Abstract
Background: Antiretroviral therapy (ART) poses challenging drug-drug interactions with immunosuppressant agents in transplant recipients. We aimed to determine the impact of specific antiretroviral regimens in clinical outcomes of HIV + kidney transplant recipients. Methods: A single-center, retrospective cohort study was conducted at a large academic center. Subjects included 58 HIV - to HIV + adult, first-time kidney transplant patients. The main intervention was ART regimen used after transplantation. The main outcomes assessed at one- and three-years were: patient survival, death-censored graft survival, and biopsy-proven acute rejection; we also assessed serious infections within the first six months post-transplant. Results: Patient and graft survival at three years were both 90% for the entire cohort. Patients receiving protease inhibitor (PI)-containing regimens had lower patient survival at one and three years than patients receiving PI-sparing regimens: 85% vs. 100% ( p=0.06) and 82% vs. 100% ( p=0.03), respectively. Patients who received PI-containing regimens had twelve times higher odds of death at 3 years compared to patients who were not exposed to PIs (odds ratio, 12.05; 95% confidence interval, 1.31-1602; p=0.02). Three-year death-censored graft survival was lower in patients receiving PI vs. patients on PI-sparing regimens (82 vs 100%, p=0.03). Patients receiving integrase strand transfer inhibitors-containing regimens had higher 3-year graft survival. There were no differences in the incidence of acute rejection by ART regimen. Individuals receiving PIs had a higher incidence of serious infections compared to those on PI-sparing regimens (39 vs. 8%, p=0.01). Conclusions: PI-containing ART regimens are associated with adverse outcomes in HIV + kidney transplant recipients.
Keywords: HIV, kidney transplant, protease inhibitor, antiretroviral therapy, infection
Introduction
More than 500 kidney transplants in human immunodeficiency virus–infected (HIV +) recipients have been performed in the United States with acceptable outcomes 1– 5. HIV infection is associated with a two- to three-fold increase in the risk of rejection 3. Reduced exposure to immunosuppressive agents is considered the main mechanism for increased predisposition to rejection 3, 6, 7.
Drug-drug interactions between antiretroviral therapy (ART) and calcineurin inhibitors (CNI), such as tacrolimus, pose a significant clinical challenge. Protease inhibitors (PI) and cobicistat increase the levels of CNI, whereas nonnucleoside reverse transcriptase inhibitors (NNRTI) reduce the levels of these agents. In contrast to PI and NNRTI, integrase strand transfer inhibitors (INSTI), which are not a substrate of CYP450, have become the preferred antiretroviral in many centers to overcome the problematic pharmacokinetic interactions 6– 9.
Although tenofovir disoproxil fumarate (TDF) has a good safety profile and is recommended as a first-line agent 10, it can cause renal tubular dysfunction in HIV + individuals 11 and tenofovir-related nephrotoxicity is always a concern in kidney transplant recipients.
Data on the impact of specific ART regimens on the clinical outcomes of HIV + kidney transplant recipients is scarce. In the present study, we compared post-transplant outcomes by ART regimens in a group of 58 HIV + kidney recipients transplanted at our institution over a 9-year period.
Methods
Study subjects
A single-center, retrospective cohort study of 58 consecutive HIV - to HIV + adult, first-time kidney transplants performed in the Miami Transplant Institute affiliated to Jackson Memorial Hospital, a 1,550-bed academic medical center, between October 2006 and October 2015. All HIV + recipients had an undetectable viral load, and all but one (a kidney-liver recipient) had a CD4 count > 200 cells/mm 3 at the time of the transplant. The study was approved by the University of Miami institutional review board (#20150614). Written consent was waived by the institutional review board due to the retrospective observational nature of the study.
Immunosuppression protocol
Immunosuppression and antimicrobial prophylaxis protocols at our center have been previously described 4, 5.
Clinical outcomes
The one- and three-year outcomes assessed were: patient survival, death-censored graft survival, and biopsy-proven acute rejection; we also assessed serious infections within the first six months post-transplant, defined as infections requiring admission to the intensive care unit during initial transplant hospitalization or re-admission to the hospital after discharge 4.
Statistics
The Fisher exact test and Wilcoxon Mann–Whitney U test were used where appropriate. Univariate analyses were performed using logistic regression with penalized likelihood estimation. Multivariable models were not pursued due to small number of events. Log-rank test was used to assess differences in time-to-event. Statistical analyses were performed using SAS University Edition (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
A total of 58 HIV + adult kidney allograft recipients were studied ( Table 1). In total, 51 subjects had at least one HIV viral load during the first year post-transplant, and except for six patients who had transient “blips” in viremia (median peak viremia, 130 copies/mL [IQR, 114–193]), all the patients had sustained ART-induced HIV viral load suppression (<50 copies/mL) post-transplant.
Table 1. Baseline characteristics of study participants *.
| Variable | All patients
n=58 (%) |
Protease inhibitor | P-value | |
|---|---|---|---|---|
| PI-sparing regimen
n=25 (%) |
PI-containing regimen
n=33 (%) |
|||
| Demographics | ||||
| Age, median (IQR) | 48 (43-54) | 49 (43-55) | 47 (42-49) | 0.13 |
| Age, older than 40 | 45 (78) | 20 (80) | 25 (76) | 0.70 |
| Male gender | 38 (66) | 15 (60) | 23 (70) | 0.44 |
| African-American | 43 (74) | 20 (80) | 23 (70) | 0.37 |
| HIV infection | ||||
| Pre-transplant CD4 count
<350 cells/mm 3 |
16 (28) | 7 (28) | 9 (27) | 0.95 |
| Pre-transplant CD4 count,
cells/mm 3, median (IQR) |
504 (351-666) | 441 (362-648) | 579 (346-666) | 0.54 |
| Pre-transplant CD4/CD8
ratio, median (IQR) |
0.7 (0.6-1) | 0.7 (0.6-0.8) | 0.7 (0.5-1.1) | 0.67 |
| Time from HIV diagnosis,
years, median (IQR) |
10 (5-16) | 12 (7-17) | 10 (5-15) | 0.24 |
| Comorbidities | ||||
| Hepatitis C | 7 (12) | 1 (4) | 6 (18) | 0.13 |
| Diabetes mellitus | 11 (19) | 8 (33) | 3 (9) | 0.04 |
| Hypertension | 38 (66) | 19 (58) | 19 (76) | 0.14 |
| HIVAN | 41 (72) | 17 (71) | 24 (73) | 0.87 |
| Overweight (BMI >25) | 28 (48) | 13 (52) | 15 (45) | 0.79 |
| Immunosuppression † | ||||
| Prednisone | 52 (90) | 24 (96) | 28 (85) | 0.22 |
| IVIG | 5 (9) | 0 | 5 (15) | 0.06 |
| Rituximab | 4 (7) | 1 (4) | 3 (9) | 0.63 |
| Tacrolimus | 57 (98) | 25 (100) | 32 (97) | >0.99 |
| MMF | 57 (98) | 25 (100) | 32 (97) | >0.99 |
| Sirolimus | 3 (5) | 0 | 3 (9) | 0.25 |
| Cyclosporine | 2 (3) | 2 (8) | 0 | 0.18 |
| Kidney allograft | ||||
| Post-transplant follow-up,
years, median (IQR) |
1.8 (0.9-4.2) | 1.7 (1-4.8) | 1.9 (0.9-3.5) | 0.40 |
| Transplant year 2006–2010 | 34 (59) | 16 (64) | 18 (55) | 0.59 |
| Living donor | 14 (24) | 6 (24) | 8 (24) | 0.98 |
| Donor age, median (IQR) | 39 (28-47) | 45 (29-47) | 37 (21-47) | 0.42 |
| Delayed graft function | 6 (11) | 1 (4) | 5 (15) | 0.38 |
| Cold ischemia time, >36h Ϯ | 9 (19) | 3 (13) | 6 (24) | 0.47 |
| HLA-ABDR mismatches, >5 Ϯ | 21 (39) | 8 (38) | 13 (39) | >0.99 |
| ABC-PRA, <5% | 48 (92) | 20 (91) | 28 (93) | >0.99 |
| DR-PRA, <5% | 49 (92) | 19 (86) | 30 (97) | 0.30 |
| CMV viremia Ϯ, >500 copies/mL | 2 (4) | 0 | 2 (6) | 0.51 |
| BK viremia ˄, >10,000 copies/mL | 5 (10) | 3 (14) | 2 (7) | 0.65 |
BMI, body mass index; CMV, cytomegalovirus; HIV, human immunodeficiency virus; HIVAN, HIV-associated nephropathy; IQR, interquartile range; IVIG, Intravenous immunoglobulin; MMF, mycophenolate mofetil; PRA, panel reactive antibody. PI, protease inhibitor.
*Data presented as absolute number (percentage), unless specified otherwise. The p-value corresponds to comparison of PI-containing and PI-sparing groups by using the Fisher exact test. Wilcoxon Mann–Whitney test was used for variables presented as median and IQR.
†All of the patients received anti–thymocyte globulin, basiliximab and methylprednisolone for induction.
ϮCold ischemia time and HLA-mismatch data available for 47 and 54 patients, respectively.
ϮDuring first year post-transplant.
Antiretroviral therapy
There were no ART restrictions in transplant eligibility for HIV + candidates during the study period. The three most common regimens post-transplant were nucleoside reverse transcriptase inhibitors (NRTI) plus PI, NRTI plus INSTI, and NRTI plus NNRTI ( Table 2).
Table 2. Distribution of ART regimens among 58 HIV + kidney transplant recipients.
| ART regimen | Pre-transplant | Post-transplant † | Post-transplant
(12 months) |
|---|---|---|---|
| n (%) | n (%) * | n (%) º | |
| Single drug class combination | |||
| NRTI | 1 (2) | 1 (2) | 1 (2) |
| Two drug class combination | |||
| NRTI + PI | 30 (52) | 23 (40) | 11 (27) |
| NRTI + INSTI | 2 (3) | 12 (21) | 8 (20) |
| NRTI + NNRTI | 15 (26) | 9 (16) | 7 (17) |
| PI + INSTI | 4 (7) | 2 (3) | 2 (5) |
| NNRTI + INSTI | 1 (2) | 1 (2) | 0 |
| NNRTI + PI | 0 | 0 | 1 (2) |
| Three drug class combination | |||
| NRTI + PI + INSTI | 2 (3) | 6 (10) | 4 (10) |
| NRTI + PI + NNRTI | 2 (3) | 1 (2) | 0 |
| NRTI + INSTI + NNRTI | 1 (2) | 2 (3) | 4 (10) |
| NNRTI + INSTI + PI | 0 | 1 (2) | 2 (5) |
| Four drug combination | |||
| NRTI + INSTI + NNRTI + PI | 0 | 0 | 1 (2) |
ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, nonnucleoside reverse transcriptase inhibitors; PI, protease inhibitors.
†Refers to the ART regimen the patient was discharged home with after the initial transplant hospitalization.
ºData only available for 41 patients (due to death, loss of follow up, or insufficient documentation in medical record).
*Individual percentage values are rounded and might not total 100%.
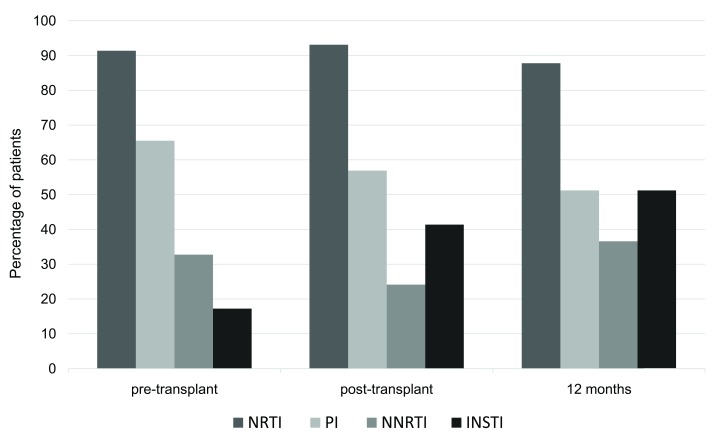
A total of 30 (52%) patients underwent ART modifications after transplant; 22 (38%) of them prior to discharge, and an additional 8 (14%) during the first year post-transplant. Adjustments in ART were primarily done to avoid drug-drug interactions or added nephrotoxicity. There was a significant increase in the proportion of patients receiving INSTI at time of discharge and at 12 months post-transplant compared to pre-transplant period: 41% ( p<0.01) and 51% ( p<0.0005) vs. 17%, respectively ( Table 2 and Figure 1).
Figure 1. Frequency of HIV + kidney transplant recipients receiving a given ART class in the pre-transplant (n=58), post-transplant (at time of discharge; n=58) and at 12 months post-transplant follow-up (n=41).
There was a significant increase in the proportion of patients receiving INSTI-containing regimens at time of discharge and 12 months post-transplant. ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, nonnucleoside reverse transcriptase inhibitors; PI, protease inhibitors.
Transplant outcomes by ART regimen
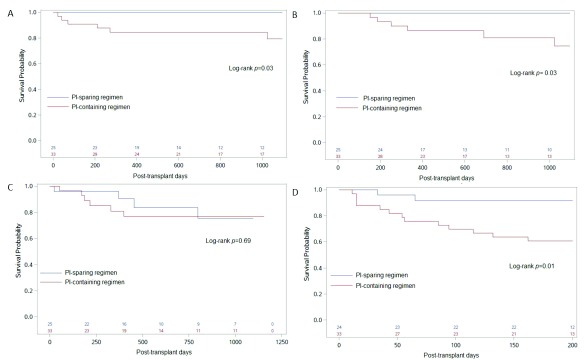
The patient and graft survival at three years were both 90% for the entire cohort. Transplant outcomes varied by ART regimen at the time of discharge after the initial transplant hospitalization. Patients receiving PI-containing regimens had lower patient survival at one and three years than patients receiving PI-sparing regimens: 85% vs. 100% ( p=0.06) and 82% vs. 100% ( p=0.03), respectively ( Table 3 and Figure 2). Patients who received PI-containing regimens had twelve times higher odds of death at three years compared to patients who were not exposed to PIs (odds ratio [OR] 12.05; 95% confidence interval [CI] 1.31-1602; p=0.02). Hepatitis C and delayed graft function also increased the odds of death, but this finding did not reach statistical significance ( Table 4). Three-year death-censored graft survival was lower in patients receiving PI vs. patients on PI-sparing regimens (82 vs 100%, p=0.03; Table 3 and Figure 2). On the contrary, patients receiving INSTI-containing regimens had higher three-year graft survival rates (100 vs. 82%, p=0.04; Table 3).
Figure 2. Transplant outcomes in HIV + kidney transplant recipients by administration of protease inhibitor (PI) at time of discharge.
Kaplan–Meier curves show the ( A) 3-year patient survival, ( B) 3-year graft survival, ( C) 3-year rejection-free survival, and ( D) 200-day infection-free survival in PI-sparing (blue) and PI-containing (red) groups. The number of patients in each group is shown in the bottom of each panel.
Table 3. One and three-year transplant outcomes by ART regimen °.
| N
(%) |
Patient survival | Death-censored graft
survival † |
Biopsy-proven acute
rejection |
Severe
infection † |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1y | p | 3y | p | 1y | p | 3y | p | 1y | p | 3y | p | 6m | p | ||
| Overall | 58 | 91.4 | 89.7 | 93.1 | 89.7 | 13.8 | 17.2 | 26.3 | |||||||
| TDF | |||||||||||||||
| Yes | 19 | 89.5 | 89.5 | 94.7 | 94.7 | 15.8 | 10.5 | 31.6 | |||||||
| No * | 35 | 94.3 | 0.6 | 91.4 | >0.99 | 91.4 | >0.99 | 85.7 | 0.4 | 14.3 | >0.99 | 22.9 | 0.4 | 23.5 | 0.52 |
| NRTI | |||||||||||||||
| Yes | 54 | 92.6 | 90.7 | 92.6 | 88.9 | 14.8 | 18.52 | 26.4 | |||||||
| No | 4 | 75 | 0.3 | 75 | 0.36 | 100 | >0.99 | 100 | >0.99 | 0 | >0.99 | 0 | >0.99 | 25 | |
| NNRTI | |||||||||||||||
| Yes | 14 | 100 | 100 | 92.9 | 92.9 | 7.14 | 21.4 | 7.14 | |||||||
| No | 44 | 88.6 | 0.32 | 86.4 | 0.32 | 93.2 | >0.99 | 88.6 | >0.99 | 15.9 | 0.66 | 15.9 | 0.69 | 32.6 | 0.08 |
| PI | |||||||||||||||
| Yes | 33 | 84.5 | 81.8 | 87.9 | 81.8 | 21.2 | 18.2 | 39.4 | |||||||
| No | 25 | 100 | 0.06 | 100 | 0.03 | 100 | 0.12 | 100 | 0.03 | 4 | 0.12 | 16 | >0.99 | 8.33 | 0.01 |
| INSTI | |||||||||||||||
| Yes | 24 | 95.8 | 95.8 | 100 | 100 | 8.33 | 8.33 | 21.7 | |||||||
| No | 34 | 88.2 | 0.39 | 85.3 | 0.38 | 88.2 | 0.13 | 82.4 | 0.04 | 17.7 | 0.45 | 23.5 | 0.17 | 29.4 | 0.5 |
| Two drug regimens ˆ | |||||||||||||||
| NRTI + INSTI | 12 | 100 | 100 | 100 | 100 | 8.3 | 8.3 | 18.1 | |||||||
| NRTI + NNRTI | 9 | 100 | 100 | 100 | 100 | 0 | 11.1 | 0 | |||||||
| NRTI + PI | 23 | 82.6 | 0.16 | 78.3 | 0.1 | 86.9 | 0.41 | 78.3 | 0.1 | 21.7 | 0.36 | 21.7 | 0.65 | 39.1 | 0.07 |
| NRTI + other * | 21 | 100 | 100 | 100 | 100 | 4.8 | 9.5 | 10 | |||||||
| NRTI + PI | 23 | 82.6 | 0.11 | 78.3 | 0.05 | 86.9 | 0.23 | 78.3 | 0.05 | 21.7 | 0.19 | 21.7 | 0.42 | 39.1 | 0.04 |
ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; PI, protease inhibitors; TDF, tenofovir disoproxil fumarate.
ºRefers to the ART regimen the patient was discharged home with after the initial transplant hospitalization.
P values correspond to Fisher 's exact test. Numbers in bold represent statistical significance.
†As defined previously 4. See main text for details.
ˆRegimens listed here were three most common ART regimens post-transplant in this cohort.
*Includes NRTI + INSTI and NRTI + NNRTI.
Table 4. Variables associated with three-year mortality.
| Variable Ϯ | Alive at 3-years
n=52 (%) |
Death at 3-years
n=6 (%) |
Odds ratio
(95% CI) |
P-value * |
|---|---|---|---|---|
| Protease inhibitor use | 27 (51.9) | 6 (100) | 12.1 (1.31-1602) | 0.02 |
| HCV co-infection | 5 (9.62) | 2 (33.3) | 4.80 (0.70-28.3) | 0.10 |
| Tacrolimus levels at 4 weeks,
median (IQR) |
6 (4.1-8.5) | 8.7 (5.9-11.9) | 1.06 (0.91-1.20) | 0.38 |
| Recipient age, years, median
(IQR) |
48 (42-54) | 48 (46-49) | 1 (0.92-1.10) | 0.91 |
| Baseline CD4 <350 cells/mm 3 | 15 (28.9) | 1 (16.67) | 0.66 (0.06-3.70) | 0.66 |
| Delayed graft function | 4 (7.84) | 2 (33.3) | 5.86 (0.84-36.70) | 0.07 |
| Type 2 diabetes | 11 (21.6) | 6 (100) | 0.27 (0.002-2.60) | 0.31 |
| Donor age, years, median (IQR) | 40 (29-47) | 24 (18-48) | 0.95 (0.88-1.02) | 0.16 |
| Living donor | 14 (26.9) | 0 | 0.20 (0.002-1.92) | 0.19 |
| Time from HIV diagnosis, years,
median (IQR) |
10 (5-16) | 12 (5-13) | 0.98 (0.86-1.09) | 0.72 |
| Morbid obesity | 9 (17.3) | 0 | 0.35 (0.003-3.45) | 0.43 |
ϮData presented as absolute number (percentage), unless specified otherwise.
* P-value calculated using logistic regression with penalized likelihood estimation (null hypothesis of beta=0).
HIV, human immunodeficiency virus; IQR, interquartile range.
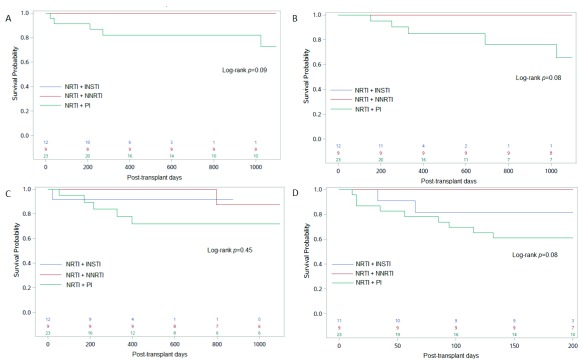
We next assessed transplant outcomes in patients receiving NRTI “backbone” combined with either NNRTI, PI or INSTI as a second drug class. Compared to a group of patients receiving NRTI plus INSTI or NRTI plus NNRTI, the 3-year patient and graft survival were lower in patients receiving NRTI plus PI (78 vs. 100%; p=0.05, Table 3 and Figure 3).
Figure 3. Transplant outcomes in HIV + kidney transplant recipients by ART regimen at time of discharge.
Kaplan–Meier curves show the ( A) 3-year patient survival, ( B) 3-year graft survival, ( C) 3-year rejection-free survival, and ( D) 200-day infection-free survival in NRTI + INSTI (blue), NRTI + NNRTI (red) and NRTI + PI (green) groups. Number of patients in each group is shown in the bottom of each panel. ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, nonnucleoside reverse transcriptase inhibitors; PI, protease inhibitors.
Causes of graft loss among patients on PI-containing regimens were acute rejection in two (33%), thrombosis/hemorrhagic complications in two (33%), CNI toxicity in one (17%), and unidentified in another patient. The cumulative incidence of biopsy-proven acute rejection was 14 and 17% at one and three years post-transplant, respectively. There were no significant differences in rejection rates by ART ( Figure 2 and Figure 3; Table 3).
Incidence of serious infections by ART
Serious non-opportunistic infections within six months post-transplant occurred in 15 (26%) patients. The etiology of such infections, mainly bacterial and fungal in nature, has been reported previously 4. In total, 13 (87%) of these patients were on PI-containing regimens. Individuals receiving PI had a higher incidence of serious infections compared to those on PI-sparing regimens (39 vs. 8%, p=0.01; Figure 2). This association remained significant in analyses restricted to patients on NRTI “backbone”: 39 vs. 10% for patients receiving NRTI + PI compared to those receiving NRTI + INSTI or NNRTI, respectively ( p=0.04; Table 3 and Figure 3).
ART and tacrolimus levels
Tacrolimus levels at 4, 12, 26 and 52 weeks post-transplant were within therapeutic range for most patient groups ( Table 5). Although we did not observe differences in tacrolimus levels by ART at these specific time points, out of 11 patients with tacrolimus levels available at the time of infection, six (54%) had supra-therapeutic levels (median, IQR: 9.2, 5.5-10.1).
Table 5. Plasma tacrolimus levels by ART regimen.
| n | Tacrolimus levels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 weeks | p | 12 weeks | p | 26 weeks | p | 52 weeks | p | |||
| Overall | 58 | 6.1 (4.1-8.5) | - | 6.3 (5.3-7.9) | - | 6.2 (4.8-8.3) | - | 6.2 (5.1-7.8) | - | |
| TDF | ||||||||||
| Yes | 19 | 6.3 (5-8.5) | 0.88 | 6.7 (5.9-7.8) | 0.25 | 6.1 (4.5-8.1) | 0.74 | 6.1 (5.8-7.8) | 0.44 | |
| No * | 35 | 6.1 (4-9) | 6.2 (4.8-7.9) | 6.2 (5.1-8.3) | 6.2 (4.4-7.6) | |||||
| NRTI | ||||||||||
| Yes | 54 | 6.3 (4.4-8.5) | 0.23 | 6.3 (5.3-7.8) | 0.51 | 6.1 (4.7-7.6) | 0.03 | 6.2 (5.2-7.7) | 0.82 | |
| No | 4 | 2.2 (0-17.2) | 8 (2.1-15) | 9.3 (8.3-19.1) | 10.9 (3.9-17.9) | |||||
| NNRTI | ||||||||||
| Yes | 14 | 5.9 (4.5-8.3) | 0.81 | 5.8 (4.8-7.1) | 0.08 | 6.2 (5.5-6.5) | 0.88 | 6.3 (5-7.6) | 0.99 | |
| No | 44 | 6.2 (4.1-8.7) | 6.4 (5.6-8.2) | 6.4 (4.7-8.7) | 6.1 (5.3-7.8) | |||||
| PI | ||||||||||
| Yes | 33 | 6.6 (4.4-9) | 0.41 | 6.1 (5.3-8.4) | 0.42 | 6.2 (4-9.3) | 0.81 | 6.4 (4.8-8.3) | 0.64 | |
| No | 25 | 5.6 (4-8.4) | 6.3 (5-7.4) | 6.2 (5.1-7.5) | 6.1 (5.6-7.5) | |||||
| INSTI | ||||||||||
| Yes | 24 | 4.6 (3.6-7.2) | 0.01 | 7.8 (6.8-9.2) | 0.62 | 7.5 (5.4-9.3) | 0.07 | 6.2 (5.8-7.6) | 0.73 | |
| No | 34 | 5.7 (4.1-7.1) | 5.9 (5.1-7.9) | 6 (4.4-7.2) | 6.1 (4.7-8.3) | |||||
| NRTI + INSTI | 12 | 4.4 (3.5-7.5) | 0.08 | 6.8 (5.3-7.8) | 0.41 | 6.7 (5.5-7.6) | 0.53 | 6.1 (5.9-7) | 0.84 | |
| NRTI + NNRTI | 9 | 7.7 (5.6-9.7) | 5.9 (4.8-7.1) | 6.1 (5-6.4) | 5.8 (5-7.6) | |||||
| NRTI + PI | 23 | 7.6 (5.7-10.9) | 6.1 (5.7-8.4) | 5.9 (3.5-7.5) | 6.6 (5.3-8.3) | |||||
| NRTI + other | 21 | 5.6 (4-8.7) | 0.17 | 6.5 (5-8.1) | 0.35 | 6.3 (5.5-7.5) | 0.49 | 6 (5.6-7.6) | 0.60 | |
| NRTI + PI | 23 | 7.6 (5.7-10.9) | 6.1 (5.7-8.4) | 5.9 (3.5-7.5) | 6.6 (5.3-8.3) | |||||
ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; PI, protease inhibitors. TDF, tenofovir disoproxil fumarate.
*Only includes patients on NRTI other than TDF.
The p-value corresponds to comparison of PI-containing and PI-sparing groups by using the Fisher exact test.
Tacrolimus target levels at our center are 6–8 ng/mL during the first three months and 5–7 ng/mL after three months post-transplant. Higher levels are targeted for highly sensitized patients.
Copyright: © 2016 Rosa R et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Discussion
Consistent with previous studies of kidney transplantation in HIV 1– 5, we observed excellent transplant outcomes without evidence of HIV disease progression. The most important finding of the present study is the association between PI use and adverse outcomes, namely reduced three-year patient and graft survival, and increased risk of serious non-opportunistic infections. These observations remained true in analyses restricted to patients receiving NRTI “backbone”; thus, even after excluding the potential influence of other agents included in the ART regimen, PI continued to be associated with poor outcomes. The immunosuppression protocol at our institution remained constant during the study period, and the proportion of patients transplanted in the 2006–2010 (and consequently the 2011–2015) eras was similar between PI and non-PI groups, suggesting that this observation was also independent of variation in transplant practices over time that might have impacted outcomes.
Biopsy-proven acute rejection and CNI toxicity accounted for half of the cases of graft loss in patients taking PI in the present study. Increased risk of allograft rejection in HIV + individuals has been largely attributed to reduced exposure to immunosuppressive agents, due to drug-drug interactions with ART 3, 6, 7. However, in this small cohort, we did not observe an association between ART regimens and the incidence of rejection. CNI levels at 4, 12, 26 and 52 weeks were comparable across ART groups. Other factors, such as infection of the allograft, previous alloimmunization and immune activation, might also play a role in predisposition to rejection 3, 5, 6.
Non-opportunistic infections within six months post-transplant are common in HIV + kidney recipients 3, especially those with marginal pre-transplant CD4 counts 4. Notably, the occurrence of serious infections in this cohort was almost five-fold higher in patients receiving PI.
This might be due to the effects of PI on tacrolimus levels, considering that the overwhelming majority of these patients were on PI-containing regimen and more-than-half had tacrolimus levels above target at the time of infection. PI could also influence the net state of immunosuppression by increasing the level or effect of other immunosuppressants, such as prednisone and mycophenolate.
Contrary to our expectations, the use of NNRTI or TDF did not influence kidney allograft survival. Tenofovir alafenamide (TAF) is a new formulation of tenofovir associated with less kidney (and bone) toxicity 12. Whether there is added clinical benefit of TAF over TDF in kidney transplant recipients remains to be established.
Consistent with recent reports 7– 9, patients receiving INSTI-containing regimens had excellent patient survival (96%) and graft survival (100%) at three years, and the lowest rejection rates in this cohort (8%). Current guidelines recommend the use of NRTI plus INSTI as a first-line therapy for HIV 10. INSTI pose no interactions with CNI or mTOR inhibitors. In addition, INSTIs have no interactions with direct-acting antivirals, which is important in the setting of hepatitis C co-infection, as that has been associated with poor outcomes 2, 3. Thus, it has become our practice to preemptively switch HIV + candidates pre-transplant or in the immediate post-transplant period to PI-sparing, preferably INSTI-based, ART regimens.
Although none of the patients studied here was on cobicistat, it is important to highlight that this pharmacokinetic enhancer, contained in several combination pills, can increase the levels of CNI 13. HIV + recipients and their community HIV providers should be educated about what ART medications to avoid, and when not possible, how to adjust CNI doses and monitor levels accordingly.
Our study is limited by the small number of patients and retrospective design; serum levels for other immunosuppressants, such as mycophenolate were not available. The association found in the present study between PI-containing ART regimens and adverse outcomes needs to be confirmed in larger studies. Until more data becomes available, the use of PI-sparing regimens in HIV + kidney recipients seems to be the most prudent approach.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2016 Rosa R et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
Dataset 1: Rosa et al. Impact of ART in KT outcomes in HIV recipients: Raw data. doi, 10.5256/f1000research.10414.d146717 14.
Acknowledgments
We thank Analucía Schneégans for technical assistance. We are indebted to all the patients that participated in the present study.
Funding Statement
This work was supported in part by a Miami Center for AIDS research (CFAR) pilot award to JFC, funded by a grant (P30AI073961) from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Locke JE, Mehta S, Reed RD, et al. : A National Study of Outcomes among HIV-Infected Kidney Transplant Recipients. J Am Soc Nephrol. 2015;26(9):2222–2229. 10.1681/ASN.2014070726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sawinski D, Forde KA, Eddinger K, et al. : Superior outcomes in HIV-positive kidney transplant patients compared with HCV-infected or HIV/HCV-coinfected recipients. Kidney Int. 2015;88(2):341–349. 10.1038/ki.2015.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stock PG, Barin B, Murphy B, et al. : Outcomes of Kidney Transplantation in HIV-Infected Recipients. N Engl J Med. 2010;363(21):2004–2014. 10.1056/NEJMoa1001197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suarez JF, Rosa R, Lorio MA, et al. : Pretransplant CD4 Count Influences Immune Reconstitution and Risk of Infectious Complications in Human Immunodeficiency Virus-Infected Kidney Allograft Recipients. Am J Transplant. 2016;16(8):2463–72. 10.1111/ajt.13782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lorio MA, Rosa R, Suarez JF, et al. : Influence of immune activation on the risk of allograft rejection in human immunodeficiency virus-infected kidney transplant recipients. Transpl Immunol. 2016;38:40–43. 10.1016/j.trim.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stock PG: Kidney infection with HIV-1 following kidney transplantation. J Am Soc Nephrol. 2014;25(2):212–215. 10.1681/ASN.2013101112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tricot L, Teicher E, Peytavin G, et al. : Safety and efficacy of raltegravir in HIV-infected transplant patients cotreated with immunosuppressive drugs. Am J Transplant. 2009;9(8):1946–1952. 10.1111/j.1600-6143.2009.02684.x [DOI] [PubMed] [Google Scholar]
- 8. Azar MM, Malinis MF, Moss J, et al. : Integrase strand transferase inhibitors: the preferred antiretroviral regimen in HIV-positive renal transplantation. Int J STD AIDS. 2016; pii: 0956462416651528, [in press]. [DOI] [PubMed] [Google Scholar]
- 9. Kershaw C, Rogers C, Pavlakis M, et al. : Impact of Integrase Inhibitor-Based Antiretroviral Regimen on Outcomes in HIV+ Renal Transplant Recipients. In: 2015 American Transplant Congress, Philadelphia, Pennsylvania. Am J Transplant. 2015;15(Suppl 3). Reference Source [Google Scholar]
- 10. Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Washington, DC: Department of Health and Human Services,2016. Reference Source [Google Scholar]
- 11. Karras A, Lafaurie M, Furco A, et al. : Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis. 2003;36(8):1070–3. 10.1086/368314 [DOI] [PubMed] [Google Scholar]
- 12. Wang H, Lu X, Yang X, et al. : The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: Meta-analysis. Medicine (Baltimore). 2016;95(41):e5146. 10.1097/MD.0000000000005146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han Z, Kane BM, Petty LA, et al. : Cobicistat Significantly Increases Tacrolimus Serum Concentrations in a Renal Transplant Recipient with Human Immunodeficiency Virus Infection. Pharmacotherapy. 2016;36(6):e50–e53. 10.1002/phar.1752 [DOI] [PubMed] [Google Scholar]
- 14. Rossana R, Suarez JF, Lorio MA, et al. : Dataset 1 in: Impact of antiretroviral therapy on clinical outcomes in HIV + kidney transplant recipients: Review of 58 cases. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed]