Abstract
A variety of neurological disorders are attractive targets for stem and progenitor cell-based therapy. Yet many conditions are not, whether by virtue of an inhospitable disease environment, poorly understood pathophysiology, or poor alignment of donor cell capabilities with patient needs. Moreover, some disorders may be medically feasible targets, but are not practicable, in light of already available treatments, poor risk-benefit and cost-benefit profiles, or resource limitations. This Perspective seeks to define those neurological conditions most appropriate for cell replacement therapy, by considering its potential efficacy and clinical feasibility in those disorders, as well as potential impediments to its application.
Keywords: neural stem cell, glial progenitor cell, oligodendrocyte progenitor cell, astrocyte, neurodegenerative disease, myelin disease, cell therapy, neurological therapeutics
Introduction
Since the advent of stem cell biology, the brain and spinal cord have been intensively investigated as potential targets of stem and progenitor cell-based therapies. The CNS would seem a promising target for cell replacement therapy, in light of the plethora of diseases of the human nervous system, the overall lack of effective therapeutic approaches for most brain diseases, and the great store of developmental information available on the ontogeny of neurons and glia that can be applied to generate clinically relevant cell types. Yet the brain is an especially difficult organ in which to employ stem cell-based therapeutics. The phenotypic heterogeneity and myriad connections of its neuronal elements, the four dimensional complexity of its synaptic architecture, and the regionally-variable and poorly understood nature of neuronal interactions with astrocytes, oligodendrocytes and glial progenitor cells, all conspire to defy precise structural reconstitution.
The limited repair capacity of the adult human brain further compounds this complexity. Despite the persistence of somatic neural stem cells and neuronal progenitor cells in the adult human brain (Arsenijevic et al., 2001; Eriksson et al., 1998; Ernst et al., 2014; Kirschenbaum et al., 1994; Pincus et al., 1998; Roy et al., 2000; Sanai et al., 2004), little evidence exists as to the contribution of these cells to structural repair in adult humans. In the early days of stem cell biology, reports appeared of context-dependent differentiation of transplanted pluripotent stem cells (PSC) or neural stem cells (NSCs) into phenotypes of interest or need (Liu et al., 2000), but realization soon grew that such demand-based differentiation was limited in scope. Rather, it became evident that for disorders of specific neuronal and glial phenotypes, that the deficient cell types or their immediate progenitors would need to be introduced to achieve structurally-accurate repair. In particular, it became clear that repair of the injured or diseased brain required the upfront determination of which cellular phenotypes, at which stages of their development, were most appropriate for treating which conditions. Fortunately, many diseases of the brain involve either single cell types or their immediate derivatives. Such conditions lend themselves to cell replacement, whether by the transplantation of single neuronal and glial phenotypes or their progenitors, or by the recruitment of new neurons or glia from endogenous stem and progenitor cells.
This Perspective will focus on identifying clinically-realistic near- and intermediate-term opportunities for cell-based repair of brain disease, using both endogenous mobilization and transplant-based strategies, with an emphasis on the latter (Figure 1). By the same token, it will indicate those disorders perhaps less suitable for near-term cell therapeutic development, whether by virtue of their multicellular or multicentric nature, their especially challenging or poorly understood disease environments, or their need for cell types refractory to clinical scale development. The emphasis of this Perspective is thus on identifying clinical targets that are realistic based not only on our ability to produce cells of defined phenotype, but also on our current understanding of the clinical tractability of each candidate disease target, and just as importantly, on our assessment of already available treatment approaches that may narrow the pool of patients for whom cell therapeutics would be appropriate. A number of excellent reviews have recently appeared that have discussed pluripotent cell-based in vitro models of neural disease (Marchetto et al., 2011; Merkle and Eggan, 2013) and CNS drug development (Sandoe and Eggan, 2013), as have broader reviews on the use of pluripotent cell derivatives in regenerative medicine (Fox et al., 2014; Steinbeck and Studer, 2015; Tabar and Studer, 2014). In contrast, this Perspective will focus solely on using CNS cells to treat CNS disease, and on defining when this approach makes the most sense, and when it does not.
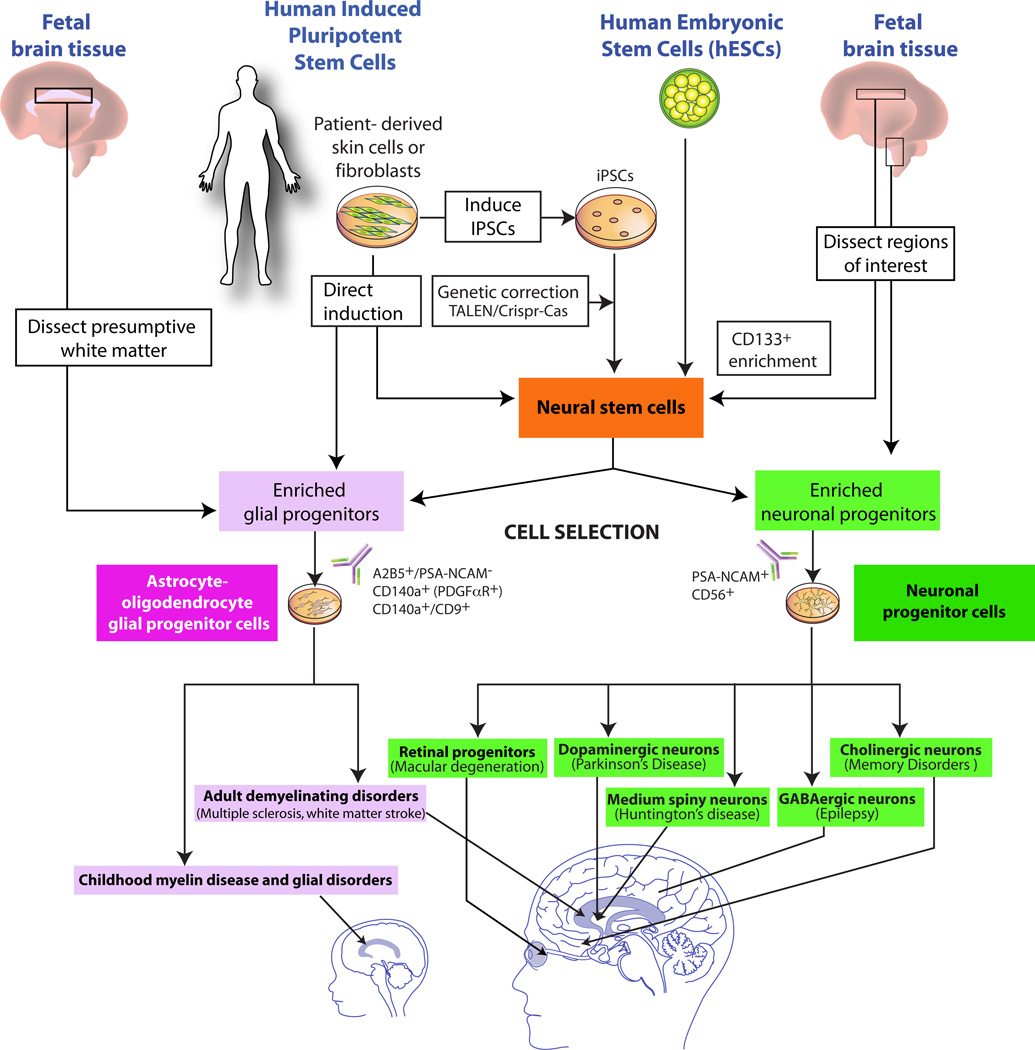
Figure 1. Neural and glial cell therapeutics and their disease targets.
This schematic illustrates the principal sources of transplantable human neural stem cells and phenotypically-restricted neuronal and glial progenitor cells, and highlights the most feasible current opportunities for their use in treating disorders of the brain.
HOPE: CNS DISEASE TARGETS FOR CELL REPLACEMENT THERAPY
Glial and myelin disorders
The white matter diseases and those of myelin, which involve the loss or dysfunction of oligodendrocytes in the brain and spinal cord, are among the most prevalent and disabling conditions in neurology, and are especially attractive targets for stem cell-based therapeutics. These disorders include the acquired diseases of myelin in adults, such as multiple sclerosis and white matter stroke, the congenital or early myelin loss of cerebral palsy and periventricular leukomalacia, and the hereditary and metabolic disorders of myelin loss, the pediatric leukodystrophies. In light of the wide range of disorders to which congenital hypomyelination or postnatal demyelination may contribute, and the relative homogeneity of oligodendrocytes and their progenitors, these conditions may be particularly appropriate targets for progenitor cell-based therapy. As a result, glial progenitor cells, which can give rise to astrocytes as well as myelinogenic oligodendrocytes (and hence are equivalently referred to as oligodendrocyte progenitor cells), have been extensively investigated as potential vectors for the restoration of myelin to the dysmyelinated brain and spinal cord (Goldman et al., 2012). These cells may be isolated from both adult and fetal human brain tissue (Armstrong et al., 1992; Dietrich et al., 2002; Nunes et al., 2003; Roy et al., 1999; Sim et al., 2011), as well as from pluripotential cells instructed to glial and oligodendrocyte progenitor phenotype (Douvaras et al., 2014; Hu et al., 2009; Izrael et al., 2007; Liu et al., 2011; Nistor et al., 2005; Stacpoole et al., 2013; Wang et al., 2013). In animal models, isolated glial progenitors derived from fetal tissue as well as from both human ESCs and hiPSCs are highly migratory, disperse throughout the neuraxis after intracerebral graft, and can differentiate as oligodendrocytes and myelinate dysmyelinated loci throughout the brain and spinal cord (Windrem et al., 2004). Perinatal transplantation of human OPCs into hypomyelinated shiverer mice, which normally die by 20 weeks, can rescue these animals and restore both normal CNS myelination and neurological phenotype, a capability that may provide a basis for their use in therapeutic remyelination across a broad range of demyelinating disorders (Wang et al., 2013; Windrem et al., 2008). In particular, one may readily envisage that efforts now underway using tissue-derived cells to treat myelin disorders as varied as Pelizaeus-Merzbacher disease in children (Gupta et al., 2012), and chronic progressive multiple sclerosis in adults, may soon be supplanted by hESC and hiPSC-derived glial progenitor cells. Beyond that, a broad set of both pediatric and adult white matter disorders, ranging from the leukodystrophies and autoimmune demyelination to vascular and age-related white matter loss, may be promising targets for glial progenitor cell-based remyelination, as might iatrogenic causes of demyelination such as radiation therapy (Fox et al., 2014; Piao et al., 2015).
Since glial progenitor cells give rise to astrocytes as well as oligodendrocytes, and are highly migratory – they typically distribute throughout the neuraxis after perinatal graft, and can do so well into adulthood – these cells may also be of great utility in rectifying the dysmyelination-associated enzymatic deficiencies of the pediatric lysosomal storage disorders, such as Krabbe disease, metachromatic leukodystrophy Tay-Sachs disease, and the mucopolysaccharidoses, among others, as well as the astrocytic pathology of vanishing white matter disease (Goldman, 2011). To be sure, substantial heterogeneity in both astroglial ontogeny and phenotype has been noted (Bayraktar et al., 2015; Chaboub and Deneen, 2012; Schitine et al., 2015; Zhang and Barres, 2010), most especially so in the adult human forebrain (Oberheim et al., 2006; Oberheim et al., 2009), and it remains unclear how precisely engrafted glial progenitors can recapitulate the pleomorphism of the host glial network they are intended to replace. In particular, the extent to which the development of astroglial morphological and functional phenotype in the adult brain is cell-autonomous or context-dependent remains unclear. Nonetheless, in all of the disorders noted, we can reasonably expect that allografted human glial progenitors may prove effective at widespread remyelination and concurrent metabolic correction, the latter having already been established by Snyder and colleagues in mouse models using allografted neural stem cells (Lacorazza et al., 1996; Lee et al., 2007; Snyder et al., 1995). Combined with gene editing technologies to correct underlying mutations (Li et al., 2014), one might then anticipate the use of gene-edited human iPSCs and their derived glial progenitor cells in autologous therapy of affected children, across a broad range of pediatric leukodystrophies.
Neurodegenerative disorders of single phenotype
Unlike the glial disorders, which involve a limited number of phenotypes of relative homogeneity, the neuronal disorders are a diverse group, which includes both hereditary and acquired disorders of the CNS, all of which share a loss of central neurons. The neurodegenerative disorders in particular comprise a heterogeneous category, that include both multicentric and diffuse disorders such as Alzheimer’s, and those in which the loss of a single phenotype predominates, such as Huntington’s and Parkinson’s diseases. The latter category, those neuronal disorders in which a single region or phenotype is differentially affected, have proven to be the most amenable to cell type-specific neuronal replacement in animal models (Lindvall, 2012; Lindvall and Bjorklund, 2011). These include classical Parkinson’s disease, in which nigrostriatal neurons are lost before other neurons, and Huntington’s disease, in which striatal atrophy becomes apparent long before the onset of more widespread cortical neuronal loss. Clinical trials of cell transplantation have already been performed for each of these prototypic neurodegenerative conditions (reviewed in (Barker et al., 2015; Barker et al., 2013; Benraiss and Goldman, 2011; Lindvall and Bjorklund, 2011)). But these trials used fetal tissues dissected from the regions of interest, which thus included all cell types in the tissue, and not just the specific populations of nigrostriatal and striatal medium spiny neurons respectively lost in PD and HD; in each of these cases, the target cell types typically comprised but a fraction of the cells delivered. Perhaps as a result, fetal tissue grafts into Parkinson’s patients have yielded variable results, with both clear successes and failures, and a disturbingly high incidence of refractory dyskinesias, in which uncontrollable movements can negate the functional gains otherwise afforded by the grafted cells (Barker et al., 2015). Similarly, fetal striatal grafts into patients with Huntington Disease have yielded mixed results, with little evidence of significant or durable functional improvement (Cicchetti et al., 2009). Thus, the specific generation of midbrain dopaminergic neurons and medium spiny neurons from human embryonic stem cells and iPSCs (An et al., 2012; Carri et al., 2013; HDConsortium, 2012; Kriks et al., 2011; Roy et al., 2006), by providing defined populations of neurons of relative phenotypic homogeneity, has enabled a leap forward in the design and therapeutic potential of cell transplantation for each of these disorders (Thompson and Bjorklund, 2015).
Parkinson’s disease
For the midbrain dopaminergic neurons lost in Parkinson’s disease in particular, preclinical animal studies using hESC and iPSC-derived neurons have proven sufficiently promising to justify both the scaled production of cells appropriate for clinical transplantation (Hallett et al., 2015; Hargus et al., 2010; Kikuchi et al., 2011; Kriks et al., 2011; Roy et al., 2006; Takahashi et al., 2009), and the design of new clinical trials by which to evaluate the efficacy of those cells in disease amelioration. To that end, new efforts have been initiated both in the US and UK, respectively by Studer and Barker and their colleagues using hESC-derived dopaminergic neurons as donor cells, and in Japan, by Takahashi and colleagues with human iPSC-derived neurons. While each study is promising, having inculcated lessons from past fetal tissue-based studies (Barker et al., 2015) (Moore et al., 2014), a number of issues may still limit the promise of this approach. As noted, past trials of fetal human midbrain tissue implanted into the striata of Parkinson’s patients yielded inconsistent results, with durable efficacy in selected cases (Kefalopoulou et al., 2014), yet many others that failed or developed refractory dyskinesias. While improved dopaminergic cell purity and acquisition of midbrain nigral phenotype have been achieved in vitro, whether a new generation of studies using these more homogeneous cell preparations will translate into more consistently improved clinical outcomes remains unknown. At the very least, PSC-derived dopaminergic populations appear as efficacious as their tissue-derived counterparts in rodent models of Parkinson’s Disease (Grealish et al., 2014). Nonetheless, the striatal site of implantation remains fundamentally heterotopic, such that putatively nigral dopaminergic donor cells are deprived of their normal afferents, and are instead engaged by cortical, intrastriatal and thalamic afferents that they would otherwise never encounter. Regardless of donor cell phenotype, it thus remains unclear – even after two decades of study in this field - whether the fundamentally non-physiological circuitry that results from intrastriatal dopaminergic engraftment is sufficiently malleable to restore normal basal ganglia function to Parkinson’s patients.
As an additional concern, several groups have reported the spread of alpha-synuclein aggregates and Lewy body pathology from Parkinsonian host to donor cells, thereby recruiting the donor neurons into the disease process (Kordower et al., 2008; Li et al., 2008). That said, the quantitative significance of this observation remains contentious (Mendez et al., 2008), and the persistence of donor cells after over a decade following transplantation suggests biological, if not clinical, durability. Nonetheless, the combination of unknown host-to-donor disease spread, unclear requirements for immunosuppression, and unclear dose optimization, necessarily suggest caution as this cell therapeutic strategy advances to the clinic. In particular, the risks and unknowns of this approach must be weighed against the ready availability of both effective pharmacotherapy for Parkinson’s disease, and of effective strategies for deep brain stimulation to mitigate disease severity. Patients refractory to each of these approaches, and not otherwise cognitively impaired, comprise a distinct minority; whether the benefits of stem cell-derived dopaminergic cell transplant to these patients will prove sufficient to justify its development, costs and risks remains to be established (Buttery and Barker, 2014).
Huntington’s disease
An overlapping but distinct set of concerns exists in regards to stem cell-based treatment of Huntington’s disease. While medium spiny neurons (MSNs) are among the first cell types lost in adult HD, the disease ultimately affects all central neuronal populations, and patients typically suffer profound cortical as well as striatal neuronal loss with disease progression, manifesting as personality changes, psychoses and ultimately dementia. Thus, while current approaches to MSN production and replacement from hESCs and hiPSCs have progressed significantly, the clinical utility of MSN addition is necessarily limited: a strategy focused on striatal neuronal replacement alone may temporize and delay, but not halt, disease progression. This concern was borne out by early clinical trials of striatal tissue and cell transplantation in patients with HD, which largely failed to achieve significant or durable benefit (reviewed in (Benraiss and Goldman, 2011). Indeed, even in experimental models, transplanting hESC and hiPSC-derived MSNs into the adult striatum has proven challenging, with little dispersal of donor MSNs into the host striatum, and thus little evidence of architecturally-appropriate neuronal integration, much less circuit reconstruction, despite MSN-appropriate antigenic expression (Arber et al., 2015). As a result, recent efforts for designing cell-based treatment approaches to HD have focused on using gene therapeutic strategies to trigger the local production of new MSNs, from endogenous subependymal neural stem cells (Chmielnicki et al., 2004). These newly generated neurons integrate into the diseased striatum and restore normal basal ganglia circuitry, and their addition is associated with the substantially prolonged survival of transgenic HD mice (Benraiss et al., 2013; Cho et al., 2007). Yet despite the development of this approach to striatal neuronal replacement, no effective strategy for inducing functional cortical neuronal replacement has yet been established, without which any treatment strategies focused solely on the striatum will be necessarily limited in efficacy.
Disorders of the hippocampus: The memory disorders
Memory disorders can derive from multicentric thalamic and cortical disease, but also from discrete structural pathology, thereby lending themselves to cell-based treatment approaches. New memory acquisition in particular may be impaired by the loss of basal forebrain cholinergic neurons projecting to the hippocampus, as well as from intrinsic hippocampal disease and disruption of the hippocampal outflow tracts. In experimental animals with disruption of the cholinergic input to the hippocampus, performance on memory tasks has responded significantly to iPSC-derived cholinergic neuronal replacement (Liu et al., 2013). That said, the common memory loss syndromes associated with early stages of Alzheimer’s and frontotemporal dementia typically herald overwhelming deterioration across all cognitive modalities as disease progression ensues; as such, isolated replacement of cholinergic neurons is likely to prove a durable clinical strategy in only very selected cases of stable or slowly-progressive memory loss – cases that remain difficult to identify upfront. One needs also to consider the plethora of central cholinesterase inhibitors already in use for the treatment of Alzheimer’s-type dementia, which act to increase central synaptic acetylcholine. Whether cholinergic neuronal replacement would ultimately prove more long-lasting and effective than small molecule cholinergic agonism remains to be established, and must be verified before transplantation of cholinergic neurons into the basal forebrains of affected patients can be reasonably considered.
Memory disorders result not only from a failure of inputs to the hippocampus, but also from loss of the dentate granule neurons upon which incoming fibers to the hippocampus synapse, or of the hippocampal pyramidal neurons to which the dentate neurons project. The dentate neuronal population is of particular interest because of its ongoing replacement by new neurons (Altman and Das, 1965; Eriksson et al., 1998) - a process that plays a complex role in both the acquisition and extinction of memories (Kempermann et al., 2015). These new adult dentate neurons arise from subgranular zone progenitors that may be isolated to purity from human tissue (Roy et al., 2000), or generated from PSCs (Yu et al., 2014a). The latter report in particular, which describes the Wnt-dependent production of functional dentate granule neurons from human iPS cells, establishes the possibility of adding new dentate neuronal progenitors to hippocampi rendered dysfunctional by regionally-restricted pathologies (Yu et al., 2014b). These may include such conditions as mesial temporal sclerosis or ischemic neuronal loss following hippocampal hypoperfusion, as may occur following cardiac arrest with prolonged resuscitation. While dentate neuronal replacement would not be so suitable for the memory loss of Alzheimer’s or Lewy body disease, which are both multicentric and preferentially involve the hippocampal pyramidal population, this strategy might well prove beneficial for treating the focal hippocampal injuries that follow ischemia and prolonged seizures.
Disorders of dispersed neuronal phenotypes: The epilepsies
Some disorders affect single, or at least relatively uniform, neuronal types, but are so dispersed throughout the CNS as to present problems of delivery. In particular, several types of primary epilepsies may derive from deficits in GABAergic interneuronal numbers and function. GABAergic neurons comprise a plethora of phenotypes, but all share a common transmitter and most derive from a common developmental source in the medial ganglionic eminence (MGE). As such, a number of investigators have asked whether the transplantation of healthy donor interneurons into epileptic cortex may provide benefit to medication-refractory epileptics (Hunt et al., 2013; Southwell et al., 2014). This potential treatment strategy was made possible by the significant migration competence of these cells in the adult cortex, a vestige of their longdistance migration in early development (Wichterle et al., 1999) - a feature shared by glial progenitors, another highly migratory, MGE-derived phenotype similarly amenable to treating diffuse disease. The recent development of protocols appropriate for the production of GABAergic interneurons from human ES and iPS cells (Liu et al., 2000; Maroof et al., 2013) should now enable the assessment of interneuronal transplantation as an approach towards seizure control, using a clinically-realistic cell source.
Yet despite these clear advances in our understanding of interneuronal biology and its importance to the stabilization of cortical circuits, the pool of epileptic patients appropriate for any interneuron-based cell therapeutic strategy might prove limited. First, a number of effective medical and surgical treatments for epilepsy are already available, such that a cell-based treatment approach would only be tenable for those patients substantially refractory to all currently approved therapeutic modalities. Second, the risk of interneuronal engraftment-associated changes in other cognitive and functional domains must be considered; the effects of interneuronal integration into existing – and already aberrant – mature neuronal networks has yet to be established in primates, much less humans. Moreover, such effects may prove both variable and unpredictable from patient to patient, depending on disease history and the local tissue environment. In light of these considerations - over and above the more generic risks of long-term intracerebral colonization with PSC-derivatives - the clinical translation of this approach to seizure management will likely be both slow and cautious. Nonetheless, its promise of ensuring durable seizure control in otherwise refractory, and often terribly disabled, patients is so attractive as to justify intense investigation of this emerging clinical opportunity.
Multicentric and diffuse neurodegenerations: ALS and FTD
Unlike the examples thus far discussed, other disorders of single phenotype are not as attractive as targets for neuronal replacement-directed therapy, due to their multicentric pathology, their non-migratory replacement cells, or both. The motor neuronopathies, such as amyotrophic lateral sclerosis and spinal muscular atrophy, are such problematic cases. Spinal motor neurons may be readily generated and purified from hESC and hiPSC cells (Davis-Dusenbery et al., 2014; Karumbayaram et al., 2009; Li et al., 2005; Roy et al., 2005; Wichterle et al., 2002), and yet their clinical utility is limited both by the multi-segmental nature of motor neuron loss in these diseases, and by our limited ability to direct long-distance axonal regrowth and target-specific innervation. As a result, the challenge of designing a cell replacement strategy appropriate for treating a whole-neuraxis multicentric neuronal disorder, even one limited to a single phenotype, has proven daunting.
In response, cell-based treatment approaches for ALS and other neurodegenerative disorders have begun to shift away from neuronal replacement, towards the delivery of astrocytes, with the goal of correcting underlying glial metabolic deficiencies that may contribute to disease progression in ALS. If successful, one may envision a rapid transition to the use of hiPSC-derived glia for this and related disorders in which neurons may prove the paracrine victims of glial dysfunction. In that respect, a number of recent reports have highlighted the causal contribution of glial cells to the pathogenesis of ALS (Di Giorgio et al., 2008; Di Giorgio et al., 2007; Meyer et al., 2014; Yamanaka et al., 2008), which has led to a number of trials and trial plans focused on spinal glial addition, whether from transplanted astrocytes or their precursors, the latter including neural stem cells (Feldman et al., 2014; Lunn et al., 2014). These efforts have thus far been limited to the use of human fetal tissue-derived glia, which have yielded acceptable safety profiles but for which therapeutic efficacy remains to be assessed; analogous trials have not yet proceeded to the use of PSC-derived glia.
Importantly, ALS is in a large proportion of cases a form fruste of the frontotemporal dementia-ALS complex, which may be associated with mutations in either the C9orf72 gene, the latter as a GGGGCC hexanucleotide repeat expansion, or in its target the TDP43 gene (DeJesus-Hernandez et al., 2011; Renton et al., 2011). Glial pathology may prove to be contributory in FTD-ALS just as in the SOD1 mutant ALS models thus far examined, and if so, FTD-ALS, like sporadic ALS, may similarly prove an amenable and appropriate target for glial cell-based therapeutics (Serio et al., 2013).
Disorders of the eye: Retinal diseases
Several phenotype-specific disorders of the eye – the retina being a distant yet integral outpost of the central nervous system – may also prove appropriate targets for cell therapy. Loss of the retinal pigment epithelium (RPE) in macular degeneration has been a particular target of interest, in that efficient protocols for generating RPE cells from both hESC and iPSCs have been developed. On that basis, hESC- and hiPSC-derived RPEs are both now in early safety trials for macular degeneration (Kamao et al., 2014; Nazari et al., 2015; Schwartz et al., 2015; Song et al., 2015; Whiting et al., 2015). More broadly, macular degeneration may be the first of a number of retinal disorders to be targeted for cell therapy; as more efficient protocols are developed for producing specific retinal phenotypes from hES and hiPS cells, a broad variety of both intrinsic retinal disorders and optic neuropathies may prove appropriate targets for phenotype-specific cell replacement. In particular, both rod and cone photoreceptors, lost in disorders as varied as macular degeneration, retinitis pigmentosa and glaucoma, among other etiologies, appear potentially replaceable by stem cell-derived photoreceptors, and may be promising adjuncts to RPE transplants as well (Jayakody et al., 2015; Warre-Cornish et al., 2014; West et al., 2012).
Importantly, in all of these eye diseases, the fate of implanted cells can be more readily visualized, and functional outcomes more clearly assessed, than with intracerebral grafts of any type. Indeed, the safety of cell therapeutics in eye disease can be more confidently ascertained, in that if tumorigenesis or other serious adverse events are realized, then enucleation of the affected eye – which would have lacked significant vision in order to necessitate treatment -would be a viable option.
As a result of these relative advantages, retinal cell replacement has been perceived as an especially promising target of CNS cell therapy, with multiple trials underway (Schwartz et al., 2015; Song et al., 2015). Yet the need for caution even with such favorable translational opportunities has already been made clear, by the discovery that hiPSC-derived RPEs prepared for autologous graft had acquired unexpected point mutations during preparation, as revealed by whole genome sequencing of the intended donor RPE cells relative to the fibroblasts from which the patient’s iPSCs were derived. While no clinical complications have been reported in the one patient transplanted before that discovery, the very appearance of mutations prompted the investigators involved to halt further recruitment, until the basis of these mutations could be better established, and the safety of the resultant cells better assured. These events highlight the need for further improvement of reprogramming strategies, while nonetheless emphasizing the power of this technology for autologous therapy once optimized.
HYPE: LIMITS TO THERAPEUTIC ADVANCE
Suboptimal disease targets
Notwithstanding the many promising targets of stem cell-based therapy thus far discussed, stem cell therapeutic are hardly a panacea, and many disorders do not yet lend themselves to cell replacement-based treatment strategies, whether by virtue of poorly understood or inimical disease environments, irreproducible circuit complexity, unfavorable risk-and cost-benefit profiles, or already available treatment strategies. This section addresses these less attractive disease targets, in terms of both current efforts at developing cell-based treatments for them, the limitations of those efforts, and - at least in some cases - the efforts being made to surmount those limitations.
The degenerative cortical dementias
Some prototypic degenerative dementias, such as Alzheimer’s disease and Lewy body disease, appear to be proteinopathies involving a multitude of neuronal phenotypes – and in some cases glia as well, as in the case of multisystem atrophy. These disorders involve a multiplicity of phenotypes, span both anatomic and functional domains as well, and may inexorably spread by both contiguous and trans-synaptic pathways within affected brains (Goedert, 2015; Kim and Holtzman, 2010; Luk et al., 2012a; Luk et al., 2012b). Alzheimer’s, mixed Alzheimer’s-Parkinson’s presentations, Lewy body disease, and multisystem atrophy are all examples of such conditions, and are all characterized by the transcellular propagation of pathogenic proteins, whether β-amyloid or alpha-synuclein. These disorders pose the shared challenges of a multiplicity of affected phenotypes, multicentric and diffuse pathology, and inexorable disease progression, through pathogenic mechanisms that are not readily modulated by cell replacement per se. These disorders would thus seem unlikely to benefit from any attempts at cell replacement-based treatment, whether neuronal or glial, at least until such time as we have learned enough about their mechanisms of pathogenesis to abrogate disease progression. Until then, these disorders would not seem especially apt targets for stem cell-based treatment approaches.
Stroke
Cerebral infarcts, traumatic brain injury and spinal cord injury all share an ischemic basis, complicated by an acute inflammatory and edematous tissue response. Stroke in particular has been assessed by many groups as a potential target for cell replacement, and was among the first targets of stem and progenitor cell-based transplantation. (Hara et al., 2008; Nelson et al., 2002; Rosado-de-Castro et al., 2013). Yet stroke occurs in a fundamentally compromised environment of ischemic infarct, often in regions that have suffered chronic hypoxic ischemia, with its attendant gliosis and inflammation. In this challenging disease environment, essentially all cell types have been severely compromised, and their interactions distorted, with neurons often disconnected from their normal inputs and/or targets, and glial support disrupted. Any cell replacement strategy designed to repopulate areas of brain lost to ischemic injury would need to provide the multiplicity of neuronal phenotypes lost, to recapitulate their specific patterns of connection to one another, and in the cortex, to reproduce the laminar organization and connectivity of the host brain. Similarly, the glial populations would need to be replaced, while the intricate relationship of parenchymal glia to neurons within their domains would need to be re-established, just as the neuronal axons would need to be remyelinated. The requirements for restoring tissue lost to stroke are thus so demanding that current strategies for cell replacement are simply insufficient. As more phenotypes can be independently generated an admixed before transplant, and as we gain more insight into the potential for self-organization among those phenotypes, we may hope for future advances in cell replacement for stroke. But for now and the decade to come, the likelihood of cell replacement evolving as a significant treatment modality for stroke and trauma would seem low. Perhaps in recognition of those difficulties, much work over the last decade has shifted to using neural and mesenchymal stem cells in stroke, not as agents for cell replacement, but rather as immunomodulators intended to suppress the post-ischemic inflammatory response (Aharonowiz et al., 2008; Kokaia et al., 2012). This is an approach that may yet prove promising, but that should not be confused with cell replacement for structural repair.
Spinal cord injury
Like stroke, spinal cord injury (SCI) has been a target of considerable interest for cell-based therapeutics. Indeed, some of the first studies of stem cell therapy of CNS disorders were focused on animal models of spinal cord injury, and the first use of human ES cell-derivatives in humans was that of hESC-derived putative oligodendrocyte progenitor cells for SCI (Priest et al., 2015). Yet these efforts, and those using OPCs in particular, were not necessarily well-matched to the reality of clinical SCI (Bretzner et al., 2011). Cord injuries are remarkably heterogeneous, and every case is unique from the standpoint of its individual pathology, which depends upon the nature and timing of the injury, the force vectors applied to the cord and surrounding vertebral bodies, the local segmental blood supply and its interruption, and the presence of local root avulsions or hemorrhage within the cord, and whether the cord was penetrated, among other considerations. At its most basic though, non-transective traumatic spinal cord injury is in large part a disorder of post-injury tissue edema occurring within a closed space – the spinal canal – with resultant compression of surface veins that drain the spinal cord, a resulting feed-forward acceleration of edema and hence ever worsening blood flow, and ultimately infarction of spinal cord tissue. The regional severity of infarct can vary both segmentally and longitudinally, and typically includes both central gray matter as well as the ascending and descending white matter tracts. Axonal injury is paramount, and rarely are the lesions of SCI limited to demyelination.
As a result of these considerations, cell-based therapy of the injured spinal cord has more to do with the issues discussed in the cellular therapy of stroke rather than of myelin disease. As such, attempts to treat spinal cord injury, especially severe spinal injuries involving complete loss of function below given segmental levels, would seem unlikely to be effective if limited simply to the delivery of restricted phenotypes, such as OPCs. That said, other approaches to treat SCI using neural stem cells, in the hope that neurons generated from those NSCs might establish multi-synaptic networks able to bypass regions of injury to restore distal innervation, have shown great promise (Nori et al., 2011; Tuszynski et al., 2014). These advances may be the harbingers of more effective approaches towards spinal cord repair, using grafted NSCs or lineage-restricted spinal neuronal progenitors (Roy et al., 2004). Once such local circuit reconstruction is accomplished, then adjunctive approaches such as olfactory ensheathing cell (Granger et al., 2012) and glial progenitor cell delivery (Buchet et al., 2011; Kawabata et al., 2016; Mozafari et al., 2015), respectively designed to generate peripheral and central myelin and hence improve conduction within locally-restored networks, may be productively deployed to potentiate recovery. Indeed, the need for multimodal biological therapies in SCI is highlighted by the additional benefit potentially gained by combining cell transplantation with matrix-modifying enzymes so as to facilitate donor cell integration (Ikegami et al., 2005).
Reprogramming is an imperfect process
Many investigators have shifted their efforts in cell therapeutic development to iPSCs, to capitalize upon the potential for autologous therapy using patient-derived cells, whether with or without genetic correction of any underlying mutations. Yet despite the attractiveness of iPS cell sources, use of these cells is not without risk. The transcription factor-induced reprogramming of somatic cells into fibroblasts can be an imperfect process, with both incomplete reprogramming to stem cell ground state, and retention of epigenetic marks referable to donor cell phenotype (Hochedlinger and Plath, 2009; Kim et al., 2010; Polo et al., 2010). The induction process may also be associated with reprogramming-associated mutations (Gore et al., 2011; Sugiura et al., 2014), which though poorly understood, have already necessitated the halt of one clinical trial, of iPSC-derived retinal pigment epithelial transplants as noted previously.
More broadly, it remains to be established whether the appearance of such mutations during cell preparation reflects a predisposition to mutation by the hiPSCs, whether during or after reprogramming. If so, despite the low probability that random mutations – if they are indeed few and random – would prove tumorigenic, the appearance of mutations with iPSC induction and differentiation could pose a significant challenge to the therapeutic development of this cell source. As a result, one might posit that hESC-derived phenotypes, rather than iPSC-derived, may be the first into the clinic for a broad variety of disease targets, pending the more rigorous assessments that may be needed to assure the safety of iPSC derivatives.
Direct induction and its limitations
For a variety of disorders, replacement cells must be available quickly, before the effects of disease and injury become irreparable, whether by ancillary cell loss or maladaptive circuit reconstruction. Depending upon the time window during which cells may be able to effectively integrate into the injured or diseased neural network, the ramp-up time between pluripotential cell deployment and the production of transplantable phenotypes of need may be problematic. This issue is especially concerning when autologous cell delivery is desired. Whereas iPSCs and their derivatives have the advantage of potential autologous use, the long time frames involved in producing iPSCs from somatic cells, and in then producing cell types of interest from those cells, can limit the disease targets for which their use is appropriate. For instance, any iPSC-based treatment of acute demyelinating injuries that might benefit from rapid introduction of OPCs would likely be hindered by the turn-around time of going from biopsied fibroblasts to iPSCs to transplantable OPCs, a sequence that currently spans well over a half-year.
To address this issue, transcription factor-mediated reprogramming has been developed to permit the direct induction of desired phenotypes from somatic cells. This technique greatly accelerates the production of desired phenotypes, potentially enabling their use in disorders for which rapid cell replacement is required. Such direct induction of selected neuronal phenotypes was first achieved by Wernig and colleagues (Vierbuchen et al., 2010; Wapinski et al., 2013), and dopaminergic neurons were subsequently induced from somatic cells using a more phenotype-specific set of transcription factors (Hargus et al., 2010; Wernig et al., 2008). These in vitro studies have enabled the production of autologously-derived transplantable neurons in time frames much shorter than achievable using PSCs. More recently, Parmar and colleagues have achieved the in vivo direct induction of striatal neuronal phenotypes from resident glial progenitors (Torper et al., 2015), an approach that may permit the in situ production of desired neuronal phenotypes in a contextually-appropriate fashion. While early in development, this approach promises to potentially negate the need for in vitro production of phenotypes of interest, at least for those diseases in which healthy resident glial progenitors persist and remain amenable to directed phenotypic induction.
A similar strategy of direct induction has enabled the production of oligodendrocytes and oligodendrocyte progenitor cells from murine fibroblasts (Najm et al., 2013; Yang et al., 2013), and although these phenotypes have yet to be directly induced from human somatic cells, one may anticipate the likely accomplishment of this goal as well as work in the field proceeds. Yet despite the promise of this approach, its drawbacks may prevent early clinical adoption. Directly induced terminal phenotypes, whether neurons or oligodendrocytes, are either post-mitotic or nearly so, limiting their expansion capacity, and thus requiring virtual 1:1 stoichiometry between successfully instructed cells and their intended product. Since the transduction and phenotypic instruction efficiencies of this technique remain relatively low, the number of cells of interest produced are similarly low, and unrealistically so for the purpose of any potential clinical use (Goldman, 2013). Moreover, the inherent heterogeneity within such an induced population would complicate assurance of their safety and hence their regulation.
To address such concerns, more recent attempts have focused on directly inducing expandable neural stem cells from somatic cells, isolating mitotic and multipotential clones of interest, and then generating cells types of interest, both neurons and glia, from those cells (Lujan et al., 2012; Thier et al., 2012). This approach may permit the production of both glial progenitor cells and their derived oligodendrocytes as well as neurons from directly induced somatic cells. By this means, the production of an expandable, homogeneous, and potentially myelinogenic cell population might be obtained from transduced somatic cells. Future studies will determine whether this approach is sufficiently predictable and efficient to enable autologous therapy, and to do so in the time frame required of those disorders for which rapid cell replacement is required for anatomic stabilization and functional recovery.
Trial design matters
Many potentially effective agents and approaches have gone by the wayside during clinical assessment, because of poorly designed trials that aggregated populations that varied in their underlying disease pathogenesis, hence diluting any therapeutic effects. Thus, designing a trial to assess the therapeutic response of cell implantation in any given disorder requires a detailed understanding of the pathogenesis and natural history of that disorder, so as to understand its phenotypic heterogeneity, and thus its optimal inclusion criteria and control populations. Yet even for diseases whose genetic bases and natural histories are reasonably well-understood, establishing the relative benefit of a cell therapeutic may prove difficult. As a case in point, the childhood leukodystrophies, which would seem exceedingly attractive targets for cell therapy, and yet have become especially difficult diseases for which to design definitive clinical trials, given the rarity of these conditions, their phenotypic heterogeneity, and our limited understanding of their natural histories and prognoses. Indeed, as exciting as these new strategies of cell therapy might be, and as strong as the preclinical animal data may be in suggesting their potential efficacy, we will be unable to establish which treatments indeed provide significant benefit, whether that benefit might outweigh the risks inherent in cell therapy, and whether the durability of their benefits justifies the effort, until we can claim a better understanding of the heterogeneity and genotype-dependent natural histories of these disorders, and design our clinical trials accordingly.
Inaccurate and non-predictive animal models
The development of cell therapeutics may be delayed by the mismatch between mouse models of disease and their actual human counterparts. Mouse models that faithfully replicate CNS disease genetics, natural history and pathology are scarce, and limited to selected monogenic hereditary disorders. More typically, mouse models are established and selected on the basis of their ability to replicate discrete aspects of disease histopathology, often without regard to pathogenesis or normal time course of disease progression. For instance, chemotoxic models of Parkinson’s disease, including both 6-hydroxydopamine and MPTP exposure, have evolved because of their selective depletion of nigral dopaminergic neurons and associated neurological dysfunction, yet neither reproduces the human-specific circuitry or disease environment of Parkinson’s patients, who may handle dopaminergic grafts very differently from their murine avatars. This distinction was made clear by the inability of experiments using these mouse models to predict the dyskinesias that plagued fetal tissue transplants into Parkinson’s patients, an unforeseen sequela that slowed progress in that field for over a decade (Barker et al., 2015).
Mouse models also fail to reflect the long periods of time often required for disease evolution in humans, particularly for the neurodegenerative disorders. For example, Huntington’s disease takes many years to evolve in humans, and to accelerate the process in mice, transgenics have been constructed that express much longer polyglutamine expansions than those appearing in nature. Perhaps as a result, none of the many types of nominally Huntington disease mice thus far developed faithfully replicate both the typical disease course and neuropathology of the disease in humans (Brooks and Dunnett, 2015; Howland and Munoz-Sanjuan, 2014; Menalled and Brunner, 2014). As such, whether cell therapeutic strategies modeled in these mice will prove as efficacious in HD patients remains an uncomfortable unknown.
Similarly, the short lifespan of mice complicates modeling diseases in which different compartments become dysfunctional at different stages. For instance, peripheral nervous system (PNS) involvement complicates many of the hereditary human leukodystrophies, but often tends to be less apparent in mice, whose short lifespans can inaccurately minimize the role of less-rapidly evolving PNS disease. For disorders such as Niemann-Pick, Krabbe disease, adrenoleukodystrophy and the mucopolysaccharidoses, all of which include significant PNS pathology, effective treatment strategies will need to include systemic enzyme replacement, or the broad dispersal of enzymatically wild-type cells throughout peripheral nerves (Hawkins-Salsbury et al., 2015). As a result, the combination of intracerebral hGPC grafts with systemic enzyme replacement will likely be necessary to achieve durable therapeutic benefit for those disorders affecting both the CNS and PNS. More broadly, this issue suggests caution in relying too heavily on mouse avatars of these disorders, lest that reliance lead to the systematic over-estimation of therapeutic response, based upon the tendency of investigators to favor simple and clearly-defined models for diseases that are rarely simple.
Another concern in regards to over-estimating therapeutic efficacy is in the use of xenografts to model cell therapy, and in particular assessing the performance of human cells in the mouse environment, diseased or otherwise. Human cells xenografted into the mouse brain retain species-specific and cell-autonomous attributes that distinguish them from their mouse hosts (Han et al., 2013; Oberheim et al., 2009). This is a specific issue in modeling the glial and myelin disorders, in which hGPCs may be used to model glial replacement-based therapeutic strategies. Yet human GPCs preferentially expand and migrate within murine hosts, out-competing resident mouse glial progenitors to ultimately dominate the glial population (Windrem et al., 2014). As a result, whether the therapeutic benefit afforded by hGPC engraftment of a myelin-deficient mouse model will prove as striking when human cells are transplanted into human hosts remains unclear. Mouse-to-mouse allografts have confirmed the ability of both healthy glial progenitors and neural stem cells to out-compete deficient glial progenitors and myelinate hypomyelinated hosts (Lachapelle et al., 1994; Mitome et al., 2001; Yandava et al., 1999), providing some assurance as to the likely efficacy of human GPCs in myelinating myelin-deficient subjects, as in the hereditary leukodystrophies. But whether allografted GPCs can effectively compete with diseased or deficient GPCs in other disease settings is unclear. Such concerns are especially germane when considering the use of hGPC allografts in treating diffuse and multicentric disorders requiring whole neuraxis glial replacement, such as vanishing white matter disease in children, or chronic progressive multiple sclerosis in adults. Indeed, these questions will likely remain unanswered until human trials are performed, as there are limits to how effectively animal allografts can ever fully model the performance of human cells introduced into a human disease environment.
Superiority or efficacy of existing therapies
The risks of employing a cell-based therapeutic in any given condition, or for any individual patient, must be must be weighed against the efficacy, risks and costs of the standard of care for that disorder. While this is an easy calculus when one is dealing with untreatable and otherwise fatal disorders, such as the childhood leukodystrophies or Huntington disease, it is a more nuanced issue when one considers disorders for which some effective treatment is already available, when lifespan is not necessarily threatened by the disease process, or for which the costs may be high for only incremental improvements in condition or lifespan. The example of Parkinson’s disease, noted previously, is especially instructive in this regard, as essentially all of these considerations come to the fore: multiple effective treatment modalities already exist that are effective for the majority of patients, lifespan is only threatened after the development of refractory disease, most typically in the late elderly, and both the costs and risks of treatment may be significant. Yet for a minority of younger patients with early refractory disease, cell-based dopaminergic replacement may be their only current hope of a productive life and lifespan. At this early stage in the development of stem cell therapeutics, little cost-benefit and risk-benefit analysis has been done to define those disease targets most appropriate for clinical development. One recent cost-benefit analysis by Barker and colleagues (Buttery and Barker, 2014), which compared the attractiveness of cell therapy in Parkinson’s disease to that of alternative standards of care, has provided both a strong precedent and roadmap by which to address this issue of comparative efficacy, which will no doubt become a critically important – and potentially contentious – issue as stem cell medicine progresses. As with several other emerging fields of medicine, stem cell neurology is a field that while still in its infancy, will demand sufficient societal resources as to suggest both the need and benefit of early prioritization of effort.
Toxicities specific to pluripotent stem cells and their derivatives
While the development of specified neural and glial cell types from hESCs and hiPSCs has been met with great enthusiasm in the stem cell community, the rush towards their clinical use must be tempered by a number of issues that remain problematic in the transition from bench-to-bedside. Issues of potential tumorigenicity, immunogenicity, heterotopic differentiation, and adventitious viral introduction have been extensively discussed in recent reviews (Kaneko and Yamanaka, 2013; Yu et al., 2013). In particular, teratoma formation from residual pluripotential cells is a well-known risk that has been mitigated by the differentiation of cells in vitro towards terminal lineages, combined with cell sorting technologies to enrich lineage-restricted progenitors and remove persistent PSCs. However, the potential for tissue-specific tumor formation, with oncogenesis from partially-differentiated hES and hiPS cells, may be an even greater concern, that is not alleviated by the absence of pluripotent cells from grafts. Neuroepithelial tumors have been found in grafts of hESC-derived neural stem cells and their derived neuronal progenitors (Roy et al., 2006), as well as in grafts of cultured NSCs expanded for prolonged periods under constant mitogenic stimulation (Amariglio et al., 2009). Nonetheless, few studies have sought to specifically rule out the presence of neural or glial tumors in hESC or hiPSC-derived graft recipients. Long-term survival studies over broad dose ranges, with rigorous and unbiased neuropathological analysis, will need to be done in experimental animals to ensure the lack of tumorigenicity of hESC and hiPSC derivatives. Importantly, these safety studies will need to be partnered with efficacy studies utilizing the same protocols, recognizing that the long in vitro differentiation protocols that may mitigate the risk of tumorigenesis do not come without a price; prolonged differentiation may limit the expansion and dispersal capability, and ultimately the utility, of the engrafted donor cells.
Besides the well-recognized risk of tumorigenesis from undifferentiated and partially differentiated pluripotent cells, lurks the risk of tumorigenesis from mutations inadvertently introduced during the intended correction of known mutations. With the advent nuclease-dependent gene editing and its potential for safely correcting genomic mutations (Hsu et al., 2014; Li et al., 2014), autologous cell transplantation of genetically-corrected phenotypes has become an exciting possibility, as first established in mouse models of sickle cell anemia (Hanna et al., 2007). Yet while the potential of any residual PSCs or their incompletely differentiated progeny for undesired expansion after transplant is already a recognized concern, less attention has been paid to the possibility of off-target mutations in the host genome imparted by Crispr-Cas or TALEN editing. The inherent risk of any off-target mutations that might be imparted by gene editing strategies will require stringent measures, such as validation of whole genome sequence, to ensure the safety of genetically-corrected cells before transplant. This is a rapidly evolving field, for which stable regulatory policies have yet to be developed.
General toxicities of introducing cells into the postnatal and adult brain
In addition to the specific risks of pluripotential cells and their derivatives, introducing cells into the postnatal and mature CNS has its own set of risks, that include heterotopic neuronal differentiation with functional disruption and epileptogenesis, as well as the structural disruption and mass effect that may accompany exuberant cell expansion. Both tissue- and hESC-derived neural stem cells have been reported to have escaped to the spinal canal and ventricular system (Amariglio et al., 2009; Steward et al., 2014), adventitious growths that auger a high risk of obstructive hydrocephalus or, in the spinal cord, syringomyelia (Steward et al., 2014; Tuszynski et al., 2014). Cells escaping to the subarachnoid space might similarly be associated with surface venous compression and consequent cerebral edema, as well as with disruption in CSF flow and metabolic waste clearance (Iliff et al., 2012).
Perhaps most worrisome of all is the risk of immune rejection and attendant inflammation and cerebritis, which may be coupled to the destruction of grafted cells and consequent transplant failure. Both tissue and cell transplantation trials have generally included transient systemic T cell-targeted immunosuppressive therapy, most typically with calcineurin inhibitors such as cyclosporine or tacrolimus. Nonetheless, the use of these agents for prophylaxis of graft rejection in the CNS has been fundamentally empiric, as optimal protocols for immunosuppressant coverage have yet to be established for intracerebral grafts; neither the degree of systemic immunosuppression required nor the length of time that it should be maintained have been systematically studied, nor has the extent to which these protocols might be varied as a function of either donor cell type or host disease environment. Furthermore, the risks of chronic immunosuppression are not insignificant, and must be considered in any risk-benefit analysis of any cell transplant strategy.
In broad terms then, the list of potential toxicities and serious adverse events that may be triggered by intracerebral cell delivery serves to highlight the care with which we must consider the use of any neural or glial cell therapeutic, as well as the degree to which we must be vigilant that any cell therapeutic strategy not trigger the laws of unintended consequences.
WISHFUL THINKING
A recurrent theme in the design of clinically-meaningful cell therapeutic strategies is ensuring the proper pairing of disease targets with the right donor phenotypes, i.e., those able to achieve functionally-effective cell replacement and circuit repair in the disease environment. Yet a number of recent efforts have promulgated the use of donor cells that may be ill-suited for the disease targets to which they have been applied. Mesenchymal stem or stromal cells are a case in point. The homogeneity and ease of production of these cells has led to substantial interest in their deployment as cellular therapeutics, and their anti-inflammatory properties have led to their assessment in a broad variety of disease models and clinical targets alike (Einstein and Ben-Hur, 2008; Fainstein et al., 2008). Yet grafted MSCs typically do not survive in the adult human CNS, and early reports of their ability to produce mature neural phenotypes have been largely discredited. As such, the use of MSCs would seem logically limited to short-term immune modulation in settings for which pharmacological immune suppression has been found ineffective or otherwise ill-advised, such as SCI and stroke (DePaul et al., 2015; Liu et al., 2014). That, as well as the unclear mechanisms by which MSCs exert their immunosuppressive actions and the variability of those effects as a function of disease environment and duration, all serve to limit the utility of MSCs as clinical therapeutics. Whereas they may well prove beneficial in accelerating recovery from acute conditions exacerbated by central inflammation, such as stroke and relapsing multiple sclerosis - in each of which they are already under clinical assessment (Cohen, 2013; Hess et al., 2014; Rosado-de-Castro et al., 2013; Vu et al., 2014) – their efficacy in preserving threatened neurons and glia after acute injury, or improving the ultimate extent of functional recovery, remains unproven. In that regard, the more recent use of MSCs and related umbilical cord stem cells in largely non-inflammatory and structural conditions such as cerebral palsy (Englander et al., 2015), and in the genetic non-metabolic disorders of myelin such as Pelizaeus-Merzbacher disease (Wishnew et al., 2014), while difficult to logicize or indeed even justify, again reinforces the point that cell therapeutics should being deployed only for those conditions in which their proposed mechanism of action is both well-founded and target-appropriate.
Similarly, neural stem cells have long been promoted as a therapeutic in a broad variety of conditions that effectively span the gamut of neurological disease. Yet while these cells may be effective for some conditions, for many others they may be suboptimal, or even inappropriate. Indeed, their intrinsic multilineage competence and phenotypic plasticity can prove counterproductive to their use in targeted cell replacement. For instance, NSCs can generate neurons that in a glial disorder might be heterotopic, potentially integrating into extant neural networks in problematic manners. The neuronal phenotypes generated by NSCs in vivo may be difficult to instruct, and evidence for their phenotype-appropriate functional integration in higher cortical networks in adults is scarce. By the same token, they may generate astrocytes in abundance and in an environmentally-modulated fashion, not necessarily producing the phenotypes of interest when cell-type specific replacement is the goal. Similarly, their migration competence in vivo is relatively restricted, compared to their glial progenitor progeny, which are considerably more effective at long-distance migration and dispersal. As a result, NSCs may not be as appropriate vectors for the treatment of glial and myelin disorders as more restricted, and hence functionally dedicated, glial progenitor cells. Yet for both MSCs and NSCs, the tendency of commercial entities in particular to pursue these phenotypes, despite their manifest limitations as therapeutic vectors, may stem not only from purely commercial considerations as operative intellectual property and freedom to operate, but also from the clinical scale homogeneity and expandability that may be achieved using these phenotypes. Such scalability is a necessary condition for the broad clinical adoption of any cell therapeutic, and yet this prerequisite has thus far proven elusive for both glial and lineage-restricted neuronal progenitor cells.
CONCLUSION
Diseases of the CNS are especially attractive targets for human stem and progenitor cell-based therapy, given the limited tissue regeneration manifested by the adult brain and spinal cord. A number of disorders, including diseases of myelin, retinal disease, Huntington Disease, Parkinson disease, and other neurodegenerative states manifested by the loss of one or more discrete phenotypes, may now be targeted for cell replacement therapy using hESC and hiPSC derivatives, in some cases with curative intent. However, other neurological disorders may not be readily amenable to cell replacement-based treatment, whether by virtue of an inhospitable disease environment, poor risk profiles of the donor cells, or a poor convergence of donor cell capabilities with host needs. Some may well be medically feasible targets, but may not be practicable, in light of already available alternative therapies, poor risk-benefit and cost-benefit profiles. In all cases, the suitability of cell therapy for any given clinical disorder - and indeed for any individual patient - will depend upon the pathogenesis of the host disorder, the homogeneity of the deficient host cell phenotype, the phenotype of the intended donor cells, and the disease environment into which transplantation is anticipated. By matching up disease targets with the phenotypes most appropriate for their treatment, stem cell neurology may hope to advance in the most rapid, resource-efficient and clinically-effective manner going forward.
Acknowledgments
Dr. Goldman is supported by NINDS, NIMH, the National Multiple Sclerosis Society, the CHDI Foundation, New York Stem Cell Science (NYSTEM), the Mathers Charitable Foundation, the Adelson Medical Research Foundation, ALS Association, Progressive MS Alliance, PML Consortium, Lundbeck Foundation and Novo Nordisk Foundation. I would like thank Dr. Maiken Nedergaard for her comments on the manuscript, Dr. Abdellatif Benraiss for figure design, and the referees for their helpful comments. I apologize to all those investigators whose relevant work could not be cited due to space constraints.
References
- Aharonowiz M, Einstein O, Fainstein N, Lassmann H, Reubinoff B, Ben-Hur T. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PloS one. 2008;3:e3145. doi: 10.1371/journal.pone.0003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comparative Neurology. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS medicine. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An MC, Zhang N, Scott G, Montoro D, Wittkop T, Mooney S, Melov S, Ellerby LM. Genetic correction of Huntington’s disease phenotypes in induced pluripotent stem cells. Cell Stem Cell. 2012;11:253–263. doi: 10.1016/j.stem.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber C, Precious SV, Cambray S, Risner-Janiczek JR, Kelly C, Noakes Z, Fjodorova M, Heuer A, Ungless MA, Rodriguez TA, et al. Activin A directs striatal projection neuron differentiation of human pluripotent stem cells. Development. 2015;142:1375–1386. doi: 10.1242/dev.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RC, Dorn HH, Kufta CV, Friedman E, Dubois-Dalcq ME. Pre-oligodendrocytes from adult human CNS. J Neurosci. 1992;12:1538–1547. doi: 10.1523/JNEUROSCI.12-04-01538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenijevic Y, Villemure JG, Brunet JF, Bloch JJ, Deglon N, Kostic C, Zurn A, Aebischer P. Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Experimental neurology. 2001;170:48–62. doi: 10.1006/exnr.2001.7691. [DOI] [PubMed] [Google Scholar]
- Barker RA, Drouin-Ouellet J, Parmar M. Cell-based therapies for Parkinson disease-past insights and future potential. Nat Rev Neurol. 2015;11:492–503. doi: 10.1038/nrneurol.2015.123. [DOI] [PubMed] [Google Scholar]
- Barker RA, Mason SL, Harrower TP, Swain RA, Ho AK, Sahakian BJ, Mathur R, Elneil S, Thornton S, Hurrelbrink C, et al. The long-term safety and efficacy of bilateral transplantation of human fetal striatal tissue in patients with mild to moderate Huntington’s disease. J Neurol Neurosurg Psychiatry. 2013;84:657–665. doi: 10.1136/jnnp-2012-302441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte development and heterogeneity. Cold Spring Harbor perspectives in biology. 2015;7:a020362. doi: 10.1101/cshperspect.a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, Goldman SA. Cellular therapy and induced neuronal replacement for Huntington’s disease. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2011;8:577–590. doi: 10.1007/s13311-011-0075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, Toner MJ, Xu Q, Bruel-Jungerman E, Rogers EH, Wang F, Economides AN, Davidson BL, Kageyama R, Nedergaard M, et al. Sustained mobilization of endogenous neural progenitors delays disease progression in a transgenic model of Huntington’s disease. Cell Stem Cell. 2013;12:787–799. doi: 10.1016/j.stem.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretzner F, Gilbert F, Baylis F, Brownstone RM. Target populations for first-inhuman embryonic stem cell research in spinal cord injury. Cell Stem Cell. 2011;8:468–475. doi: 10.1016/j.stem.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Brooks SP, Dunnett SB. Mouse Models of Huntington’s Disease. Current topics in behavioral neurosciences. 2015;22:101–133. doi: 10.1007/7854_2013_256. [DOI] [PubMed] [Google Scholar]
- Buchet D, Garcia C, Deboux C, Nait-Oumesmar B, Baron-Van Evercooren A. Human neural progenitors from different foetal forebrain regions remyelinate the adult mouse spinal cord. Brain : a journal of neurology. 2011;134:1168–1183. doi: 10.1093/brain/awr030. [DOI] [PubMed] [Google Scholar]
- Buttery PC, Barker RA. Treating Parkinson’s disease in the 21st century: can stem cell transplantation compete? J Comp Neurol. 2014;522:2802–2816. doi: 10.1002/cne.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carri AD, Onorati M, Lelos MJ, Castiglioni V, Faedo A, Menon R, Camnasio S, Vuono R, Spaiardi P, Talpo F, et al. Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP-32+ medium-sized spiny neurons. Development. 2013;140:301–312. doi: 10.1242/dev.084608. [DOI] [PubMed] [Google Scholar]
- Chaboub LS, Deneen B. Developmental origins of astrocyte heterogeneity: the final frontier of CNS development. Developmental neuroscience. 2012;34:379–388. doi: 10.1159/000343723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. Journal of Neuroscience. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SR, Benraiss A, Chmielnicki E, Samdani A, Economides A, Goldman SA. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. Journal of Clinical investigation. 2007;117:2889–2902. doi: 10.1172/JCI31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F, Saporta S, Hauser RA, Parent M, Saint-Pierre M, Sanberg PR, Li XJ, Parker JR, Chu Y, Mufson EJ, et al. Neural transplants in patients with Huntington’s disease undergo disease-like neuronal degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12483–12488. doi: 10.1073/pnas.0904239106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA. Mesenchymal stem cell transplantation in multiple sclerosis. Journal of the neurological sciences. 2013;333:43–49. doi: 10.1016/j.jns.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Dusenbery BN, Williams LA, Klim JR, Eggan K. How to make spinal motor neurons. Development. 2014;141:491–501. doi: 10.1242/dev.097410. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p–linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaul MA, Palmer M, Lang BT, Cutrone R, Tran AP, Madalena KM, Bogaerts A, Hamilton JA, Deans RJ, Mays RW, et al. Intravenous multipotent adult progenitor cell treatment decreases inflammation leading to functional recovery following spinal cord injury. Scientific reports. 2015;5:16795. doi: 10.1038/srep16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell stem cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Noble M, Mayer-Proschel M. Characterization of A2B5+ glial precursor cells from cryopreserved human fetal brain progenitor cells. Glia. 2002;40:65–77. doi: 10.1002/glia.10116. [DOI] [PubMed] [Google Scholar]
- Douvaras P, Wang J, Zimmer M, Hanchuk S, O’Bara MA, Sadiq S, Sim FJ, Goldman J, Fossati V. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem cell reports. 2014;3:250–259. doi: 10.1016/j.stemcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Arch Neurol. 2008;65:452–456. doi: 10.1001/archneur.65.4.452. [DOI] [PubMed] [Google Scholar]
- Englander ZA, Sun J, Laura C, Mikati MA, Kurtzberg J, Song AW. Brain structural connectivity increases concurrent with functional improvement: evidence from diffusion tensor MRI in children with cerebral palsy during therapy. NeuroImage Clinical. 2015;7:315–324. doi: 10.1016/j.nicl.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Fainstein N, Vaknin I, Einstein O, Zisman P, Sasson Ben SZ, Baniyash M, Ben-Hur T. Neural precursor cells inhibit multiple inflammatory signals. Mol Cell Neurosci. 2008;39:335–341. doi: 10.1016/j.mcn.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Feldman EL, Boulis NM, Hur J, Johe K, Rutkove SB, Federici T, Polak M, Bordeau J, Sakowski SA, Glass JD. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Annals of neurology. 2014;75:363–373. doi: 10.1002/ana.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox IJ, Daley GQ, Goldman SA, Huard J, Kamp TJ, Trucco M. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science (New York, NY) 2014;345:1247391. doi: 10.1126/science.1247391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. Neurodegeneration. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science (New York, NY) 2015;349:1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- Goldman SA. Progenitor cell-based treatment of the pediatric myelin disorders. Archives of neurology. 2011;68:848–856. doi: 10.1001/archneurol.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA. White matter from fibroblasts. Nature biotechnology. 2013;31:412–413. doi: 10.1038/nbt.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nedergaard M, Windrem MS. Glial progenitor cell-based treatment and modeling of neurological disease. Science (New York, NY) 2012;338:491–495. doi: 10.1126/science.1218071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger N, Blamires H, Franklin RJ, Jeffery ND. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain : a journal of neurology. 2012;135:3227–3237. doi: 10.1093/brain/aws268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, Bramoulle Y, Van Camp N, Perrier AL, Hantraye P, Bjorklund A, et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15:653–665. doi: 10.1016/j.stem.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T, et al. Neural stem cell engraftment and myelination in the human brain. Science translational medicine. 2012;4:155ra137. doi: 10.1126/scitranslmed.3004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PJ, Deleidi M, Astradsson A, Smith GA, Cooper O, Osborn TM, Sundberg M, Moore MA, Perez-Torres E, Brownell AL, et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell stem cell. 2015;16:269–274. doi: 10.1016/j.stem.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science (New York, NY) 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- Hara K, Yasuhara T, Maki M, Matsukawa N, Masuda T, Yu SJ, Ali M, Yu G, Xu L, Kim SU, et al. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Progress in neurobiology. 2008;85:318–334. doi: 10.1016/j.pneurobio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Hargus G, Cooper O, Deleidi M, Levy A, Lee K, Marlow E, Yow A, Soldner F, Hockemeyer D, Hallett PJ, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins-Salsbury JA, Shea L, Jiang X, Hunter DA, Guzman AM, Reddy AS, Qin EY, Li Y, Gray SJ, Ory DS, et al. Mechanism-based combination treatment dramatically increases therapeutic efficacy in murine globoid cell leukodystrophy. J Neurosci. 2015;35:6495–6505. doi: 10.1523/JNEUROSCI.4199-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HDConsortium. Induced pluripotent stem cells from patients with Huntington’s disease show CAG-repeat-expansion-associated phenotypes. Cell Stem Cell. 2012;11:264–278. doi: 10.1016/j.stem.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Sila CA, Furlan AJ, Wechsler LR, Switzer JA, Mays RW. A double-blind placebo-controlled clinical evaluation of MultiStem for the treatment of ischemic stroke. International journal of stroke : official journal of the International Stroke Society. 2014;9:381–386. doi: 10.1111/ijs.12065. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland DS, Munoz-Sanjuan I. Mind the gap: models in multiple species needed for therapeutic development in Huntington’s disease. Movement disorders : official journal of the Movement Disorder Society. 2014;29:1397–1403. doi: 10.1002/mds.26008. [DOI] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Du ZW, Li XJ, Ayala M, Zhang SC. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 2009;136:1443–1452. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nature neuroscience. 2013;16:692–697. doi: 10.1038/nn.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Nakamura M, Yamane J, Katoh H, Okada S, Iwanami A, Watanabe K, Ishii K, Kato F, Fujita H, et al. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth-associated protein-43-positive fibers after rat spinal cord injury. The European journal of neuroscience. 2005;22:3036–3046. doi: 10.1111/j.1460-9568.2005.04492.x. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Science translational medicine. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, Itskovitz-Eldor J, Chebath J, Revel M. Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neurosci. 2007;34:310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Jayakody SA, Gonzalez-Cordero A, Ali RR, Pearson RA. Cellular strategies for retinal repair by photoreceptor replacement. Prog Retin Eye Res. 2015;46:31–66. doi: 10.1016/j.preteyeres.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem cell reports. 2014;2:205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Yamanaka S. To be immunogenic, or not to be: that’s the iPSC question. Cell stem cell. 2013;12:385–386. doi: 10.1016/j.stem.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, Conway AE, Clark AT, Goldman SA, Plath K, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–811. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata S, Takano M, Numasawa-Kuroiwa Y, Itakura G, Kobayashi Y, Nishiyama Y, Sugai K, Nishimura S, Iwai H, Isoda M, et al. Grafted Human iPS Cell-Derived Oligodendrocyte Precursor Cells Contribute to Robust Remyelination of Demyelinated Axons after Spinal Cord Injury. Stem cell reports. 2016;6:1–8. doi: 10.1016/j.stemcr.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalopoulou Z, Politis M, Piccini P, Mencacci N, Bhatia K, Jahanshahi M, Widner H, Rehncrona S, Brundin P, Bjorklund A, et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014;71:83–87. doi: 10.1001/jamaneurol.2013.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Med. 2015;5:a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Morizane A, Doi D, Onoe H, Hayashi T, Kawasaki T, Saiki H, Miyamoto S, Takahashi J. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson’s disease. Journal of Parkinson’s disease. 2011;1:395–412. doi: 10.3233/JPD-2011-11070. [DOI] [PubMed] [Google Scholar]
- Kim J, Holtzman DM. Medicine. Prion-like behavior of amyloid-beta. Science (New York, NY) 2010;330:918–919. doi: 10.1126/science.1198314. [DOI] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser RA, Goldman SA. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cerebral Cortex. 1994;4:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Martino G, Schwartz M, Lindvall O. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nature neuroscience. 2012;15:1078–1087. doi: 10.1038/nn.3163. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy bodylike pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nature medicine. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachapelle F, Duhamel-Clerin E, Gansmuller A, Baron-Van Evercooren A, Villarroya H, Gumpel M. Transplanted transgenically marked oligodendrocytes survive, migrate and myelinate in the normal mouse brain as they do in the shiverer mouse brain. The European journal of neuroscience. 1994;6:814–824. doi: 10.1111/j.1460-9568.1994.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Lacorazza HD, Flax JD, Snyder EY, Jendoubi M. Expression of human beta-hexosaminidase alpha-subunit gene (the gene defect of Tay-Sachs disease) in mouse brains upon engraftment of transduced progenitor cells. Nature medicine. 1996;2:424–429. doi: 10.1038/nm0496-424. [DOI] [PubMed] [Google Scholar]
- Lee JP, Jeyakumar M, Gonzalez R, Takahashi H, Lee PJ, Baek RC, Clark D, Rose H, Fu G, Clarke J, et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nature medicine. 2007;13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nature medicine. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Li M, Suzuki K, Kim NY, Liu GH, Izpisua Belmonte JC. A cut above the rest: targeted genome editing technologies in human pluripotent stem cells. The Journal of biological chemistry. 2014;289:4594–4599. doi: 10.1074/jbc.R113.488247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nature biotechnology. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Lindvall O. Dopaminergic neurons for Parkinson’s therapy. Nature biotechnology. 2012;30:56–58. doi: 10.1038/nbt.2077. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. Cell therapeutics in Parkinson’s disease. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2011;8:539–548. doi: 10.1007/s13311-011-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, McDonald JW. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6126–6131. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, Fan X, Jiang Y, Stetler RA, Liu G, et al. Cell based therapies for ischemic stroke: from basic science to bedside. Progress in neurobiology. 2014;115:92–115. doi: 10.1016/j.pneurobio.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jiang P, Deng W. OLIG gene targeting in human pluripotent stem cells for motor neuron and oligodendrocyte differentiation. Nature protocols. 2011;6:640–655. doi: 10.1038/nprot.2011.310. [DOI] [PubMed] [Google Scholar]
- Liu Y, Weick JP, Liu H, Krencik R, Zhang X, Ma L, Zhou GM, Ayala M, Zhang SC. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nature biotechnology. 2013;31:440–447. doi: 10.1038/nbt.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science (New York, NY) 2012a;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012b;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JS, Sakowski SA, Feldman EL. Concise review: Stem cell therapies for amyotrophic lateral sclerosis: recent advances and prospects for the future. Stem Cells. 2014;32:1099–1109. doi: 10.1002/stem.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Brennand KJ, Boyer LF, Gage FH. Induced pluripotent stem cells (iPSCs) and neurological disease modeling: progress and promises. Human molecular genetics. 2011;20:R109–R115. doi: 10.1093/hmg/ddr336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menalled L, Brunner D. Animal models of Huntington’s disease for translation to the clinic: best practices. Movement disorders : official journal of the Movement Disorder Society. 2014;29:1375–1390. doi: 10.1002/mds.26006. [DOI] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nature medicine. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Eggan K. Modeling human disease with pluripotent stem cells: from genome association to function. Cell Stem Cell. 2013;12:656–668. doi: 10.1016/j.stem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Meyer K, Ferraiuolo L, Miranda CJ, Likhite S, McElroy S, Renusch S, Ditsworth D, Lagier-Tourenne C, Smith RA, Ravits J, et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:829–832. doi: 10.1073/pnas.1314085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitome M, Low HP, van Den Pol A, Nunnari JJ, Wolf MK, Billings-Gagliardi S, Schwartz WJ. Towards the reconstruction of central nervous system white matter using neural precursor cells. Brain : a journal of neurology. 2001;124:2147–2161. doi: 10.1093/brain/124.11.2147. [DOI] [PubMed] [Google Scholar]
- Moore SF, Guzman NV, Mason SL, Williams-Gray CH, Barker RA. Which patients with Parkinson’s disease participate in clinical trials? One centre’s experiences with a new cell based therapy trial (TRANSEURO) J Parkinsons Dis. 2014;4:671–676. doi: 10.3233/JPD-140432. [DOI] [PubMed] [Google Scholar]
- Mozafari S, Laterza C, Roussel D, Bachelin C, Marteyn A, Deboux C, Martino G, Baron-Van Evercooren A. Skin-derived neural precursors competitively generate functional myelin in adult demyelinated mice. The Journal of clinical investigation. 2015;125:3642–3656. doi: 10.1172/JCI80437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm FJ, Lager AM, Zaremba A, Wyatt K, Caprariello AV, Factor DC, Karl RT, Maeda T, Miller RH, Tesar PJ. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nature biotechnology. 2013;31:426–433. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari H, Zhang L, Zhu D, Chader GJ, Falabella P, Stefanini F, Rowland T, Clegg DO, Kashani AH, Hinton DR, et al. Stem cell based therapies for age-related macular degeneration: The promises and the challenges. Prog Retin Eye Res. 2015;48:1–39. doi: 10.1016/j.preteyeres.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Kondziolka D, Wechsler L, Goldstein S, Gebel J, DeCesare S, Elder EM, Zhang PJ, Jacobs A, McGrogan M, et al. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. The American journal of pathology. 2002;160:1201–1206. doi: 10.1016/S0002-9440(10)62546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistor G, Totoiu M, Haque NS, Carpenter M, Keirstead H. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nature medicine. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Oberheim N, Wang X, Goldman SA, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends in Neurosciences. 2006;29:1–10. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, Gutin P, Uryu K, Tchieu J, Soulet D, et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell. 2015;16:198–210. doi: 10.1016/j.stem.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus DW, Keyoung HM, Harrison-Restelli C, Goodman RR, Fraser RA, Edgar M, Sakakibara S, Okano H, Nedergaard M, Goldman SA. Fibroblast growth factor-2/brain-derived neurotrophic factor-associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol. 1998;43:576–585. doi: 10.1002/ana.410430505. [DOI] [PubMed] [Google Scholar]
- Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nature biotechnology. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest CA, Manley NC, Denham J, Wirth ED, 3rd, Lebkowski JS. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regenerative medicine. 2015;10:939–958. doi: 10.2217/rme.15.57. [DOI] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado-de-Castro PH, Pimentel-Coelho PM, da Fonseca LM, de Freitas GR, Mendez-Otero R. The rise of cell therapy trials for stroke: review of published and registered studies. Stem cells and development. 2013;22:2095–2111. doi: 10.1089/scd.2013.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N, Nakano T, Xuing L, Kang J, Nedergaard M, Goldman SA. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp Neurology. 2005;196:224–234. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nature medicine. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Roy NS, Nakano T, Keyoung HM, Windrem M, Rashbaum WK, Alonso ML, Kang J, Peng W, Carpenter MK, Lin J, et al. Telomerase immortalization of neuronally restricted progenitor cells derived from the human fetal spinal cord. Nature biotechnology. 2004;22:297–305. doi: 10.1038/nbt944. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Harrison-Restelli C, Benraiss A, Fraser RA, Gravel M, Braun PE, Goldman SA. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, Fraser RA, Couldwell WT, Kawaguchi A, Okano H, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nature medicine. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia VJ, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sandoe J, Eggan K. Opportunities and challenges of pluripotent stem cell neurodegenerative disease models. Nature neuroscience. 2013;16:780–789. doi: 10.1038/nn.3425. [DOI] [PubMed] [Google Scholar]
- Schitine C, Nogaroli L, Costa MR, Hedin-Pereira C. Astrocyte heterogeneity in the brain: from development to disease. Frontiers in cellular neuroscience. 2015;9:76. doi: 10.3389/fncel.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- Serio A, Bilican B, Barmada SJ, Ando DM, Zhao C, Siller R, Burr K, Haghi G, Story D, Nishimura AL, et al. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4697–4702. doi: 10.1073/pnas.1300398110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim FJ, McClain CR, Schanz SJ, Protack TL, Windrem MS, Goldman SA. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nature biotechnology. 2011;29:934–941. doi: 10.1038/nbt.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, Shim SH, Del Priore LV, Lanza R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem cell reports. 2015;4:860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Nicholas CR, Basbaum AI, Stryker MP, Kriegstein AR, Rubenstein JL, Alvarez-Buylla A. Interneurons from embryonic development to cell-based therapy. Science (New York, NY) 2014;344:1240622. doi: 10.1126/science.1240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole SR, Spitzer S, Bilican B, Compston A, Karadottir R, Chandran S, Franklin RJ. High yields of oligodendrocyte lineage cells from human embryonic stem cells at physiological oxygen tensions for evaluation of translational biology. Stem cell reports. 2013;1:437–450. doi: 10.1016/j.stemcr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck JA, Studer L. Moving stem cells to the clinic: potential and limitations for brain repair. Neuron. 2015;86:187–206. doi: 10.1016/j.neuron.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Sharp KG, Matsudaira Yee K. Long-distance migration and colonization of transplanted neural stem cells. Cell. 2014;156:385–387. doi: 10.1016/j.cell.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kasama Y, Araki R, Hoki Y, Sunayama M, Uda M, Nakamura M, Ando S, Abe M. Induced pluripotent stem cell generation-associated point mutations arise during the initial stages of the conversion of these cells. Stem cell reports. 2014;2:52–63. doi: 10.1016/j.stemcr.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabar V, Studer L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nature reviews Genetics. 2014;15:82–92. doi: 10.1038/nrg3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J, Takagi Y, Saiki H. Transplantation of embryonic stem cell-derived dopaminergic neurons in MPTP-treated monkeys. Methods in molecular biology. 2009;482:199–212. doi: 10.1007/978-1-59745-060-7_13. [DOI] [PubMed] [Google Scholar]
- Thier M, Worsdorfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nothen MM, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Thompson LH, Bjorklund A. Reconstruction of brain circuitry by neural transplants generated from pluripotent stem cells. Neurobiol Dis. 2015;79:28–40. doi: 10.1016/j.nbd.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Torper O, Ottosson DR, Pereira M, Lau S, Cardoso T, Grealish S, Parmar M. In Vivo Reprogramming of Striatal NG2 Glia into Functional Neurons that Integrate into Local Host Circuitry. Cell reports. 2015;12:474–481. doi: 10.1016/j.celrep.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Wang Y, Graham L, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, et al. Neural stem cell dissemination after grafting to CNS injury sites. Cell. 2014;156:388–389. doi: 10.1016/j.cell.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82:1277–1286. doi: 10.1212/WNL.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–264. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapinski OL, Vierbuchen T, Qu K, Lee QY, Chanda S, Fuentes DR, Giresi PG, Ng YH, Marro S, Neff NF, et al. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell. 2013;155:621–635. doi: 10.1016/j.cell.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warre-Cornish K, Barber AC, Sowden JC, Ali RR, Pearson RA. Migration, integration and maturation of photoreceptor precursors following transplantation in the mouse retina. Stem cells and development. 2014;23:941–954. doi: 10.1089/scd.2013.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EL, Gonzalez-Cordero A, Hippert C, Osakada F, Martinez-Barbera JP, Pearson RA, Sowden JC, Takahashi M, Ali RR. Defining the integration capacity of embryonic stem cell-derived photoreceptor precursors. Stem Cells. 2012;30:1424–1435. doi: 10.1002/stem.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P, Kerby J, Coffey P, da Cruz L, McKernan R. Progressing a human embryonic stem-cell-based regenerative medicine therapy towards the clinic. Philos Trans R Soc Lond B Biol Sci. 2015:370. doi: 10.1098/rstb.2014.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nature neuroscience. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, Roy NS, Goldman SA. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nature medicine. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Morrow C, Munir J, Chandler-Militello D, Wang S, Goldman SA. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J Neurosci. 2014;34:16153–16161. doi: 10.1523/JNEUROSCI.1510-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishnew J, Page K, Wood S, Galvin L, Provenzale J, Escolar M, Gustafson K, Kurtzberg J. Umbilical cord blood transplantation to treat Pelizaeus-Merzbacher Disease in 2 young boys. Pediatrics. 2014;134:e1451–e1457. doi: 10.1542/peds.2013-3604. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nature neuroscience. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandava BD, Billinghurst LL, Snyder EY. “Global” cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7029–7034. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Zuchero JB, Ahlenius H, Marro S, Ng YH, Vierbuchen T, Hawkins JS, Geissler R, Barres BA, Wernig M. Generation of oligodendroglial cells by direct lineage conversion. Nature biotechnology. 2013;31:434–439. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DX, Di Giorgio FP, Yao J, Marchetto MC, Brennand K, Wright R, Mei A, McHenry L, Lisuk D, Grasmick JM, et al. Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem cell reports. 2014a;2:295–310. doi: 10.1016/j.stemcr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DX, Marchetto MC, Gage FH. Therapeutic translation of iPSCs for treating neurological disease. Cell stem cell. 2013;12:678–688. doi: 10.1016/j.stem.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Yu DX, Marchetto MC, Gage FH. How to make a hippocampal dentate gyrus granule neuron. Development. 2014b;141:2366–2375. doi: 10.1242/dev.096776. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Current opinion in neurobiology. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]