Abstract
Neuroendocrine tumors of the breast are very rare accounting for less than 0.1% of all breast cancers and less than 1% of all neuroendocrine tumors. Focal neuroendocrine differentiation can be found in different histologic types of breast carcinoma including in situ and invasive ductal or invasive lobular. However, primary neuroendocrine carcinoma of the breast requires the expression of neuroendocrine markers in more than 50% of the cell population, the presence of ductal carcinoma in situ, and the absence of clinical evidence of concurrent primary neuroendocrine carcinoma of any other organ. Reports discussing the imaging characteristics of this rare carcinoma in different breast imaging modalities are scarce. We present 2 cases of primary neuroendocrine carcinoma of the breast for which mammography, ultrasound, and magnetic resonance imaging findings and pathology findings are described. A review of the medical literature on this particular topic was performed, and the results are presented.
Keywords: Neuroendocrine, Carcinoma, Breast, Mammogram, Ultrasound, MRI
Case report #1
A 58-year-old asymptomatic female presented for an annual mammogram.
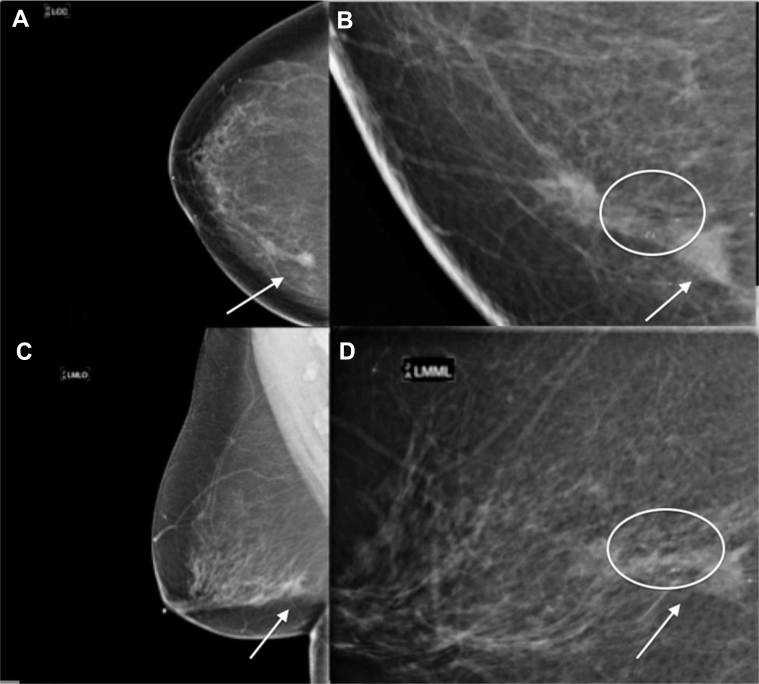
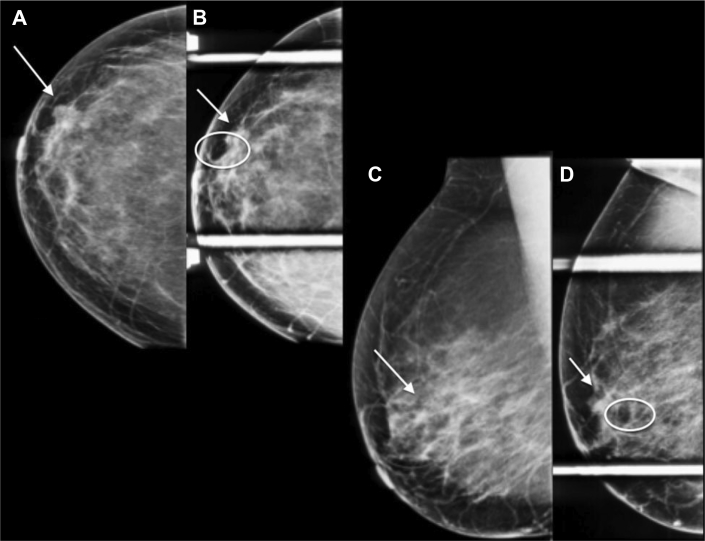
Mammography (Figs. 1A–D) demonstrated a persistent focal asymmetry with associated amorphous, indistinct, and coarse, heterogeneous calcifications spanning approximately 5.0 cm in maximum length in the left breast lower inner quadrant. There were additional coarse heterogeneous calcifications in the immediate left retroareolar region spaced approximately 5.0 cm from the anterior margin of the focal asymmetry.
Fig. 1.
A 58-year-old female with left primary neuroendocrine carcinoma of the breast. Findings: left CC (A), left spot compression CC (B), left MLO (C), and left spot compression MLO (D) views demonstrate a focal asymmetry (arrow) with associated heterogeneous calcifications spanning 5 cm in length in the left breast lower inner quadrant (circle) and retroareolar heterogeneous calcifications (circle). Technique: (A) left breast full field digital mammographic craniocaudal (kVp 30; mAs 78), (B) Spot compression craniocaudal views (kVp 32; mAs 34), (C) left breast mediolateral oblique (kVp 30; mAs 80), and (D) spot compression mediolateral (kVp 32; mAs 52) projections. CC, craniocaudal; MLO, mediolateral oblique.
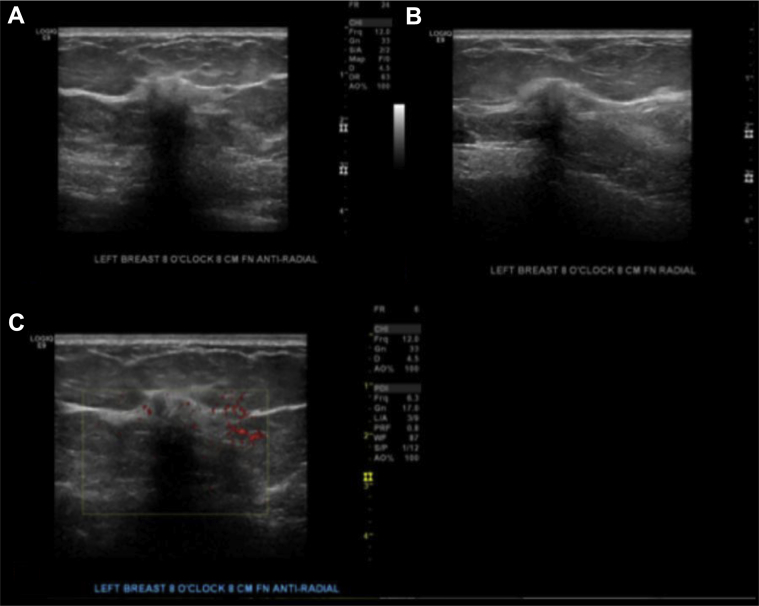
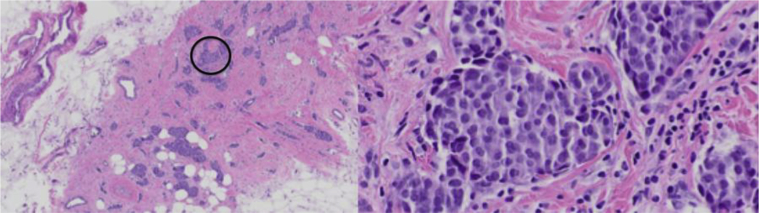
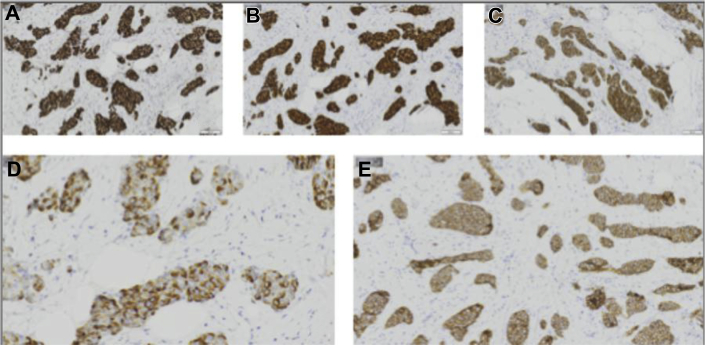
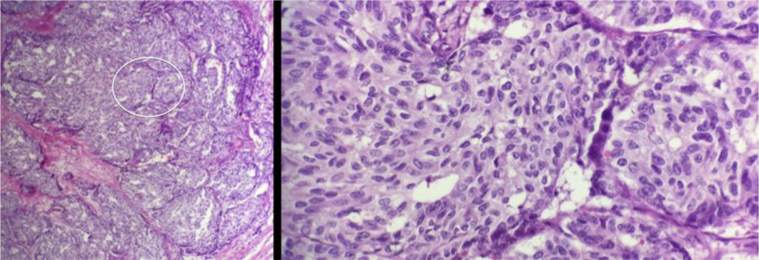
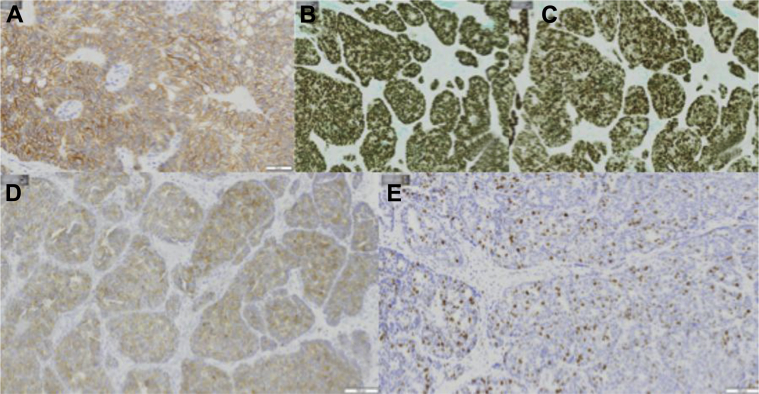
Breast ultrasound (Figs. 2A–C) showed a 1.0 × 0.8 × 0.7 cm irregular, spiculated, hypoechoic not parallel mass with posterior acoustic shadowing in the left breast at the 8 o'clock axis, 8.0 cm from the nipple, in the area of focal asymmetry on mammogram. Ultrasound-guided core needle biopsy of the mass at 8 o’clock was performed, and the results showed well-differentiated neuroendocrine carcinoma (Fig. 3). Immunohistochemistry showed tumor cells to be positive for E-cadherin, estrogen receptor (ER), progesterone receptor (PR), gross cystic disease fluid protein-15, cytokeratin 7, chromogranin, and synaptophysin (Figs. 4A–E). Immunohistochemistry was negative for cytokeratin 20 and human epidermal growth factor receptor 2.
Fig. 2.
A 58-year-old female with left primary neuroendocrine carcinoma of the breast. Findings: gray scale images (A) and (B) and power Doppler images (C) show a 1.0 × 0.8 × 0.7 cm in the left breast at in the area of focal asymmetry on mammogram. Technique: (A and B) gray scale and (C) power Doppler ultrasound images of the left breast using a high-frequency linear probe.
Fig. 3.
A 58-year-old female with left primary neuroendocrine carcinoma of the breast. Low (left) and high (right) magnification hematoxylin and eosin stains from left breast core biopsy at 8 o’clock. Low magnification demonstrates nests and solid sheets of cells with rounded margins separated by fibrovascular stroma. High magnification demonstrates neoplastic cells which display large polygonal, granulomas, and eosinophilic cytoplasm with salt and pepper like nuclei. These pathologic findings are highly characteristic of a primary neuroendocrine carcinoma of the breast.
Fig. 4.
A 58-year-old female with left primary neuroendocrine carcinoma of the breast. Left breast core biopsy at 8 o’clock positive immunohistochemistry shown above include the following: (A) ER, (B) PR, (C) synaptophysin, (D) chromogranin, and (E) E-cadherin. ER, estrogen receptor; PR, progesterone receptor.
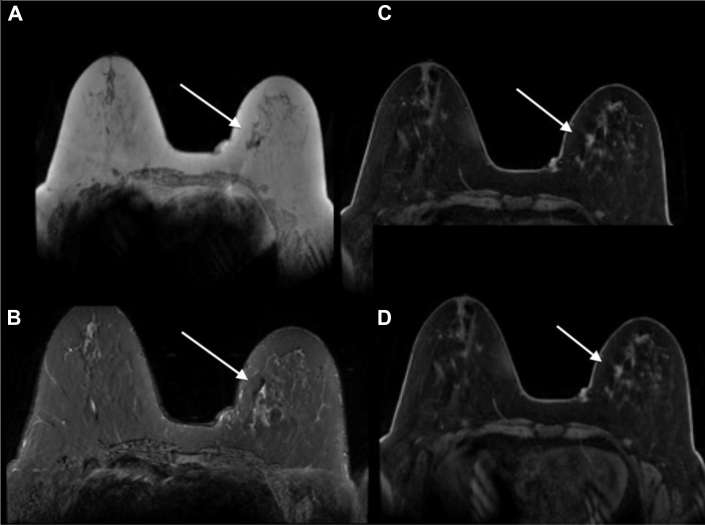
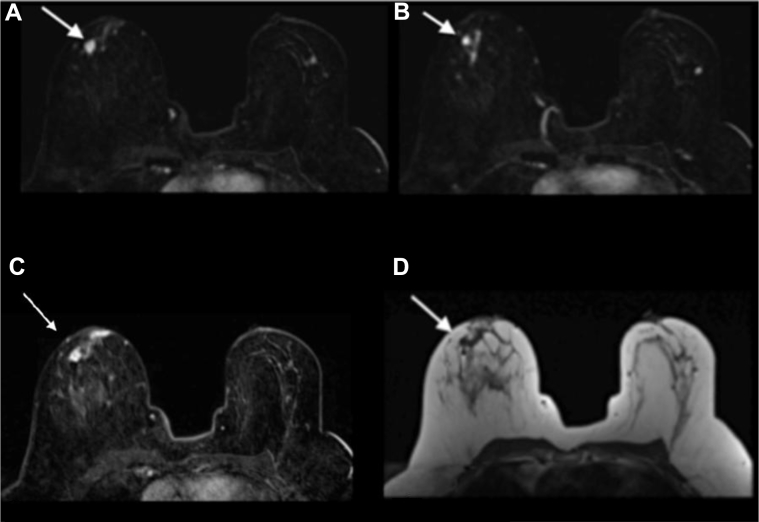
The patient then underwent presurgical breast magnetic resonance imaging (MRI) (Figs. 5A–D) which demonstrated 2 confluent spiculated enhancing masses with rapid wash-in and delayed washout enhancement (progressive kinetics), heterogeneous on T2, isointense on T1 located in the left lower inner quadrant corresponding to area of mammographic and ultrasound findings. There was a focus of signal void artifact corresponding to a biopsy clip adjacent to the most posterior mass. There were several associated adjacent small sub centimeter enhancing satellite lesions. The entire area of abnormality on breast MRI measured approximately 5.5 × 3.5 × 2.5 cm.
Fig. 5.
A 58-year-old female with left primary neuroendocrine carcinoma of the breast. Findings: (A) axial T1 precontrast image, (B) axial inversion recovery precontrast image, and (C and D) axial T1 postcontrast subtracted images of both breasts demonstrate 2 confluent spiculated masses (arrows show better) isointense on T1, heterogeneous on T2, enhance with rapid wash-in and delayed washout enhancement located in the left lower inner quadrant, in the area of suspicious mammographic and ultrasound findings. There is a biopsy clip adjacent to the most posterior mass. There are several associated adjacent small enhancing satellite lesions. The entire area of abnormality on breast MRI measures approximately 5.5 × 3.5 × 2.5 cm. Technique: breast magnetic resonance images obtained in a 3.0 Tesla magnet using a dedicated breast coil. (A) Axial MRI T1 precontrast image, TR 4.728 TE 2.292 1 mm slice thickness. (B) Axial MRI T2 stir inversion recovery precontrast image TR 5475 TE 66.816, 4 mm slice thickness. (C) Axial MRI T1 postcontrast subtracted image 1 minute, TR 6.429 TE 2.556, 2 mm slice thickness, 18-mL Prohance. (D) Axial MRI T1 postcontrast subtracted image (5 minutes), TR 6.429 TE 2.556, 2 mm slice thickness, 18 ml Prohance. MRI, magnetic resonance imaging.
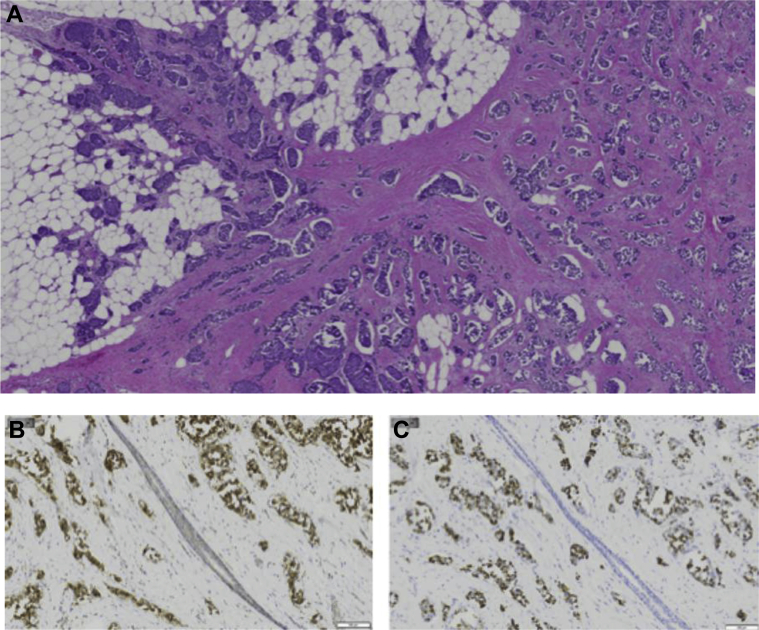
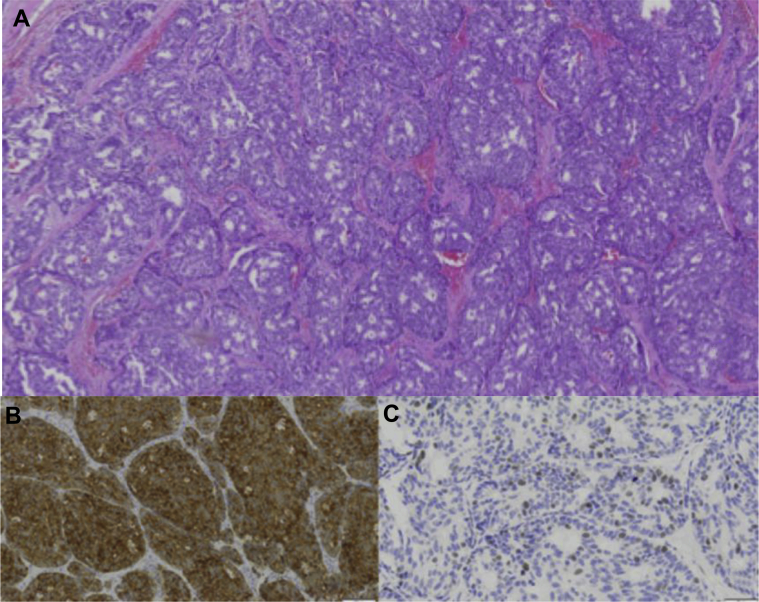
Subsequently, the patient underwent a left breast lumpectomy with sentinel lymph node biopsy. The final pathology (Figs. 6A–C) revealed 2 different foci of well-differentiated neuroendocrine carcinoma of the breast measuring 15 mm and 8 mm in greatest microscopic dimension with negative margins and negative sentinel nodes (stage: pT1cN0M0).
Fig. 6.
A 58-year-old female with left primary neuroendocrine carcinoma of the breast. Left breast lumpectomy with sentinel lymph node dissection. (A) Low magnification hematoxylin and eosin stain demonstrates nests and solid sheets of cells with rounded margins separated by fibrovascular stroma. Positive immunohistochemistry shown above includes the following: (B) synaptophysin and (C) chromogranin.
Case report #2
A 62-year-old asymptomatic female presented for an annual mammogram.
Mammography (Figs. 7A and B) demonstrated a 0.9 cm oval partially obscured mass and an adjacent 0.5 cm oval circumscribed mass in the right breast upper outer quadrant at 10 o’clock.
Fig. 7.
A 62-year-old female with right primary neuroendocrine carcinoma of the breast. Findings: right CC (A), right spot compression CC (B), right MLO (C), and right spot compression MLO (D) views demonstrate a 0.9-cm oval partially obscured mass with spiculated margins (arrow) with an adjacent 0.5-cm oval circumscribed mass in the anterior right upper outer quadrant at 10 o’clock (circle). Technique: right breast digital mammogram (A) full field craniocaudal (kVp 30; mAs 72) and spot compression craniocaudal (kVp 32; mAs 38) and (B) full field mediolateral oblique (kVp 30; mAs 80) and spot compression mediolateral (kVp 32; mAs 57). CC, craniocaudal; MLO, mediolateral oblique.
Breast ultrasound, (Figs. 8A–C) showed a 1.0 × 0.5 × 0.7 cm oval hypoechoic circumscribed parallel mass without posterior acoustic features in the right breast 11 – 12 o'clock axis in the retroareolar region, corresponding to the largest mass on mammogram. Deep to this mass, there was a 0.6 × 0.4 × 0.8 cm oval anechoic circumscribed mass consistent with a simple cyst corresponding to the smallest mass on mammogram.
Fig. 8.
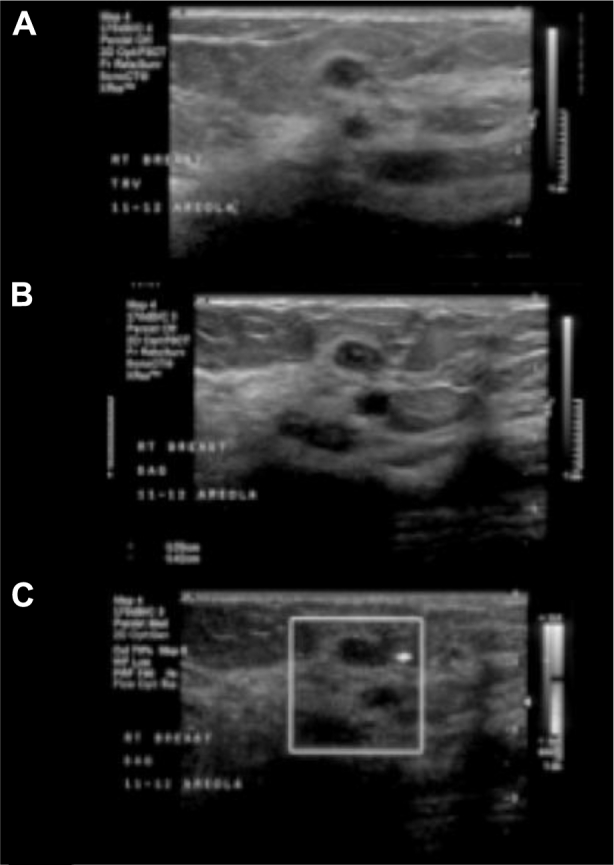
A 62-year-old female with right primary neuroendocrine carcinoma of the breast. Findings: right breast ultrasound using a high-frequency linear probe (A and B) gray scale and (C) power Doppler images shows a 1.0 × 0.5 × 0.7 cm oval hypoechoic circumscribed parallel mass with normal sound transmission in the right breast 11–12 o'clock axes in the retroareolar region, corresponding to the largest mass on mammogram. Just deeper to this mass is a 0.6 × 0.4 × 0.8 cm oval anechoic circumscribed mass, consistent with a simple cyst, corresponding to the smallest mass on mammogram. Technique: right breast ultrasound using a high-frequency linear probe (A and B) gray scale and (C) power Doppler.
Ultrasound guided core needle biopsy was performed and yielded invasive ductal carcinoma (IDC) with neuroendocrine features, Nottingham grade 1, measuring up to 0.5 cm (Fig. 9). Immunohistochemistry (Figs. 10A–E) showed tumor cells to be positive for E-cadherin, ER, PR, and synaptophysin. Immunohistochemistry was negative for human epidermal growth factor receptor 2.
Fig. 9.
A 62-year-old female with right primary neuroendocrine carcinoma of the breast. Low (left) and high (right) magnification hematoxylin and eosin stains from 11 o’clock right breast core biopsy. Low magnification demonstrates nests and solid sheets of cells with rounded margins separated by fibrovascular stroma. High magnification demonstrates neoplastic cells which are uniform in shape and size with increased nuclear cytoplasmic ratio, hyperchromatic nucleus, and scant cytoplasm. These pathologic findings are highly characteristic of a primary neuroendocrine carcinoma of the breast.
Fig. 10.
A 62-year-old female with right primary neuroendocrine carcinoma of the breast. Right breast core biopsy at 11 o’clock positive immunohistochemistry shown above includes the following: (A) E-cadherin, (B) ER, (C) PR, (D) synaptophysin, and (E) ki67. ER, estrogen receptor; PR, progesterone receptor.
The patient then underwent presurgical breast MRI, (Figs. 11A–D) which demonstrated a 1.2 × 1.3 cm oval heterogeneously enhancing spiculated mass in the right retroareolar region with an adjacent biopsy clip corresponding to the largest mass on mammogram, ultrasound and to the biopsy-proven carcinoma. Adjacent to this mass there was a 3.8 cm in maximum length area of ductal clumped non-mass-like enhancement corresponding to pathology-proven DCIS at lumpectomy.
Fig. 11.
A 62-year-old female with right primary neuroendocrine carcinoma of the breast. Findings: (A) axial T1 dynamic 1 minute postcontrast fat-suppressed subtracted image demonstrates a 1.2 × 1.3 cm oval heterogeneously enhancing spiculated mass in the right retroareolar region (arrow), with an adjacent biopsy clip, corresponding to the largest mass on mammogram and ultrasound and to the biopsy-proven carcinoma. (B) axial T1 dynamic 1 minute postcontrast fat-suppressed subtracted image demonstrates a 3.8 cm in maximum length area of ductal clumped non–mass-like enhancement adjacent to the mass (arrow), without mammographic or sonographic correlate, corresponding to pathology-proven DCIS at lumpectomy. (C) Axial T1 dynamic 6 minute postcontrast fat-suppressed subtracted image demonstrates delayed washout of the right retroareolar 1.2 × 1.3 cm spiculated mass (arrow). (D) Axial T1 precontrast image shows the right retroareolar 1.2 × 1.3 cm spiculated mass (arrow). Technique: breast magnetic resonance images obtained in a 1.5 Tesla magnet using a dedicated breast coil: (A) axial MRI T1 postcontrast subtracted image 1 minute, TR 3.87 TE 1.05, 0.9 mm slice thickness, 18 mL Magnevist image 108/208. (B) Axial MRI T1 postcontrast subtracted image 1 minute, TR 3.87 TE 1.05, 0.9 mm slice thickness, 18 mL Magnevist image 112/208. (C) Axial MRI T1 postcontrast subtracted image 6 minutes, TR 3.87 TE 1.05, 0.9 mm slice thickness, 18 ml Magnevist. (D) Axial MRI T1 precontrast image, TR 449 TE 12 4 mm slice thickness. MRI, magnetic resonance imaging.
Subsequently, the patient underwent right breast lumpectomy with sentinel lymph node biopsy which showed ductal carcinoma in situ and invasive mammary carcinoma with neuroendocrine and focal mucinous features, measuring up to 1.7 cm in greatest microscopic dimension, without margin involvement and negative sentinel lymph nodes (Figs. 12A–C), (stage: pT1cN0Mx).
Fig. 12.
A 62-year-old female with right primary neuroendocrine carcinoma of the breast. Right breast lumpectomy with sentinel lymph node dissection. (A) Low magnification hematoxylin and eosin stain demonstrates nests and solid sheets of cells with rounded margins separated by fibrovascular stroma. Positive immunohistochemistry shown above includes the following: (B) synaptophysin and (C) ki 67.
Discussion
Etiology and demographics
Neuroendocrine carcinoma of the breast (NECB) is rare, accounting for less than 0.1% of all breast cancers and less than 1% of all neuroendocrine tumors (Table 1) [1]. In a retrospective study of 381,786 cases of invasive mammary carcinoma recorded from 2003 to 2009 in the surveillance, epidemiology and end results database (SEER), Wang et al. (2014) reported only 142 cases of primary neuroendocrine breast carcinomas [2]. This study calculated an incidence of <0.1% of total invasive carcinomas with most patients presenting in the sixth decade of life (mean age 64 years) [2].
Table 1.
Summary table of primary neuroendocrine carcinoma of the breast.
| Etiology | Uncertain |
|---|---|
| Incidence | <0.1% of all mammary carcinomas |
| Gender ratio | Female predominance |
| Age predilection | Sixth decade of life |
| Risk factors | Not known |
| Treatment | Surgical resection and chemotherapy (optimal adjuvant therapy is still unknown) |
| Prognosis | Worse than invasive mammary carcinoma Wang et al. 2014 SEER study showed median survival duration of NEC cases was much shorter than that of IMC-NOS cases (26 months in NEC; 34 months in IMC-NOS) |
| Imaging findings | Nonspecific and cannot be differentiated from in situ breast carcinoma without biopsy. Mammogram: high-density spiculated mass, ultrasound: hypoechoic or heterogeneous irregular mass with normal sound transmission, MRI: heterogeneous low T1, high T2, enhancing mass with rapid initial enhancement and delayed washout |
IMC-NOS, invasive mammary carcinoma not otherwise specified case; MRI, magnetic resonance imaging.
The histogenesis of neuroendocrine breast tumors is unclear, with 2 leading theories: that the tumor arises from endocrine differentiation of breast carcinoma rather than preexisting endocrine cells in the breast; or that the tumor arises from multi potential stem cells that differentiate along neuroendocrine carcinoma phenotype [3].
Clinical and imaging findings
In 2003, the World Health Organization established formal diagnostic criteria for NECB requiring: the expression of neuroendocrine markers in more than 50% of the cell population, no evidence of other primary sites, and histologic evidence of a carcinoma in situ component [4]. Neuroendocrine markers include chromogranin, synaptophysin, and neuron-specific enolase. Neuroendocrine carcinomas of the breast are further histologically defined as solid, atypical/carcinoid-like, large cell-type, and small/oat cell-type. The most defining histologic features are cellular monotony, nuclear palisading, and pseudorosette formation [3].
The number of cases with radiology findings has been too small to allow generalization of the imaging features. The imaging features of Primary Neuroendocrine Tumor of the breast have been previously described by only a small number of case reports [1], [5], [6], [7], [8], [9], [10]. The published cases describe nonspecific suspicious findings and do not provide ground for generalization of the imaging characteristics of this particular carcinoma (Table 1). The described mammographic characteristics of this carcinoma include a high-density mass with spiculated, lobulated, or indistinct margins. The typical sonographic appearance of this cancer has been reported as a hypoechoic or heterogeneous mass, with irregular shape or microlobulated margins, and with normal sound transmission [7], [11]. On breast MRI, the tumor has been described as a mass of heterogeneously low signal intensity on T1-weighted images, and heterogeneously high signal intensity on T2-weighted images, and heterogeneous contrast enhancement with rapid initial enhancement and delayed washout [11].
Given that the presence of carcinoma in situ is one of the required criteria for the diagnosis of NECB, it is of interest that in our case 1, there is presence of associated suspicious calcifications, which from an imaging/mammographic point of view suggests the presence of carcinoma in situ (later proved by pathology) and that in case 2, there is presence of associated linear clumped non–mass-like enhancement, which from an imaging/MRI point of view suggests the presence of carcinoma in situ (later proved by pathology).
The imaging features demonstrated by our 2 cases, although suspicious for malignancy, are not specific. This was also noted in other published case reports of NECB (Table 2). This precludes the possibility of diagnosing NECB solely based on imaging characteristics. In these cases, biopsy is warranted.
Table 2.
Literature review table of breast imaging characteristics of primary neuroendocrine carcinoma of the breast.
| Source | Ultrasound | Mammogram | MRI |
|---|---|---|---|
| Park Y et al., 2014 [12] | Irregular Hypoechoic Indistinct margins No or enhanced posterior acoustic features |
High density Round or oval or lobular mass with nonspiculated margins |
Irregular mass Irregular margins Washout kinetics |
| Chang E et al, 2013 [11] | Irregularly shaped Heterogeneous Lobulated margins Posterior enhancement Increased vascularity |
High-density mass with ill-defined margin | Heterogeneously low T1 high T2 Washout kinetics |
| Valentim M et al, 2014 [13] | Irregular and ill defined, solid Hypoechoic |
Ovoid well-defined mass | Irregular Peripheral ring enhancement Washout kinetics |
| Stita W et al. 2009 [14] | Ill-defined mass Hypoechoic Microlobulated |
Ovoid high-density mass w/ ill-defined margins | N/A |
| Angarita F et al. 2013 [15] | N/A | Distinctive mass w/ microscopic calcifications and spiculations | N/A |
| Jeon C et al 2014 [16] | Solid irregular, ill defined Hypoechoic Posterior enhancement Increased vascularity Cystic components |
Mass, circumscribed, isodense | Isointense on T2 Irregular, indistinct margins Washout kinetics |
| Gunhan-Bilgen et al 2003 [7] | Irregular Hypoechoic Microlobulated |
Dense, round, speculated, or lobulated margins | N/A |
| Kim J et al 2008 [17] | Oval Heterogeneous Microlobulated |
Well-demarcated lobulated mass | N/A |
MRI, magnetic resonance imaging.
Differential diagnosis
Primary neuroendocrine carcinoma of the breast is an in situ breast carcinoma, which expresses neuroendocrine markers in more than 50% of the cell population. Therefore, imaging findings carry the same differential as in situ breast carcinoma. A definitive diagnosis of primary neuroendocrine carcinoma of the breast can only be made by biopsy. In both cases, the imaging finding differential diagnosis includes invasive ductal carcinoma, fibroadenoma/phyllodes tumor, focal adenosis, abscess, invasive lobular carcinoma, radial scar, and stromal fibrosis (Table 3). Most importantly, the findings found on all imaging modalities were considered suspicious for malignancy and required biopsy.
Table 3.
Differential diagnosis table for primary neuroendocrine carcinoma of the breast.
| Diagnosis | Mammogram | Ultrasound | MRI |
|---|---|---|---|
| Primary neuroendocrine carcinoma of the breast |
|
|
|
| Invasive ductal carcinoma |
|
|
|
| Fibroepithelial lesion |
|
|
|
| Focal adenosis |
|
|
|
| Abscess |
|
|
|
| Invasive lobular carcinoma |
|
|
|
| Radial scar |
|
|
|
| Stromal fibrosis |
|
|
MRI, magnetic resonance imaging; FS, fat suppressed.
Treatment and prognosis
To date, there is no standard treatment protocol for primary neuroendocrine carcinoma of the breast. Optimal treatment requires simultaneous consideration of both the neuroendocrine and breast in situ carcinoma features [4]. Most cases are treated like adenocarcinoma of the breast with radical mastectomy and axillary clearance considered as a first line of treatment with adjuvant chemotherapy (Table 1) [18]. Differentiating “primary” from “metastatic” NECB is crucial because the latter does not justify submitting a patient to mastectomy and axillary node dissection [19].
There is no consensus on the optimal adjuvant chemotherapeutic regimen. The most commonly used chemotherapeutic regimens include cisplatin and etoposide, adriamycin and cyclophosphamide, or 5 fluorouracil, epirubicin, and cyclophosphamide [20]. Wang et al. [2] found that radiation therapy did not prolong survival.
The prognosis for this rare carcinoma is controversial (Table 1). Patient outcome is affected by histologic traits, which include the following: grade, mucin production, and apocrine differentiation [21]. Better prognosis is seen with well-differentiated carcinomas (eg, solid neuroendocrine carcinoma and atypical carcinoids), mucin production (eg, solid papillary carcinomas mucinous carcinomas), and apocrine differentiation [22], [23], [24].
Wei et al. [18] reported in a retrospective study of 74 patients with NECB that hormonal therapy, chemotherapy, and radiation therapy have not demonstrated an advantage in overall survival when compared to ductal carcinoma.
Recent reports on small cell neuroendocrine carcinoma indicate that the size, stage of disease at the time of diagnosis, and expression of the ER and PR are important determinants of the prognosis [25], [26]. Higher grade, increased tumor size, and regional lymph node metastasis are associated with poor prognosis and decreased disease-free survival [27]. Mucinous differentiation and ER/PRs positivity are favorable prognostic factors [3].
Clinical outcomes in the literature report a 15% local and 34% distant recurrence risk by 5 years among NECB patients [3]. The common sites for distant metastasis are bone and liver [18]. Wang et al. [2] reported median survival of patients with NECB cases to be shorter than that of invasive mammary carcinoma not otherwise specified cases (26 months in NEC; 34 months in IMC-NOS).
Significance to clinical practice
Imaging features of primary neuroendocrine carcinoma of the breast are suspicious for malignancy, but are not specific, precluding the possibility of arriving at the diagnosis solely based on imaging characteristics. The diagnosis requires biopsy showing expression of neuroendocrine markers in more than 50% of the cell population, the presence of ductal carcinoma in situ, and the absence of clinical evidence of concurrent primary neuroendocrine carcinoma of any other organ.
Acknowledgment
The authors are thankful to Dr. Ayse Burcu Ergonul for her assistance with pathology photographs.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Ogawa H., Nishio A., Satake H., Naganawa S., Imai T., Sawaki M. Neuroendocrine tumor in the breast. Radiat Med. 2008;26(1):28–32. doi: 10.1007/s11604-007-0182-y. [DOI] [PubMed] [Google Scholar]
- 2.Wang J., Wei B., Albarracin C., Hu J., Abraham S., Wu Y. Invasive neuroendocrine carcinoma of the breast: a population-based study from the surveillance, epidemiology and end results (SEER) database. Biomedcentral Cancer. 2014;14:147. doi: 10.1186/1471-2407-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murthy V., Geethamala K., Kumar B., Sudharao M. Primary neuroendocrine carcinoma of breast: a rare case report. Ann Med Health Sci Res. 2013;3(1):S35–S37. doi: 10.4103/2141-9248.121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernando A., Rodríguez J., Meek E., Sanchez J., Tawil M., Torregrosa L. Locally-advanced primary neuroendocrine carcinoma of the breast: case report and review of literature. World Journal of Surgical Oncology. 2013;11:128. doi: 10.1186/1477-7819-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajisaka H., Maeda K., Miwa A. Breast cancer with endocrine differentiation: report of two cases showing different histologic patterns. Surg Today. 2003;22:909–912. doi: 10.1007/s00595-003-2612-5. [DOI] [PubMed] [Google Scholar]
- 6.An J., Woo J., Kang J., Kim E. Small-cell neuroendocrine carcinoma of the breast. J Korean Surg Society. 2012;82(2):116–119. doi: 10.4174/jkss.2012.82.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Günhan-Bilgen I., Zekioglu O., Ustün E., Memis A., Erhan Y. Neuroendocrine differentiated breast carcinoma: imaging features correlated with clinical and histopathological findings. Eur Radiol. 2003;13:788–793. doi: 10.1007/s00330-002-1567-z. [DOI] [PubMed] [Google Scholar]
- 8.Irshad A., Ackerman S., Pope T., Moses C., Rumboldt T., Panzegrau B. Rare breast lesions: correlation of imaging and histologic features with WHO classification. Radiographics. 2008;28:1399–1414. doi: 10.1148/rg.285075743. [DOI] [PubMed] [Google Scholar]
- 9.Mariscal A., Balliu E., Díaz R., Casas J., Gallart A. Primary oat cell carcinoma of the breast: imaging features. Am J Roentgenol. 2004;183:1169–1171. doi: 10.2214/ajr.183.4.1831169. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J., Chen W. Bilateral primary breast neuroendocrine carcinoma in a young woman: report of a case. Surg Today. 2011;41(11):1575–1578. doi: 10.1007/s00595-010-4516-5. [DOI] [PubMed] [Google Scholar]
- 11.Chang E.D., Kim M.K., Kim J.S., Whang I.Y. Primary neuroendocrine tumor of the breast: imaging features. Korean J Radiol. 2013;14(3):395–399. doi: 10.3348/kjr.2013.14.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park Y., Wu Y., Wei W., Yang W. Primary neuroendocrine carcinoma of the breast: clinical, imaging, and histologic features. AJR. 2014;203(2):W221–W230. doi: 10.2214/AJR.13.10749. [DOI] [PubMed] [Google Scholar]
- 13.Valentim M., Monteiro V., Marques J. Primary neuroendocrine breast carcinoma: a case report and literature review. Radiol Brasileira. 2014;47(2):125–127. doi: 10.1590/S0100-39842014000200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stita W., Trabelsi A., Gharbi O., Mokni M., Korbi S. Primary solid neuroendocrine carcinoma of the breast. Can J Surg. 2009;52(6):289–290. [PMC free article] [PubMed] [Google Scholar]
- 15.Angarita F., Rodriguez J., Meek E., Sanchez J., Tawil M., Torregrosa L. Locally-advanced primary neuroendocrine carcinoma of the breast: case report and review of the literature. World J Surg Oncol. 2013;11:128. doi: 10.1186/1477-7819-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon C., Kim S., Jang M., Yun B., Ahn H., Kim S. Clinical and radiologic features of neuroendocrine breast carcinomas. J Ultrasound Med. 2014;33(8):1511–1518. doi: 10.7863/ultra.33.8.1511. [DOI] [PubMed] [Google Scholar]
- 17.Kim J., Woo O., Cho K., Seo B., Yong H., Kim A. Primary large cell neuroendocrine carcinoma of the breast: radiologic and pathologic findings. J Korean Med Sci. 2008;23:1118–1120. doi: 10.3346/jkms.2008.23.6.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei B., Ding T., Xing Y., Wei W., Tian Z., Tang F. Invasive neuroendocrine carcinoma of the breast: a distinctive subtype of aggressive mammary carcinoma. Cancer. 2010;116(19):4463–4473. doi: 10.1002/cncr.25352. [DOI] [PubMed] [Google Scholar]
- 19.Richter-Ehrenstein C., Arndt J., Buckendahl A., Euker J., Weichert W., Kasajima A. Solid neuroendocrine carcinomas of the breast: metastases or primary tumors? Breast Cancer Res Treat. 2010;124:413–417. doi: 10.1007/s10549-010-1178-3. [DOI] [PubMed] [Google Scholar]
- 20.Ghanem S., Kabaj H., Naciri S., Glaoui M., Ismaili N., Benjaafar N. Primary neuroendocrine carcinoma of the breast: a rare and distinct entity. J Cancer Res Exp Oncol. 2011;3(5):50–54. [Google Scholar]
- 21.Miremadi A., Pinder S., Lee A., Bell J.A., Paish E.C., Wencyk P. Neuroendocrine differentiation and prognosis in breast adenocarcinoma. Histopathology. 2002;40(3):215–222. doi: 10.1046/j.1365-2559.2002.01336.x. [DOI] [PubMed] [Google Scholar]
- 22.Sapino A., Righi L., Cassoni P., Papotti M., Gugliotta P., Bussolati G. Expression of apocrine differentiation markers in neuroendocrine breast carcinomas of aged women. Mod Pathol. 2001;14(8):768–776. doi: 10.1038/modpathol.3880387. [DOI] [PubMed] [Google Scholar]
- 23.Diab S., Clark G., Osborne C., Libby A., Allred D., Elledge R. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol. 1999;17(5):1442–1448. doi: 10.1200/JCO.1999.17.5.1442. [DOI] [PubMed] [Google Scholar]
- 24.Pagani A., Sapino A., Eusebi V., Bergnolo P., Bussolati G. Pip/gcdfp-15 gene expression and apocrine differentiation in carcinomas of the breast. Virchows Arch. 1994;425(5):459–465. doi: 10.1007/BF00197548. [DOI] [PubMed] [Google Scholar]
- 25.Adegbola T., Connolly C., Mortimer G. Small cell neuroendocrine carcinoma of the breast: a report of three cases and review of the literature. J Clin Pathol. 2005;58(7):775–778. doi: 10.1136/jcp.2004.020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi R., Furusawa H., Nakahara H., Inomata M., Namba K., Tanaka M. Clinicopathological study of invasive ductal carcinoma with large central acellular zone: special reference to magnetic resonance imaging findings. Pathol Int. 2008;58(1):26–30. doi: 10.1111/j.1440-1827.2007.02184.x. [DOI] [PubMed] [Google Scholar]
- 27.Lu C., Huang S., Ho C., Chen J., Chao T. Primary neuroendocrine carcinoma of the breast. J Balkan Union Oncol. 2014;19(2):419–429. [PubMed] [Google Scholar]