Abstract
Background
Lateral Organ Boundaries Domain (LBD) genes arise from charophyte algae and evolve essential functions in land plants in regulating organ development and secondary metabolism. Although diverse plant species have been investigated to construct the phylogeny of LBD gene family, a detailed and reliable ancestry that characterizes their evolutionary patterns has not been revealed.
Results
We develop an improved bioinformatic method that allows robust detection of 431 LBD genes in 11 high-quality land plant genomes. Phylogenetic analysis classifies the LBD genes into six subfamilies which support the existence of 7 ancient gene lineages. Phylogenetic relationship and gene collinearity are combined to retrace 11 ancestor genes for seed plants and 18 ancestor genes for angiosperms, which improves the resolution of LBD gene ancestry. The ancient gene lineages are strictly preserved in current plant genomes, including the previously controversial class IB gene in Selaginella moellendorphii, suggesting extreme reluctance of LBD genes to be lost during evolution. Meanwhile, whole-genome and dispersed gene duplications substantially expand LBD gene family in angiosperms, and elaborate functions of LBD genes through frequent expression pattern change and protein sequence variation.
Conclusions
Through phylogenetic and gene collinearity analyses, we retrace the landscape of LBD gene ancestry which lays foundation for elucidating evolutionary diversification of LBD genes in land plants.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-016-3264-3) contains supplementary material, which is available to authorized users.
Keywords: LBD gene family, Phylogenetic analysis, Land plant, Evolution
Background
Lateral Organ Boundaries (LOB) Domain (LBD) proteins define a group of plant-specific transcription factors that originated from charophyte algae [1, 2]. They share a characteristic LOB domain (also referred to as AS2 domain) with a conserved C-motif (CX2CX6CX3C), a Gly–Ala–Ser (GAS) block, and a leucine-zipper-like coiled-coil motif [3–5]. The C-motif is predicted to bind to DNA sequence, while the coiled-coil motif functions in mediating protein-protein interaction. In Arabidopsis, LBD genes were first identified for the specific expression at the base of lateral organs and the noticeable function in regulating leaf development [3, 4]. Subsequent studies showed diverse functions of LBD genes in regulating plant organ development and secondary metabolism: AtLBD6/AtAS2 regulates leaf formation [3]; AtLBD16, AtLBD18 and AtLBD29 control lateral root formation [6]; AtLBD27/SCP is required for microspore cell divisions [7]; AtLBD37, AtLBD38 and AtLBD39 are negative regulators of anthocyanin biosynthesis and N availability signals [8]. It is frequently observed that LBD genes exhibit similar biological functions in different angiosperm species. For example, AtLBD16 orthologs control lateral root formation in Arabidopsis, rice and maize [6, 9, 10], and AtLBD6/AtAS2 orthologs repress meristematic cell formation and regulate abaxial-adaxial leaf polarity in Arabidopsis and maize [11–14]. The conserved roles of LBD gene orthologs suggest their functions may have been established prior to angiosperms emergence.
Extensive efforts have been exerted to classify LBD gene family in diverse plant species. In Arabidopsis, two major classes of LBD genes are traditionally classified according to the LOB domain structure [3, 4]. Class II proteins are distinctive to the class I due to the absence of the coiled-coiled motif. Further subdivisions of the two major classes reveal highly dynamic patterns in different species. In rice and maize, five subgroups are divided among class I LBD genes [15, 16]. In Arabidopsis and Malus, four and nine subgroups of class I LBD genes are classified respectively [5, 17]. The inconsistent subgroup number in these studies is likely caused by extensive duplications of LBD genes in angiosperms, and a single plant genome may not encompass their full diversity.
Recent studies performed more comprehensive analysis of phylogenetic distribution of LBD genes in multiple species spanning bryophytes, lycophytes and seed plants [1, 2]. Their results accordantly subdivide LBD genes into class IA, IB, IC1/ID, IC2, IE and class II, but there are still debates on whether class IB LBD genes are present in the genome of lycophytes [1, 2]. Moreover, angiosperm genomes retain large-scale collinear gene blocks which provides direct evidence to identify orthologous genes descendent from a common ancestor [18]. But none of above studies investigates collinearity of LBD genes to infer their ancestors at each stage of land plant evolution. Therefore, a detailed and reliable ancestry that describes evolutionary history of LBD genes has not been revealed. Here we develop an improved method for LBD gene detection in 11 representative plant species. Through investigating gene collinearity and phylogenetic relationships, we present a detailed ancestry of LBD genes which characterizes their diversifying patterns in land plants.
Results and discussion
An improved method to identify LBD genes
Previous strategy to detect LBD genes is primarily based on database query or BLAST search of Arabidopsis protein sequences [1, 2], which heavily depends on Arabidopsis sequence features and has limited applications for evolutionarily divergent genomes of land plants. Therefore we develop a new method that can be widely employed to detect LBD genes. As sequences of LBD proteins are highly variable except the conserved LOB domain [3, 4], we use the PFAM profile hidden Markov models of the LOB domain (PFAM database, http://pfam.xfam.org/) to query each plant genome with a cutoff of gathering threshold (E-value 1e-5). Only proteins with matched sequence covering at least 80% of the LOB domain are regarded as LBD proteins. This improved method would be effective to preclude partial sequence matches outside the LOB domain, while allowing identification of LBD genes with relative sequence variance. We identify 431 LBD genes in 11 high-quality genomes of land plants spanning bryophytes to angiosperms (Fig. 1 and Additional file 1). The lycophyte Selaginella contains 15 LBD genes, ranking the least in land plants. The number of LBD genes nearly doubles in bryophyte Physcomitrella which has a genome duplication event [19]. Apart from basal eudicot Aquilegia, more than 34 LBD genes are identified in the angiosperm genomes (Fig. 1), suggesting extensive expansion of LBD gene family in angiosperms. Noticeably, compared with the previous study [1], our improved method identifies more LBD genes in basal-node bryophyte Physcomitrella (28 vs 26) and lycophyte Selaginella (15 vs 11).
Fig. 1.
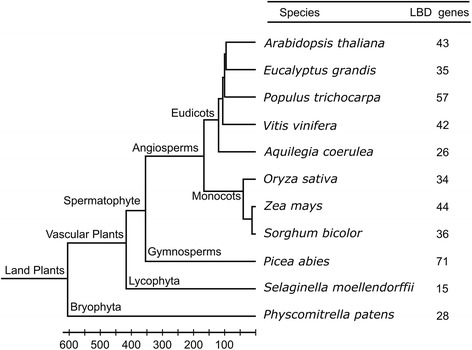
Summary of LBD genes in the analyzed land plant species. The order of tree branches and divergence time are derived from the TimeTree database (http://timetree.org/)
Expansion patterns of LBD genes in angiosperms
The expansion of LBD genes in angiosperms suggests they are likely influenced by whole-genome duplications (WGDs) that substantially increase gene content [18]. Therefore, we analyze each angiosperm genome to identify different types of gene duplications that contribute to the diversity of LBD genes. According to genome positions of the affected genes, gene duplication events are categorized into different sorts: WGD, dispersed duplication, and tandem/proximal duplications. On average 84% of LBD genes in angiosperms are affected by WGD and dispersed duplications (Fig. 2a). WGD events influenced 35–45% of LBD genes in most angiosperm species, while the ratio can vary dynamically from 28% in Arabidopsis to 77% in Populus. This could be explained by the fact that Arabidopsis has lost many LBD gene duplicates following two recent WGDs within the crucifer lineage, whereas Populus retained more duplicated genes after the Salicaceae WGD event [20, 21]. Compared with WGD event, tandem duplications in Populus only affect 5.2% of LBD genes. In contrast, up to 40% of LBD genes in Vitis are influenced by tandem duplications, suggesting tandem duplication in Vitis is an important driving force for the expansion of LBD genes.
Fig. 2.
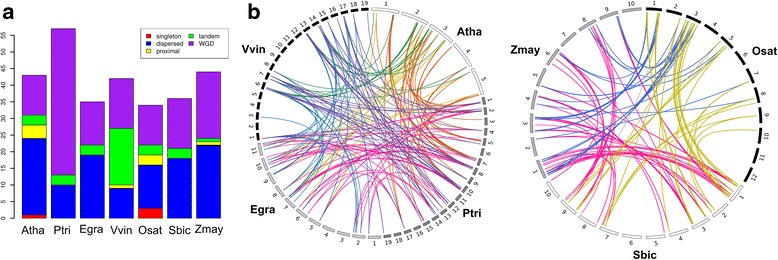
A category of LBD gene duplication events and inter-species collinearity. a The histogram indicates the occurrence of LBD gene duplication events in representative angiosperm species. b Inter-species collinearity of LBD genes in dicots and monocots. Colored lines represent pairs of orthologous LBD genes in the conserved syntenic blocks of plant genomes
Previous studies demonstrate genes descendent from a common ancestor often share chromosomal collinearity in angiosperms [18]. Therefore, we analyze collinearity relationship of LBD genes to infer their inter-species orthology. Through iterative clustering of collinear LBD genes, we merge 133 LBD genes of dicots into 21 collinear groups and 92 LBD genes of monocots into 23 collinear groups (Fig. 2b and Additional file 2). The collinear groups contain 77% of total LBD genes in the analyzed angiosperms and each group represents a set of LBD gene orthologs originated from a common ancestor. Merging collinear groups into a higher hierarchy is not feasible because extensive genome fragmentation and rearrangement obscured syntenic blocks between dicots and monocots [18]. Although the result identifies less than 23 members of LBD gene ancestors, additional phylogenetic information is needed to collapse the collinear groups of dicots and monocots to reflect a common angiosperm ancestor.
Retracing LBD gene ancestors in land plants
A maximum likelihood (ML) tree is constructed with the aligned LOB domain in the 11 species to reveal phylogenetic relationships of LBD genes. The phylogenetic tree classifies LBD genes into class IA, IB, IC1/ID, IC2, IE, and class II, a topology similar with previously reported [1, 2] (Fig. 3). Through scrutinizing the phylogenetic tree, we identify 7 independent gene clusters of Physcomitrella and Selaginella genes that neighbor with LBD genes of all analyzed seed plants, suggesting they are ancient gene lineages that give rise to LBD genes in modern plant genomes. We detect two ancient lineages in class IA, and one ancient lineage in each of the five remaining classes (Additional file 3). Therefore, class IA is actually composed of two founder genes in early land plants. To yield a better resolution, we generate another ML tree using complete protein sequences of class IA. The phylogenetic tree verifies the existence of two ancient lineages with high support values (Shimodaira-Hasegawa-like approximate likelihood-ratio test (SH-aLRT) > 94%) (Additional file 4).
Fig. 3.
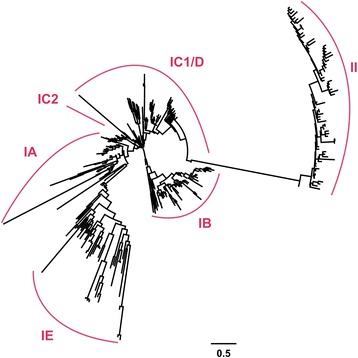
ML tree of LBD genes in land plants. The phylogenetic tree is constructed using the aligned LOB domains
We then search gene clusters of seed plants to retrace ancestor LBD genes predating seed plants emergence. As phylogenetic tree often suffers from long branch attraction in which distant protein sequences are incorrectly grouped together [22], we employ stringent criteria in the analysis, requiring that LBD genes of gymnosperm Picea should be clustered with angiosperm genes from all the analyzed species. In total 11 gene clusters of seed plants were identified from the ML tree: three in class IC1/ID, two in class IA, IB and II, one in class IC2 and IE (Fig. 4 and Additional file 3). Because each cluster of seed plant LBD genes share the same phylogenetic origin, 11 LBD gene ancestors likely existed prior to seed plant divergence. Compared with the ancient lineages, class IB, IC1/ID and II LBD genes experience gene content expansion before seed plant evolution.
Fig. 4.
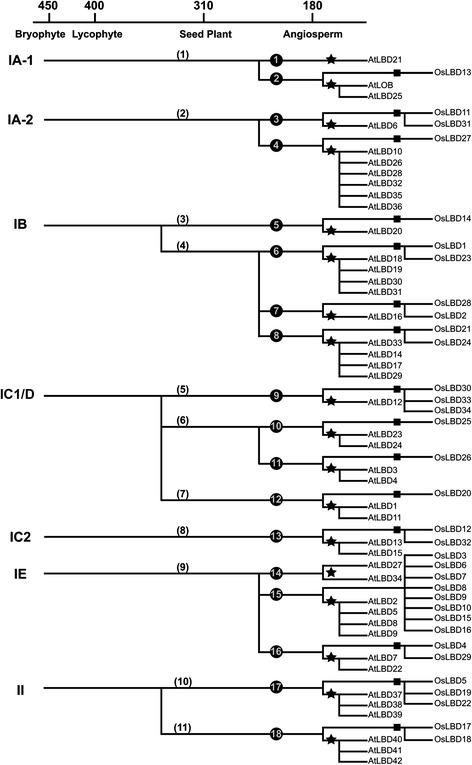
Reconstruction of LBD gene ancestry in land plants. The LBD genes in Arabidopsis and rice are mapped to the ancestral lineages at each key node of land plant evolution. Each line indicates a lineage of LBD genes. Asterisks and boxes and indicate the support from collinear gene groups in dicots and monocots. Note that the exact timing of duplication events is not estimated in the analysis due to uncertainties of the molecular clock assumption. The LBD genes in Arabidopsis are named in accordance with the previous study [4]
Gene collinearity and phylogenetic relationships are combined to retrace angiosperm ancestors of LBD genes. The ML tree categorizes 18 angiosperm gene clusters that include both dicot and monocot LBD genes (Fig. 4 and Additional file 3). Most of them are supported by inter-species gene collinearity except one cluster in class IE, which may be caused by massive gene duplications that likely obscure the syntenic block detection. Therefore, we propose that 18 LBD gene ancestors have been established prior to angiosperm emergence.
Reconstruct the ancestry of LBD gene family
We reconstruct evolutionary history of LBD genes with the deduced ancestor genes. In early land plants, 7 ancient lineages of LBD genes were established and remained in a stable amount until the divergence of seed plants (Fig. 4). Two rounds of gene duplication occurred before the emergence of seed plants and angiosperms, expanding LBD gene family to 11 and 18 members respectively. Further expansion of LBD genes in individual angiosperm species is highly associated with WGDs. The basal eudicot Aquilegia contains 26 LBD genes, while the amount could be increased to over 40 members in other eudicots which have undergone genome triplication or additional WGD events (Fig. 1) [23]. The analyzed monocots have survived from two successive WGDs and contain more than 34 LBD genes in the genome [24]. Noticeably, all of the major lineages of LBD genes could be detected in current angiosperm genomes, indicating they are extremely reluctant to be lost during evolution.
The reconstructed ancestry describes detailed evolutionary routes for 43 LBD genes in Arabidopsis and 34 LBD genes in rice. RNA-seq data of rice developmental atlas was further analyzed to investigate expression patterns of LBD genes from different subfamily [25]. The heatmap shows that different subfamily genes exhibit variable expression enrichment in different tissues (Fig. 5). For example, class IA LBD genes are more abundant in leaf and flower tissues, while class II LBD genes are universally expressed in diverse tissues. Meanwhile, it is often observed that LBD genes from a same subfamily genes display differential expression patterns. A noticeable case is in class IE which contains eight LBD genes (OsLBD3, OsLBD6, OsLBD7, OsLBD8, OsLBD9, OsLBD10, OsLBD15 and OsLBD16) sharing a same angiosperm ancestor. Only four of these LBD genes show abundant expression in normal growth tissues, while the other genes are detected with very weak expression level (Fig. 5), suggesting expression pattern has been shifted during these genes evolution. Another case is the class IA-2 subfamily which contains OsLBD11, OsLBD31 and OsLBD27 paralog genes. Expression of OsLBD11 is enriched in leaves, shoots and panicle (Fig. 5). Meanwhile, OsLBD31 and OsLBD27 display complementary expression patterns in either leaves or shoot and panicle, suggesting the two paralog genes underwent expression specialization after gene duplication. In Arabidopsis, similar expression divergence is also observed for class IA-2 LBD genes. For example, AtLBD6/AS2 is specifically expressed in the adaxial side of leaves [3], while AtLBD10 is only detected in the pollen grains, and AtLBD36 is expressed in a variety of tissues, including leaf vasculature, flower organs and seeds [26, 27]. These observations suggest alteration of expression level and tissue specificity occurred during LBD genes evolution.
Fig. 5.
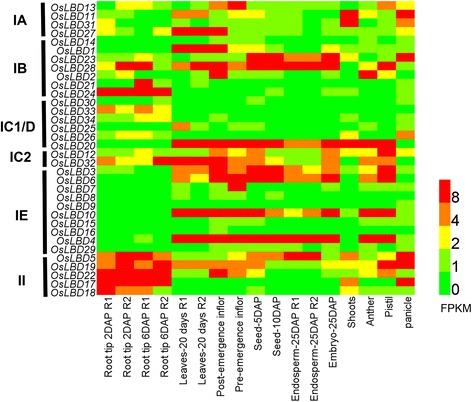
Tissue expression patterns of LBD genes in rice. The heatmap is constructed to illustrate expression level of 34 rice LBD genes. The RNA-seq data is obtained from the CARMO database [25]. The gene order is sorted according to their affiliated subfamilies
Diversification of LBD gene subfamily
To deepen annotation and classification of LBD gene family, we developed sequence profile features for each LBD gene class using whole protein sequences alignment. The result shows that, besides the well recognized LOB domains, different gene classes possess specific characteristic protein sequences (Additional file 5). The location of these characteristic sequences varies among different classes. They can lie immediately downstream the LOB domain (class IA), present at C-terminal regions (class IB and IC1/ID), or extend flanking the LOB domain (class IC2).
In seed plants, class IB LBD genes display prominent functions in regulating root development [6, 9, 10]. Previous study detected none of class IB LBD gene exists in Selaginella moellendorphii [1], leading to the assumption that genetic programs of root development in lycophytes are distinctive to the seed plants. In contrast, our method is sufficient to identify a basal-node LBD gene in S. moellendorphii (SmoeLBD007), which clusters with class IB genes with high support (aLRT = 95%) (Fig. 6). This ancient gene lineage is preserved in all of the land plant genomes analyzed, and its direct descendant in Arabidopsis is AtLBD20, which was demonstrated to participate in pathogen defense [28]. Realtime PCR shows SmoeLBD007 is mainly expressed in root and leaf tissues (Additional file 6), suggesting it is functional during these tissues development. Meanwhile, phylogenetic analysis identifies two LBD gene groups that are specific to seed plants. These gene groups contain some key regulators of lateral root development in angiosperms, including AtLBD16, AtLBD18 and AtLBD29 in Arabidopsis, OsLBD21/CRL1 in rice and ZmayLBD002/RTCS in maize [6, 9, 10]. Selection pressure analysis shows that the seed plant-specific gene lineages exhibit a higher nonsynonymous/synonymous substitution ratio (ka/ks, p-value = 0.0039) (Fig. 6), suggesting they accumulated more nonsynonymous amino acid changes during seed plants evolution. Therefore, although class IB LBD genes are present in S. moellendorphii, they are extensively duplicated in seed plants, and recruited to root regulations through sequence change and functional specialization.
Fig. 6.
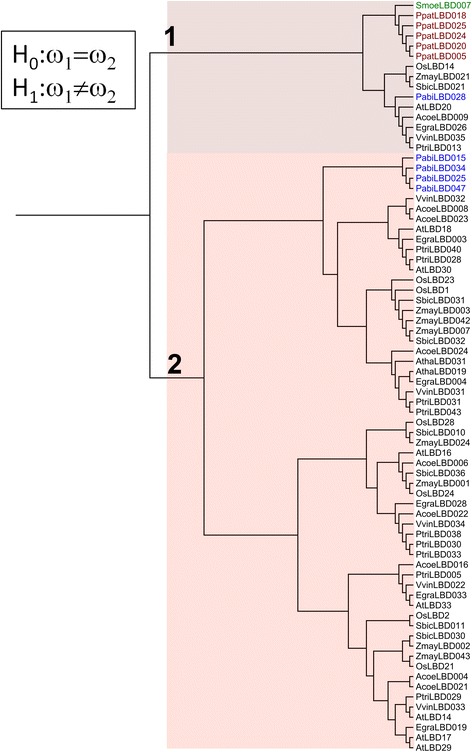
Selection pressure analysis of class IB LBD genes. Phylogenetic tree is constructed by maximum likelihood method. Genes of bryophytes, lycophytes, gymnosperms and angiosperms are indicated by red, green, blue and black color, respectively. Differences in selection pressure are modeled by specifying different ω ratios for ancestral lineages and seed plant-specific lineages. Two hypotheses of variable selection pressure are specified as H0 and H1. H0: ω1 = ω2 = 0.03395, l = -8133.64; H1: ω1 = 0.0220, ω2 = 0.0382, l = -8129.48; p-value = 0.0039 (df = 1)
Class IA is composed of two ancient lineages, namely IA-1 and IA-2, which give rise to AtLOB and AtLBD6/AS2 in Arabidopsis respectively (Fig. 4). Consistent with the independent gene ancestry, AtLOB and AtLBD6/AS2 exhibit divergent biological functions even with highly similar amino acid compositions [5]. Class IA-2 experienced a duplication event before angiosperm emergence and generates AtLBD6/AS2 lineage and AtLBD10 lineage. Sequence alignment identifies a “SKYQ” motif immediately downstream of the LOB domain in AtLBD6/AS2 orthologs (Additional file 7). In contrast, AtLBD10 orthologs contain a totally different sequence featured with “AAYIGP”. This phenomenon suggests protein sequence change is also accompanied with evolution of class IA LBD genes in angiosperms.
Class II subfamily contains two LBD gene ancestors before seed plant appearance. In Arabidopsis, AtLBD37, AtLBD38 and AtLBD39 are derived from a common ancestor (Fig. 4) and participate in a same biological process to repress anthocyanin biosynthesis and nitrogen responsive genes [8]. Through sequence alignment, we identify a featured pattern of LxLxL motif in these proteins (Additional file 7) which is required to recruit TPL/TPR co-repressors and fulfill transcriptional repression activity [29]. The LxLxL motif is also conserved in class II LBD genes of Physcomitrella, suggesting the ability to recruit TPL/TPR co-repressors has been acquired by early land plants.
Conclusions
In this study we present an improved method for LBD gene detection, and identify 431 LBD genes in 11 high-quality genomes of land plants. Through gene collinearity and phylogenetic analyses, we retrace 7 ancient LBD gene lineages in early land plants, which gave rise to 11 ancestor genes for seed plants and 18 ancestor genes for angiosperms through gene duplications. All of the ancient gene lineages are preserved by current genomes of land plant, including the previously controversial class IB gene in S. moellendorphii, suggesting LBD genes are extremely reluctant to be lost during evolution. On the other hand, whole-genome and dispersed gene duplications, accompanied with frequent protein sequence change and expression pattern alteration, account for the major expansions of LBD genes in angiosperms, which illustrates an important scheme for LBD gene family diversification.
Methods
Sequence retrieval
LBD protein sequences are retrieved from public genome databases, including Aquilegia coerulea (JGI v1.1); Arabidopsis thaliana (TAIR 10); Eucalyptus grandis (JGI v2.0); Oryza sativa subsp. japonica (MSU v7.0); Picea abies (ConGenIE v1.0); Physcomitrella patens subsp. patens (JGI v3.0); Populus trichocarpa (JGI v3.0); Sorghum bicolor (JGI v2.1); Selaginella moellendorffii (JGI v1.0); Vitis vinifera (Genoscope 12X); Zea mays (MaizeSequence Release 6a). The LOB domain (designated as DUF260 in the PFAM database) is searched to identify putative LBD proteins. Hmmsearch (HMMer package version3.1b1) is used to search the PFAM profile hidden Markov model (pHMM) DUF260.hmm (http://pfam.xfam.org/) [30] against protein sequences from each genome. To ensure the searching reliability, domain hits beyond the gathering threshold (E-value 1e-5) and less than 80% of the coverage are filtered out before downstream analysis. We also remove redundant protein sequences which are alternatively spliced from the same locus. LBD genes in Arabidopsis and maize were named according to previous studies, while gene sequences from other species were renamed for simplicity (Additional file 1).
Gene duplication events and collinearity relationship analysis
MCscanX is used to detect gene duplication types and collineartiy relationships [31]. For seven angiosperms (A. thaliana, E. grandis, P. trichocarpa, V. vinifera, O. sativa, S. bicolor and Z. mays), all annotated proteins in each genome were self-to-self compared by BLASTP (version 2.2.21) program with E-value 1e-10. The top 5 BLASTP hits of each gene were retained for downstream analysis of syntenic regions. The duplicate_gene_classifier program incorporated in MCscanX is used to identify different duplication types in a genome: whole genome duplication (collinear genes in syntenic blocks), tandem duplication (consecutive repeat genes), proximal duplication (genes spanning less than 20 genes in nearby chromosomal region) and dispersed duplication (other modes than whole genome, tandem and proximal duplications).
MCscanX is used to detect collinear blocks within dicot or monocot separately. Protein sequences of dicots (A. thaliana, E. grandis, P. trichocarpa and V. vinifera) or monocots (O. sativa, S. bicolor and Z. mays) were pooled independently to conduct self-self comparison by BLASTP (version 2.2.21) program with same parameters as described above (‘-e 1e-10 -b5 -v5 -m8’). PERL script detect_collinearity_within_gene_families.pl incorporated in MCscanX is used to detect collinearity within LBD gene family. LBD genes derived from collinear blocks are recursively merged into collinear groups using custom PERL script.
Phylogenetic analysis
To generate phylogenetic tree of LBD genes in land plants, the protein sequences of LBD genes are aligned to PFAM profile hidden Markov models of the LOB domain (pHMM DUF260.hmm) using HMMalign (HMMer package version 3.1b1) [30]. Terminal tails of non-aligned residues are trimmed using parameter ‘--trim’ and only unambiguous alignment of each sequence is subjected for subsequent phylogenetic analyses. The Jones, Taylor, and Thorton (JTT) model is selected as the best-fitting amino acid substitution model according to the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) scores estimated by ProtTest (v3.3) [32]. The maximum likelihood (ML) analysis is performed by the program PhyML (version 3.1) using the JTT model of amino acid substitution, four gamma-distributed rate categories and the Shimodaira-Hasegawa-like approximate likelihood-ratio test (SH-aLRT) [33]. Reliability of the internal branches is evaluated based on SH-aLRT supports. The tree is started from BIONJ tree and the topology of the tree is improved by subtree pruning and regrafting (SPR) method from 10 random starting trees. The output tree is visualized in the program Figtree (http://tree.bio.ed.ac.uk/software/figtree/).
To analyze class IA LBD genes, complete protein sequences were aligned with MUSCLE (v3.7) with default parameters [34]. Multiple sequence alignments were trimmed by removing poorly aligned regions using TRIMAL (v1.4.rev15) with the option ‘-automated1’ [35]. The ML analysis is performed by the program PhyML (version 3.1) using the JTT model of amino acid substitution, four gamma-distributed rate categories and SH-aLRT test. The tree is started from BIONJ tree and the topology of the tree is improved by SPR method from 10 random starting trees.
Sequence logos of LBD proteins
For each LBD class, MUSCLE (v3.7) was used to align the complete protein sequences with default parameters [34]. Nucleotide conservation was hereafter analyzed and shown with WebLogo (v3.3) (http://weblogo.threeplusone.com/).
Selection pressure analysis for class IB LBD genes
Selection pressure analysis is measured by ω parameter in PAML (v4.6) package [36], which is the nonsynonymous/synonymous substitution rate ratio. Two hypotheses of variable selection pressure are modeled as H0 and H1. While the null model (H0) assigns only one ω for the whole tree, the branch model (H1) assigns two independent ω values for two branches. Codeml program is used to obtain the log likelihood by performing multiple analyses with a range of initial values for the ω parameter. Significant likelihood ratio tests (LRTs) is used to conduct the significance of difference between two models by chi2 program.
Acknowledgments
Not applicable.
Declaration
This article has been published as part of BMC Genomics Volume 18 Supplement 1, 2016: Proceedings of the 27th International Conference on Genome Informatics: genomics. The full contents of the supplement are available online at http://bmcgenomics.biomedcentral.com/articles/supplements/volume-18-supplement-1.
Funding
This work is supported in part by grants from the National Key Basic Research Program of China (2012CB316501, 2013CB127005 and 2012CB114502), the National Natural Science Foundation of China (31571310, 31401128, 31271409 and 31130012), Ministry of Agriculture of China (2016ZX08010-002) and special Fund for strategic pilot technology Chinese Academy of Sciences (XDA08020104). Publication costs for this article were funded by the National Key Basic Research Program in China (Nos. 2012CB316501) and the National Natural Science Foundation of China (31130012).
Availability of data and material
The data sets supporting the results of this article are included within the article and its additional file.
Authors’ contributions
YK performed the data collection and analysis. PX designed the study and composed the manuscript. XJ and LC performed the realtime PCR analysis and participated in data analysis. XL and LL directed the research project and edited the manuscript. All the authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Additional files
LBD genes identified in this study. (PDF 19 kb)
Collinear LBD gene groups in dicots and monocots. (PDF 50 kb)
ML tree of LBD genes in land plants. (PDF 74 kb)
ML tree of class IA LBD genes. (TIF 1275 kb)
Sequence logo of LBD proteins in each class. (TIF 2487 kb)
Expression of class IB LBD gene in S. moellendorphii. (TIF 381 kb)
Representative motifs for class IA and class II genes. (TIF 4927 kb)
Contributor Information
Laigeng Li, Email: lgli@sibs.ac.cn.
Xuan Li, Email: lixuan@sippe.ac.cn.
References
- 1.Coudert Y, Dievart A, Droc G, Gantet P. ASL/LBD Phylogeny Suggests that Genetic Mechanisms of Root Initiation Downstream of Auxin Are Distinct in Lycophytes and Euphyllophytes. Mol Biol Evol. 2012;30(3):569–572. doi: 10.1093/molbev/mss250. [DOI] [PubMed] [Google Scholar]
- 2.Chanderbali AS, He F, Soltis PS, Soltis DE. Out of the Water: Origin and Diversification of the LBD Gene Family. Mol Biol Evol. 2015;32(8):1996–2000. doi: 10.1093/molbev/msv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwakawa H, Ueno Y, Semiarti E, Onouchi H, Kojima S, Tsukaya H, Hasebe M, Soma T, Ikezaki M, Machida C, et al. The ASYMMETRIC LEAVES2 Gene of Arabidopsis thaliana, Required for Formation of a Symmetric Flat Leaf Lamina, Encodes a Member of a Novel Family of Proteins Characterized by Cysteine Repeats and a Leucine Zipper. Plant Cell Physiol. 2002;43(5):467–478. doi: 10.1093/pcp/pcf077. [DOI] [PubMed] [Google Scholar]
- 4.Shuai B, Reynaga-Pena CG, Springer PS. The Lateral Organ Boundaries Gene Defines a Novel, Plant-Specific Gene Family. Plant Physiol. 2002;129(2):747–761. doi: 10.1104/pp.010926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumura Y, Iwakawa H, Machida Y, Machida C. Characterization of genes in the ASYMMETRIC LEAVES2/LATERAL ORGAN BOUNDARIES (AS2/LOB) family in Arabidopsis thaliana, and functional and molecular comparisons between AS2 and other family members. Plant J. 2009;58(3):525–537. doi: 10.1111/j.1365-313X.2009.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. ARF7 and ARF19 Regulate Lateral Root Formation via Direct Activation of LBD/ASL Genes in Arabidopsis. Plant Cell. 2007;19(1):118–130. doi: 10.1105/tpc.106.047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh SA, Park KS, Twell D, Park SK. The SIDECAR POLLEN gene encodes a microspore-specific LOB/AS2 domain protein required for the correct timing and orientation of asymmetric cell division. Plant J. 2010;64(5):839–850. doi: 10.1111/j.1365-313X.2010.04374.x. [DOI] [PubMed] [Google Scholar]
- 8.Rubin G, Tohge T, Matsuda F, Saito K, Scheible WR. Members of the LBD Family of Transcription Factors Repress Anthocyanin Synthesis and Affect Additional Nitrogen Responses in Arabidopsis. Plant Cell. 2009;21(11):3567–3584. doi: 10.1105/tpc.109.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M. Crown rootless1, Which Is Essential for Crown Root Formation in Rice, Is a Target of an AUXIN RESPONSE FACTOR in Auxin Signaling. Plant Cell. 2005;17(5):1387–1396. doi: 10.1105/tpc.105.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taramino G, Sauer M, Stauffer JL, Multani D, Niu X, Sakai H, Hochholdinger F. The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J. 2007;50(4):649–659. doi: 10.1111/j.1365-313X.2007.03075.x. [DOI] [PubMed] [Google Scholar]
- 11.Semiarti E, Ueno Y, Tsukaya H, Iwakawa H, Machida C, Machida Y. The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of meristem-related homeobox genes in leaves. Development. 2001;128(10):1771–1783. doi: 10.1242/dev.128.10.1771. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Xu Y, Dong A, Sun Y, Pi L, Huang H. Novel as1 and as2 defects in leaf adaxial-abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development. 2003;130(17):4097–4107. doi: 10.1242/dev.00622. [DOI] [PubMed] [Google Scholar]
- 13.Evans MMS. The indeterminate gametophyte1 Gene of Maize Encodes a LOB Domain Protein Required for Embryo Sac and Leaf Development. Plant Cell. 2007;19(1):46–62. doi: 10.1105/tpc.106.047506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phelps-Durr TL, Thomas J, Vahab P, Timmermans MC. Maize rough sheath2 and Its Arabidopsis Orthologue ASYMMETRIC LEAVES1 Interact with HIRA, a Predicted Histone Chaperone, to Maintain knox Gene Silencing and Determinacy during Organogenesis. Plant Cell. 2005;17(11):2886–2898. doi: 10.1105/tpc.105.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Yu X, Wu P. Comparison and evolution analysis of two rice subspecies LATERAL ORGAN BOUNDARIES domain gene family and their evolutionary characterization from Arabidopsis. Mol Phylogenet Evol. 2006;39(1):248–262. doi: 10.1016/j.ympev.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Zhang YM, Zhang SZ, Zheng CC. Genomewide analysis of LATERAL ORGAN BOUNDARIES Domain gene family in Zea mays. J Genet. 2014;93(1):79–91. doi: 10.1007/s12041-014-0342-7. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Zhang S, Su L, Liu X, Hao Y. A genome-wide analysis of the LBD (LATERAL ORGAN BOUNDARIES domain) gene family in Malus domestica with a functional characterization of MdLBD11. PLoS One. 2013;8(2):e57044. doi: 10.1371/journal.pone.0057044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrouk M, Murat F, Pont C, Messing J, Jackson S, Faraut T, Tannier E, Plomion C, Cooke R, Feuillet C. Palaeogenomics of plants: synteny-based modelling of extinct ancestors. Trends Plant Sci. 2010;15(9):479–487. doi: 10.1016/j.tplants.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud PF, Lindquist EA, Kamisugi Y, et al. The Physcomitrella Genome Reveals Evolutionary Insights into the Conquest of Land by Plants. Science. 2008;319(5859):64–69. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- 20.Tuskan GA, DiFazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A, et al. The Genome of Black Cottonwood, Populus trichocarpa (Torr. & Gray) Science. 2006;313(5793):1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- 21.Tang H, Wang X, Bowers JE, Ming R, Alam M, Paterson AH. Unraveling ancient hexaploidy through multiply-aligned angiosperm gene maps. Genome Res. 2008;18(12):1944–1954. doi: 10.1101/gr.080978.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergsten J. A review of long-branch attraction. Cladistics. 2005;21(2):163–193. doi: 10.1111/j.1096-0031.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- 23.Tang H, Bowers JE, Wang X, Ming R, Alam M, Paterson AH. Synteny and Collinearity in Plant Genomes. Science. 2008;320(5875):486–488. doi: 10.1126/science.1153917. [DOI] [PubMed] [Google Scholar]
- 24.Tang H, Bowers JE, Wang X, Paterson AH. Angiosperm genome comparisons reveal early polyploidy in the monocot lineage. Proc Natl Acad Sci U S A. 2009;107(1):472–477. doi: 10.1073/pnas.0908007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Qi M, Liu J, Zhang Y. CARMO: a comprehensive annotation platform for functional exploration of rice multi-omics data. Plant J. 2015;83(2):359–374. doi: 10.1111/tpj.12894. [DOI] [PubMed] [Google Scholar]
- 26.Chalfun-Junior A, Franken J, Mes JJ, Marsch-Martinez N, Pereira A, Angenent GC. ASYMMETRIC LEAVES2-LIKE1 gene, a member of the AS2/LOB family, controls proximal-distal patterning in Arabidopsis petals. Plant Mol Biol. 2005;57(4):559–575. doi: 10.1007/s11103-005-0698-4. [DOI] [PubMed] [Google Scholar]
- 27.Mangeon A, Lin W-c, Springer PS. Functional divergence in the Arabidopsis LOB-domain gene family. Plant Signal Behav. 2012;7(12):1544–1547. doi: 10.4161/psb.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thatcher LF, Powell JJ, Aitken EAB, Kazan K, Manners JM. The Lateral Organ Boundaries Domain Transcription Factor LBD20 Functions in Fusarium Wilt Susceptibility and Jasmonate Signaling in Arabidopsis. Plant Physiol. 2012;160(1):407–418. doi: 10.1104/pp.112.199067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagale S, Links MG, Rozwadowski K. Genome-wide analysis of ethylene-responsive element binding factor-associated amphiphilic repression motif-containing transcriptional regulators in Arabidopsis. Plant Physiol. 2010;152(3):1109–1134. doi: 10.1104/pp.109.151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eddy SR. Profile hidden Markov models. Bioinformatics. 1998;14(9):755–763. doi: 10.1093/bioinformatics/14.9.755. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27(8):1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 34.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25(15):1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Z. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol Biol Evol. 2007;24(8):1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]


