Abstract
We report a rare case of disseminated coccidioidomycosis with multifocal musculoskeletal involvement. The patient presented to the emergency department with left shoulder pain and swelling. Magnetic resonance imaging of the left shoulder revealed enhancing soft tissue masses, bony lesions, and fluid collections in and around the glenohumeral joint with involvement of the proximal humerus, glenoid, and rotator cuff musculature. Multiple additional areas of involvement were subsequently discovered. Fungal cultures confirmed coccidioidomycosis infection at all surgical sites with superimposed polymicrobial bacterial infection in the left shoulder.
Keywords: Coccidioidomycosis, Disseminated, Coccidioides immitis
Introduction
Coccidioides immitis is a soil inhabiting, spore forming fungus found in the southwestern United States, Mexico, and parts of Central and South America. It is common in the San Joaquin Valley of California and the pulmonary infection due to inhalation of the spores is commonly referred to as San Joaquin Valley Fever or Valley Fever [1], [2]. Pulmonary infection is common in regions where the spores are endemic. Sixty percent of people who are infected are asymptomatic [2]. Less than one percent of cases lead to disseminated infection, with the most common sites being the bones, joints, meninges, and skin. Twenty to 50% of disseminated infections have skeletal involvement [3]. Risk factors for disseminated infection include male gender, African American or Filipino race, or immunocompromised state, as well as living in an endemic region [1].
We report a case of multifocal disseminated coccidioidomycosis in an immunocompetent African American male. The extensive nature of the infection was demonstrated with both computed tomography (CT) and magnetic resonance imaging (MRI), and cultures confirmed the etiologic agent. Several features of this case make it unique. Disseminated coccidioidomycosis infection is extremely rare [3], particularly in immunocompetent patients. This patient, on review of the medical record, had prior disseminated coccidioidomycosis infection and was being treated with suppressive antibiotics at the time of the recurrence. This patient also had multiple sites of disease involvement including multiple joints, intraosseous lesions, soft tissue masses and abscesses, and spinal disease. The multiple manifestations of coccidioidomycosis infection in this patient provide a remarkable example of the imaging appearance of disseminated coccidioidomycosis.
Case report
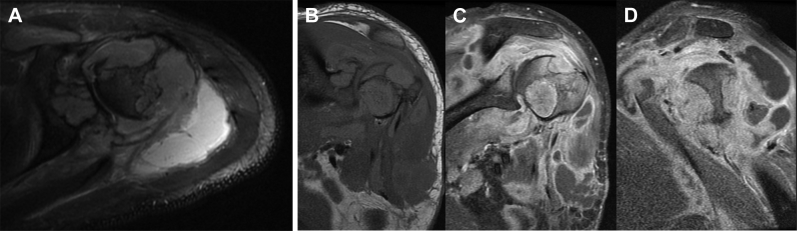
A 33-year-old African American male presented to the emergency department complaining of left shoulder pain, swelling, and oozing of purulent material from the shoulder. The patient had surgery at an outside facility 5 months before due to an infection and the purulent material traversed through an area of dehiscence at the surgical site. MRI was obtained to determine the extent of infection in the left shoulder (Fig. 1). Multiple masses were seen involving the proximal left humerus, glenoid, and within the glenohumeral joint and adjacent musculature. Bone lesions were homogenously mildly to moderately hyperintense on T2WI, isointense to muscle on T1WI, and demonstrated avid but slightly heterogenous postcontrast enhancement. Several of the bone lesions broke through the cortex into the adjacent soft tissues. Fluid collections were also seen adjacent to the glenohumeral joint and tracking anteriorly from the shoulder to a defect along the skin surface. In addition, a large destructive lesion was seen in a left lateral rib extending into the adjacent soft tissues of the left chest wall with similar MRI characteristics to the bone lesions in the humerus and glenoid.
Fig. 1.
(A) Axial T2WI with fat saturation of the left shoulder at the level of the humeral head shows destructive soft tissue masses in the humeral head, glenoid, and within the glenohumeral joint and adjacent musculature. A fluid collection is seen posteriorly. Coronal T1WI (B), postcontrast coronal (C), and sagittal (D) T1WI with fat saturation demonstrate avid enhancement within the soft tissue masses and peripheral enhancement of the fluid collections.
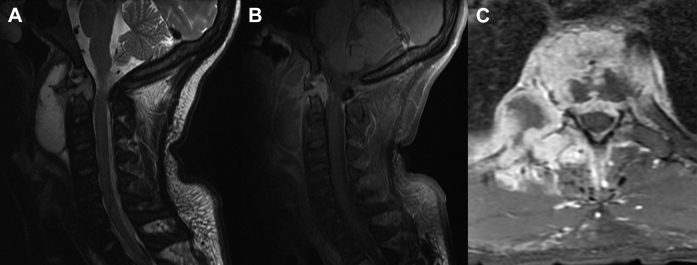
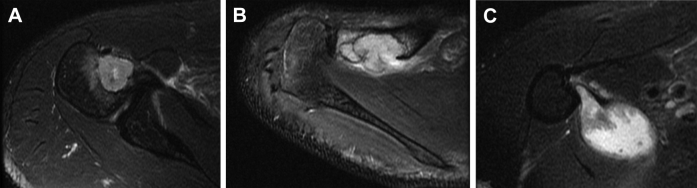
A lesion was seen in the upper thoracic spine on a chest CT to rule out pulmonary embolism obtained while the patient was in the emergency department. MRI of the cervical and thoracic spine was obtained (Fig. 2) to evaluate the lesion seen on the CT. MRI revealed a destructive mass involving the T2 vertebral body with associated pathologic fracture and epidural abscess. A lesion in the cervical spine was also seen with destruction of the odontoid process of C2 and epidural abscess as well as a large prevertebral abscess anterior to C2 and C3. The CT of the chest also partially imaged lesions in the right shoulder, which were confirmed on MRI (Fig. 3).
Fig. 2.
Sagittal T2WI with fat saturation (A) shows a lesion in the odontoid process of C2 and extending to the epidural space and with a large prevertebral abscess anterior to C2-C3. Postcontrast sagittal T1WI with fat saturation shows enhancement of the lesion and peripheral enhancement of the prevertebral abscess. (C) Axial postcontrast T1WI with fat saturation demonstrates a destructive enhancing process involving the T2 vertebral body, adjacent intervertebral discs and epidural space, and adjacent paravertebral muscles and ribs.
Fig. 3.
(A) Axial T2WI with fat saturation reveals a lesion in the right humeral head, similar in imaging appearance to lesions seen in the left shoulder. (B) Axial T2WI with fat saturation of the right shoulder shows a lesion in the distal clavicle. (C) Axial T2WI with fat saturation of the right shoulder demonstrates a fluid collection along the medial aspect of the proximal right humerus.
Initial review of the imaging and clinical information was consistent with a multifocal infectious process. The intraosseous masses were not consistent with a typical pyogenic infection, which was highly suspected clinically in the left shoulder. The appearance of the intraosseous masses was more consistent with either a granulomatous type infection, such as tuberculosis or fungal infection, or an underlying metastatic malignancy.
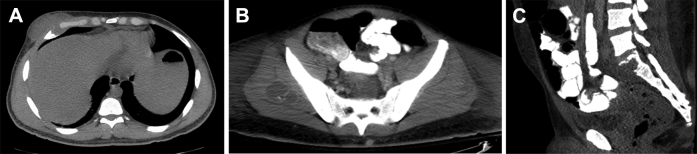
On review of the medical record, the patient had a history of soft tissue mass in his right chest wall and abscess in his right gluteal region and presacral region with involvement of the S1 vertebra due to biopsy-proven infection with C immitis (Fig. 4). Given the history, the imaging findings were likely a combination of recurrent multifocal disseminated coccidioidomycosis with superimposed bacterial infection in the left shoulder related to the recent surgery.
Fig. 4.
(A) Axial CT image of the chest without contrast demonstrates a low attenuation mass in the right anterior chest wall with an adjacent destructive right anterior rib lesion. (B) Axial CT image of the pelvis demonstrates an abscess in the right gluteal musculature. (C) Sagittal reformatted CT image of showing the presacral abscess with destruction of the S1 vertebral body.
The patient was taking oral fluconazole at the time of the recurrence, and therefore the antifungal medication was switched to high dose intravenous liposomal amphotericin B. The patient was immediately started on intravenous antibiotics with vancomycin and piperacillin and/or tazobactam in the emergency department for presumed superimposed bacterial infection in the left shoulder. Surgical incision and drainage of the infected left shoulder was performed with irrigation of the shoulder with an amphotericin B impregnated solution. Curettage of the lesions in the left humeral head was performed with plan to return to surgery for definitive humeral head resection and placement of an antifungal spacer mold created with cement and amphotericin B, which was accomplished 5 days later. The right shoulder was also treated with incision and drainage of the abscess along the medial aspect of the proximal humerus and curettage of the lesion in the humeral head, followed by irrigation with amphotericin B-impregnated solution.
The lesions in the spine were treated surgically, with laminectomy for the spinal epidural abscess at T1-T2 and T2-T3, corpectomy of T2 with diskectomy at T1-T2 and T2-T3, placement of an expandable cage at T1-T2, and posterior spinal fusion with posterior spinal instrumentation and bone graft at C5 through T5. Decompressive laminectomies at C1-C2 and C2-C3 were performed as a second stage of the spinal procedure 1 week later. The prevertebral abscess anterior to C2 and C3 was subsequently drained surgically.
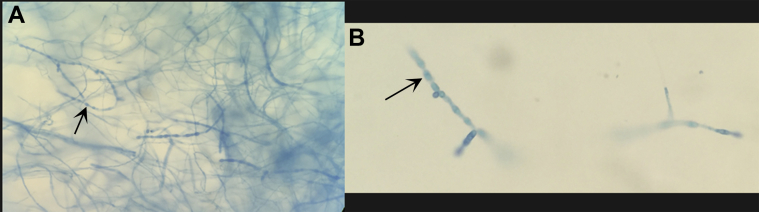
Superimposed bacterial infection was confirmed when cultures from the open wound in the left shoulder were positive for Staphylococcus aureus, Klebsiella pneumonia, and Enterobacter cloacae complex. Subsequent surgically obtained fungal cultures from the left shoulder, right shoulder, and thoracic spine were all positive for Coccidioides immitis/posadasii (Fig. 5).
Fig. 5.
Lactophenol cotton blue slide mount at 40× magnification from culture obtained from the right shoulder abscess. Mature barrel shaped arthroconidia (arrow) with intervening disjunctor cells are seen surrounded by masses of immature hyphal elements. Identification of Coccidioides immitis/posadasii was further confirmed by DNA probe.
Discussion
This case demonstrates extensive recurrent multifocal musculoskeletal involvement of coccidioidomycosis infection. The skeletal lesions had a solid, enhancing appearance on MRI and, given their multifocality, could have been presumed to be metastatic disease in the absence of the clinical history of infection. Other differential considerations would have included tuberculosis or other granulomatous diseases. In our case, most of the bony lesions are well circumscribed, with a punched-out appearance, which is the most common appearance of bony involvement, usually seen in the long and flat bones. Permeative and expansive patterns of bony destruction have also been described [4], [5], [6].
The infection had a second prominent feature of abscess formation involving both glenohumeral joints. Abscesses were also seen during the initial infection in the right gluteal region. A prevertebral abscess in the cervical spine was also noted in our case. Soft tissue abscess can occur without associated osteomyelitis [7].
Involvement of the joints, as was seen in our case, is seen with disseminated coccidioidomycosis, and the arthritis most commonly results from direct extension of osteomyelitis, and rarely due to direct hematogenous spread [8]. The findings of articular involvement include synovitis, joint effusion, periarticular bony destruction, erosions, and juxta-articular osteopenia. Joint space destruction happens late in the disease [8].
The spine is one of the more frequent sites of musculoskeletal disease [9], [10], and our case had extensive disease in the upper cervical and upper thoracic spine with involvement of the vertebra, adjacent disks, and epidural extension. Vertebral osteomyelitis is common in coccidioidomycosis [7]. Extensive paraspinal soft tissue disease with abscess and phlegmon formation as well as disk space involvement is often present, as in our case. Epidural disease, subligamentous spread of infection, nerve root impingement, and cord compression are frequently seen. The degree of disk space narrowing is often minimal; this finding, in combination with significant paravertebral soft tissue involvement may also help favor coccidioidal disease over other granulomatous processes [7].
Disseminated infection always requires prolonged antifungal treatment, commonly with amphotericin B, ketoconazole, fluconazole, or itraconazole [1]. Lifelong suppressive treatment is often needed [11]. Surgery is indicated for musculoskeletal infections to drain abscesses and resect sequestra of disease [1].
This case illustrates several points. First, although immunocompromised patients are more susceptible to the disseminated form of coccidioidomycosis, immunocompetent patients can be affected. In particular, African American and Filipino patients have a higher incidence of disseminated infection than the general population [1]. Second, musculoskeletal coccidioidomycosis infection is difficult to treat and has a high incidence of recurrence. Our patient had been treated successfully and developed a recurrence 2 years later despite taking suppressive oral antifungal treatment. Third, the multifocal destructive skeletal lesions could easily be confused with metastatic disease, but the extent of periarticular and paraspinal soft tissue involvement and abscess formation help favor an infectious process. This infection should be considered in the differential for skeletal lesions in patients who have lived in an endemic area, particularly if there has been any history of coccidioidomycosis infection or if the patient is immunocompromised.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Stockamp N., Thompson G. Coccidioidomycosis. Infect Dis Clin North Am. 2016;30(1):229–246. doi: 10.1016/j.idc.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Stevens D. Coccidioidomycosis. N Engl J Med. 1995;332(16):1077–1082. doi: 10.1056/NEJM199504203321607. [DOI] [PubMed] [Google Scholar]
- 3.Blair J. State-of-the-art treatment of coccidioidomycosis skeletal infections. Ann N Y Acad Sci. 2007;1111(1):422–433. doi: 10.1196/annals.1406.000. [DOI] [PubMed] [Google Scholar]
- 4.Garvin G., Peterfy C. Soft tissue coccidioidomycosis on MRI. J Comp Assist Tomogr. 1995;19(4):612–614. doi: 10.1097/00004728-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Sheldon P., Forrester D., Learch T. Imaging of intraarticular masses. RadioGraphics. 2005;25(1):105–119. doi: 10.1148/rg.251045050. [DOI] [PubMed] [Google Scholar]
- 6.Nidhi A., Gupta M.D., Iv M., Pandit R.P., Patel M.R. Imaging manifestations of primary and disseminated coccidioidomycosis. Appl Radiol. 2015;44(2):9–21. [Google Scholar]
- 7.Olson E., Duberg A., Herron L., Kissel P., Smilovitz D. Coccidioidal spondylitis: MR findings in 15 patients. Am J Roentgenol. 1998;171(3):785–789. doi: 10.2214/ajr.171.3.9725317. [DOI] [PubMed] [Google Scholar]
- 8.Resnick D. Osteomyelitis, septic arthritis, and soft tissue infection: organisms. In: Resnick D., editor. Diagnosis of bone and joint disorders. 4th ed. Saunders; Philadelphia, PA: 2002. pp. 2510–2624. [Google Scholar]
- 9.Reach P., Paugam A., Kahan A., Allanore Y., Wipff J. Coccidioidomycosis of the spine in an immunocompetent patient. Joint Bone Spine. 2010;77(6):611–613. doi: 10.1016/j.jbspin.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 10.Zeppa M., Laorr A., Greenspan A., McGahan J., Steinbach L. Skeletal coccidioidomycosis: imaging findings in 19 patients. Skeletal Radiol. 1996;25(4):337–343. doi: 10.1007/s002560050092. [DOI] [PubMed] [Google Scholar]
- 11.Galgiani J., Ampel N., Catanzaro A., Johnson R., Stevens D., Williams P. Practice guidelines for the treatment of coccidioidomycosis. Clin Infect Dis. 2000;30(4):658–661. doi: 10.1086/313747. [DOI] [PubMed] [Google Scholar]