Abstract
Primary intracranial choriocarcinoma (PICCC), a type of germ-cell tumor, is a very rare primary tumor of the central nervous system that generally arises in the pineal or suprasellar region. We present a case of a teenage boy with PICCC of the bilateral basal ganglia, an anatomic site for which we were unable to find the previous reports. We offer discussion of the differential diagnosis, imaging characteristics, and prognosis of PICCC and germ-cell tumors of the basal ganglia, in the hope that it will increase awareness and allow for early detection.
Keywords: Primary intracranial choriocarcinoma, Germ-cell tumor
Introduction
Primary intracranial choriocarcinoma (PICCC) is a very rare, highly malignant type of germ-cell tumor (GCT). It accounts for a small fraction of all intracranial GCTs and is typically located in the pineal and suprasellar regions [1]. Rarely, PICCC and other GCTs can arise in other sites, such as the basal ganglia and thalami [2], [3]. PICCC involving the bilateral basal ganglia is exceedingly rare and to our knowledge has not been previously reported in the literature. We report a patient with bilateral PICCC in whom magnetic resonance imaging (MRI) was performed.
Case report
A 14-year-old previously healthy Vietnamese boy with no significant past medical history developed behavioral changes, including inattention, 2 years prior to presentation which lead to an initial diagnosis of attention-deficit hyperactivity disorder. The patient’s symptoms did not improve with medical therapy, and he was subsequently referred to a psychiatrist due to deteriorating academic and social functioning, apathy, and anhedonia. The psychiatrist noted left ptosis, disconjugate gaze, and subtle left lower facial paresis, and the patient was referred to a neurologist. Approximately three and one-half weeks later, a neurologist noted subtle left hemiparesis and hyperreflexia, concerning for an upper motor neuron lesion. An urgent MRI brain with contrast was obtained.
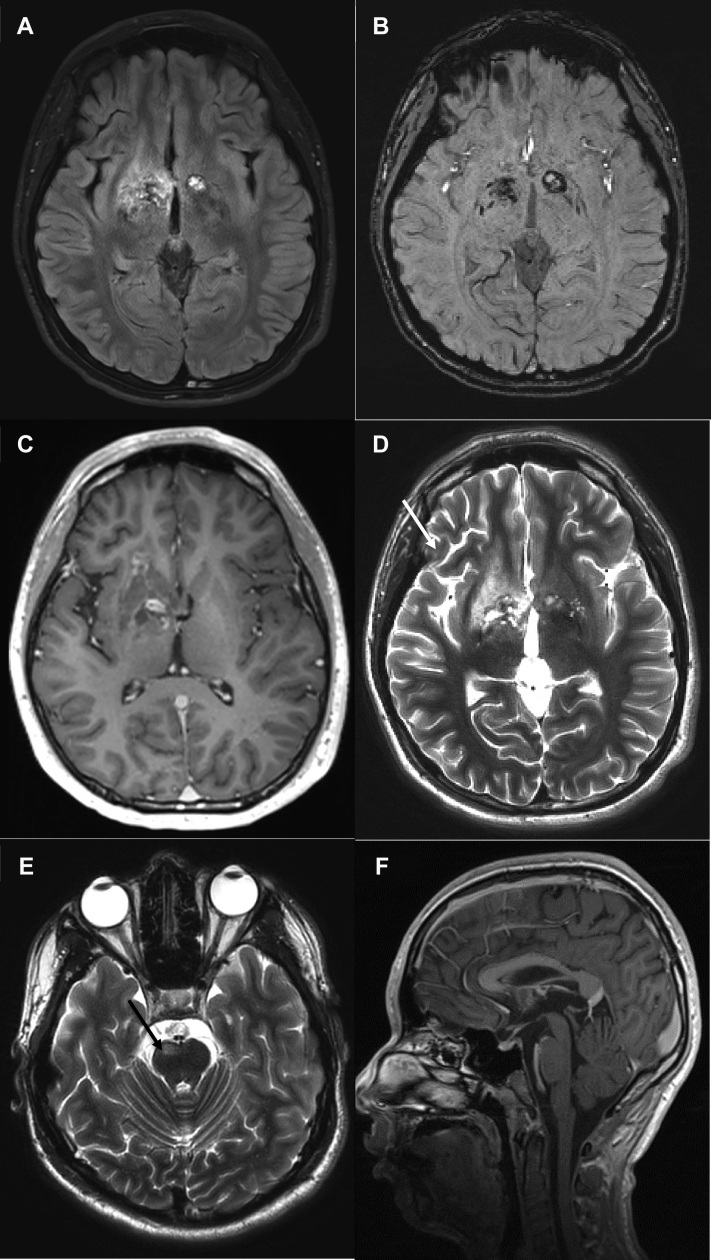
Initial MRI demonstrated non–mass-like, T2/FLAIR signal hyperintense, partially cystic-appearing lesions of the right greater than left basal ganglia, with associated bilateral signal abnormality on susceptibility weighted imaging (SWI) indicating iron or hemosiderin, patchy right basal ganglia contrast enhancement, and right cerebral and pontine volume loss (imaging findings are summarized in Fig. 1). Based on these imaging findings, a workup for metabolic, neurodegenerative, and autoimmune disease was initiated. Leading differential diagnoses included neurodegenerative disorders with brain iron accumulation, particularly pantothenate kinase-associated neurodegeneration given the cystic degeneration and possible iron deposition, as well as disorders of amino acid and organic acid metabolism. Initial laboratory data were normal. As part of the diagnostic workup, the patient underwent a lumbar puncture, approximately 4 weeks following initial presentation. Cerebral spinal fluid (CSF) analysis showed no malignant cells, elevated albumin of 107 mg/dL, normal alpha-fetoprotein (AFP), and a very elevated beta-human chorionic gonadotropin (hCG) of 30,800 mIU/mL. Blood beta-hCG was also elevated at 22,000 mIU/mL. This elevated beta-hCG suggested a nongerminomatous GCT. No extracranial masses were present on CT chest, abdomen, and pelvis.
Fig. 1.
Baseline MRI in a 14-year-old boy with progressive cognitive decline, behavioral changes, and left hemiparesis. Axial FLAIR image demonstrates FLAIR hyperintense lesions in the bilateral basal ganglia (A) with associated signal abnormality on axial SWI image indicating iron or hemosiderin (B). The right basal ganglia lesion demonstrates abnormal contrast enhancement on axial postcontrast T1 images (C). There is evidence of right cerebral volume loss (arrows in D) and abnormal signal and volume loss in the right pons (arrows in E) on axial T2 images. No suprasellar or pineal region lesions were present on sagittal postcontrast T1 images (F). MRI, magnetic resonance imaging; SWI, susceptibility weighted imaging.
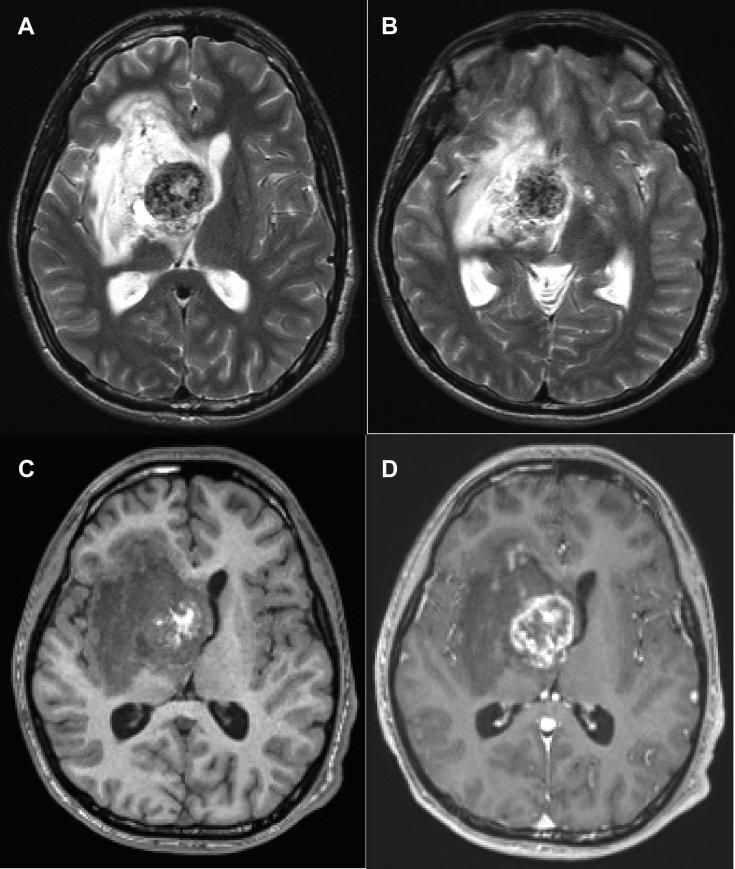
Five weeks after presentation, the patient experienced clinical decompensation due to an acute right basal ganglia hemorrhage, resulting in mass effect and hydrocephalus (Fig. 2). An intraventricular catheter was placed, the right basal ganglia hematoma was partially evacuated, and the tumor was debulked. Intraoperatively, the tumor was noted to be infiltrative without clear borders, fibrous, and highly vascular. Final pathologic diagnosis from multiple separate resection specimens was choriocarcinoma (histologic findings summarized in Fig. 3).
Fig. 2.
Follow-up axial MRI images approximately 5 weeks following initial presentation. T2-weighted (A and B), T1-weighted (C), and T1-weighted postcontrast (D) images demonstrate marked interval enlargement of the mass centered in the right basal ganglia. Extensive interval T2/FLAIR signal hyperintensity and mass effect with right to left midline shift. The T2 hyperintense left basal ganglia lesion (B, arrow) is similar in size.
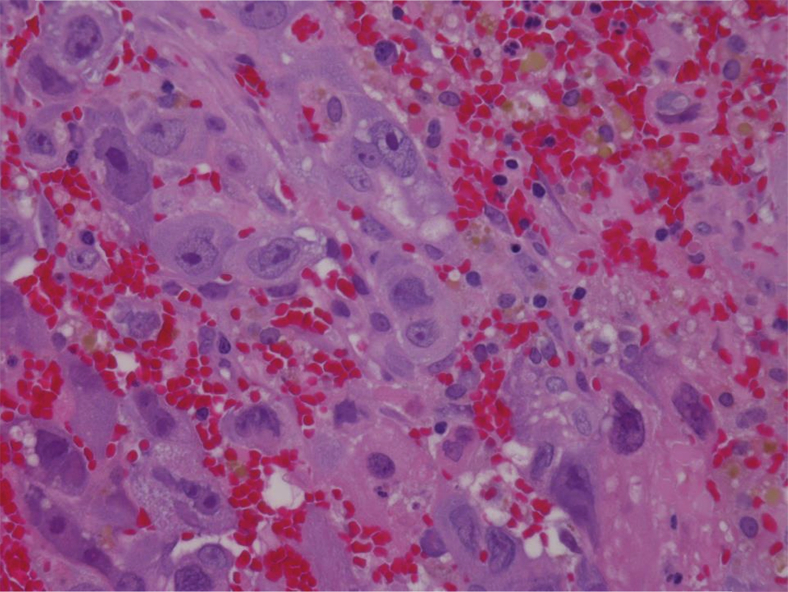
Fig. 3.
Hematoxylin and eosin staining 400×. The tumor is composed largely of sheets of large, pleomorphic syncytiotrophoblast cells, admixed with blood clot and abundant hemosiderin laden macrophages. Mononuclear cytotrophoblasts are relatively rare. The neoplastic cells are immunoreactive for cytokeratin and negative for GFAP, SALL-4, CD30, and CD117. The neoplasm had a high proliferative index, with 60%–70% of nuclei staining positive with Ki-67. Final pathologic diagnosis from all specimens was choriocarcinoma.
Postoperatively, the patient had persistent profound neurologic disability. He subsequently received craniospinal photon radiation therapy as well as chemotherapy with carboplatin and etoposide and survived for 1.5 years after presentation.
Discussion
GCTs are uncommon primary tumors of the central nervous system accounting for 0.4%–3.0% of all intracranial tumors in children in Western countries and up to 11% in Asian countries [2]. GCTs are classified based on histology into three main categories: (1) germinomas; (2) nongerminomatous germ cell tumors (NGGCTs) including choriocarcinoma, teratoma, and embryonal carcinoma; and (3) mixed tumors. Approximately 65% of intracranial GCTs are germinomas, while NGGCTs and mixed tumors make up 35% [4].
Intracranial GCTs are typically located in the pineal and suprasellar regions. Less than 10% of germinomas arise from the basal ganglia region, so called “ectopic germinomas” [2], [3], [4], [5]. Case reports of bilateral basal ganglia germinomas and yolk sac tumors have been described [2], [6]; however, due to their rarity, the prevalence of NGGCTs and mixed tumors arising in the basal ganglia have not been reported in the literature.
PICCC is the rarest and most malignant of the primary intracranial GCTs, accounting for approximately 3%–5% of all intracranial GCTs [1]. PICCC presents between 3 and 22 years of age with a male predominance [7]. Choriocarcinoma is typically thought of in the context of gestational trophoblastic disease with development in the uterus. Nongestational choriocarcinoma arises from germ cells in gonadal or extragonadal regions and is composed of mononuclear cytotrophoblasts and multinucleated syncytiotrophoblasts. Multiple sites of PICCC have been described, with the most common locations being the pineal and suprasellar regions. Uncommon sites include the septum pellucidum, lateral ventricle, and basal ganglia [1]. This is the first reported case of bilateral basal ganglia PICCC to our knowledge.
Bilateral basal ganglia lesions often present a diagnostic dilemma for the radiologist, as the findings are often nonspecific. When presented with bilateral basal ganglia lesions, the typical differential includes the broad categories of ischemic, toxic, metabolic, and neurodegenerative causes. This classical thinking tends to lead one away from neoplasm as a potential cause for bilateral basal ganglia lesions, possibly resulting in delay in diagnosis.
Moreover, GCTs of the basal ganglia are difficult radiologic diagnoses due to very low incidence and relatively nonspecific radiological findings. However, certain imaging features can aid in diagnosis. Early GCT on CT will generally appear as an irregularly defined, slightly hyperdense area without mass effect, as in this case, often with faint calcification [2], [4]. On MRI, basal ganglia GCT generally appears iso- to hyperintense to gray matter on T2/FLAIR, often without a discrete mass lesion early in the course of the disease. Restricted diffusion is often seen in germinomas (due to high cellularity) and variably seen in other GCTs [2], [3], [4], [5]. Other clues include cystic changes, heterogeneous contrast enhancement, and intratumoral hemorrhage. Additionally, ipsilateral cerebral or brain stem hemiatrophy can be seen in up to 33% of cases of GCT of the basal ganglia or thalamus [2], [4], [5].
MRI findings typical of PICCC, described in the literature [1], [5], are of an ovoid or irregular mass with a prominent hemorrhagic component. PICCC will usually demonstrate marked heterogeneous contrast enhancement. In a series of seven PICCCs reported by Lv et al. in 2010 (six in the pineal region and one in the suprasellar region), T2-weighted images demonstrated heterogeneous hyperintensity and hypointensity. Patchy hypointensity on T2-weighted images corresponded histologically to intratumoral blood products. Hemorrhage within PICCC is typical due to the fragility of its vessels and is often best seen on gradient-recalled echo T2* or SWI images. On contrast-enhanced T1-weighted imaging, there is typically heterogeneous, ring-like, and intratumoral nodular enhancement. In most cases, the most useful information to distinguish PICCC from other GCTs is a high serum and CSF level of beta-hCG.
In our case of bilateral basal ganglia lesions, we were unable to appreciate any restricted diffusion, likely due to the significant hemosiderin deposition and a small solid component. There was subtle right hemisphere atrophy which was likely a result of Wallerian degeneration caused by tumor infiltration into the internal capsule with interruption of thalamicocortical tracts [4], [6].
PICCC has a very poor prognosis, with median survival of 22 months [7]. Intratumoral hemorrhage and extraneural and CSF metastasis are common in PICCC; therefore, routine biopsy is not recommended [8]. The optimal treatment regimen for NGGCTs, including PICCC, is unclear. However, a combination of chemotherapy, radiation, and surgery is typical.
In summary, when presented with a pediatric patient with bilateral basal ganglia lesions, especially with associated cystic degeneration and hemosiderin deposition, one should not limit the differential diagnosis to toxic, ischemic, metabolic, autoimmune, and neurodegenerative diseases. Although rare, GCTs and NGGCTs can present as infiltrative unilateral or bilateral basal ganglia lesions without a discrete mass lesion early in their course. Early CSF and plasma tumor marker (AFP and beta-HCG) analysis can aid in the diagnosis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Lv X.F., Qiu Y.W., Zhang X.L., Han L.J., Qiu S.J., Xiong W. Primary intracranial choriocarcinoma: MR imaging findings. ANJR Am J Neuroradiol. 2010;31:1994–1998. doi: 10.3174/ajnr.A2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oyama N., Terae S., Saitoh S., Sudoh A., Sawamura Y., Miyasaka K. Bilateral germinoma involving the basal ganglia and cerebral white matter. ANJR Am J Neuroradiol. 2005;26:1166–1169. [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D.I., Yoon P.H., Ryu Y.H., Jeon P., Hwang G.J. MRI of germinomas arising from the basal ganglia and thalamus. Neuroradiology. 1998;40:507–511. doi: 10.1007/s002340050634. [DOI] [PubMed] [Google Scholar]
- 4.Rasalkar D.D., Chu W.C.W., Cheng F.W.T., Paunipagar B.K., Shing M.K., Li C.K. Atypical location of germinoma in basal ganglia in adolescents: radiological features and treatment outcomes. Br J Radiol. 2010;83:261–267. doi: 10.1259/bjr/25001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang L., Korogi Y., Sugahara T., Ikushima I., Shigematsu Y., Okuda T. MRI of intracranial germ-cell tumours. Neuroradiology. 2002;44:382–388. doi: 10.1007/s00234-001-0752-0. [DOI] [PubMed] [Google Scholar]
- 6.Wang C.H., Hsu T.R., Yang T.Y., Wong T.T., Chang F.C., Ho D.M. Primary yolk sac tumor of bilateral basal ganglia. J Chin Med Assoc. 2010;73:444–448. doi: 10.1016/S1726-4901(10)70096-4. [DOI] [PubMed] [Google Scholar]
- 7.Shinoda J., Sakai N., Yano H., Hattori T., Ohkuma A., Sakaguchi H. Prognostic factors and therapeutic problems of primary intracranial choriocarcinoma/germ-cell tumors with high levels of HCG. J Neurooncol. 2004;66:225–240. doi: 10.1023/b:neon.0000013499.74404.81. [DOI] [PubMed] [Google Scholar]
- 8.Kageji T., Nagahiro S., Matsuzaki K., Kanematsu Y., Nakatani M., Okamoto Y. Successful neoadjuvant synchronous chemo- and radiotherapy for disseminated primary intracranial choriocarcinoma: case report. J Neurooncol. 2007;83:199–204. doi: 10.1007/s11060-006-9311-1. [DOI] [PubMed] [Google Scholar]