Sozzi et al. 2011, 2012). The length of the time constants suggests that the reweighing process requires cortical supervision, as also found under sensory (haptic) steady-state conditions (Bolton et al. 2011). However, the time constants were not affected by the counting-backward task, on either addition or withdrawal of vision. Therefore, dual tasking does not seem to interfere with the process whereby sway is being progressively minimized after the delay connected with vision integration or progressively increased to the level determined by the absence of the stabilizing visual information. In line with our previously mentioned assumptions, we suggest that whereas the sensory integration (in our case, addition of vision) occurs at the cortical level and is affected by the cognitive task, the subsequent reweighting process may not be supervised by the regions occupied by the counting-backward task.
It is not unlikely that different parts of the central nervous system contribute to the reweighting process, as has been shown in connection to different phases in a dynamic locomotor task (Taube et al. 2008). For instance, the cerebellar vermis, site of convergence of information relevant for standing (proprioceptive, vestibular, visual), is seemingly not implicated in the timing of the integration of vision, whereas it is definitely implicated in the control of sway amplitude under steady state (Colnaghi et al. 2016). It could be responsible for the progressive adjustment of the sway level to the new sensory condition.
Problems and limitations.
In this study, the latencies obtained under the control (mentally idle) condition were somewhat shorter than those measured in previous research (Sozzi et al. 2011, 2012) and just slightly so compared with those of Honeine et al. (2015). In studies reported by Sozzi et al. (2011, 2012), a threshold algorithm based on the Student's t-test was used to measure the latencies to the changes in CoP oscillation. This algorithm depended much on the variability of the mean level of sway preceding the sensory change, likely resulting in an overestimation of the latencies, as discussed by Honeine and Schieppati (2014).
The minor difference compared with the findings of Honeine et al. (2015) has two sources. One is the larger number of trials that were performed by the subjects (about 90 with respect to about 60), which enhances the sharpness of the analytical procedure. The other is the choice of the signal considered for the computation, i.e., the mean velocity of the CoP oscillation (Assländer and Peterka 2016) instead of the mean level of displacement. We consider that increasing the trial number and the selecting the velocity allows us to get closer to the “true locus” in the central nervous system, where this time-consuming sensorimotor integration process takes place.
We found a relatively large variability across subjects in the levels of oscillation under constant visual conditions and a relatively variable effect of the cognitive task on those levels, as well as on the duration of the latencies and the time constants. In addition to the different individual abilities of standing in tandem position, an uncontrolled source of variability can be traced to the level of cognitive activity of the subjects during the task of standing mentally idle or to the attention devoted to counting backward. We did not check for either the cognitive engagement devoted to the mentally idle task (the “internal focus of attention”; Nafati and Vuillerme 2011) or the quality of the subtraction task itself (such as velocity of computing or exactness of the calculus, as mentioned in Rougier and Bonnet 2016). Although we and the subjects were broadly satisfied with the counting results, it proved to be unrealistic to identify any relationship between features of counting and effects on integration latencies. As a note, the effects of our arithmetic cognitive task may not generalize to other cognitive tasks, and engagement of other executive functions or sensory channels may have produced different effects.
Conclusion.
To our knowledge, this is the first study to show a delaying effect of performing a cognitive task on the timing of the sensorimotor integration process that occurs following addition of vision. Interestingly, the cognitive task has instead no effect on the latency and on the time constant following withdrawal of vision. Consequently, it seems plausible that, on one hand, cortical processing is involved in the computational process involved in the sensorimotor integration on addition of a stabilizing input. On the other hand, the sensorimotor integration following withdrawal of a stabilizing input may be a more “automatic” process, prompted under a critical balance state to deal with the increasing postural threat. The following reweighting period, in which vision is either fully incorporated in the postural control system or its effects are removed and balance is maintained by proprioceptive and vestibular feedback, is unaffected by the subtraction cognitive task. We take this as being the expression of the operation of a subcortical mechanism. These results should be helpful for future work in clinical populations, such as older adults or patients with disorders that affect balance.
GRANTS
This study was supported in part by “Ricerca Finalizzata” Grant RF-2011-02352379 from the Italian Ministry of Health and by “PRIN” Grant 2010MEFNF7 from the Italian Ministry of University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.-L.H. and M.S. conceived and designed research; J.-L.H. and O.C. performed experiments; J.-L.H., O.C., and M.S. analyzed data; J.-L.H. and M.S. interpreted results of experiments; J.-L.H. prepared figures; J.-L.H. and M.S. drafted manuscript; J.-L.H. and M.S. edited and revised manuscript; J.-L.H., O.C., and M.S. approved final version of manuscript.
REFERENCES
- Amblard B, Carblanc A. Role of foveal and peripheral visual information in maintenance of postural equilibrium in man. Percept Mot Skills 51: 903–912, 1980. [DOI] [PubMed] [Google Scholar]
- Andersson G, Hagman J, Talianzadeh R, Svedberg A, Larsen HC. Effect of cognitive load on postural control. Brain Res Bull 58: 135–139, 2002. [DOI] [PubMed] [Google Scholar]
- Asai Y, Tasaka Y, Nomura K, Nomura T, Casadio M, Morasso P. A model of postural control in quiet standing: robust compensation of delay-induced instability using intermittent activation of feedback control. PLoS One 4: e6169, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assländer L, Peterka RJ. Sensory reweighting dynamics in human postural control. J Neurophysiol 111: 1852–1864, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assländer L, Peterka RJ. Sensory reweighting dynamics following removal and addition of visual and proprioceptive cues. J Neurophysiol 116: 272–285, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier MP, Beets IA, Duysens J, Nieuwboer A, Krampe RT, Swinnen SP. Age-related differences in attentional cost associated with postural dual tasks: increased recruitment of generic cognitive resources in older adults. Neurosci Biobehav Rev 37: 1824–1837, 2013. [DOI] [PubMed] [Google Scholar]
- Bolton DA. The role of the cerebral cortex in postural responses to externally induced perturbations. Neurosci Biobehav Rev 57: 142–155, 2015. [DOI] [PubMed] [Google Scholar]
- Bolton DA, McIlroy WE, Staines WR. The impact of light fingertip touch on haptic cortical processing during a standing balance task. Exp Brain Res 212: 279–291, 2011. [DOI] [PubMed] [Google Scholar]
- Bonnet CT. Advantages and disadvantages of stiffness instructions when studying postural control. Gait Posture 46: 208–210, 2016. [DOI] [PubMed] [Google Scholar]
- Brandt T, Bartenstein P, Janek A, Dieterich M. Reciprocal inhibitory visual-vestibular interaction. Visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain 121: 1749–1758, 1998. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Hood JD, Gresty MA, Panagi C. Visual control of balance in cerebellar and parkinsonian syndromes. Brain 113: 767–779, 1990. [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, McLaren DG. Cortical network for vibrotactile attention: a fMRI study. Hum Brain Mapp 29: 207–221, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver S, Kiemel T, Jeka JJ. Modeling the dynamics of sensory reweighting. Biol Cybern 95: 123–134, 2006. [DOI] [PubMed] [Google Scholar]
- Cenciarini M, Peterka RJ. Stimulus-dependent changes in the vestibular contribution to human postural control. J Neurophysiol 95: 2733–2750, 2006. [DOI] [PubMed] [Google Scholar]
- Ceyte H, Lion A, Caudron S, Kriem B, Perrin PP, Gauchard GC. Does calculating impair postural stabilization allowed by visual cues? Exp Brain Res 232: 2221–2228, 2014. [DOI] [PubMed] [Google Scholar]
- Chong RK, Mills B, Dailey L, Lane E, Smith S, Lee KH. Specific interference between a cognitive task and sensory organization for stance balance control in healthy young adults: visuospatial effects. Neuropsychologia 48: 2709–2718, 2010. [DOI] [PubMed] [Google Scholar]
- Colnaghi S, Honeine JL, Sozzi S, Schieppati M. Body sway increases after functional inactivation of the cerebellar vermis by cTBS. Cerebellum. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE. Physiology of central pathways. Handb Clin Neurol 137: 17–40, 2016. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol 91: 1919–1933, 2004. [DOI] [PubMed] [Google Scholar]
- Cutfield NJ, Scott G, Waldman AD, Sharp DJ, Bronstein AM. Visual and proprioceptive interaction in patients with bilateral vestibular loss. Neuroimage Clin 4: 274–282, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dault MC, Geurts AC, Mulder TW, Duysens J. Postural control and cognitive task performance in healthy participants while balancing on different support-surface configurations. Gait Posture 14: 248–255, 2001. [DOI] [PubMed] [Google Scholar]
- De Nunzio AM, Schieppati M. Time to reconfigure balancing behaviour in man: changing visual condition while riding a continuously moving platform. Exp Brain Res 178: 18–36, 2007. [DOI] [PubMed] [Google Scholar]
- Dreher T, Teasdale N. Editorial. Gait Posture 46: 208, 2016. [DOI] [PubMed] [Google Scholar]
- Elias LA, Watanabe RN, Kohn AF. Spinal mechanisms may provide a combination of intermittent and continuous control of human posture: predictions from a biologically based neuromusculoskeletal model. PLoS Comput Biol 10: e1003944, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MO, Bülthoff HH. Merging the senses into a robust percept. Trends Cogn Sci 8: 162–169, 2004. [DOI] [PubMed] [Google Scholar]
- Fattori P, Pitzalis S, Galletti C. The cortical visual area V6 in macaque and human brains. J Physiol (Paris) 103: 88–97, 2009. [DOI] [PubMed] [Google Scholar]
- Fehr T, Code C, Herrmann M. Common brain regions underlying different arithmetic operations as revealed by conjunct fMRI-BOLD activation. Brain Res 1172: 93–102, 2007. [DOI] [PubMed] [Google Scholar]
- Grangeon M, Guillot A, Collet C. Postural control during visual and kinesthetic motor imagery. Appl Psychophysiol Biofeedback 36: 47–56, 2011. [DOI] [PubMed] [Google Scholar]
- Honeine JL, Crisafulli O, Sozzi S, Schieppati M. Processing time of addition or withdrawal of single or combined balance-stabilizing haptic and visual information. J Neurophysiol 114: 3097–3110, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeine JL, Schieppati M. Time-interval for integration of stabilizing haptic and visual information in subjects balancing under static and dynamic conditions. Front Syst Neurosci 8: 190, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Agada P, Kiemel T, Jeka JJ. Dynamic reweighting of three modalities for sensor fusion. PLoS One 9: e88132, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai Y, Saito K, Sasaki R, Kotan S, Nakagawa M, Onishi H. Influence of Transcranial Direct Current Stimulation To The Cerebellum On Standing Posture Control. Front Hum Neurosci 10: 325, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigaki T, Imai R, Morioka S. Cathodal transcranial direct current stimulation of the posterior parietal cortex reduces steady-state postural stability during the effect of light touch. Neuroreport 27: 1050–1055, 2016. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm (Vienna) 114: 1339–1348, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K, Deutschländer A, Stephan T, Strupp M, Wiesmann M, Brandt T. Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. Neuroimage 22: 1722–1731, 2004. [DOI] [PubMed] [Google Scholar]
- Jonsson E, Seiger A, Hirschfeld H. Postural steadiness and weight distribution during tandem stance in healthy young and elderly adults. Clin Biomech (Bristol, Avon) 20: 202–208, 2005. [DOI] [PubMed] [Google Scholar]
- Kelly LA, Kuitunen S, Racinais S, Cresswell AG. Recruitment of the plantar intrinsic foot muscles with increasing postural demand. Clin Biomech (Bristol, Avon) 27: 46–51, 2012. [DOI] [PubMed] [Google Scholar]
- Kerr B, Condon SM, McDonald LA. Cognitive spatial processing and the regulation of posture. J Exp Psychol Hum Percept Perform 11: 617–622, 1985. [DOI] [PubMed] [Google Scholar]
- Kiemel T, Zhang Y, Jeka JJ. Identification of neural feedback for upright stance in humans: stabilization rather than sway minimization. J Neurosci 31: 15144–15153, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Wang C, Kwong K, Vangel M, Chua E, Gollub R. The neural substrate of arithmetic operations and procedure complexity. Brain Res Cogn Brain Res 22: 397–405, 2005. [DOI] [PubMed] [Google Scholar]
- Lacour M, Bernard-Demanze L, Dumitrescu M. Posture control, aging, and attention resources: models and posture-analysis methods. Neurophysiol Clin 38: 411–421, 2008. [DOI] [PubMed] [Google Scholar]
- Lajoie Y, Teasdale N, Bard C, Fleury M. Attentional demands for static and dynamic equilibrium. Exp Brain Res 97: 139–144, 1993. [DOI] [PubMed] [Google Scholar]
- Logan D, Kiemel T, Jeka JJ. Asymmetric sensory reweighting in human upright stance. PLoS One 9: e100418, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso E, Maravita A. The representation of space near the body through touch and vision. Neuropsychologia 48: 782–795, 2010. [DOI] [PubMed] [Google Scholar]
- Mahboobin A, Loughlin PJ, Redfern MS. A model-based approach to attention and sensory integration in postural control of older adults. Neurosci Lett 429: 147–151, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. Influence of arousal and attention on the control of postural sway. J Vestib Res 6: 53–59, 1996. [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. Cognitive demands and cortical control of human balance-recovery reactions. J Neural Transm (Vienna) 114: 1279–1296, 2007. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Allison S, Wing AM. Effects of spatial and nonspatial cognitive activity on postural stability. Br J Psychol 92: 319–338, 2001. [PubMed] [Google Scholar]
- McIlroy WE, Norrie RG, Brooke JD, Bishop DC, Nelson AJ, Maki BE. Temporal properties of attention sharing consequent to disturbed balance. Neuroreport 10: 2895–2899, 1999. [DOI] [PubMed] [Google Scholar]
- Menon V, Rivera SM, White CD, Glover GH, Reiss AL. Dissociating prefrontal and parietal cortex activation during arithmetic processing. Neuroimage 12: 357–365, 2000. [DOI] [PubMed] [Google Scholar]
- Mergner T, Maurer C, Peterka RJ. Sensory contributions to the control of stance: a posture control model. Adv Exp Med Biol 508: 147–152, 2002. [DOI] [PubMed] [Google Scholar]
- Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Role of the prefrontal cortex in human balance control. Neuroimage 43: 329–336, 2008. [DOI] [PubMed] [Google Scholar]
- Mirelman A, Maidan I, Bernad-Elazari H, Nieuwhof F, Reelick M, Giladi N, Hausdorff JM. Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. J Neuroeng Rehabil 11: 85, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Fraizer EV. Effects of explicit sway-minimization on postural-suprapostural dual-task performance. Hum Mov Sci 23: 1–20, 2004. [DOI] [PubMed] [Google Scholar]
- Mitra S, Knight A, Munn A. Divergent effects of cognitive load on quiet stance and task-linked postural coordination. J Exp Psychol Hum Percept Perform 39: 323–328, 2013. [DOI] [PubMed] [Google Scholar]
- Murnaghan CD, Squair JW, Chua R, Inglis JT, Carpenter MG. Cortical contributions to control of posture during unrestricted and restricted stance. J Neurophysiol 111: 1920–1926, 2014. [DOI] [PubMed] [Google Scholar]
- Nafati G, Vuillerme N. Decreasing internal focus of attention improves postural control during quiet standing in young healthy adults. Res Q Exerc Sport 82: 634–643, 2011. [DOI] [PubMed] [Google Scholar]
- Nardone A, Schieppati M. Inhibitory effect of the Jendrassik maneuver on the stretch reflex. Neuroscience 156: 607–617, 2008. [DOI] [PubMed] [Google Scholar]
- Negahban H, Karimi M, Goharpey S, Mehravar M, Namnik N. Posture-cognition interaction during quiet standing in patients with knee osteoarthritis. Physiother Theory Pract 31: 540–546, 2015. [DOI] [PubMed] [Google Scholar]
- Norrie RG, Maki BE, Staines WR, McIlroy WE. The time course of attention shifts following perturbation of upright stance. Exp Brain Res 146: 315–321, 2002. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Okada H, Yoshikawa E, Nobezawa S, Futatsubashi M. Brain activation during maintenance of standing postures in humans. Brain 122: 329–338, 1999. [DOI] [PubMed] [Google Scholar]
- Papegaaij S, Taube W, van Keeken HG, Otten E, Baudry S, Hortobágyi T. Postural challenge affects motor cortical activity in young and old adults. Exp Gerontol 73: 78–85, 2016. [DOI] [PubMed] [Google Scholar]
- Pellecchia GL. Postural sway increases with attentional demands of concurrent cognitive task. Gait Posture 18: 29–34, 2003. [DOI] [PubMed] [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol 88: 1097–1118, 2002. [DOI] [PubMed] [Google Scholar]
- Pinar S, Kitano K, Koceja DM. Role of vision and task complexity on soleus H-reflex gain. J Electromyogr Kinesiol 20: 354–358, 2010. [DOI] [PubMed] [Google Scholar]
- Polastri PF, Barela JA, Kiemel T, Jeka JJ. Dynamics of inter-modality re-weighting during human postural control. Exp Brain Res 223: 99–108, 2012. [DOI] [PubMed] [Google Scholar]
- Rankin JK, Woollacott MH, Shumway-Cook A, Brown LA. Cognitive influence on postural stability: a neuromuscular analysis in young and older adults. J Gerontol A Biol Sci Med Sci 55: M112–M119, 2000. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Jennings JR, Martin C, Furman JM. Attention influences sensory integration for postural control in older adults. Gait Posture 14: 211–216, 2001. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Muller ML, Jennings JR, Furman JM. Attentional dynamics in postural control during perturbations in young and older adults. J Gerontol A Biol Sci Med Sci 57: B298–B303, 2002. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Talkowski ME, Jennings JR, Furman JM. Cognitive influences in postural control of patients with unilateral vestibular loss. Gait Posture 19: 105–114, 2004. [DOI] [PubMed] [Google Scholar]
- Reynolds RF. The ability to voluntarily control sway reflects the difficulty of the standing task. Gait Posture 31: 78–81, 2010. [DOI] [PubMed] [Google Scholar]
- Rickard TC, Romero SG, Basso G, Wharton C, Flitman S, Grafman J. The calculating brain: an fMRI study. Neuropsychologia 38: 325–335, 2000. [DOI] [PubMed] [Google Scholar]
- Rougier PR, Bonnet CT. How providing more or less time to solve a cognitive task interferes with upright stance control; a posturographic analysis on healthy young adults. Hum Mov Sci 47: 106–115, 2016. [DOI] [PubMed] [Google Scholar]
- Saffer M, Kiemel T, Jeka J. Coherence analysis of muscle activity during quiet stance. Exp Brain Res 185: 215–226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saradjian AH, Tremblay L, Perrier J, Blouin J, Mouchnino L. Cortical facilitation of proprioceptive inputs related to gravitational balance constraints during step preparation. J Neurophysiol 110: 397–407, 2013. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A, Siliotto R, Grasso M. Early and late stretch responses of human foot muscles induced by perturbation of stance. Exp Brain Res 105: 411–422, 1995. [DOI] [PubMed] [Google Scholar]
- Schmid M, Conforto S, Lopez L, D'Alessio T. Cognitive load affects postural control in children. Exp Brain Res 179: 375–385, 2007. [DOI] [PubMed] [Google Scholar]
- Sciadas R, Dalton C, Nantel J. Effort to reduce postural sway affects both cognitive and motor performances in individuals with Parkinson's disease. Hum Mov Sci 47: 135–140, 2016. [DOI] [PubMed] [Google Scholar]
- Sepulcre J. Functional streams and cortical integration in the human brain. Neuroscientist 20: 499–508, 2014. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott M. Attentional demands and postural control: the effect of sensory context. J Gerontol A Biol Sci Med Sci 55: M10–M16, 2000. [DOI] [PubMed] [Google Scholar]
- Siu KC, Woollacott MH. Attentional demands of postural control: the ability to selectively allocate information-processing resources. Gait Posture 25: 121–126, 2007. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Hallett M, Stanhope S, Shibasaki H. Role of cerebral cortex in human postural control: an EEG study. Clin Neurophysiol 116: 315–323, 2005. [DOI] [PubMed] [Google Scholar]
- Soto O, Valls-Solé J, Shanahan P, Rothwell J. Reduction of intracortical inhibition in soleus muscle during postural activity. J Neurophysiol 96: 1711–1717, 2006. [DOI] [PubMed] [Google Scholar]
- Sozzi S, Do MC, Monti A, Schieppati M. Sensorimotor integration during stance: processing time of active or passive addition or withdrawal of visual or haptic information. Neuroscience 212: 59–76, 2012. [DOI] [PubMed] [Google Scholar]
- Sozzi S, Honeine JL, Do MC, Schieppati M. Leg muscle activity during tandem stance and the control of body balance in the frontal plane. Clin Neurophysiol 124: 1175–1186, 2013. [DOI] [PubMed] [Google Scholar]
- Sozzi S, Monti A, De Nunzio AM, Do MC, Schieppati M. Sensori-motor integration during stance: time adaptation of control mechanisms on adding or removing vision. Hum Mov Sci 30: 172–189, 2011. [DOI] [PubMed] [Google Scholar]
- Sozzi S, Nardone A, Schieppati M. Calibration of the leg muscle responses elicited by predictable perturbations of stance and the effect of vision. Front Hum Neurosci 10: 419, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmach GE, Zelaznik HN, Lowe D. The influence of aging and attentional demands on recovery from postural instability. Aging (Milano) 2: 155–161, 1990. [DOI] [PubMed] [Google Scholar]
- Stins JF, Michielsen ME, Roerdink M, Beek PJ. Sway regularity reflects attentional involvement in postural control: effects of expertise, vision and cognition. Gait Posture 30: 106–109, 2009. [DOI] [PubMed] [Google Scholar]
- Sveljo O, Culić M, Koprivšek K, Lučić M. The functional neuroimaging evidence of cerebellar involvement in the simple cognitive task. Brain Imaging Behav 8: 480–486, 2014. [DOI] [PubMed] [Google Scholar]
- Swan L, Otani H, Loubert PV, Sheffert SM, Dunbar GL. Improving balance by performing a secondary cognitive task. Br J Psychol 95: 31–40, 2004. [DOI] [PubMed] [Google Scholar]
- Taube W, Gruber M, Beck S, Faist M, Gollhofer A, Schubert M. Cortical and spinal adaptations induced by balance training: correlation between stance stability and corticospinal activation. Acta Physiol (Oxf) 189: 347–358, 2007. [DOI] [PubMed] [Google Scholar]
- Taube W, Leukel C, Schubert M, Gruber M, Rantalainen T, Gollhofer A. Differential modulation of spinal and corticospinal excitability during drop jumps. J Neurophysiol 99: 1243–1252, 2008. [DOI] [PubMed] [Google Scholar]
- Taube W, Schubert M, Gruber M, Beck S, Faist M, Gollhofer A. Direct corticospinal pathways contribute to neuromuscular control of perturbed stance. J Appl Physiol 101: 420–429, 2006. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Bard C, LaRue J, Fleury M. On the cognitive penetrability of posture control. Exp Aging Res 19: 1–13, 1993. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Simoneau M. Attentional demands for postural control: the effects of aging and sensory reintegration. Gait Posture 14: 203–210, 2001. [DOI] [PubMed] [Google Scholar]
- Tokuno CD, Taube W, Cresswell AG. An enhanced level of motor cortical excitability during the control of human standing. Acta Physiol (Oxf) 195: 385–395, 2009. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Alvarez R, Tolosa ES. Responses of the soleus muscle to transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 93: 421–427, 1994. [DOI] [PubMed] [Google Scholar]
- Van Impe A, Coxon JP, Goble DJ, Doumas M, Swinnen SP. White matter fractional anisotropy predicts balance performance in older adults. Neurobiol Aging 33: 1900–1912, 2012. [DOI] [PubMed] [Google Scholar]
- Varghese JP, Beyer KB, Williams L, Miyasike-daSilva V, McIlroy WE. Standing still: is there a role for the cortex? Neurosci Lett 590: 18–23, 2015. [DOI] [PubMed] [Google Scholar]
- Vuillerme N, Nougier V, Teasdale N. Effects of a reaction time task on postural control in humans. Neurosci Lett 291: 77–80, 2000. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 16: 1–14, 2002. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Smith CE, Suzuki Y, Kiyono K, Tanahashi T, Sakoda S, Morasso P, Nomura T. Universal and individual characteristics of postural sway during quiet standing in healthy young adults. Physiol Rep 3: e12329, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zok M, Mazzà C, Cappozzo A. Should the instructions issued to the subject in traditional static posturography be standardised? Med Eng Phys 30: 913–916, 2008. [DOI] [PubMed] [Google Scholar]
- Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol Aging 33: 1073–1084, 2012. [DOI] [PubMed] [Google Scholar]