Abstract
Two thiophene-based monocyclic receptors L1 and L2 have been studied for phosphate binding in solutions (D2O and DMSO-d6) by 1H NMR and 31P NMR titrations, and in the solid state by single crystal X-ray analysis. Results from 1H NMR titrations suggest that the ligands bind phosphate anions in a 1:2 binding mode in DMSO-d6, with the binding constants of 5.25 and 4.20 (in log K), respectively. The binding of phosphate to L1 and L2 was further supported by 31P NMR in D2O at pH = 5.2. The crystal structure of the phosphate complex of L1 reveals unambiguous proof for the formation of a ditopic complex via multiple hydrogen bonds from NH···O and CH···O interactions.
Keywords: Azamacrocycle, Ditopic complex, Anion binding, Phosphate complex
Phosphate is a key building block of nucleic acids, playing critical roles in many biochemical processes [1]. The translocation of phosphate between DNA and proteins in living cells is an essential step in the regulation of metabolic processes [2]. Energy production and storage processes within the body are regulated by phosphorylated compounds, such as adenosine triphosphate [3]. It is known that the phosphate level in serum and saliva is linked to several diseases including hyperparathyroidism, vitamin D deficiency and Fanconi syndrome [4]. It is also widely used in fertilizer and drug-related industries [5]. Because of its significant roles in environment, healthcare and biochemical applications, molecular recognition of phosphate by synthetic molecules is a growing area of current research in supramolecular chemistry [6]. However, phosphate binding in water is unfavorable due to its high free energy of hydration (ΔG0 = −465 J/mol) [7]. Polyamine-based receptors that are water soluble, are often used to bind phosphates in water over a wide range of pH [8]. For examples, Martell and coworkers synthesized hexaazamacrocyclic ligands and used them to encapsulate a pyrophosphate via four hydrogen bonds [9]. A hexaprotonated 26-membered polyammonium macrocycle reported by Bowman-James and coworkers was shown to form a complex with six di-hydrogen phosphate anions and two neutral phosphoric acid molecules, illustrating dipotic behavior of the receptor [10]. Bianchi, García-España, Paoletti and coworkers reported a tetraprotonated macrocycle [18]aneN6 that binds two pyrophosphate anions via NH···O and CH···O bonds [11]. Lu and coworkers showed that an octa-protonated p-xylyl-based cryptand encapsulated a phosphate anion via multiple hydrogen bonds [12]. Other reported receptors that are neutral molecules including amides [13], thioamides [14], ureas [15], thioureas [16], pyrroles [17] and indoles [18] were shown to bind phosphate in organic solvents. Our recent studies on various macrocycle-based receptors indicated that hydrogen bonding and electrostatic interactions are major binding forces in stabilizing complexes; thus providing insight into the binding modes of anions and conformations of host molecules [19-21]. Herein, we report the binding aspects of two thiophene-based macrocycles L1 and L2 via 1H NMR and 31P NMR in solutions. We also report the structural characterization of a phosphate complex with L1, forming a ditopic complex with one dihydrogen phosphate and one monohydrogen phosphate.
The hexamine ligands L1 and L2 were synthesized through Schiff-base condensation reaction (Scheme 1) of the corresponding dipodal amine with two equivalents of 2,5-thiophendicarboxaldehyde followed by the reduction with NaBH4, as reported earlier [20-22]. The tosylate salts were prepared by reacting the free ligands with p-toluenesulfonic acid in methanol. The analysis of 1H NMR data suggested the formation of an adduct with six tosylate groups providing six positive charges on the macrocyclic moiety. The phosphate complex was synthesized by the addition of a few drops of aqueous phosphoric acid to L1 dissolved in methanol. Crystals suitable for X-ray analysis were grown from the slow evaporation of the solution at room temperature.
Scheme 1.
Synthetic scheme for the macrocycles L1 and L2: Reaction conditions: (i) methanol, 0 °C, 24 hr stirring; (ii) NaBH4, methanol, overnight stirring.
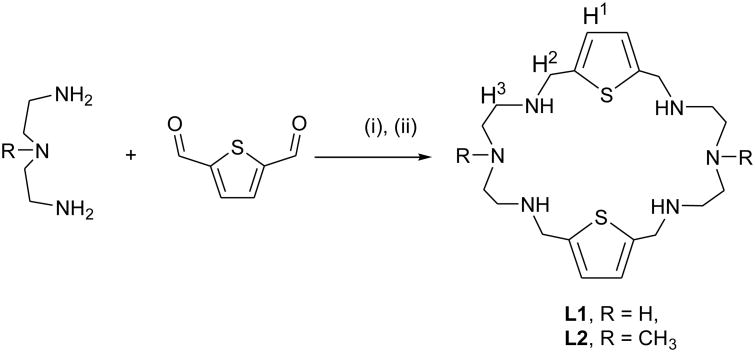
1H NMR titrations of [H6L1]6+ and [H6L2]6+ were performed to study the binding interactions with phosphate using n-Bu4N+H2PO4− (TBAH2PO4) in D2O as well as in DMSO-d6. The incremental addition of the anion (20 mM) to [H6L1]6+ (2 mM) in D2O (pH = 5.2) resulted in a downfield shift of aliphatic protons. The non-linear regression analysis of the shift change of several independent protons of [H6L1]6+ showed moderate binding for phosphate (log K = 2.0 M−1), providing a good fit of a 1:1 binding model [23]. However, under identical conditions, there was no significant change in any protons of [H6L2]6+ upon the addition of TBAH2PO4 to the host solution, indicating weak host-guest interactions in D2O. We, therefore, proceeded to investigate the binding properties of [H6L1]6+ and [H6L2]6+ in DMSO-d6. Figure 1 displays the stacking of 1H NMR titration spectra of [H6L1]6+ obtained after the increasing amount of phosphate anion (ranging 0 to 10 equivalents), showing a gradual upfield shift of both the aromatic and the aliphatic protons of the macrocyclic moiety at room temperature. The changes in the chemical shift of the aromatic proton (ArH) as a function of the anion concentration are displayed in Figure 1. The non-linear regression analysis of the changes in the chemical shift of the ligand as recorded with an increasing amount of anionic solution provided the best fit for a 1:2 binding model [24]. The ligand [H6L2]6+ also shows a similar binding trend with phosphate in DMSO-d6. Association constants presented in Table 1 suggest that the binding process of each ligand involves the formation of both a 1:1 (ligand:anion) complex and a 1:2 (ligand:anion) complex. However, a 1:2 complex is stronger than a 1:1 complex in DMSO-d6, supporting the X-ray structure (discussed later). A similar binding mode was previously reported for phosphate with [26]aneN6C6 [10]. As shown in Table 1, the overall binding constant of L1 for phosphate in DMSO-d6 is slightly higher than that of L2 which could be due to the reduced hydrogen bonding ability of the methylated compound.
Figure 1.
(a) 1H NMR spectra of [H6L1] (Ts)6 (2 mM) with an increasing amount of TBAH2PO4 (20 mM) in DMSO-d6. (b) Titration curve of [H6L1] (Ts)6 with TBAH2PO4 showing the change in the chemical shift of Ar-H (H1) against an increasing concentration of phosphate in DMSO-d6.
Table 1.
Association constants of the ligands for phosphate as determined by 1H NMR titrations.a
| Ligand | Log K | |
|---|---|---|
| D2O | DMSO-d6 | |
| L1 | 2.0 (1:1) | 1.54 (1:1) 5.25 (1:2) |
| L2 | b | 1.92 (1:1) 4.20 (1:2) |
Estimated error was less than 15%
No significant NMR shift was observed.
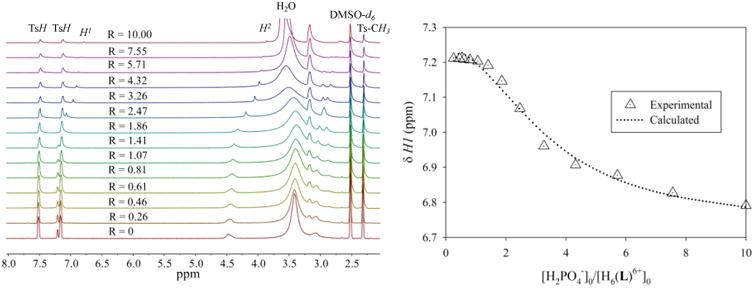
The interaction of [H6L1]6+ and [H6L2]6+ with TBAH2PO4 in D2O was also investigated by phosphorus 31P NMR at room temperature. Because of the lower sensitivity of 31P NMR compared to 1H NMR, a higher concentration of TBAH2PO4 (10 mM) was loaded to an NMR tube, and a host solution (25 mM) was prepared as a titrant. The 31P resonance was calibrated against an aqueous phosphoric acid used in a sealed capillary tube. Figure 2 shows the 31P NMR spectra of TBAH2PO4 (10 mM) after the addition of two equivalents of [H6L1]6+ and [H6L2]6+ in D2O. As clearly shown in Figure 2a, the signal at δP = 0.218 ppm for the free TBAH2PO4 shifts upfield to δP = −1.635 ppm (ΔδP = 1.853 ppm) after the addition of [H6L1]6+ (2 equivalents). The other ligand [H6L2]6+ also shows similar shifting to δP = −1.404 ppm but to a lesser extent (ΔδP = 1.622 ppm), indicating a lower affinity for the phosphate anion than that with [H6L1]6+. This upfield shift suggest the formation of the phosphate complex due to the strong electrostatic interactions, where the bound phosphate is shielded by the interacting macrocycle. Similar upfield shifts of phosphorus resonance were reported due to the complexation by polyamine-based hosts [12,25].
Figure 2.
31P NMR spectra of n-Bu4N+H2PO4− in D2O recorded at room temperature (a) free TBAH2PO4 (δP = 0.218 ppm) (10 mM) (b) TBAH2PO4 + 2 equivalents of [H6L2] (Ts)6 (δP = − 1.404 ppm) and (c) TBAH2PO4 + 2 equivalents of [H6L1] (Ts)6 (δP = − 1.6357 ppm)
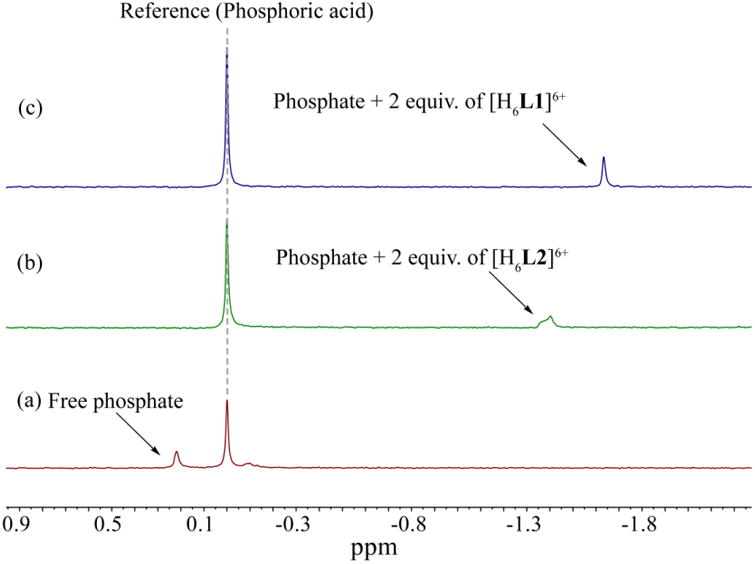
The phosphate complex of L1 was obtained by reacting the free macrocycle with phosphoric acid. Crystals suitable for X-ray analysis were obtained by recrystallization of the salt in methanol/water. Crystallographic data are presented in Table 2. The structural analysis of the phosphate complex reveals that the salt crystallizes in the triclinic space group (P1) and the asymmetric unit consists of two hexaprotonated macrocycles, three monohydrogen phosphate (HPO42−), six di-hydrogen phosphate (H2PO4−) and eight water molecules to yield a molecular formula of 2(C20H40N6S2)6+·3(HPO4)2−·6(H2PO4)−·8(H2O). One hydrogen phosphate (P9) is disordered about 90:10 into two orientations. One water molecule (O8W) is also found to be disordered about 80:20. Each macrocycle is fully protonated and the total positive charges (12+) from the two hexaprotonated receptors are balanced by the negative charges provided by three doubly-charged monohydrogen phosphates and six singly-charged dihydrogen phosphate anions.
As shown in Figure 3, two identical macrocycles in the asymmetric unit possess a non-crystallographic inversion center, forming a sandwich type complex with four bound phosphate groups and two water molecules. Two water molecules serve as linkers to macrocycles as well as to bound dihydrogen phosphates locating between the macrocycles (Figure 3a). Each macrocycle adopts the shape of a flat ellipsoid and binds two phosphate groups; one is monohydrogen phosphate and the other is dihydrogen phosphate, located above and below the elliptical plane. The complex is stabilized through electrostatic interactions and multiple H-bonds. A list of H-bond distances with bond angles are shown in Table 3. Figure 3c shows one macrocycle of the asymmetric unit, with two bound phosphates held by hydrogen bonding interactions with the macrocyclic cation. The monohydrogen phosphate (P76) is held by two NH···O (2.684 and 2.747 Å) and two CH···O (3.266 and 3.388 Å) bonds, while the dihydrogen phosphate interacts through two strong NH···O bonds (N15H···O70 = 2.932 and N15H···O73 = 2.677 Å) and one weak NH···O bonds (N39H···O80 = 3.266 Å). As a result, the monohydrogen phosphate is closer to the cavity than the dihydrogen phosphate. The involvement of CH···O interactions is well documented in the literature [21]. Extensive hydrogen bonds between the macrocycle and water molecules are also present.
Figure 3.
Top: crystal structure of the phosphate complex showing [(H6L1)2·(HPO4)2·(H2PO4)2·(H2O)2]6+ moiety in capped-stick view (a), and space filling view (b). Bottom: crystal structure of the phosphate complex showing [(H6L1)·(HPO4)·(H2PO4)]3+ moiety in capped-stick view (c), and space filling view (d).
In conclusion, we report the phosphate binding properties of two simple thiophene-based monocyclic receptors L1 and L2 by 1H NMR and 31P NMR studies in two different polar solvents (D2O and DMSO-d6). Both ligands show lessened interactions with the anion in competitive D2O due to their weak hydrogen bonding ability in the highly polar solvent. However, after switching the solvent from D2O to DMSO-d6, the binding affinity is significantly increased, exhibiting the formation of 1:2 complexes. The 31P NMR has been successfully used as a probe to identify the chemical environment of bound and free phosphates in D2O. Structural characterization of the phosphate complex with L1 suggests that both NH···O and CH···O interactions play roles in stabilizing the host-guest complex.
Supplementary Material
Acknowledgments
The National Science Foundation is acknowledged for a CAREER award (CHE-1056927) to MAH. This research was supported by the National Institutes of Health (G12MD007581). R. B. was supported by NSF REU site (CHE 1156111) for an undergraduate research at Jackson State University. M. A. Saaed is thanked for assisting summer research of R. B. The authors thank the Louisiana Board of Regents for funds to upgrade the diffractometer used in this work (LEQSF(2011-2012)-ENH-TR-01).
Footnotes
Appendix A. Supplementary material CCDC 1035333 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_ request/cif. Supplementary data associated with this article can be found, in the online version, at doi: ???.
References
- 1.(a) Mason CF. Biology of Freshwater Pollution. Longman; New York: 1991. [Google Scholar]; (b) Liebschner D, Elias M, Moniot S, Fournier B, Scott K, Jelsch C, Guillot B, Lecomte C, Chabrière E. J Am Chem Soc. 2009;131:7879–7886. doi: 10.1021/ja901900y. [DOI] [PubMed] [Google Scholar]
- 2.Smith E, Morowitz H. Proc Natl Acad Sci USA. 2004;101:13168–73. doi: 10.1073/pnas.0404922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kivlehan F, Mace WJ, Moynihan HA, Arrigan DWM. Analytica Chimica Acta. 2007;585:154–160. doi: 10.1016/j.aca.2006.11.078. [DOI] [PubMed] [Google Scholar]
- 4.Berchmans S, Issa TB, Singh P. Analytica Chimica Acta. 2012;729:7–20. doi: 10.1016/j.aca.2012.03.060. [DOI] [PubMed] [Google Scholar]
- 5.Tiessen H. Phosphorus in the Global Environment: Transfers, Cycles, and Management. Vol. 1995 Wiley; New York: [Google Scholar]
- 6.(a) Hargrove AE, Nieto S, Zhang T, Sessler JL, Anslyn EV. Chem Rev. 2011;111:6603–6782. doi: 10.1021/cr100242s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tobey SL, Anslyn EV. J Am Chem Soc. 2003;125:14807–14815. doi: 10.1021/ja030507k. [DOI] [PubMed] [Google Scholar]; (c) Barker JE, Liu Y, Martin ND, Ren T. J Am Chem Soc. 2003;125:13332–13333. doi: 10.1021/ja036407w. [DOI] [PubMed] [Google Scholar]; (d) Iverson BL, Shreder K, Kral V, Sessler JL. J Am Chem Soc. 1993;115:11022–11023. [Google Scholar]; (e) Arranz P, Bianchi A, Cuesta R, Giorgi C, Godino ML, Gutiérrez MD, López R, Santiago A. Inorg Chem. 2012;51:4883–4883. doi: 10.1021/ic100929f. [DOI] [PubMed] [Google Scholar]; (f) Murugavel R, Kuppuswamy S, Randoll S. Inorg Chem. 2008;47:6028–6039. doi: 10.1021/ic8003239. [DOI] [PubMed] [Google Scholar]; (g) Barker JE, Liu Y, Yee GT, Chen WZ, Wang G, Rivera VM, Tong R. Inorg Chem. 2006;45:7973–7980. doi: 10.1021/ic0611140. [DOI] [PubMed] [Google Scholar]; (h) Warden AC, Warren M, Hearn MTW, Spiccia L. Inorg Chem. 2004;43:6936–6943. doi: 10.1021/ic049633v. [DOI] [PubMed] [Google Scholar]; (i) Harris WR, Wang Z, Hamada YZ. Inorg Chem. 2003;42:3262–3273. doi: 10.1021/ic026027w. [DOI] [PubMed] [Google Scholar]; (j) Jurček O, Cametti M, Pontini M, Kolehmainen E, Rissanen K. Org Biomol Chem. 2013;11:4585–4590. doi: 10.1039/c3ob40724a. [DOI] [PubMed] [Google Scholar]; (k) Arranz-Mascarós P, Bazzicalupi C, Bianchi A, Giorgi C, Godino-Salido ML, Gutiérrez-Valero MD, Lopez-Garzón R, Valtancoli B. New J Chem. 2011;35:1883–1891. [Google Scholar]; (l) Rajbanshi A, Wan S, Custelcean R. Cryst Growth Des. 2013;13:2233–2237. [Google Scholar]; (k) Gavette JV, Mills NS, Zakharov LN, Johnson CA, Johnson DW, Haley MM. Angew Chem. 2013;125:10460–10464. doi: 10.1002/anie.201302929. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Law al AT, Adeloju SB. Talanta. 2013;114:191–203. doi: 10.1016/j.talanta.2013.03.031. [DOI] [PubMed] [Google Scholar]; (l) Liu J, Wu K, Li X, Han Y, Xia M. RSC Adv. 2013;3:8924–8928. [Google Scholar]
- 7.Blades AT, Ho Y, Kebarle P. J Phys Chem. 1996;100:2443–2446. [Google Scholar]
- 8.García-España E, Díaz P, Llinares JM, Bianchi A. Coord Chem Rev. 2006;250:2952–2988. [Google Scholar]
- 9.Nation DA, Reibenspies J, Martell AE. Inorg Chem. 1996;35:4597–4603. [Google Scholar]
- 10.Gerasimchuk OA, Mason S, Llinares JM, Song M, Alcock NW, Bowman-James K. Inorg Chem. 2000;39:1371–1375. doi: 10.1021/ic9911116. [DOI] [PubMed] [Google Scholar]
- 11.Bazzicalupi C, Bencini A, Bianchi A, Cecchi M, Escuder B, Fusi V, Garcia-España E, Giorgi C, Luis SV, Maccagni G, Marcelino V, Paoletti P, Valtancoli B. J Am Chem Soc. 1999;121:6807–6815. [Google Scholar]
- 12.Yang LZ, Li Y, Jiang L, Feng XL, Lu TB. CrystEngComm. 2009;11:2375–2380. [Google Scholar]
- 13.Bondy CR, Loeb SJ. Coord Chem Rev. 2003;240:77–99. [Google Scholar]
- 14.(a) Hossain MA, Kang SK, Llinares LM, Powell D, Bowman-James K. Inorg Chem. 2003;42:5043–5045. doi: 10.1021/ic034735r. [DOI] [PubMed] [Google Scholar]; (b) Inoue Y, Kanbara T, Yamamoto T. Tetrahedron Lett. 2003;44:5167–5169. [Google Scholar]
- 15.(a) Custelcean R, Moyer BA, Hay BP. Chem Commun. 2005:5971–5973. doi: 10.1039/b511809c. [DOI] [PubMed] [Google Scholar]; (b) Ravikumar I, Lakshminarayanan PS, Arunachalam M, Suresh E, Ghosh P. Dalton Trans. 2009:4160–4168. doi: 10.1039/b820322a. [DOI] [PubMed] [Google Scholar]; (c) Bell KJ, Westra AN, Warr RJ, Chartres J, Ellis R, Tong CC, Blake AJ, Tasker PA, Schroder M. Angew Chem Int Ed. 2008;47:1745–1748. doi: 10.1002/anie.200703272. [DOI] [PubMed] [Google Scholar]; (d) Busschaert N, Wenzel M, Light ME, Iglesias-Hernandez P, Perez-Tomas R, Gale PA. Am J Chem Soc. 2011;133:14136–14148. doi: 10.1021/ja205884y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Bose P, Dutta R, Ghosh P. Org Biomol Chem. 2013;11:4581–4584. doi: 10.1039/c3ob41071d. [DOI] [PubMed] [Google Scholar]; (f) Basu A, Das G. J Org Chem. 2014;79:2647–2656. doi: 10.1021/jo500102e. [DOI] [PubMed] [Google Scholar]; (g) Pramanik A, Powell DR, Wong BM, Hossain MA. Inorg Chem. 2012;51:4274–4284. doi: 10.1021/ic202747q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Khansari ME, Wallace KD, Hossain MA. Tetrahedron Lett. 2014:438–440. [Google Scholar]; (b) Bose P, Dutta R, Santra S, Chowdhury B, Ghosh P. Eur Inog J Chem. 2012:5791–5801. [Google Scholar]
- 17.(a) Sessler JL, Cho DG, Stepien M, Lynch V, Waluk J, Yoon ZS, Kim D. J Am Chem Soc. 2006;128:12640–12641. doi: 10.1021/ja064675z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Katayev EA, Sessler JL, Khrustalev VN, Ustynyuk YA. J Org Chem. 2007;72:7244–7252. doi: 10.1021/jo071106g. [DOI] [PubMed] [Google Scholar]
- 18.(a) Jiang QQ, Darhkijav B, Liu H, Wang F, Li Z, Jiang YB. Chem An Asian J. 2010;5:543–549. doi: 10.1002/asia.200900519. [DOI] [PubMed] [Google Scholar]; (b) Liu WX, Yang R, Li AF, Li Z, Gao YF, Luo XX, Ruan YB, Jiang YB. Org Biomol Chem. 2009;7:4021–4028. doi: 10.1039/b910255h. [DOI] [PubMed] [Google Scholar]
- 19.(a) Gibson D, Dey KR, Fronczek FR, Hossain MA. Tetrahedron Lett. 2009;50:6537–6539. doi: 10.1016/j.tetlet.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Saeed MA, Wong BM, Fronczek FR, Venkatraman R, Hossain MA. Cryst Growth Des. 2010;10:1486–1488. doi: 10.1021/cg100161a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mendy JS, Saeed MA, Fronczek FR, Powell DR, Hossain MA. Inorg Chem. 2010;49:7223–7225. doi: 10.1021/ic100686m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saeed MA, Thompson JJ, Fronczek FR, Hossain MA. CrystEngComm. 2010;12:674–676. doi: 10.1039/b918152k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Mendy JS, Pilate M, Horne T, Day VW, Hossain MA. Chem Commun. 2010;46:6084–6086. doi: 10.1039/c0cc01699c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Saeed MA, Fronczek FR, Powell DR, Hossain MA. Tetrahedron Lett. 2010;51:4233–4236. doi: 10.1016/j.tetlet.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rhaman MM, Ahmed L, Wang J, Powell DR, Leszczynski J, Hossain MA. Org Biomol Chem. 2014;12:2045–2048. doi: 10.1039/c4ob00116h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saeed MA, Pramanik A, Hossain MA. Inorg Chem Commun. 2012;21:32–24. doi: 10.1016/j.inoche.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider HJ, Kramer R, Simova S, Schneider U. J Am Chem Soc. 1988;110:6442–6448. [Google Scholar]
- 24.Hynes MJ. J Chem Soc Dalton Trans. 1993:311–312. [Google Scholar]
- 25.Hossain MA, Işıklan M, Pramanik A, Saeed MA, Fronczek FR. Cryst Growth Des. 2012;12:567–571. doi: 10.1021/cg201464k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.