Abstract
Neuromuscular disorders such as Pompe disease (glycogen storage disease, type II), result in early and potentially irreversible cellular damage with a very limited opportunity for intervention in the newborn period. Pompe disease is due to deficiency in acid α-glucosidase (GAA) leading to lysosomal accumulation of glycogen in all cell types, abnormal myofibrillogenesis, respiratory insufficiency, neurological deficits, and reduced contractile function in striated muscle. Previous studies have shown that fetal delivery of recombinant adeno-associated virus (rAAV) encoding GAA to the peritoneal cavity of Gaa–/– mice resulted in high-level transduction of the diaphragm. While progression of other genetic disorders may occur later in life, the potential of fetal gene delivery to avoid the onset of irreversible damage suggests it is an attractive option for many inherited diseases. In this study, rhesus monkey fetuses were administered 4.5 × 1012 particles of rAAV type 1 expressing human GAA (rAAV1-CMV-hGAA), human α-1-antitrypsin (rAAV1-CBA-hAAT), or human mini-dystrophin (rAAV1-CMV-miniDMD) in the late first trimester using an established intraperitoneal ultrasound-guided approach. Fetuses were monitored sonographically and newborns delivered at term for postnatal studies. All animals remained healthy during the study period (growth, hematology, and clinical chemistry), with no evidence of adverse effects. Tissues were collected at a postnatal age of 3 months (∼7 months post-fetal gene transfer) for immunohistochemistry (IHC) and quantitative PCR. Both the diaphragm and peritoneum from vector-treated animals were strongly positive for expression of human GAA, AAT, or dystrophin by IHC, similar to findings when reporter genes were used. Protein expression in the diaphragm and peritoneum correlated with high vector copy numbers detected by real-time PCR. Other anatomical areas were negative, although the liver showed minimal evidence of human GAA, AAT, and DMD, vector genomes. In summary, delivery of rAAV vectors provided stable transduction of the muscular component of the diaphragm without any evidence of adverse effects.
Keywords: : AAV, fetal gene transfer, rhesus monkey, Pompe disease, neuromuscular disorders
Introduction
Fetal gene transfer may be advantageous for the treatment of congenital disorders that cause irreversible damage prior to birth leading to significant infant mortality. Delivery of a therapeutic gene prenatally and prior to development of the immune system could prevent disease progression and avoid immune responses that can occur postnatally. Neuromuscular disorders such as Pompe disease (glycogen storage disease, type II; GSDII) result in early and potentially irreversible cellular damage with a very limited opportunity for intervention in the newborn period. Deficiency in the lysosomal enzyme acid α-glucosidase (GAA) impacts both the accumulation of glycogen in cardiac and skeletal muscle and reduced contractile function. Pompe disease, for example, is an often fatal inherited disorder resulting in glycogen accumulation in lysosomes as a result of GAA deficiency, with most infant deaths from cardiac or respiratory complications before 1 year of age. Typical findings include poor weight gain, muscle weakness, respiratory complications, and cardiomyopathy. The only currently approved therapy is biweekly infusions of recombinant enzyme.1,2 Enzyme replacement therapy (ERT) has been shown to be effective in prolonging the survival of treated patients. However, the course of the disease is delayed rather than prevented, suggesting that this therapeutic may have limited access to some cell types.3,4
Contractile function of the diaphragm is severely affected in both infantile- and late-onset Pompe disease, with death often resulting from respiratory insufficiency. Prior studies have shown that in utero delivery of recombinant adeno-associated virus (rAAV) encoding GAA to the peritoneal cavity of Gaa–/– mice resulted in high-level transduction of the diaphragm. In addition, in utero transduction of the diaphragm of Gaa–/– mice with rAAV-GAA demonstrated near-normal muscle function when assayed at a postnatal age of 6 months.5 Studies have also shown the efficiency of lentiviral vector–mediated gene transfer in fetal monkeys using both systemic and organ-targeting approaches.6–9 These studies have indicated that intraperitoneal (i.p.) administration of reporter genes (e.g., enhanced green fluorescent protein [eGFP] or firefly luciferase) in early gestation results in long-term transgene expression in the muscular component of the diaphragm and peritoneum.6–9 This study used a similar technique for early gestation fetal gene delivery of rAAV type 1 expressing human GAA (rAAV1-CMV-hGAA), human α-1-antitrypsin (rAAV1-CBA-hAAT), or mini-dystrophin (rAAV1-CMV-miniDMD). The results show high levels of hGAA, hAAT, or DMD expression within the muscular component of the diaphragm and peritoneum in treated animals, without any evidence of adverse effects.
Results and Discussion
Clinical trial
This preclinical study has supported at least three different clinical trials by inclusion of some of the data in Investigational New Drug (IND) submissions. One is an ongoing open-label, single center, sequential two-arm, Phase I/II clinical study to evaluate the safety and potential therapeutic benefit of a single administration of rAAV1-CMV-hGAA injected into the diaphragm (ClinicalTrials.gov Identifier NCT00976352). Two dose levels (1.0 × 1012 and 5.0 × 1012 vector genomes) were administered to nine subjects each, with the total dose distributed over six injection sites. The study consisted of nine subjects diagnosed with Pompe disease, male or female, aged 2–18 upon enrollment, actively receiving standard of care ERT and retaining some residual diaphragm function as assessed by pulmonary function studies. The primary outcome measure was safety. This was determined by evaluations of serum chemistry, hematology, urinalysis, vector genomes, antibody response to GAA and AAV1, and any observable symptoms or adverse events. Two other completed clinical trials included the use of AAV1 to express alpha-sarcoglycan or α-1-antitrypsin in adult patients.10–12
Objectives and study design
The objective of this study was to demonstrate that in vivo gene transfer using rAAV type 1 expressing either human GAA (rAAV1-CMV-hGAA), human α-1-antitrypsin (rAAV1-CBA-hAAT), or mini-dystrophin (rAAV1-CMV-miniDMD) to developing rhesus monkeys in the late first trimester is efficient in providing long-term transgene expression and does not result in adverse effects. As many genetic disorders display early and potentially irreversible cellular damage, it is important to establish the safety of fetal gene delivery in order to explore the possibility of early gene replacement therapies for humans. In this study, three groups of two fetal rhesus monkeys were administered 4.5 × 1012 vector genomes of rAAV1-CMV-hGAA, rAAV1-CBA-hAAT, or rAAV1-CMV-miniDMD via the i.p. route of delivery during early gestation.13 Prior studies have demonstrated therapeutic levels of correction following muscle-directed delivery of these vectors in their respective mouse models of disease.5,14 Thus, it was of interest to test the efficacy and translation of those results in a larger, more complex primate model. The CMV promoter was included in the GAA vector for several reasons: (1) the genome size limitations when packaging AAV vectors made CMV an optimal choice based upon its ideal length, (2) the efficient ability of this promoter to transduce striated muscle (the tissue primarily affected in these diseases), and (3) because CMV is potentially less promiscuous than the chicken β-actin (CBA) promoter and therefore has been suggested to reduce potential adverse immune responses. Following gene delivery, ultrasound evaluations were included to assess fetal growth and development. After Cesarean section delivery at term, newborns were raised in the nursery for postnatal studies until a postnatal age of 3 months. Tissue harvests were performed, and collected specimens were evaluated for transgene expression, biodistribution of the vector, and histological assessments.
Summary of data
Fetal growth and development
Six rhesus monkey fetuses were administered 4.5 × 1012 particles of rAAV1 in a total volume of ∼300 μL by ultrasound-guided i.p. delivery at 50 days of gestation (late first trimester) using established methods.13 Two fetuses received rAAV1-CMV-hGAA, two fetuses received rAAV1-CBA-hAAT, and two fetuses received rAAV1-CMV-miniDMD. Post-gene transfer assessment of the fetal head, abdomen, and limbs were conducted at regular intervals using established sonographic methods approximately every 2 weeks, as previously described.13 All parameters evaluated were compared to normative growth charts13 and found to be within normal limits (data not shown).
Therapeutic protein expression: immunohistochemistry (IHC)
This study determined transgene expression levels in several striated muscle groups (e.g., diaphragm, heart, muscular component of the peritoneum, skeletal muscle). The liver, which is a potential source for secreted proteins such as GAA and AAT, and cerebral hemispheres were also evaluated. The diaphragm was removed intact, and three sections of the right and three sections of the left were collected from each animal at the time of tissue harvest.
rAAV1-hGAA
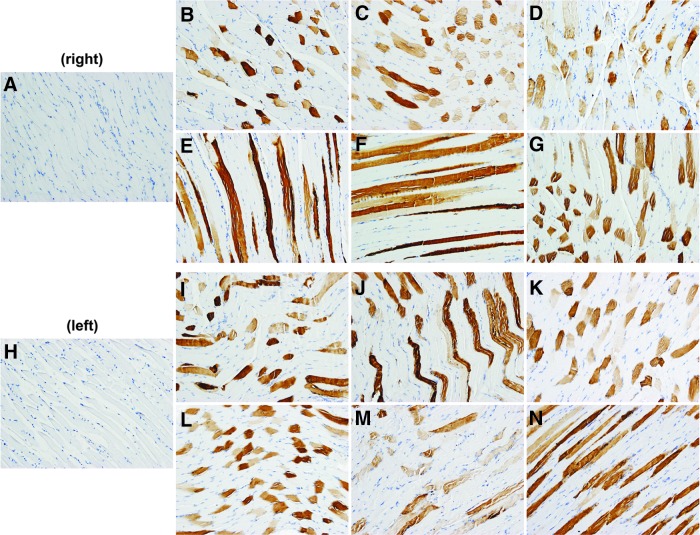
A representative section from each of six sections from the diaphragm (Fig. 1) and peritoneum (not shown) from the rAAV1-hGAA-treated animals was stained, and all displayed widespread, evenly distributed GAA expression. Some localized areas of GAA expression were observed in skeletal muscle, heart, and liver sections, but these areas were not highly abundant or evenly distributed (not shown). Sections of the cerebral hemispheres did not show any detectible GAA expression and were indistinguishable from control specimens.
Figure 1.
GAA protein expression. The diaphragm of each rAAV1-CMV-hGAA-treated animal was harvested in six sections (right and left). Representative images are from control (A, H) and treated animal #1 right (B–D) and left (I–K), and animal #2 right (E–G) and left (L–N). Color images available online at www.liebertpub.com/humc
rAAV1-hAAT
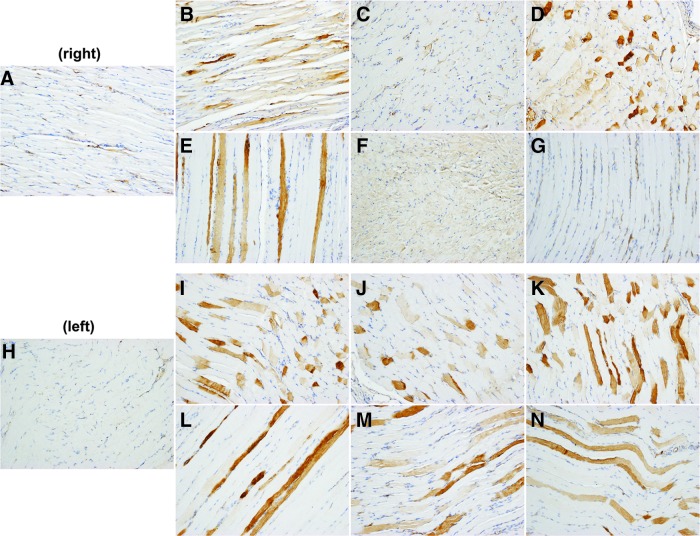
A representative section from each of six sections from the diaphragm (Fig. 2) from the rAAV1-hAAT-treated animals similarly displayed widespread, evenly distributed AAT expression. Sections from the peritoneum of treated animals exhibited evenly distributed AAT expression (not shown), and modest, low-level AAT expression was present in skeletal muscle from one animal and a section of the heart from one animal. All other tissue sections showed no detectible AAT expression and were indistinguishable from control tissues.
Figure 2.
AAT protein expression. The diaphragm of each rAAV1-CMV-hAAT-treated animal was harvested in six sections (right and left). Representative images are shown from control (A, H), and treated animal #1 right (B–D) and left (I–K), and animal #2 right (E–G) and left (L–N). While most of the sections show widespread AAT expression, three sections of the right diaphragm (C, F, G) showed more modest AAT expression levels. Color images available online at www.liebertpub.com/humc
rAAV-miniDMD
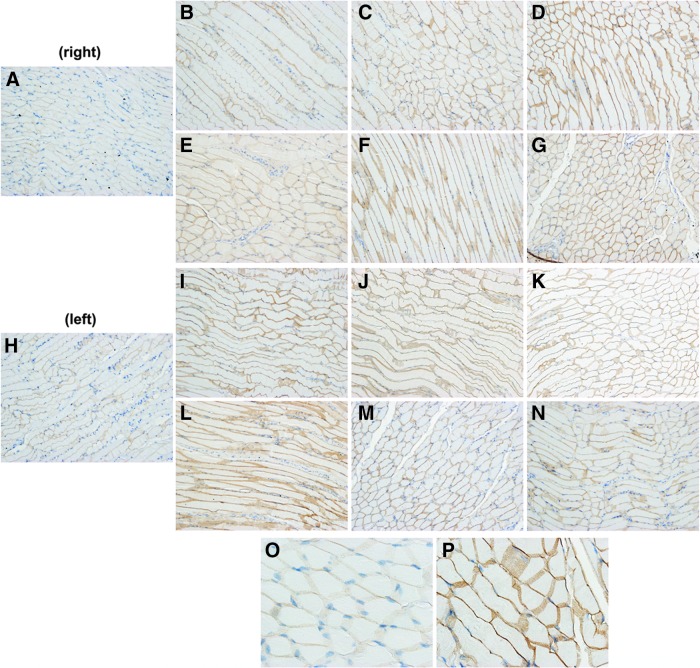
A representative section from each of six sections from the diaphragm from the rAAV1-miniDMD-treated animals also displayed widespread, evenly distributed dystrophin expression (Fig. 3), which was similar to the right and left peritoneum (not shown). All other tissues showed no detectable dystrophin expression and were indistinguishable from control specimens.
Figure 3.
Mini-dystrophin protein expression. The diaphragm of each rAAV1-CMV-miniDMD-treated animal was harvested in six sections (right and left). Representative images are shown from control (A, H), and treated animal #1 right (B–D) and left (I–K), and animal #2 right (E–G) and left (L–N). All sections showed even and widespread DMD expression. Representative higher magnification images (O, P) indicate the dramatic increase in dystrophin expression observed at the muscular component of the diaphragm of the treated (P) versus the control (O) tissue. Color images available online at www.liebertpub.com/humc
Evaluation of transgene expression: real-time PCR
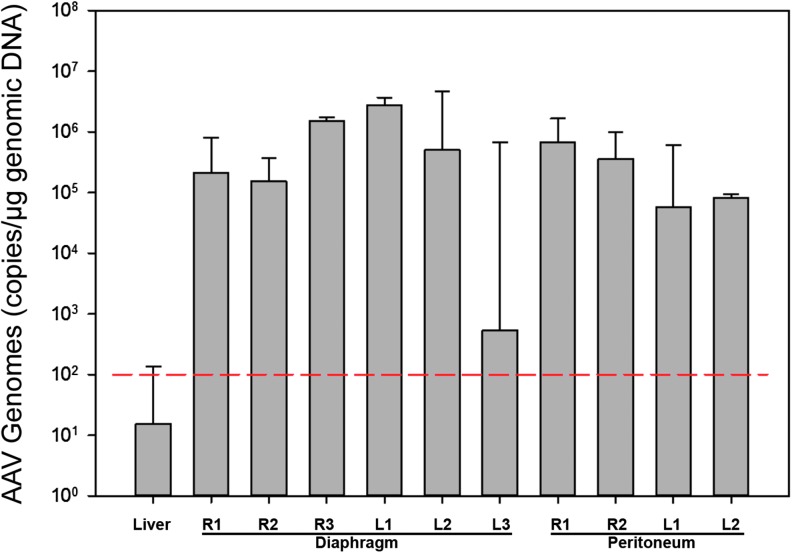
Vector genomes were detected in a wide spectrum of tissues from all animals. Approximately 30 different tissues were analyzed in triplicate for the presence of vector genomes by real-time PCR. In the diaphragm and peritoneum, the concentration of vector genomes reached into the hundreds of thousands of vector copies/μg of DNA (Fig. 4), while the liver remained below the limit of detection. One rAAV1-CBA-hAAT-treated diaphragm section contained an average of 489 vector copies/μg of DNA (AAT-2 R3 diaphragm). This suggests that the staining method used was sufficiently stringent to prevent detection of background rhesus monkey AAT expression but too stringent to detect low-level hAAT transgene expression.
Figure 4.
Real-time PCR for vector genomes. The total copies of vector DNA detected in the liver, diaphragm, and peritoneum are shown. The positive cutoff limit is ≥100 vector genomes/μg of gDNA, and is indicated by the horizon trend line (dashed). R, right; L, left. Color images available online at www.liebertpub.com/humc
Peripheral tissues other than the target organs (e.g., diaphragm, peritoneum, liver) were also analyzed for vector genome biodistribution. The reproductive organs (uterus, right and left seminal vesicles, prostate, gonads)15 were all below the limit of detection (<100 copies/μg of gDNA) in all animals. Results for vector dissemination to the heart, skeletal muscle, and brain (cerebral hemispheres, cerebellum) were minimal in all cases, but varied between each animal (data not shown). Samples from the myocardium contained the most significant amount of vector DNA (up to 6,400 vector genomes/μg DNA in one animal) followed by the liver (up to 323 vector genomes/μg DNA in one animal). The cerebral hemispheres in both AAV1-CBA-hAAT animals were positive (<500 copies/μg). Cerebellum and skeletal muscle tissue sampled from the quadriceps were all negative.
Immune responses to AAV1 and GAA transgenes
Three of four fetuses that received rAAV1 vectors expressing either human AAT or human GAA did not elicit a humoral immune response against the vector-derived protein, as determined by ELISA specific for circulating rhesus anti-human AAT or rhesus anti-human GAA antibodies. Blood samples were collected at birth then weekly for the first month and monthly until 3 months of age. No circulating antibodies were detected at any time point in either rAAV1-hAAT-treated animal (data not shown) or in one rAAV1-hGAA-treated animal. However, the second animal that received rAAV1-hGAA during gestation elicited an immune response against vector-derived human GAA protein. The response was initiated prior to 1 month of age, peaked at 2 months, and decreased to background levels by 3 months. Previous reports on the expression of human AAT in nonhuman primates has been shown to elicit a humoral immune response to human AAT, despite the high degree of protein sequence homology.16
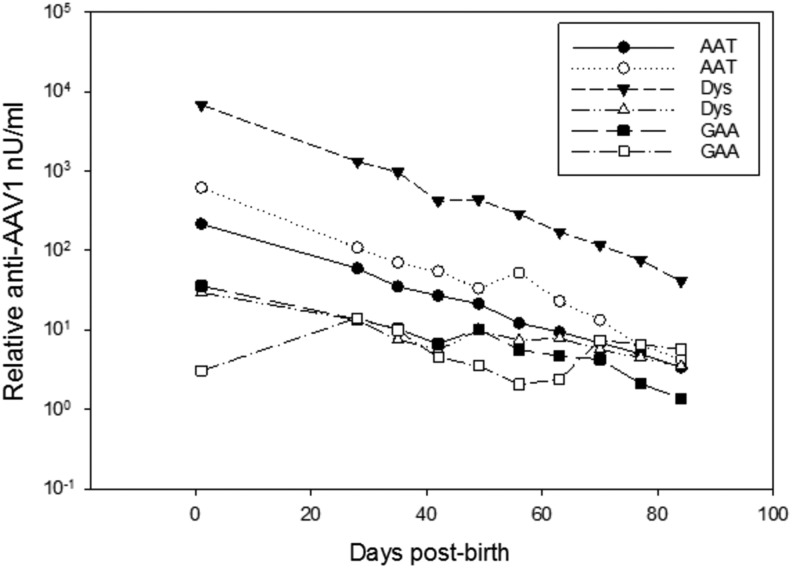
Following i.p. delivery of 4.5 × 1012 particles of rAAV1 to fetal rhesus monkeys at ∼50 days of gestation (late first trimester), the dams did not show a significant increase in humoral immune responses against rAAV1 viral capsid proteins as determined by enzyme-linked immunosorbent assay (ELISA) (data not shown), although all six dams had a positive baseline response, possibly due to prior exposure to AAV1. The first blood sample collected from each dam (N = 6) prior to vector delivery served as a baseline for each animal. In contrast, circulating antibodies against rAAV1 viral capsid proteins were detected at birth in five out of six newborns that received rAAV1 vector in the late first trimester (Fig. 5). The circulating antibody levels gradually decreased over time. This may represent maternal pre-existing antibodies against viral proteins. In support of this was the finding that 98% of the anti-capsid antibodies present in the newborn circulation immediately following birth were the IgG isoform as opposed to IgM isoform. IgG is known to cross the placenta, and prior studies have shown that maternal antibodies can be detected in the fetal circulation in the early second trimester in dams that are seropositive for rhesus cytomegalovirus prior to pregnancy.17
Figure 5.
Presence of anti-AAV1 circulating antibodies in newborns. Postnatal serum samples were assayed for the presence of IgG antibodies by ELISA.
Conclusions
These studies have shown that direct in vivo gene transfer with rAAV type 1 expressing human GAA (rAAV1-CMV-hGAA), human α-1-antitrypsin (rAAV1-CBA-hAAT), or minidystrophin (rAAV1-CMV-miniDMD) to early gestation fetal rhesus monkeys is efficient and does not result in adverse effects. Results also showed high levels of hGAA, hAAT, or miniDMD expression that persisted over time within the muscular component of the diaphragm and peritoneum. In a prior study using HIV-1-derived lentiviral vectors, a quantitative real-time PCR assay was used to calculate the level of lentiviral transduction and the eGFP mRNA transcripts in transduced tissues.6 This prior study focused on direct comparisons between fetuses under a variety of experimental conditions, including gene transfer at different gestational ages using different routes of administration and lentiviral vector constructs. The findings from these studies were similar to those in the study described herein, namely that i.p. administration of lentiviral and AAV vectors to early gestation rhesus monkey fetuses provides consistent transduction of the muscular component of the diaphragm and the peritoneum that persists long-term. This has been further demonstrated in studies with in vivo imaging where long-term transgene expression has been monitored using bioluminescence imaging (BLI) and firefly luciferase as the reporter gene.9,18
In this study, some hepatocytes were found to be positive for hGAA or hAAT expression. Previous studies in rodents have suggested that i.p. delivery results primarily in transduction of the serosa of abdominal organs such as the liver capsule,19 although in these studies transduction was found to be concentrated in the peritoneum and peritoneal serosa. Other studies by these authors with AAV220 and comparing AAV2 to AAV521 after i.p. administration (3 × 1011 vector genomes) showed luciferase expression for >15 months with expression from AAV5 found to be greater than AAV2. These studies also demonstrated that by modifying the promoter and serotype, an increase in the efficiency of AAV-directed expression was achieved. Both of the promoters included in these studies are well established for driving high levels of persistent transgene expression. Alternatively, a strong muscle-specific promoter driving expression of GAA and minidystrophin could also provide a more targeted therapy.
Another rodent study used a knockout mouse model of GSDII (Gaa–/–) that survives into adulthood, despite the gradual weakening of striated muscle groups. Using this model, this study investigated the in utero delivery of rAAV vectors encoding the human GAA cDNA and showed high-level transduction of the diaphragm with restoration of normal contractile function. Up to an estimated 50 vector copies/diploid genome were found.5 Glycogen staining also revealed prevention of lysosomal glycogen accumulation in almost all fibers when compared with untreated controls. In this study, a loss of expression in the liver was proposed to be related to dispersion of unintegrated vector genomes, as the cells proliferate and divide during development, with a loss of effective copy numbers per cell. This finding contrasts the hypothesis that has been proposed for the high and persistent levels of transduction in the diaphragm related to fusion of myoblasts to form multi-nucleated myotubes.
The diaphragm is a musculotendinous partition that separates the thoracic and abdominal cavities early in gestation. The primitive diaphragm develops from four structures including the septum transversum, pleuroperitoneal membranes, dorsal mesentery of the esophagus, and lateral body walls.22 The septum transversum is composed of mesoderm, which forms the future central tendon of the diaphragm and initially an incomplete partition between the pericardial and peritoneal cavities, which fuse dorsally. The pleuroperitoneal membranes fuse with the dorsal mesentery of the esophagus and the septum transversum, which completes the partition between the thoracic and abdominal cavities and forms the primitive diaphragm. The lateral body walls are invaded by the pleural cavities during weeks 9–12 in humans, when the body wall splits off medially to form the peripheral portions of the diaphragm with the pleuroperitoneal membranes. The muscular ingrowth from the body wall of the developing fetus increases substantially over time. At the time of gene transfer (50 days of gestation, end of the first trimester), the diaphragm is fully formed, and the muscular component is modest relative to birth. The results of these and related studies suggest that muscle is a target area for transduction, and that the initial cells transduced are effective in maintaining the expression of the transgene(s) as the cells proliferate and the diaphragm grows and matures.
In conclusion, the results of these studies further support that fetal administration of rAAV vector is highly efficient for the transduction of muscles of the diaphragm. These findings parallel those with lentiviral vectors as noted, and provide evidence that therapeutic genes can be effectively inserted into developing fetal tissues without evidence of adverse effects. The outcome of these studies also further supports the potential of fetal gene delivery to be developed into a therapeutic modality for the treatment of congenital disorders such as skeletal myopathies, which can prove lethal in the early postnatal period, and may be ameliorated by the prenatal administration of corrective genes. (Supplementary Data are available online at www.liebertpub.com/humc)
Supplementary Material
Acknowledgments
The authors wish to thank the animal care and clinical laboratory staff at the California National Primate Research Center (CNPRC) for expert technical assistance and the University of Florida Powell Gene Therapy Center Toxicology Core. These studies were supported by NIH grants #HL069748 (NHLBI Center for Fetal Monkey Gene Transfer for Heart, Lung, and Blood Diseases), HL59412, DK58327, HD052682, and the CNPRC base operating grant (#OD011107).
Author Disclosure
B.J.B., The Johns Hopkins University, and the University of Florida could be entitled to patent royalties for inventions described in this manuscript. No competing financial interests exist for the remaining authors.
References
- 1.Katzin LW, Amato AA. Pompe disease: a review of the current diagnosis and treatment recommendations in the era of enzyme replacement therapy. J Clin Neuromuscul Dis 2008;9:421–431 [DOI] [PubMed] [Google Scholar]
- 2.Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology 2007;68:99–109 [DOI] [PubMed] [Google Scholar]
- 3.Deruisseau LR, Fuller DD, Qiu K, et al. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci U S A 2009;106:9419–9424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolino M, Byrne B, Wraith JE, et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med 2009;11:210–219 [DOI] [PubMed] [Google Scholar]
- 5.Rucker M, Fraites TJ, Jr, Porvasnik SL, et al. Rescue of enzyme deficiency in embryonic diaphragm in a mouse model of metabolic myopathy: Pompe disease. Development 2004;131:3007–3019 [DOI] [PubMed] [Google Scholar]
- 6.Jimenez DF, Lee CI, Kohn DB, et al. HIV-1-derived lentiviral vectors and fetal route of administration on transgene biodistribution and expression in rhesus monkeys. Gene Ther 2005;12:821–830 [DOI] [PubMed] [Google Scholar]
- 7.Tarantal AF, McDonald RJ, Jimenez DF, et al. Intrapulmonary and intramyocardial gene transfer in rhesus monkeys: safety and efficiency of lentiviral vectors for fetal gene delivery. Mol Ther 2005;12:87–98 [DOI] [PubMed] [Google Scholar]
- 8.Tarantal AF, Lee CCI. Long-term luciferase expression monitored by bioluminescence imaging after adeno-associated virus-mediated fetal gene delivery in rhesus monkeys (Macaca mulatta). Hum Gene Ther 2010;21:143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarantal AF, Skarlatos SI. Center for Fetal Monkey Gene Transfer for Heart, Lung, and Blood Diseases: an NHLBI resource for the gene therapy community. Hum Gene Ther 2012;23:1130–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brantly ML, Chulay JD, Wang L, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A 2009;106:16363–16368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendell JR, Rodino-Klapac LR, Rosales-Qunitero X, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol 2009;66:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendell JR, Rodino-Klapac LR, Rosales-Qunitero X, et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol 2010;68:629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarantal AF. Ultrasound Imaging in Rhesus and Long-tailed Macaques: Reproductive and Research Applications. In: Wolfe-Coote S, ed. The Laboratory Primate. San Diego, CA: Elsevier Academic Press, 2005:317–351 [Google Scholar]
- 14.Lu Y, Choi YK, Campbell-Thompson M, et al. Therapeutic level of functional human alpha 1 antitrypsin (hAAT) secreted from murine muscle transduced by adeno-associated virus (rAAV1) vector. J Gene Med 2006;8:730–735 [DOI] [PubMed] [Google Scholar]
- 15.Lee CC, Jimenez DF, Kohn DB, et al. Fetal gene transfer using lentiviral vectors and the potential for germ cell transduction in rhesus monkeys (Macaca mulatta). Hum Gene Ther 2005;16:417–425 [DOI] [PubMed] [Google Scholar]
- 16.Hernandez YJ, Wang J, Kearnes WG, et al. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J Virol 1999;73:8549–8558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry PA, Lockridge KM, Salamat S, et al. Nonhuman primate models of intrauterine cytomegalovirus infection. ILAR J 2006;47:49–64 [DOI] [PubMed] [Google Scholar]
- 18.Tarantal AF, Lee CC, Jimenez DF, et al. Fetal gene transfer using lentiviral vectors: in vivo detection of gene expression by microPET and optical imaging in fetal and infant monkeys. Hum Gene Ther 2006;17:1254–1261 [DOI] [PubMed] [Google Scholar]
- 19.Lipshutz GS, Sarkar R, Flebbe-Rehwaldt L, et al. Short-term correction of factor VIII deficiency in a murine model of hemophilia A after delivery of adenovirus murine factor VIII in utero. Proc Natl Acad Sci U S A 1999;96:13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipshutz GS, Gruber CA, Cao Y, et al. In utero delivery of adeno-associated viral vectors: intraperitoneal gene transfer produces long-term expression. Mol Ther 2001;3:284–292 [DOI] [PubMed] [Google Scholar]
- 21.Lipshutz GS, Titre D, Brindle M, et al. Comparison of gene expression after intraperitoneal delivery of AAV2 or AAV5 in utero. Mol Ther 2003;8:90–98 [DOI] [PubMed] [Google Scholar]
- 22.Moore KL, Persaud TVN, Torchia MG. The Developing Human: Clinically Oriented Embryology. 9th ed. Philadelphia, PA: Elsevier Saunders, 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.