Abstract
The neurotransmitter serotonin (5-HT) plays a central role in brain development, regulation of mood, stress reactivity and risk of psychiatric disorders, and thus alterations in 5-HT signaling early in life have critical implications for behavior and mental health across the life span. Drawing on preclinical and emerging human evidence this narrative review paper will examine three key aspects when considering the consequences of early life changes in 5-HT: (1) developmental origins of variations of 5-HT signaling; (2) influence of genetic and epigenetic factors; and (3) preclinical and clinical consequences of 5-HT-related changes associated with antidepressant exposure (SSRIs). The developmental consequences of altered prenatal 5-HT signaling varies greatly and outcomes depend on an ongoing interplay between biological (genetic/epigenetic variations) and environmental factors, both pre and postnatally. Emerging evidence suggests that variations in 5-HT signaling may increase sensitivity to risky home environments, but may also amplify a positive response to a nurturing environment. In this sense, factors that change central 5-HT levels may act as ‘plasticity’ rather than ‘risk’ factors associated with developmental vulnerability. Understanding the impact of early changes in 5-HT levels offers critical insights that might explain the variations in early typical brain development that underlies behavioral risk.
Keywords: 5-HT, fetal development, epigenetics, neurodevelopment, SSRI exposure, maternal depression
INTRODUCTION
Serotonin (5-hydroxytryptamine, 5-HT) is a phylogenetically ancient neurotransmitter that appears early in development and extends widely throughout the brain. In fact, in the human brain, serotonergic neurons are first evident as early as 5 weeks of gestation (Sundstrom et al., 1993) and by 15 weeks, the raphe nuclei already contain a typical arrangement of serotonin neurons (Takahashi et al., 1986). Advances over the past 60 years have illustrated multiple roles 5-HT plays shaping health and illness. And more recently, this research has helped elucidate the role 5-HT plays in moderating the impact of early life experience on behavior and mental health across the life span. Long before birth, 5-HT is already setting developmental pathways that contribute to learning, thinking, and stress reactivity. 5-HT signaling is regulated by a complex network of genes, that code for transcription factors, transporters, receptors, and synthetic metabolic enzymes (for review see: (Homberg et al., 2006)). Serotonin plays two key roles: first, during early developmental periods, it acts as a growth factor, regulating the development of its own and related neural systems (Whitaker-Azmitia et al., 1996; Bonnin et al., 2007). In its developmental role as a trophic factor, 5-HT regulates diverse and developmentally critical processes such as cell division, differentiation, migration, myelination, synaptogenesis, and dendritic pruning (Gaspar et al., 2003) before taking on its second role as a neurotransmitter in the mature brain regulating cognition, attention, emotion, pain, sleep and arousal. 5-HT is also central to the development and function of two key stress response systems – the hypothalamic-pituitary–a drenal (HPA) and the locus–coeruleus–norepinephrine (LC–NE) systems (Chaoulo et al., 1999) that shape self-regulation and mental health across the lifespan (Lupien et al., 2009). Alterations in HPA function that are characteristic of anxiety and depressive disorders (Fuller, 1996; Lowry, 2002) point to altered serotonergic tone as a risk factor for psychopathology (Chrousos, 2000; McEwen, 2005; Homberg and Contert, 2009). Disruption of 5-HT signaling is considered a key developmental component underlying a number of neuropsychiatric disorders, such as schizophrenia, affective disorders, anxiety, and autism (Chugani et al., 1999; Whitaker-Azmitia, 2001; Sodhi and Sanders-Bush, 2004; Bonnin and Levitt, 2012). With this perspective, the importance of understanding the implications of changing 5-HT signaling during critical periods of neurodevelopment becomes particularly evident. This has been highlighted by increased use of SSRI antidepressants to treat maternal depression during pregnancy, which has raised critical and yet unanswered questions about the impact of altering 5-HT levels in utero and the implications for subsequent behavior and mental health (Gaspar et al., 2003; Homberg et al., 2010; Homberg and Lesch, 2011) across the early life span. However, one has to keep in mind, that serotonergic signaling per se has not been investigated electrophysiologically and/ or neurochemically in intact brains of developing human fetuses. The serotonergic and other neurotransmitter systems are endowed with enormous plasticity and thus may be able to adapt to mild or moderate developmental pressures, such as therapeutic drug exposure or maternal depression with little to no consequence for actual serotonergic signaling, however the functional consequences in the fetus remain an open question.
Serotonin also acts as a mediator between early adverse life experience and subsequent behavior (Way and Taylor, 2010), shaping individual differences in susceptibility to mental health or illness. In this sense variations in 5-HT signaling – either due to genetic variations, epigenetic modifications or drug exposure – set developmental pathways that predispose some individuals to succumb in the face of contextual adversity while permitting others to benefit from an advantageous environment. Understanding how early 5-HT signaling influences brain development and function that is subsequently reflected in behavioral and social development offers critical insights into what underlies vast variations in human development. To understand 5-HT’s developmental role, this paper will review three key aspects central to understanding the consequences of early life changes in 5-HT: (1) developmental origins of variations of 5-HT signaling; (2) influence of genetic and epigenetic factors; and (3) the preclinical and clinical consequences of early changes in 5-HT levels associated with exposure to selective serotonin reuptake inhibitor antidepressants (SSRIs). Increased use of antidepressants to manage mood disorders during pregnancy raises critical and unanswered questions about the risks and potential benefits for the infants and children associated with maternal treatment with an SSRI antidepressant. This paper reviews current evidence within a perspective suggesting that factors which change central 5-HT levels during sensitive periods may act as ‘plasticity factors’ rather than ‘risk factors’ associated with vulnerability that predicts disordered development and behavior. Understanding the impact of early changes in serotonergic levels offers important insights that may explain why variations in early typical brain development are associated with both developmental risk and resilience.
DEVELOPMENTAL ORIGINS OF VARIATIONS OF 5-HT SIGNALING
The concept that classical neurotransmitters such as 5-HT also work as hormones/growth and differentiation factors in the fetal brain, even before neural circuits are functional, emerged decades ago (Lauder and Krebs, 1976; Buznikov, 1984). However, 5-HT functions may not always be strictly separable between early development and later postnatal, childhood, or adult time frames. Shortly after 5-HT is detectable during early development, the capacity for “neurotransmission”, as we conceptualize it in adulthood, is already available, but the presence of serotonergic synapses does not necessarily indicate that 5-HT modulates the electric activity of target neurons in the fetal brain as they do in the adult (Lauder, 1990). Thus, there are clear developmental differences and studies in animal models have shown that 5-HT modulates neuronal progenitor cell proliferation, neuronal migration, and axonal wiring during fetal and early postnatal development – processes that do not typically take place in the adult brain (Brezun and Daszuta, 1999, 2000; Azmitia, 2001; Kindt et al., 2002; Bansr et al., 2004; Bonnin et al., 2007). In particular, altering 5-HT signaling simultaneously through multiple receptors (i.e. 5-HT1B and 1D receptors) during restricted time periods of fetal brain development significantly disrupts thalamocortical axon pathway formation (Bonnin et al., 2007). The diversity of 5-HT developmental functions is also illustrated by the fact that genetic or pharmacological disruption of 5-HT receptors or transporter function during fetal brain development in mice can lead to an array of atypical behaviors compared to untreated or wild-type animals, such as increased anxiety, later in life (Gaspar et al., 2003; Holmes et al., 2003a,b; Ansorge et al., 2004; Nordquist and Oreland, 2010; Morelli et al., 2011). Therefore, the trophic vs neuromodulatory roles of 5-HT appears to be temporally distinct (at least between early prenatal and adult). Postnatal functions may be less separable, since 5-HT may be acting as a modulator of neuronal activity, and still play trophic functions such as refinement of thalamocortical axon terminal arborization. Speculatively, some trophic functions may still be present in the adult brain (for neurogenesis in the dentate gyrus for instance). In fact, recent research suggests that serotonin modulates adult neurogenesis rates and that this is maybe a mechanism by which SSRIs improve depressive symptoms (e.g. Mahar et al., 2014; Imoto et al., 2015).
Despite the significant effects of 5-HT transporter or receptor gene expression manipulations in basic animal models, there are very few specific associations between any 5-HT-related gene mutation or dysfunction and mental illness in humans (Gingrich and Hen, 2001; Gaspar et al., 2003; Segman et al., 2003). A likely reason for this discrepancy is the existence of 15 different receptor subtypes for 5-HT, many of which share similar pharmacological characteristics. Thus the impact of genetic alteration of one specific receptor subtype on overall serotonergic function may be compensated for, in the long term, by the presence of other pharmacologically and functionally related receptors (e.g. 5-HT1B and 5-HT1D receptors; see: (van Kleef et al., 2012)). From a pathological standpoint, it follows that disruption of 5-HT signaling through multiple receptor subtypes simultaneously may be necessary to trigger significant outcomes.
Global disruption of signaling through all receptor subtypes simultaneously can occur when the availability of their common ligand, 5-HT, is altered. This in fact leads to much more dramatic phenotypes than would the genetic alteration of a single serotonergic system component (receptor or transporter). This is illustrated by the significant effects of either pharmacological disruption of 5-HT synthesis or genetic disruption of 5-HT metabolism in early development (see details in: van Kleef et al., 2012). In terms of developmental effects, specific outcomes may depend on the particular complement of 5-HT receptor subtypes expressed during the time of altered 5-HT availability. For example, recent results show that 5-HT signaling, and thus extracellular levels of 5-HT, play a crucial role in the thalamocortical wiring of the fetal forebrain by affecting netrin-1-mediated axonal guidance (Bonnin et al., 2007; Bonnin and Levitt, 2011). Noteworthy, extracellular 5-HT levels were not directly measured in the study (Bonnin et al., 2007), however, the effect of 5-HT signaling on thalamocortical axon formation was demonstrated by manipulating the expression levels of two specific 5-HT receptors that are specifically expressed in thalamic neurons during fetal development. The assumption is that increasing or decreasing extracellular 5-HT concentration should directly affect signaling through those receptors and lead to disruptions similar to what is observed after disrupting receptor expression directly. Importantly, the effect of 5-HT on thalamocortical axons’ response to guidance cues was shown to be cAMP-dependent, 5-HTreceptor-dependent (blocked by antagonists, mimicked by agonists) and therefore resulting from actual receptor-mediated signaling (Bonnin et al., 2007). However, there are potentially other mechanisms such as alterations in the amount of 5-HT uptake intracellularly via SERT or altered SERT expression levels that could potentially alter serotonylation and other downstream pathways; but unlike the effect of receptor-based signaling mentioned above, there is no evidence yet that serotonylation takes place or influences fetal brain development.
The establishment of the thalamocortical axonal pathway involves several phases (e.g. in chronological order: thalamic neuron differentiation, migration, axon elongation, guidance, final arborization and refinement of axonal arbors in the cortex). Depending on the timing and duration of altered 5-HT availability in the fetal brain, one or more of these developmental phases may be disrupted. Therefore, it is critically important to understand precisely when and how 5-HT is provided to the fetal brain, and which genetic or environmental factors can disrupt it. A recent study showed that the placenta synthesizes 5-HT from maternally derived tryptophan precursor. This placentally derived 5-HT accumulates in the fetal forebrain during early/mid pregnancy (Bonnin et al., 2011), a period when thalamocortical axons express the 5-HT1B/1D receptors and are experiencing active outgrowth and guidance. Since the growth and guidance of fetal thalamocortical axons is finely tuned by 5-HT signaling (Bonnin et al., 2007), alterations of placentally derived 5-HT could have direct consequences on fetal brain axonal wiring. More generally, genetic and environmental perturbations of the placental metabolism of 5-HT from maternally derived tryptophan could have significant downstream consequences on fetal brain development, with specific outcomes depending on the timing of the insult.
Genetic mouse models have shown that excess levels of 5-HT in the brain lead to abnormal development of topographically organized whisker-barrel fields in the somatosensory cortex (Cases et al., 1996; Persico et al., 2001; Salichon et al., 2001). These phenotypes were observed in mice lacking the gene SLC6A4 encoding the 5-HT transporter (SERT), that is responsible for the re-uptake of 5-HT or lacking the gene for the enzyme monoamine oxidase (MOA)-A, that is involved in the degradation of 5-HT. More generally, genetic manipulations generating either excess or reduction of 5-HT lead to later behavioral and functional deficits (Hendricks et al., 2003; Savelieva et al., 2008; Alenina et al., 2009; Yadav, 2009). These findings will be further discussed in the next section. Furthermore, pharmacological manipulations that mimic an increase of the serotonergic pathway activity lead to abnormal cortical development and neuronal migration (Janusonis et al., 2004; Riccio et al., 2009). In line with this, we could recently show that infants that were exposed to SSRIs in utero had lower levels of the neurodevelopmental marker reelin (Brummelte et al., 2012). As reelin, a protein responsible for proper neuron migration (Tissir and Goffinet, 2003), usually decreases with increasing gestational age (Ikeda and Terashima, 1997; Brummelte et al., 2012) the lower levels in SSRI exposed infants may indicate accelerated neurodevelopment by the SSRI-induced increased activity of the serotonergic system. On the other hand, 5-HT depletion resulting from tryptophan hydroxylase 2 gene deletion showed significant abnormalities in serotonergic innervation in several rostral brain regions (Migliarini et al., 2013). These data suggest that precise levels of 5-HT are required for the fine tuning of specific brain circuits initial formation, including the serotonergic system itself. It is therefore crucial to determine if, and how, 5-HT signaling during development is impacted by genetic and environmental perturbations known to be associated with increased risk of neuropsychiatric disorders.
INFLUENCE OF GENETIC, AND EPIGENETIC FACTORS
5-HT signaling is dependent on multiple receptors, enzymes, transporters, other neurotransmitters and related genes, thus it is not surprising that a single or cluster of genes cannot account for outcomes related to variations in 5-HT levels. The 5-HT system is comprised of multiple classes of receptors (5-HT1–5-HT7) and related subtypes (Hoyer et al., 1994; Glennon et al., 1995) with related genetic variations that influence receptor function (e.g. David et al., 2005). A case in point is the inhibitory 5-HT1A receptor that acts as both an autoreceptor and post synaptic heteroreceptor regulating 5-HT and other neurotransmitters’ release (Glennon et al., 1995). Variations in receptor 5-HT1A availability and binding have been associated with depression and the therapeutic efficacy of antidepressants (Blier and Bergeron, 1995; Drevets et al., 1999; Parsey et al., 2006). Further, epigenetic modification of the 5-HT3A receptor has been shown to mediate the effect of childhood maltreatment on the severity of several psychiatric disorders (Perroud et al., 2015). However, considering the number of serotonergic receptors and their variations, it is beyond the scope of this paper to review all of them here. For a review of 5-HT receptor-dependent signaling and environmental impact on brain development see: Holloway and Gonzalez-Maeso (2015).
Instead, we are going to focus on allelic variations in a promoter region of the gene that codes for SERT that have been widely studied in relation to behavioral and neuropsychiatric disorders (Caspi and Moffitt, 2006; Munafo et al., 2009) with different genetic variations carrying different risks. The polymorphism of the SLC6A4 gene influences gene transcription, SERT levels, and thus 5-HT reuptake (Heils et al., 1996), therefore it is believed to be a “master controller” of intrasynaptic 5-HT concentrations. Though it should be noted, that intrasynaptic 5-HT has, to our knowledge, not been directly measured in humans or animals yet, it can be assumed that it will be affected based on altered transporter levels and efficiency. Variations in the way SERT protein controls extracellular and synaptic 5-HT may therefore play a critical role in moderating environmental influences and developmental risk (Murphy et al., 2008; Homberg and Lesch, 2011).
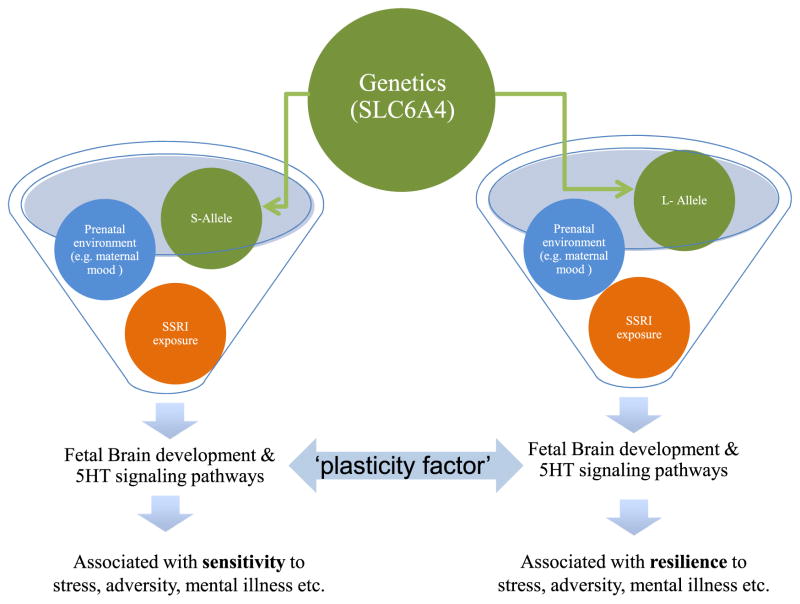
Allelic variations for SLC6A4 may shape sensitivity to negative as well as positive environments (Way and Taylor, 2010) suggesting that not all factors that affect 5-HT signaling will influence outcomes in the same way. In this way allelic variations offer critical insights into how altered early 5-HT signaling might have lasting developmental consequences. Two functional alleles, a long (l) (16 repeats of 20–23 base pair sequence) and a short (s) (14 repeats) have been identified (Heils et al., 1996). The short allele is associated with reduced SERT binding in the limbic region of the brain, lower SERT expression, and decreased 5-HT reuptake into presynaptic neurons (Collier et al., 1996). Compared with the long form, the short allele is associated with reduced SERT protein availability and function (Luddington et al., 2009) pointing to a higher effective “5-HT dose”. Considerable evidence points to associations between the short allele and an increased stress sensitivity, emotional disturbances and risks for cognitive, mood and personality disorders (Schinka et al., 2004; Sen et al., 2004; Karg et al., 2011), though not all studies have replicated these relationships (Willis-Owen et al., 2005). See Fig. 1.
Fig. 1.
There is a complex interaction between genetic phenotype (s or l alleles for the SLC6A4 gene), in utero SSRI exposure, prenatal and postnatal environment to influence the 5-HT system. Depending on this interaction, certain conditions or environments can be protective or associated with an increased risk of mental illness later in life. Changes in central 5-HT levels during developmentally sensitive periods may act as ‘plasticity factors’ rather than ‘risk factors’ associated with vulnerability.
Environmental influences
Developmental susceptibility to early life exposures to adversity varies greatly putting some but not all children are at risk. Thus incorporating information about interactions between genetic variations and the postnatal (and possibly prenatal) environment offers ways to explain individual heterogeneity in developmental outcomes (Boyce and Ellis, 2005; Moffitt et al., 2005). Allelic variations also influence a differential susceptibility to environmental context (i.e. Gene × Environment (G × E) interactions) (Moffitt et al., 2005; Caspi and Moffitt, 2006; Belsky and Pluess, 2009), but not every environmental influence that affects 5-HT signaling carries the same developmental risk. Allelic variations in SLC6A4 have been widely studied as a key moderator of early life environmental influences. In a seminal paper, Caspi and colleagues reported that childhood maltreatment predicted adult depression among individuals with at least one copy of the short allele (Caspi et al., 2003). Since then, the short allele has been widely studied as a crucial moderator of risk of mental illness later in adulthood (Schinka et al., 2004; Sen et al., 2004; Kendler, 2005; Kendler et al., 2005; Lesch, 2007, 2011; Karg et al., 2011). However, not all outcomes associated with having the short allele are negative (Risch et al., 2009) and not all studies have replicated these relationships (Willis-Owen et al., 2005), raising questions about the role of other factors that may underlie G × E interactions (Duncan, 2013).
To further elucidate G × E interactions, attention has turned to understanding the role of concurrent environment, as distinct from prenatal exposures. While adults with two short alleles may be at increased risk of depression following early adversity, those raised in a nurturing environment may have a lower risk of depressive symptoms (Taylor et al., 2006), suggesting heightened susceptibility to the environmental conditions, whether positive or negative. In this way increased central extracellular 5-HT associated with the SLC6A4 short allele may contribute to an increased sensitivity to environmental stimuli or hypervigilance, leading to adaptation on the one hand or increased risk of mental health disorders on the other. In other words, in a low reward or low adversity setting, such hypervigilance may confer an actual benefit that increases processing of relevant stimuli which improves learning and social cognition (Homberg and Lesch, 2011). In contrast, the long allele has been associated with a bias toward positive affective material and an avoidance of negative (Fox et al., 2009), which may be a disadvantage in some environments.
Changes in 5-HT signaling early in life, either directly associated with allelic variations in SLC6A4 or via an early caregiving context, may shape a susceptibility to both negative and positive outcomes. Allelic variations in SLC6A4 in newborns have been shown to influence neonatal behavior, revealing that both increased and decreased 5-HT levels can influence developmental outcomes. Even before birth, the impact of self-report of maternal anxiety at 20-week gestation on mother-rated infant-negative emotionality is already taking shape, moderated by the SLC6A4 genotype. A case in point is the finding that greater antenatal maternal anxiety and higher levels of infant negative emotionality were observed in infants with two short alleles, suggesting an element of context-dependent prenatal programing which is not present under conditions of low maternal stress (Pluess et al., 2011). Neonates with two short alleles undergoing a painful event (heel lance) have significantly higher cortisol responses than those with two long alleles (Mueller et al., 2010). Among neonates with in utero SSRI antidepressant exposure – which conceivably increases central 5-HT levels – behavioral disturbances (as reflected by lower 5 min Apgar scores and risk of neuromotor symptoms) are more evident among those with the short allele (Oberlander et al., 2008a). In contrast, risk of respiratory distress was higher in SSRI exposed neonates with two copies of the long allele (Oberlander et al., 2008a).
Everyday context during infancy also counts. At 6 months postpartum, mothers with allelic variations that reduce 5-HT reuptake (the s alleles) were found to be more sensitive to their infants than mothers with two l alleles. In contrast to the G × E studies cited above, an interaction emerged whereby mothers with the l allele within a negative care experience were more likely to orient away from their infants, while mothers with the s allele and a positive care experience reported better perceived attachment (Mileva-Seitz et al., 2011). These findings raise intriguing questions about the differential effects of the allelic variations s and l alleles during the antenatal period, and how maternal 5-HT might mediate postnatal relationships with her infant and how maternal genotype shapes the infant’s early environment. Importantly, infant genotype was not reported in this study, but the impact of the maternal genotype may not only depend on environmental interaction but will also depend on the infant’s allelic variations. These findings may reflect 5-HT “signaling by proxy”, whereby maternal allelic variations shape the postnatal environment during a maternal–infant interaction. While our understanding of the impact of maternal allelic variations and her 5-HT signaling has on infant development still remains largely unknown, these findings raise intriguing questions about how maternal 5-HT signaling-meditated changes in an early environment might also influence early infant development. Together, such evidence illustrates that changes in serotonergic signaling, either directly associated with allelic variations in SLC6A4 or via an early experience or context, may affect early developmental vulnerability.
By 3 years of age, in children with reduced SERT expression (two short alleles), antenatal exposure to maternal mood predicted increased anxious and depressed behavior (Oberlander et al., 2010). In contrast, 3-year olds with two copies of the long allele, presumably leading to increased 5-HT reuptake, coupled with late gestational maternal mood disturbance, were more likely to exhibit externalizing behaviors. Here the impact of antenatal maternal mood on child mood was moderated by child SLC6A4 genotype, possibly reflecting “fetal serotonergic programing” and the impact of early changes in central 5-HT signaling via child or even maternal genetic variations. The idea of fetal programing of the stress response system due to prenatal glucocorticoid exposure has been well established for over a decade (Seckl and Meaney, 2004; Turecki and Meaney, 2016). More recently, evidence is accumulating suggesting that other systems may be similarly ‘programmable’, i.e., that neonatal experiences can have a long-lasting effect on brain development and thus offspring behavior later in life. In line with this, a recent study in 54 healthy human mothers revealed an association between glucocorticoid-related placental mRNA levels and infant regulatory behaviors, but, more importantly, they also found that higher placental mRNA levels of SLC6A4 were associated with more regulatory behavioral challenges of the infants (Raikkonen et al., 2015).
Together such findings illustrate processes whereby allelic variations for SLC6A4, may confer differential vulnerability to depressive or other disorders associated with stressful events in early life (Devlin et al., 2010). Individuals with two short alleles have been observed to be more affected by both negative and positive environments. In the face of early life adversity such genetic variants may both increase risks of depression as well as improve cognitive function and enhance sensitivity to relevant environmental stimuli (Eley et al., 2004; Schinka et al., 2004; Belsky and Pluess, 2009). Such hypervigilance may be adaptive and enhance emotional response to environmental conditions, drawing attention away from stimuli predicting adversity (or reward) (Homberg and Lesch, 2011). Alternatively, in a non-threatening environment, such responses may be maladaptive or even become antecedent to psychopathology. While the short alleles may reflect an increased sensitivity to negative social contexts, they also confer sensitivity to positive life circumstances. In this sense, allelic variations function less like factors predicting ‘vulnerability’, but rather like a ‘plasticity factor’ (Belsky and Pluess, 2009).
Such findings are consistent with a conceptual framework of fetal programing. Two models have been proposed to explain how genotype interacts with maternal mood to predict infant temperament. In the diathesis-stress model a particular allele (i.e. s) carries with it an increased risk of negative outcomes when exposed to an adverse event (maternal mood, SSRIs). Individuals without the ‘risk allele’ would not have this sensitivity to such an environmental exposure. In contrast, a differential susceptibility model (Belsky and Pluess, 2009) has been proposed that offers another perspective to account for why some but not all individuals may be susceptible to negative consequences of adverse experiences (Burmeister et al., 2008). This conceptual framework proposes that some individuals are more susceptible to both positive as well as negative environments (Belsky and Pluess, 2009). With this perspective SLC6A4 can be regarded as a “plasticity gene”, rather than a “vulnerability” gene.
Epigenetic influences
The interactions between SLC6A4 alleles and environmental influences offers a promising mechanism to explain how experience ‘gets under the skin’ (Hertzman and Boyce, 2010), however not all G × E interactions lead to adverse outcomes and increasing attention has been paid to epigenetic mechanisms that also influence the function of SLC6A4 (Booij et al., 2013). Beyond the fixed sequence that comprises DNA, epigenetic processes – chemical modifications to DNA that influence gene expression without altering nucleotide sequences – are triggered by external environmental influences (Weaver et al., 2004; Whitelaw and Whitelaw, 2006; Szyf et al., 2007; Oberlander et al., 2008c) and are central to operational genetic function. DNA methylation is a stable epigenetic marker, though it is potentially reversible (Ramchandani et al., 1999). DNA methylation influences gene expression by interrupting binding of transcription factors and indirectly by targeting proteins that suppress gene expression (Booij et al., 2013). Notable changes are those that arise through changes in methylation at DNA cytosine–phosphate–guanosine (CpG) sites, often found in proximity to transcription promoter regions.
Epigenetic mechanisms are increasingly recognized as a critical factor in the relationship between early life experience and risk of psychopathology (Roth and Sweatt, 2011). For instance, McGowan et al. (2009) could link childhood abuse to increased methylation and decreased transcription of the glucocorticoid receptor gene using human brain tissue from suicide victims. This study was the first to translate findings from animal studies (showing a link between increased methylation and decreased expression of the glucocorticoid receptor after early adversity) to human brain tissue (McGowan et al., 2009). Previously, Oberlander et al. had shown a correlation between maternal mood and neonatal methylation status of glucocorticoid receptor (NR3C1) using human cord blood (Oberlander et al., 2008c). Further, altered DNA methylation patterns for several genes involved in important steps of development such as growth and forebrain formation were found in older adults who were exposed to famine during their perinatal development, compared to their siblings that did not experience in utero famine (Tobi et al., 2014). In line with this, Essex et al. (2013) reported an association between maternal stress during early childhood and altered methylation levels (increased or decreased) in multiple genes in adolescent boys and girls. Interestingly, this study also demonstrated a gender effect, with fathers’ reported stressors more strongly associated with girls’ epigenetic modifications and mothers’ more related to those of both sexes (Essex et al., 2013). Greater methylation of cytosine nucleotides are associated with reduced gene transcription and have been shown in response to a wide variety of environmental exposures such as toxins, diet, and stressful life events (Caldji et al., 2011). Thus, not surprisingly, recent research has also focused on methylation of 5-HT-related genes to try to further explain how regulation of gene expression results in behavioral changes.
Methylation patterns for SLC6A4 in peripheral tissues, such as blood and saliva have been associated with early life adversity and psychiatric disorders such as depression (Booij et al., 2015a) in both animal models and human. In rhesus monkeys reared in a nursery, increased SLC6A4 methylation and increased separation anxiety was observed compared with mother-reared infants (Kinnally et al., 2010). Using lymphoblast cells, Philibert et al. (2008) found a trend for links between increased SLC6A4 methylation and a life history of depression (Philibert et al., 2008). Further, people with a history of many traumatic events were at an increased risk of PTSD but only at lower methylation levels. At higher methylation levels, individuals with more traumatic events were protected from this disorder (Koenen et al., 2011).
Childhood adversities such as child abuse or aggression also appears to be related with subsequently increased SLC6A4 methylation in peripheral blood of adults (Beach et al., 2010; Wang et al., 2012; Kang et al., 2013). Further, 10-year-old twins that experienced bullying had higher DNA methylation of SLC6A4 in buccal cells compared to their non-bullied twins (Ouellet-Morin et al., 2013). An increase in maternal depressed mood mid-gestation has been associated with a lower methylation status in the key promoter region for SLC6A4 (Caldji et al., 2011), indicating that in utero maternal experience may epigenetically alter key components of serotonergic signaling. Taken together, early adverse experiences may be associated with methylation levels in the HPA axis stress related genes as well as the SLC6A4 gene (Booij et al., 2013) and altered methylation may reflect a developmental mechanism altering 5-HT signaling that shapes the risk of self-regulatory behaviors and mood disorders.
Importantly, higher SLC6A4 methylation levels in T cells and monocytes have been associated with lower in vivo brain 5-HT synthesis (Wang et al., 2012), thus supporting the relevance of peripheral markers of DNA methylation for central 5-HT function (Wang et al., 2012). In line with this, greater SLC6A4 methylation in whole blood was associated with lower hippocampal volumes (Booij et al., 2015a) and brain activity in response to emotional stimuli in relevant brain regions (Frodl et al., 2015). However, methylation levels may differ by genotype, thus adding another level of complexity to the association of childhood experiences, epigenetic mechanisms and behavioral outcome. For instance, higher mean methylation and lower SLC6A4 levels were found in peripheral blood mononuclear cells (PBMCs) of ss carrier rhesus monkeys (Kinnally et al., 2010). In humans, depressive symptoms were more commonly observed in adolescent carries of the s allele with elevated methylation in buccal cells (Olsson et al., 2010). Further evidence for this link between genotype, methylation status and mental illness comes from a study investigating the association between SERT polymorphism, methylation and dealing with loss or trauma. Increased methylation of the SERT promoter region in lymphoblast cells was associated with increased risk of unresolved trauma in people carrying the l allele, while the opposite was true for carriers of two short alleles, suggesting that the genotype predicted more unresolved trauma when methylation levels were low (van et al., 2010). Thus, it is essential to take the genotype into consideration when investigating the epigenetic profile of the 5-HT system after early adversity.
PHARMACOLOGICAL MANIPULATIONS: DEVELOPMENTAL CONSEQUENCES OF PRENATAL SSRI ANTIDEPRESSANT EXPOSURE
Since the late 1980s maternal mood disorders (depression and anxiety) during pregnancy have been treated with SSRI antidepressants and more recently with ‘non-selective’ serotonin reuptake inhibitors that may also target the re-uptake of other neurotransmitters such as norepinephrine (SNRIs) or dopamine. SSRIs primarily act by blocking SERT at the presynaptic neuron, which regulates extracellular 5-HT levels (DE Montigny et al., 1990). Between 8% and 13% of pregnant women are prescribed antidepressants with SSRIs being the most common type (Cooper et al., 2007; Andrade et al., 2008; Patil et al., 2011) and given that they readily cross the placenta and enter the fetal circulation (Kim et al., 2006; Rampono et al., 2009) questions arise about the impact of altering fetal 5-HT levels during developmentally sensitive periods on early brain growth and function.
Direct impact of transplacental SSRI exposure may not be the only source of altered fetal 5-HT signaling though. High expression of SERT in the placenta supports the notion that SSRIs could have high binding affinity sites in this organ (Yavarone et al., 1993; Shearman et al., 1998; Verhaagh et al., 2001). If blocking placental SERT function alters 5-HT synthesis and/or transport to the fetus, or maternal 5-HT degradation, SSRIs could affect fetal brain development via altered placental physiology. In a rodent model, 4 h of sertraline exposure increased placental phosphoglycoprotein efflux resulting in decreased drug transfer to the fetus. However, sertraline decreased phosphoglycoprotein-mediated efflux through the blood–brain barrier thus resulting in increased drug transfer into maternal and fetal brain tissue (Bhuiyan et al., 2012). The impact of SSRIs on fetal brain development may therefore result from direct actions on the fetal brain, indirect actions on placental or maternal physiology or, more likely, a combination of all these routes.
5-HT and the impact of maternal mood and stress: animal and human findings
At the outset we need to recognize that stress and depression may be two different phenomena in animal models and these terms cannot be used interchangeably. While chronic stress can be a susceptibility factor for the development of depression in some humans, not all individuals that experience stress develop depression. Chronic stress is often used in rodents to model certain aspects of depression, however, even here, responses across individual animals vary quite greatly. Animal studies investigating the effects of the impact of maternal stress alone provide support of a link between maternal stress and 5-HT signaling alterations. Using a rodent model of chronic mild unpredictable stress before pregnancy, thus mimicking a depressive phenotype before gestation, researchers found increased levels of 5-HT in the hippocampus and hypothalamus of fetuses (gestational day 20) in the stressed group compared to controls. Further, hippocampal 5-hydroxyindoleacetic acid (5-HIAA) levels, the ratio of 5-HIAA to 5-HT as well as 5-HT1A receptor and SERT levels were significantly lower for fetuses in the pre-gestational stress group (Huang et al., 2012). However, preliminary data from our group using a corticosterone-induced model of maternal depression (Brummelte et al., 2006) indicate no difference in neonatal 5-HT levels in brain tissue between rat pups from dams pre-conceptionally exposed to high levels of corticosterone compared to controls, but lower 5-HT levels in the frontal cortex of newborn pups from SSRI treated dams (Mooney-Leber et al., 2014). Similarly, animal studies investigating prenatal stress have found varying results such as reduced SERT levels and 5-HT1A receptor binding in the hippocampus (Van den Hove et al., 2006; Mueller and Bale, 2008), but increased 5-HT and tryptophan hydroxylase expression within the dorsal raphe nuclei (Miyagawa et al., 2011). Taken together, animal studies using depression models suggest that maternal distress and mood may have a significant impact on the serotonergic system but more research is needed to better understand the exact mechanisms and direction of the alterations.
In humans, 15–20% of women experience mood disorders (e.g. depression) during their pregnancy and a third are treated with an SSRI antidepressant (Oberlander et al., 2006). A key challenge to understanding the developmental consequences of SSRI exposure is appreciating how maternal mental health that leads to SSRI treatment also affects neurodevelopment (Glover, 2011) as well as fetal serotonergic signaling (Field et al., 2004b, 2008). However, up to 50% stop medication within the first 60 days of pregnancy (Vesga-Lopez et al., 2008; Warburton et al., 2010). Women who discontinue antidepressant medication early in gestation have a higher risk of relapse (68%) compared to those who maintain treatment (26%) (Cohen et al., 2006). Even in the presence of prenatal SSRI treatment, for some women, maternal mood disturbances continue and there are ongoing developmental risks. Antenatal maternal stress disrupts fetal neurobehavioral development (DiPietro et al., 1996; Tronick and Weinberg, 1997), alters behavioral reactivity in utero (Monk et al., 2000; Allister et al., 2001), reduces birth weight, and increases risks for prematurity (Talge et al., 2007; Glover, 2011). At present, there are limited data linking antenatal maternal depression to changes in serotonergic signaling in the fetus or newborn. Brain extracellular serotonin levels cannot to be directly studied in newborns, however indirect proxy measures, such as s100B or reelin (see below), provide some evidence that might reflect central serotonin signaling. Elevated cortisol and norepinephrine and low levels of 5-HT and dopamine were observed in neonates of depressed mothers (Field et al., 2004a, 2008) indicating that maternal depression affects fetal serotonergic signaling. The exact mechanisms by which antenatal depression influences fetal brain development and the role of 5-HT in this remain mainly unclear, yet the magnitude of the effect is clinically significant, as approximately 15% of emotional and behavioral problems in childhood are attributable to antenatal stress/anxiety (Talge et al., 2007). Importantly, by school-age a third of children of depressed mothers suffer from depression and anxiety disorders (Pilowsky et al., 2006). A detailed review of the developmental impact of maternal antenatal stress is beyond the scope of this paper, for a systematic review of the effects of untreated antenatal depression on the offspring see Gentile (2017).
Preclinical models of 5-HT manipulations: impact of SSRI exposure
Animal models provide an opportunity to investigate the effect of early manipulations of 5-HT and the consequences of antidepressant exposure thereby enabling us to distinguish the influence of antenatal maternal stress from the impact of the psychotropic medication. Early studies reported increased depressive-like and anxiety-like behavior, but reduced exploratory tendencies in rats exposed to SSRI in the postpartum period (Hansen et al., 1997; Ansorge et al., 2004). However, there is now significant variation in the reported results, i.e., some studies found negative associations, while others found no differences or beneficial outcomes (Kiryanova et al., 2013; Altieri et al., 2015). These variations may be due to numerous factors, but it is clear that SSRIs and early 5-HT manipulations can alter brain development and thus adult behavioral outcome (Kiryanova et al., 2013). In particular, for early fluoxetine exposure decreased exploration and social interaction was associated with anxiety and depressive-like behaviors (Kiryanova et al., 2013). Though other behavioral alterations including, but not limited to, locomotor activity, sexual behavior, aggression, sleep and reward responses have also been observed (Olivier et al., 2011).
Further, physiological changes, altered birth outcomes and mortality rates have been observed with gestational antidepressant exposure. For instance, in utero SSRI exposure decreases uterine and fetal brain blood flow, oxygen saturation and rapid eye movement during sleep in sheep (Morrison et al., 2001) and results in permanent reductions in novelty investigation, impaired social behavior, increased anxiety in conflict tasks, and anhedonia in mice exposed to fluoxetine (Ansorge et al., 2004, 2008; Popa et al., 2008; Simpson et al., 2011). In rodents, several studies have reported lower gestational age, smaller litter sizes and increased mortality rates with prenatal fluoxetine exposure, though others failed to find significant differences (for review see: Kiryanova et al., 2013). The great heterogeneity of the reported data underlines the complexity of studying early adversity and that it is important to take timing, dosing, type of drug, genetic and other environmental factors into consideration. For instance, Pawluski and colleagues reported that differences in drug administration methods and doses affected hippocampal plasticity in the offspring in opposite directions (Pawluski et al., 2014). Further, animal studies using low doses of SSRIs that are more clinically relevant, showed that neonatal pups actually had lower 5-HT levels in the frontal cortex after maternal SSRI treatment (Mooney-Leber et al., 2014). Similarly, a study using chronic (P8–P21) neonatal citalopram treatment at doses that resulted in serum drug concentrations comparable to those obtained in clinical practice, reported a reduction of tryptophan hydroxylase expression in the raphe nucleus complex and a reduction in the density of SERT-expressing fibers in the cortex of male rats (Maciag et al., 2006a). Importantly, in these studies, drug serum levels for dams and their offspring were within the low clinical range, demonstrating that even subtle exposure of offspring to maternal SSRI treatment (many animal studies use high doses that are not clinically relevant) can lead to alterations in the neonatal serotonergic system.
Besides the dosing, it is essential to take the developmental stage in which the fetus or neonate is exposed into consideration when trying to understand the impact of developmental SSRI exposure (Olivier et al., 2011). Animal studies suggest, that early gestational exposure may have a very different outcome compared to neonatal SSRI administration. In fact, the highest risk of negative effects may be in the first trimester when it is most likely for women to be taking antidepressants (Louik et al., 2007), though other studies have shown more profound effects during the postnatal period in rodents (akin to the 3rd trimester in humans) (e.g. Simpson et al., 2011). Biobehavioral and physiological effects are apparent even before acute in utero exposure ends and can persist beyond birth (Hanley and Oberlander, 2012). For instance, neonatal administration of citalopram led to aberrant axon and oligodendrite soma morphology and altered myelination of callosal axons, which could indicate abnormalities in neural network wiring and interhemispheric connectivity (Simpson et al., 2011). However, the precise effects and even the direction of the effects cannot be predicted yet based on the available animal or human data and the underlying mechanisms remain to be resolved (Kiryanova et al., 2013). As indicated in the first part of this review this diversity in outcomes may be linked to the diverse timing of 5-HT receptors, synthetic and metabolic enzymes developmental patterns of expression (Booij et al., 2015b). Further, molecular studies showed that the different 5-HT receptors each modulate specific developmental processes such as neurogenesis and dendritogenesis during particular developmental stages (Gaspar et al., 2003).
Interestingly, there seems to be a sex difference in 5-HT maturation as well as in the vulnerability to 5-HTmediated disruptions in brain development. Maciag et al. (2006b) reported that neonatal citalopram only affected males, but not females, while Simpson et al. (2011) reported that males seemed to be more severely affected than females. In line with this, high-performance liquid chromatography results suggest that males and females differ in their early 5-HT levels with male pups having much higher 5-HT levels and showing a prominent peak at P3, while female pups have a more gradual elevation at P5 (Connell et al., 2004). Further, in vivo micro dialysis studies in adult mice have shown that stimulated 5-HT release differs in males and females and depends on the estrous cycle phase of the female (Yang et al., 2015). Thus, it is conceivable that males and females have different sensitivities to elevated levels of 5-HT depending on their age, which further underlines the importance of taking age and sex into consideration when studying SSRI exposure. This is in line with findings in humans, where SSRI-exposed female neonates revealed lower reelin levels compared to SSRI-exposed males or controls (discussed below, Brummelte et al., 2012). Such sex differences suggest that SSRI-exposure may affect males and females differently depending on the sex-specific maturation of the 5-HT system.
Taken together, both, animal models using SSRI exposure, as well as genetic models that lack SERT which affect overall 5-HT uptake in the brain and periphery, have tremendous potential to help improve our understanding of the consequences of early 5-HT disturbances. Altered 5-HT during development leads to behavioral, neurophysiological and neuroanatomical changes (Charney and Manji, 2004; Homberg et al., 2010). Perinatal exposure to SSRI antidepressants during critical developmental periods affects gene expression, alter neuronal signaling, protein metabolism and neural plasticity in the rat brain, which may lead to functional disturbances (Homberg et al., 2010; Liao and Lee, 2011; Olivier et al., 2011; Simpson et al., 2011; Zheng et al., 2011). Genetic and pharmacological manipulations in animal models reveal a link between 5-HT and cortical development which in turn may be linked to anxiety and altered explorative behavior, via decreased serotonergic tone in adult animals (Hensler, 2006). Paradoxically, increased intrasynaptic 5-HT during developmentally sensitive times appears to lead to a down-regulation or constrained maturation of the 5-HT system, which in turn could contribute to an increased predisposition to affective disorders later in life (Ansorge et al., 2004, 2008).
However, not all SSRI-induced changes in early serotonergic signaling lead to negative outcomes. For instance, though SERT knockout models have shown profound changes in serotonergic receptor levels and function that are associated with higher anxiety, negative emotionality and reduced social behaviors, these rodents also displayed increased memory and decision making when faced with ambiguity (Kalueff et al., 2010). This is consistent with improved spatial learning observed following prenatal SSRI exposure (Bairy et al., 2007). Most importantly in relation to the clinical context, early SSRI exposure in animal models appears to also reverse some of the adverse effects of prenatal stress exposure. Postnatal fluoxetine treatment during week 1– 3, but not later, ‘rescued’ corticosterone stress response and hippocampal morphology (CA3 dendritic spine and synapse density) otherwise observed after prenatal stress (Ishiwata et al., 2005). This also partially restored the ability to learn spatial information compared with the effects of exposure to prenatal stress alone. Consistent with these observations, Rayen et al. (2011) showed that postnatal maternal fluoxetine treatment reversed the negative effect of prenatal stress on hippocampal neurogenesis and immobility in the FST in adolescent offspring (Rayen et al., 2011). Moreover, post-operative pain was normalized in prenatally stressed adult offspring when their dams were given fluoxetine (Knaepen et al., 2013). These findings emphasize the importance of studying SSRI exposure in the light of maternal stress in animal models which allow us to clearly discriminate the developmental effects of SSRIs or other 5-HT manipulations from the impact of in utero exposure to maternal psychological distress.
Animal models highlight three key points regarding early-manipulations of 5-HT signaling associated with early SSRI exposure. First, biobehavioral physiological effects are apparent even before acute in utero exposure ends and persist beyond birth. Second, early altered 5-HT signaling during developmentally sensitive periods – either from genetic variations or pharmacologically induced 5-HT reuptake blockade alters the maturation and function of the 5-HT system (Ansorge et al., 2004) either for better and/or worse. These changes are associated with alterations in brain structure leading to altered anxiety, exploratory and depression-like behavior during adulthood. Third, SSRIs also appear to alter stress regulation and cognitive functions that reflect both risk and resilience that could shape mental and physical health across the life span. These findings might help guide our understanding of the impact (both positive and negative) of early SSRI exposure in humans.
Developmental SSRI exposure: human clinical evidence
To date, gross structural neuroteratogenic effects have not been identified in humans following in utero SSRI exposure, yet evidence of sustained functional behavioral disturbances is emerging (Hanley et al., 2015) possibly reflecting sustained effects related to changes in early 5-HT signaling. During the 3rd trimester, SSRI exposure reduces Doppler brain flow resistance indices (pulsatility index) and fetal heart rate variability (Rurak et al., 2011). Reduced middle cerebral artery cross-sectional areas have been observed with SSRI exposure and coupled with increased cord red blood cell indices at birth suggest fetal hypoxia associated with altered early blood flow. Importantly, these effects were observed both before and following a typical daily maternal SSRI dose and none were associated with antenatal maternal mood, suggesting an early and sustained medication-related effect, rather than a transient finding. Whether these changes relate to 5-HT function and represent the early origins of altered neonatal or childhood neurobehavior remains to be determined.
Soon after the introduction of SSRIs in the late 1980s, their off label use to manage mood disorders during pregnancy led to reports of neonatal neurobehavioral disturbances (irritability, weak or absent cry, increased motor activity), shorter mean gestational age and lower birth weight appeared (Zeskind and Stephens, 2004; Moses-Kolko et al., 2005). Subsequent studies reported that SSRI-exposed neonates were more motorically active and tremulous, and had fewer variations in heart rate and state regulation. While many of these effects may be mediated through the effects of SSRI exposure on reduced gestational age at birth (Zeskind and Stephens, 2004), neonatal behavioral disturbances have been directly linked with increased cord drug levels (Oberlander et al., 2004) and monoamine neurotransmitter alterations (Laine et al., 2003; Hilli et al., 2009). In a recent meta-analysis examining the impact of prenatal SSRI exposure, Ross et al. (2013), reported that significant decreased gestational age, birth weight, and Apgar scores were observed among infants exposed to antidepressant medications in utero, though the clinical impact of these effects were small.
In utero SSRI exposure also appears to change neonatal and infant stress regulation. In response to an acute painful event, the duration of facial action and cardiac responses – particularly parasympathetic cardiac activity – is shorter and less intense in SSRI exposed neonates (Oberlander et al., 2002). Such altered pain reactivity persists at 2 months of age, after controlling for drug level and maternal mood (Oberlander et al., 2005). Such neurobehavioral changes have been associated with cord blood levels of the 5-HT metabolite 5-HIAA (Laine et al., 2003), reflecting altered in utero serotonergic metabolism. SSRIs, possibly via increased central 5-HT activity, may also “normalize” the hypercortisolism that characterizes depression (Barden et al., 1995). In an animal model, Ishiwata et al. (2005) observed that early fluoxetine treatment of prenatally stressed mice “normalized” corticosterone responses to a subsequent stressor, increased 5-HT turnover in the hippocampus and restored the ability to learn spatial information compared with the effects of exposure to prenatal stress alone. At 3 months of age, in prenatally SSRI-exposed infants, altered HPA stress response patterns and early evening basal cortisol levels have been reported (Oberlander et al., 2008b). In response to a non-noxious challenge, SSRI-exposed infants responded with increased salivary cortisol levels similar to infants of healthy mothers and it was only when method of feeding was taken into account that the effects of prenatal exposure became apparent, namely, in comparison with SSRI- and non SSRI-exposed infants who breastfed, non-breastfeeding infants without prenatal SSRI exposure had a blunted post-stress cortisol pattern. Interestingly, among SSRI-exposed infants, where stress reactivity patterns did not differ with mode of feeding, these findings suggesting an early SSRI “programing” effect on the HPA stress system that was only apparent in a particular postnatal maternal care giving context.
Direct evidence linking altered neonatal behavior and SSRI-induced alterations of the 5-HT system remains limited but emerging reports point to possible links. It remains unclear whether alterations in infant behavior and stress reactivity observed following in utero SSRI exposure are the direct effect of altered 5-HT signaling. The severity of neonatal behavioral disturbances in SSRI-exposed newborns has been associated with reduced levels of 5-HIAA in cord blood (Laine et al., 2003). Reduced cord blood 5-HT, homovanillic acid (HVA, a major catecholamine metabolite), norepinephrine levels, reductions in 3,4-dihydroxyphenylglycol (DHPG, a norepinephrine metabolite) and dihydroxyphenylacetic acid (DOPAC, a major dopamine metabolite) have been reported in SSRI-exposed neonates suggesting alterations in catecholamine function (Hilli et al., 2009).
More indirect evidence for 5-HT alterations associated with SSRI exposure reveals that SSRI-exposed neonates have lower cord blood levels of the astroglial-specific calcium-binding protein S100B, a biomarker of early brain maturation and central serotonergic function (Pawluski et al., 2009). In addition, SSRI-exposed infants have lower umbilical levels of the glycoprotein reelin at birth (Brummelte et al., 2012). Reelin is essential during neurodevelopment as it is involved in neuron migration and positioning (Tissir and Goffinet, 2003). Reelin-expressing Cajal-Retzius cells are one of the early targets of the in-growing 5-HT afferents during brain development and express 5-HT3 receptors (Chameau et al., 2009), which may directly or indirectly control the amount of reelin release. The link between early 5-HT manipulations and the reelin system is further evidenced by a study using pregnant mice injected with a non-specific serotonergic receptor agonist: neonatal pups exposed to the serotonergic agonist in utero had lower cortical reelin levels and altered cortical arrangements (Janusonis et al., 2004), which may suggest that SSRI-induced alterations in early 5-HT levels may affect cortical developmental via the reelin signaling pathway.
Reports of lower levels of biomarkers of brain maturation such as s100b and reelin proteins with SSRI exposure suggest a negative impact of the antidepressant drug exposure during development. However, they may also be interpreted as support to the notion that in utero SSRI exposure and elevated levels of 5-HT accelerate brain development (also see discussion below). Reelin levels usually decrease with increasing brain maturation (Ikeda and Terashima, 1997; Meyer et al., 2002) and increasing gestational age (Brummelte et al., 2012), so that more mature brains would express less of this biomarker. If SSRI exposure in utero led to increased 5-HT levels which in turn triggered earlier or increased release of reelin, then that may have accelerated or shortened key neurodevelopmental processes, which in turn may be reflected in lower reelin levels in cord blood at birth (Brummelte et al., 2012).
Emerging evidence suggests that long-term development and behavior during infancy and childhood may also be affected by in utero SSRI exposure. SSRI-exposed infants display altered stress and pain regulation and significantly lower scores on gross motor, social-emotional, and adaptive behavior, even controlling for pre- and postnatal depressed maternal mood (Hanley et al., 2013). Further, SSRI-exposed toddlers displayed poorer social behavior and psychomotor development (Casper et al., 2003; Hanley et al., 2013), however, as they grow into early childhood a more nuanced picture of development begins to emerge.
In early childhood, behavioral outcomes continue to reflect a “main effect” for some but not all children (Galbally et al., 2015) with prenatal SSRI exposure (Oberlander et al., 2002). Beyond infancy, typical patterns of development have been reported (Nulman et al., 1997) but life with a depressed mother continues to influence outcomes (Oberlander et al., 2010). Poor psychomotor development (PDI, Bayley) was observed in 6–40 months olds (Oberlander et al., 2007), though the influence of maternal mood remained unclear in that study. Increased duration of maternal depression and number of depressive episodes predicted lower IQ and language scores in fluoxetine-exposed children (15–70 months) (Nulman et al., 1997; Croen et al., 2011). At 3 years of age, prenatal antidepressant exposure and current maternal mood equally predicted increased reports of internalizing behaviors (Oberlander et al., 2010). Further, increased internalizing behavioral disturbances (Oberlander et al., 2007) in prenatally SSRI exposed 4-year olds were associated with increased maternal depressive symptoms. Externalizing behaviors in SSRI-exposed children were also associated with increased cord drug levels and using a direct observation of behavior in a laboratory setting reduced persistence and increased aggressiveness was observed, particularly in children with a history of neonatal withdrawal symptoms (Oberlander et al., 2007). Such findings suggest that early changes in 5-HT signaling as well as neonatal neurobehavioral disturbances associated with in utero SSRI exposure increase childhood behavioral vulnerability for some but not all children.
In line with this, evidence is emerging indicating that among some infants and children for certain behaviors, prenatal SSRI exposure may “buffer” against the effects of maternal mood disturbances. For instance, in a study investigating infants’ response to a social emotional task at 3 months of age higher levels of maternal depression symptoms predicted poorer infant readiness to interact with mothers during the toy play session. However, for SSRI-exposed infants, higher prenatal depression predicted greater infant readiness to interact at 3 months (Weikum et al., 2012). These findings may be among the first pointing to a “SSRI exposure programing effect” that may buffer an infant from adversity inherent to a particular postnatal environment with a depressed mother.
Importantly not all outcomes reflect patterns of adverse development and SSRIs may, in fact, accelerate key milestones of neurodevelopment, however, such premature closing of critical developmental windows may not necessarily be advantageous. Studies of fetal auditory and visual discrimination, revealed that SSRI-exposed fetuses showed accelerated language development by discriminating both, vowels and consonants at 36-week gestation while non exposed fetuses only responded to a change in vowel but not the more difficult consonant sounds as expected at that age (Weikum et al., 2012). Later in infancy, these control infants responded as expected on a non-native speech and visual language discrimination task at 6 and 10 months, but the SSRI-exposed infants failed to discriminate the language differences, suggesting a developmental shift in speech perception and an already ‘closed’ window for language discrimination. In other words, while non exposed infants of the non-depressed mothers showed the typical pattern of language discrimination, the SSRI-exposed infants displayed an accelerated timing of perceptual development. In contrast, infants of depressed mothers exhibited a delay, or a shift, in early language perception development that lasted to at least 10 months. While our understanding of what such developmental shifts might mean for long-term development remains uncertain, a recent population-based cohort study reported that the use of SSRI during pregnancy was associated with lower language skills at age three, independent of depression raising critical questions about the long-term developmental implications of early mistiming in language development (Levitt, 2011).
These concerns have been further raised by recent studies linking in utero SSRI exposure to an increased risk of complex disorder development such as autism spectrum disorders (Gentile, 2015; Boukhris et al., 2016) (Croen et al., 2011; Rai et al., 2013; Sorensen et al., 2013; Gidaya et al., 2014; Clements et al., 2015; Man et al., 2015). An initial report found a twofold increase in risk in SSRI exposed children (6.7% vs 3.3%) (Croen et al., 2011), even when controlling for maternal psychiatric history, demographics and co-morbidities. This has been confirmed in recent findings suggesting that boys may be at increased risk (Harrington et al., 2014) however maternal mood equally continues to have an impact (El et al., 2014). Others report that the effect was no longer significant when controlling for the maternal depression status (Sorensen et al., 2013; Clements et al., 2015). Moreover, a recent study reported a 30% increase in risk of autism spectrum disorders if fathers were taking an SSRI (Sorensen et al., 2013), suggesting a more complex relationship between paternal depression, treatment and the risk of autism beyond a simple teratogenic effect. One proposed mechanism, influenced by finding that approximately a third of children with autism have elevated serotonin levels, is that increased serotonergic activity in utero can impact the subsequent emotional and behavioral development in children; presenting as symptoms of autism (Whitaker-Azmitia, 2005; Gentile, 2015). Despite a number of studies reporting an association between SSRI use and autism, findings are far from conclusive. Research is still limited by sample size, lack of person specific clinical diagnostic data, variations in diagnostic criteria, limited follow-up beyond childhood, the ongoing difficulty distinguishing the severity of maternal mood disturbance from a direct medication effects (confounding by indication), and residual confounding secondary to yet unmeasured maternal health and social characteristics.
Gene × drug exposure effects
Beyond the impact of genetic variations and prenatal medication exposure alone, examining the interaction between SLC6A4 variations and the child’s environment may provide additional insight into risk associated with in utero SSRI exposure. Importantly allelic variations may shape sensitivity to negative as well as positive environments (Way and Taylor, 2010) and thus could add additional risk or even resilience against the effects of in utero SSRI exposure. Variations in the SERT alleles have been shown to influence the efficacy of SSRIs in adults (Welberg aand Seckl, 2001; Van den Bergh et al., 2008) and thus it is conceivable that the short or long allele may also play a role in mediating the effect of SSRI exposure in utero on the fetus. For instance, SSRI exposed neonates with the short allele of SLC6A4 had lower 5 min Apgar scores and risk of neuromotor symptoms, while neonates with two copies of the long allele had a higher risk of respiratory distress (Oberlander et al., 2008a). Further, infants with at least one short allele had higher levels of the developmentally important protein reelin at birth (Brummelte et al., 2012).
Beyond infancy, evidence of a sustained effect of in utero SSRI exposure on childhood behavior remains limited. Maternal depression during pregnancy can also act as an environmental factor that influences the impact of fetal genotype. At 3 years, children with two short alleles of SLC6A4 – presumably reflecting reduced 5-HT re-uptake, showed more anxious and depressed symptoms if their mothers experienced antenatal depression (Oberlander et al., 2010). In contrast, children with the long alleles and exposed to maternal gestational anxiety were more likely to exhibit aggressive or externalizing behaviors. Consistent with animal models (Ansorge et al., 2004; Kiryanova et al., 2013) increased reports of anxiety and depressive behaviors have emerged in preschool and early school aged children (Hanley et al., 2015), though the impact of concurrent maternal mood remains evident and distinguishing in utero medication effects from multiple genetic and pregnancy related factors (i.e. changes in volume of distribution, weight gain, drug metabolism etc.) which also influence therapeutic efficacy and contribute to increased relapse remains challenging. At 6 years, the impact of prenatal SSRI exposure on executive functioning (EF) varied depending on the child’s SLC6A4 genotype and mother’s concurrent mood (Weikum et al., 2013). For prenatally SSRI-exposed children, regardless of maternal mood, executive control remained stable. As well, even with increasing maternal depressive symptoms, executive control of children with the genetic variant associated with a reduced level of the SERT (at least one short allele) also remained stable and was comparable to children with the same genotype whose mothers reported few, if any, depressive symptoms. In this sense, these children showed resilience in the face of increased maternal depression at 6 years of age. In contrast, non-SSRI-exposed children with the genetic variant that was associated with more of the SERT protein (two long alleles) were more sensitive to context with a depressed mother. When mothers had few depressive symptoms, children with the two long alleles showed extremely good executive function performance – better than any other group. However, when mothers reported more depressive symptoms, executive function performance of children with the l alleles was worse than that of any other group. Thus, in the face of a mother with a more depressed mood, executive functions were best preserved in children prenatally exposed to SSRIs and with at least one short SLC6A4 allele. These findings suggest that even with prenatal exposure to an SSRI, children with two long alleles may have superior EF if their mother’s mood remains stable or improves. This finding may highlight a possibility that increased 5-HT signaling related to SSRI exposure and having one short allele, confers a biological sensitivity to or adaptation to life with a depressed mother (Fig. 2a, b). Here risk was only realized in a particular context and not merely inherent to prenatal SSRI exposure or having a particular genetic variation alone. Together it appears that pharmacological, genetic and environmental factors both together and independently can influence 5-HT signaling and predict increased behavioral vulnerability for some, but not all children. Further, these findings might reflect a process of “serotonergic programing” associated with early changes in central 5-HT either via environmental stressors, genetic variations or fetal SSRI exposure (Oberlander, 2012).
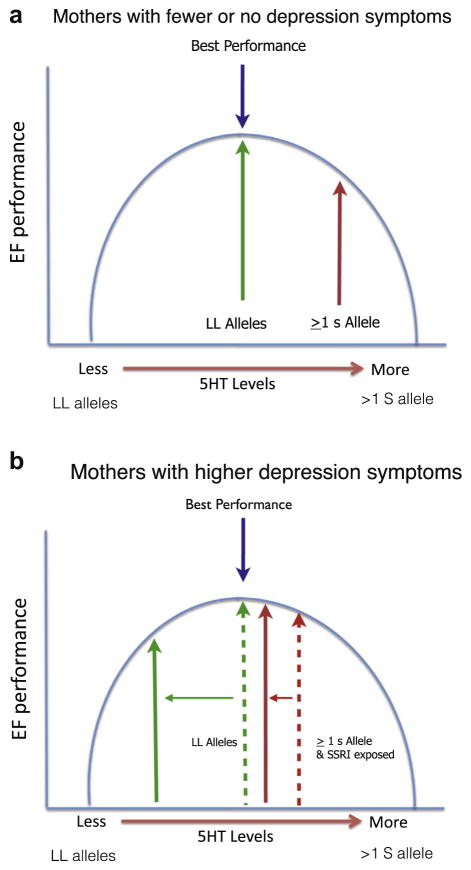
Fig. 2.
The impact of prenatal SSRI exposure on executive functioning (EF) at 6 years varied depending on the child’s SLC6A4 genotype and mother’s concurrent mood (Weikum et al., 2013). When mothers had few or no depression symptoms, children with two long alleles (LL) did extremely well (Fig. 2a). However, when mothers were highly symptomatic (Fig. 2b), LL children performed worse than any other group. In contrast, SSRI-exposed children with at least one short (S) allele showed resilience (no impairment in inhibition and attention), and even in the face of a more symptomatic mothers, accuracy among children with ≥ 1 S allele did not diminish. If a mother had a more depressed mood, executive functions were best preserved in children prenatally exposed to SSRIs and with at least one short SLC6A4 allele, whereas LL children were far more sensitive to the context of life with a depressed mother. These findings suggest that even with prenatal SSRI exposure, LL children may have superior EFs if their mother’s mood remains stable or improves. This finding may highlight a possibility that increased 5-HT signalling (related to SSRI exposure) and having one S allele, confers a biological sensitivity or adaptation to life with a depressed mother. Here, risk only became apparent in a particular context and was not merely inherent to prenatal SSRI exposure or having a particular genetic variation alone. These findings could reflect the impact of “serotonergic programing” associated with early changes in central 5-HT either via environmental stressors, genetic variations or fetal SSRI exposure (Oberlander, 2012).
SUMMARY AND FUTURE DIRECTIONS
5-HT plays a central role in brain development long before regulating mood, stress reactivity and mental health. Developmental alterations in 5-HT signaling have critical implications for behavior and mental health across the life span with multiple metabolic, genetic and epigenetic factors influencing 5-HT signaling. Importantly, not all developmental changes in 5-HT are associated with negative outcomes and recognition of interactions between nature (genetic, medication exposure), nurture (maternal mood) and niche (where the child lives) are critical to understanding the variations in development associated with “programing” or shaping fetal 5-HT levels for health and behavior across the life span.
Recent scientific (Glover, 2011) and popular (Murphy Paul, 2010) attention has focused on “fetal programing” whereby the in utero environment shapes fetal development with consequences for health and disease across the life span (Barker, 1995). The concept of fetal programing implies that changes in the fetal environment may shape a “predictive adaptive response” in which fetal development sets a forecast for a place in the world ahead (Gluckman et al., 2005, 2007). Such adaptations might alter vigilance or a capacity to respond to stress that may be either maladaptive in one context or quite adaptive in another, shaping pathways to health or illness (Glover, 2011). In this way, 5-HT plays a critical component of fetal programing that may set a sensitivity to negative and positive life experience that predicts long risks for behavioral and psychiatric health (Homberg et al., 2010).
Multiple factors contribute to finely tuning serotonergic tone for healthy brain development (Morrison et al., 2001; Munafo et al., 2009) and this process starts long before birth (Homberg et al., 2010) and is shaped by social context (antenatal maternal stress, mood), exposure to antidepressants and genetic variations (maternal and fetal) that all contribute to regulating fetal 5-HT tone. In this way adaptive changes in 5-HT function, whether through genetic or epigenetic mechanisms, receptor sensitivity, maternal and placental factors as well early life experience itself may generate developmental risks for some, but not all, individuals (Barden et al., 1995; Fox et al., 2005; Oberlander et al., 2008a; Way and Taylor, 2010). Reducing antenatal maternal depressive symptoms using SSRIs may alter fetal 5-HT levels thereby restricting serotonergic tone (Munafo et al., 2009), leading to abnormal 5-HT signaling and predisposing to increased risk of psychopathology later in life (Munafo et al., 2009) (see (Gobbi et al., 2001) for review). Functional polymorphisms in 5-HTrelated genes, environmental adversity or pharmacologic exposures collectively contribute to “calibrating” emerging 5-HT-related stress regulatory systems (e.g. neuroendocrine stress regulation, pain reactivity, arousal regulation). Depending on the environmental context, fluctuating levels of 5-HT in the offspring, secondary to prenatal maternal depression, SSRI exposure or genetic variations, may conceivably have either neutral, detrimental or even beneficial effects on early neurodevelopment.
Importantly, not all factors that influence 5-HT signaling confer the same developmental risk. Early life experiential variables influence susceptibility to environmental factors (Moffitt et al., 2005; Caspi and Moffitt, 2006) and not all outcomes associated with the short allele are necessarily negative (Risch et al., 2009). While adults with two short alleles may be at increased risk of depression (Caspi et al., 2003) following early life adversity, those raised in a nurturing environment may ultimately have a lower risk of depressive symptoms (Taylor et al., 2006). Increased central 5-HT associated with the SLC6A4 short allele may therefore contribute to an increased sensitivity to environmental stimuli or hyper vigilance, leading to adaptation in one setting or an increased risk of poor mental health in another. In other words, in a low reward or low adversity setting, such hyper vigilance may confer an actual benefit that increases processing of relevant stimuli improving learning and social cognition (Homberg and Lesch, 2011). In this context, serotonergic tone, via either prenatal SSRI exposure or SLC6A4 allelic variations, appeared to affect a self-regulatory capacity that might heighten sensitivity to a world with a depressed mother. Highly vigilant individuals may therefore either become vulnerable or resilient, depending on the particular demands of their social environment.
With prenatal SSRI exposure, changes in fetal 5-HT signaling are still subject to multiple causal factors such as maternal mood, the drug, genetics, placenta, maternal pharmacological factors. Development and behavior in this setting represents an ongoing interplay between psychological, pharmacological, genetic and social factors inherent to both the mother and her child. Rather than being a case where 5-HT levels are “too high” or “too low”, this is a setting where 5-HT finely tunes neurogenic processes during critical periods to meet a particular developmental demand. Timing of exposure to changes in 5-HT signaling also appears to matter. During the first year of life infant psychomotor and mental development appeared to be enhanced when there was congruence between prenatal and postnatal maternal mood, regardless of whether maternal mood conditions were optimal or adverse (Sandman et al., 2012). Interestingly, postpartum euthymic mothers conferred no benefit to their infants who had been exposed to antenatal anxiety. Together, these findings illustrate the impact of the continuity between pre and postnatal environments and how such stability may prepare the fetus for a postnatal world ahead (Serretti et al., 2006). Within this conceptual framework, maternal signals (e.g., antenatal stress, depressed mood) interact with the fetus’ SLC6A4 genotype to influence fetal 5-HT signaling. Such fetal differences in signaling might influence the fetus’ sensitivity to its postnatal environment (e.g. postnatal care giving, maternal mood, supportive parenting). Such sensitivity could go on to be expressed as a particular endophenotype (child reactivity to stress, mood, internalizing behaviors) or phenotype (depression, anxiety disorders) depending on postnatal environmental demands. In this sense prenatal environment influences plasticity that spans both gestation and postnatal contexts.
In the context of combined early (fetal/prenatal) and ongoing postnatal and childhood social experience, variations that alter 5-HT signaling appear to set up a serotonergic “differential susceptibility” (Belsky and Pluess, 2009) or “biological sensitivity” (Boyce and Ellis, 2005) that contribute to shaping individual differences, leading some to being affected by both negative and positive life experiences (Belsky and Pluess, 2009). In this way a susceptibility to environmental influences reflects differences in developmental plasticity (Belsky and Pluess, 2009).
In summary, increasing evidence supports critical roles for fetal 5-HT signaling in shaping early human brain development that sets pathways for behavior in childhood and adolescence. From animal and human studies three themes emerge: First, prenatal influences alter central 5-HT levels in the context of maternal prenatal stress and developmental outcomes represent interactions between psychological, pharmacological, genetic and social factors. Second, variations in 5-HT signaling shape a wide spectrum of typical and atypical development. Not all variations lead to negative outcomes. Finally, changes in 5-HT signaling may confer sensitivity to life experiences that may contribute a developmental benefit associated with positive social environments. Thus beyond identifying prenatal exposures that might put child development at risk, our challenge is to learn more about who, even in the face of adversity, might thrive in a particular postnatal environment, or even benefit from prenatal maternal SSRI treatment. In this sense, early alterations in 5-HT signaling can be seen to potentially confer a “predictive adaptive response” (Gluckman et al., 2005) that forecasts that under some an advantage in a particular postnatal stressful environment. The opposite also may be possible, whereby an individual may be at an increased risk even in a favorable postnatal environment (Hariri and Holmes, 2006), and thus the challenge is to elucidate the impact of interactions between risk factors and context. Longitudinal studies are needed that integrate a maternal and infant/child developmental perspective, helping us move away from characterizing factors that alter 5-HT signaling (i.e. prenatal SSRI exposure, or genetic variations) as “bad” or “harmful” and regard these as factors that heighten or lessen vulnerability associated with early experience. With this perspective, this should enable us to identify “plasticity” factors and move us beyond regarding maternal depressed mood, prenatal drug exposure or even allelic variations as categorical early “environmental pathogens”. Understanding the impact of early changes in serotonergic signaling offers critical insights that might explain patterns of individual differences in childhood developmental risk and resilience.
Acknowledgments
We are grateful to Ursula Brain for her thoughtful and diligence in editing and preparation of this manuscript. T.F.O. is the R. Howard Webster Professor in Brain Imaging and Early Child Development (UBC) and his work is supported by the Child and Family Research Institute CFRI (UBC) and the Canadian Institutes for Health Research (CIHR).
Abbreviations
- 5-HIAA
5-hydroxyindoleacetic acid, main 5-HT metabolite
- 5-HT
5-hydroxytryptamine, serotonin
- EF
executive functioning
- G × E
Gene × Environment (interactions)
- HPA
hypotha lamic-pituitary–adrenal
- MAO
monoamine oxidase
- SERT
5-HT transporter
- SLC6A4
5-HT transporter gene (Solute Carrier Family 6, Member 4 gene)
- s/l allele
short/long allele
- SNRI
serotonin-norepinephrine reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
References
- Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allister L, Lester BM, Carr S, Liu J. The effects of maternal depression on fetal heart rate response to vibroacoustic stimulation. Dev Neuropsychol. 2001;20:639–651. doi: 10.1207/S15326942DN2003_6. [DOI] [PubMed] [Google Scholar]
- Altieri SC, Yang H, O’Brien HJ, Redwine HM, Senturk D, Hensler JG, Andrews AM. Perinatal vs genetic programming of serotonin states associated with anxiety. Neuropsychopharmacology. 2015;40(6):1456–1470. doi: 10.1038/npp.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, Rolnick SJ, Roblin D, Smith DH, Willy ME, Staffa JA, Platt R. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198:194–195. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- Bairy KL, Madhyastha S, Ashok KP, Bairy I, Malini S. Developmental and behavioral consequences of prenatal fluoxetine. Pharmacology. 2007;79:1–11. doi: 10.1159/000096645. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Barden N, Reul JM, Holsboer F. Do antidepressants stabilize mood through actions on the hypothalamic-pituitary– adrenocortical system? Trends Neurosci. 1995;18:6–11. doi: 10.1016/0166-2236(95)93942-q. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Intrauterine programming of adult disease. Mol Med Today. 1995;1:418–423. doi: 10.1016/s1357-4310(95)90793-9. [DOI] [PubMed] [Google Scholar]
- Beach SRH, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at SLC6A4 is linked to family history of child abuse: an examination of the Iowa Adoptee sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:710–713. doi: 10.1002/ajmg.b.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bhuiyan M, Petropoulos S, Gibb W, Matthews SG. Sertraline alters multidrug resistance phosphoglycoprotein activity in the mouse placenta and fetal blood–brain barrier. Reprod Sci. 2012;19:407–415. doi: 10.1177/1933719111424438. [DOI] [PubMed] [Google Scholar]
- Blier P, Bergeron R. Effectiveness of pindolol with selected antidepressant drugs in the treatment of major depression. J Clin Psychopharmacol. 1995;15:217–222. doi: 10.1097/00004714-199506000-00011. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience. 2011;197:1–7. doi: 10.1016/j.neuroscience.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Levitt P. Placental source for 5-HT that tunes fetal brain development. Neuropsychopharmacology. 2012;37:299–300. doi: 10.1038/npp.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, Levitt P. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Wang D, Levesque ML, Tremblay RE, Szyf M. Looking beyond the DNA sequence: the relevance of DNA methylation processes for the stress-diathesis model of depression. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120251. doi: 10.1098/rstb.2012.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij LM, Carballedo A, Frey EM, Morris D, Dymov S, Vaisheva F, Ly V, Fahey C, Meaney J, Gill M, Frodl T. DNA methylation of the serotonin transporter gene in peripheral cells and stress-related changes in hippocampal volume: a study in depressed patients and healthy controls. PLoS One. 2015a;10:e0119061. doi: 10.1371/journal.pone.0119061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Tremblay RE, Szyf M, Benkelfa C. Genetic and early environmental influences on the serotonin system: consequences for brain development and risk for psychopathology. J Psychiatry Neurosci. 2015b;40:5–18. doi: 10.1503/jpn.140099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhris T, Sheehy O, Mottron L, Berard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr. 2016;170(2):117–124. doi: 10.1001/jamapediatrics.2015.3356. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonergic reinnervation reverses lesion-induced decreases in PSA-NCAM labeling and proliferation of hippocampal cells in adult rats. Hippocampus. 2000;10:37–46. doi: 10.1002/(SICI)1098-1063(2000)10:1<37::AID-HIPO4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Pawluski JL, Galea LA. High post-partum levels of corticosterone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: a model of post-partum stress and possible depression. Horm Behav. 2006;50:370–382. doi: 10.1016/j.yhbeh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LAM, Devlin AM, Oberlander TF. Antidepressant use during pregnancy and serotonin transporter genotype (SLC6A4) Affect newborn serum reelin levels. Dev Psychobiol. 2012 doi: 10.1002/dev.21056. [DOI] [PubMed] [Google Scholar]
- Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nat Rev Genet. 2008;9:527–540. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- Buznikov GA. The action of neurotransmitters and related substances on early embryogenesis. Pharmacol Ther. 1984;25:23–59. doi: 10.1016/0163-7258(84)90023-8. [DOI] [PubMed] [Google Scholar]
- Caldji C, Hellstrom IC, Zhang TY, Diorio J, Meaney MJ. Environmental regulation of the neural epigenome. FEBS Lett. 2011;585:2049–2058. doi: 10.1016/j.febslet.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De ME, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, Hoyme HE. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142:402–408. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Opinion – gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chameau P, Inta D, Vitalis T, Monyer H, Wadman WJ, Van Hooft JA. The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc Natl Acad Sci U S A. 2009;106:7227–7232. doi: 10.1073/pnas.0810764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacology. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic-pituitary– adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S50–S55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Niimura K, Chaturvedi S, Muzik O, Fakhouri M, Lee ML, Chugani HT. Increased brain serotonin synthesis in migraine. Neurology. 1999;53:1473–1479. doi: 10.1212/wnl.53.7.1473. [DOI] [PubMed] [Google Scholar]
- Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, Erb JL, Churchill SE, Kaimal AJ, Doyle AE, Robinson EB, Smoller JW, Kohane IS, Perlis RH. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry. 2015;20:727–734. doi: 10.1038/mp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrick V, Reminick AM, Loughead A, Vitonis AF, Stowe ZN. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- Collier DA, Stober G, Li T, Heils A, Catalano M, Di Bella D, Arranz MJ, Murray RM, Vallada HP, Bengel D, Muller CR, Roberts GW, Smeraldi E, Kirov G, Sham P, Lesch KP. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry. 1996;1:453–460. [PubMed] [Google Scholar]
- Connell S, Karikari C, Hohmann CF. Sex-specific development of cortical monoamine levels in mouse. Brain Res Dev Brain Res. 2004;151:187–191. doi: 10.1016/j.devbrainres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196:544–545. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- David SP, Murthy NV, Rabiner EA, Munafo MR, Johnstone EC, Jacob R, Walton RT, Grasby PM. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J Neurosci. 2005;25:2586–2590. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE Montigny C, Chaput Y, Blier P. Modification of serotonergic neuron properties by long-term treatment with serotonin reuptake blockers. J Clin Psychiatry. 1990;51(Suppl B):4–8. [PubMed] [Google Scholar]
- Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS One. 2010;5:e12201. doi: 10.1371/journal.pone.0012201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Hodgson DM, Costigan KA, Johnson TR. Fetal antecedents of infant temperament. Child Dev. 1996;67:2568–2583. [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Duncan LE. Paying attention to all results, positive and negative. J Am Acad Child Adolesc Psychiatry. 2013;52:462–465. doi: 10.1016/j.jaac.2013.02.007. [DOI] [PubMed] [Google Scholar]
- El MH, White TJ, van der Knaap NJ, Homberg JR, Fernandez G, Schoemaker NK, Jaddoe VW, Hofman A, Verhulst FC, Hudziak JJ, Stricker BH, Tiemeier H. Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population-based study of young children. Br J Psychiatry. 2014;205:95–102. doi: 10.1192/bjp.bp.113.127746. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Boyce WT, Hertzman C, Lam LL, Armstrong JM, Neumann SM, Kobor MS. Epigenetic vestiges of early developmental adversity: childhood stress exposure and DNA methylation in adolescence. Child Dev. 2013;84:58–75. doi: 10.1111/j.1467-8624.2011.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Vera Y, Gil K, Schanberg S, Kuhn C, Gonzalez-Garcia A. Prenatal maternal biochemistry predicts neonatal biochemistry. Int J Neurosci. 2004a;114:933–945. doi: 10.1080/00207450490461305. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, Yando R, Bendell D. Prenatal depression effects on the fetus and the newborn. Infant Behav Dev. 2004b;27:216–229. [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Figueiredo B, Deeds O, Ascencio A, Schanberg S, Kuhn C. Prenatal dopamine and neonatal behavior and biochemistry. Infant Behav Dev. 2008;31:590–593. doi: 10.1016/j.infbeh.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin K, Schmidt L, Hamer D, Ernst M, Pine DS. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychol Sci. 2005;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Fox E, Ridgewell A, Ashwin C. Looking on the bright side: biased attention and the human serotonin transporter gene. Proc Biol Sci. 2009;276:1747–1751. doi: 10.1098/rspb.2008.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Szyf M, Carballedo A, Ly V, Dymov S, Vaisheva F, Morris D, Fahey C, Meaney J, Gill M, Booij L. DNA methylation of the serotonin transporter gene (SLC6A4) is associated with brain function involved in processing emotional stimuli. J Psychiatry Neurosci. 2015;40:296–305. doi: 10.1503/jpn.140180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW. Mechanisms and functions of serotonin neuronal systems: opportunities for neuropeptide interactions. Ann NY Acad Sci. 1996;780:176–184. doi: 10.1111/j.1749-6632.1996.tb15122.x. [DOI] [PubMed] [Google Scholar]
- Galbally M, Lewis AJ, Buist A. Child developmental outcomes in preschool children following antidepressant exposure in pregnancy. Aust N Z J Psychiatry. 2015;49:642–650. doi: 10.1177/0004867415569800. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gentile S. Prenatal antidepressant exposure and the risk of autism spectrum disorders in children. Are we looking at the fall of Gods? J Affect Disord. 2015;182:132–137. doi: 10.1016/j.jad.2015.04.048. [DOI] [PubMed] [Google Scholar]
- Gentile S. Untreated depression during pregnancy: short- and long-term effects in offspring. A systematic review. Neuroscience. 2017;342:154–166. doi: 10.1016/j.neuroscience.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Gidaya N, Lee B, Burstyn I, Yudell M, Mortensen E, Newschaffer C. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J Autism Dev Disord. 2014;44:2558–2567. doi: 10.1007/s10803-014-2128-4. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Dukat M, Westkaemper RB. Serotonin receptor subtypes and ligands. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology-4th generation of progress. New York, New York: Raven Press; 1995. [Google Scholar]
- Glover V. Annual research review: prenatal stress and the origins of psychopathology: an evolutionary perspective. J Child Psychol Psychiatry. 2011;52:356–367. doi: 10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20:527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Murphy DL, Lesch K, Blier P. Modifications of the serotonergic system in mice lacking serotonin transporters: an in vivo electrophysiological study. J Pharmacol Exp Ther. 2001;296:987–995. [PubMed] [Google Scholar]
- Hanley GE, Oberlander TF. Neurodevelopmental outcomes following prenatal exposure to serotonin reuptake inhibitor antidepressants: a “social teratogen” or moderator of developmental risk? Birth Defects Res A Clin Mol Teratol. 2012;94:651–659. doi: 10.1002/bdra.23032. [DOI] [PubMed] [Google Scholar]
- Hanley GE, Brain U, Oberlander TF. Infant developmental outcomes following prenatal exposure to antidepressants, and maternal depressed mood and positive affect. Early Hum Dev. 2013;89:519–524. doi: 10.1016/j.earlhumdev.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Hanley GE, Brain U, Oberlander TF. Prenatal exposure to serotonin reuptake inhibitor antidepressants and childhood behavior. Pediatr Res. 2015;78:174–180. doi: 10.1038/pr.2015.77. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Sanchez C, Meier E. Neonatal administration of the selective serotonin reuptake inhibitor Lu 10–134-C increases forced swimming-induced immobility in adult rats: a putative animal model of depression? J Pharmacol Exp Ther. 1997;283:1333–1341. [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Harrington RA, Lee LC, Crum RM, Zimmerman AW, Hertz-Picciotto I. Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics. 2014;133:e1241–e1248. doi: 10.1542/peds.2013-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Serotonergic modulation of the limbic system. Neurosci Biobehav Rev. 2006;30:203–214. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annu Rev Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- Hilli J, Heikkinen T, Rontu R, Lehtimaki T, Kishida I, Aklillu E, Bertilsson L, Vahlberg T, Laine K. MAO-A and COMT genotypes as possible regulators of perinatal serotonergic symptoms after in utero exposure to SSRIs. Eur Neuropsychopharmacol. 2009;19:363–370. doi: 10.1016/j.euroneuro.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Holloway T, Gonzalez-Maeso J. Epigenetic mechanisms of serotonin signaling. ACS Chem Neurosci. 2015;6:1099–1109. doi: 10.1021/acschemneuro.5b00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Li Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behaviour in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003a;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003b;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Contet C. Deciphering the interaction of the corticotropin-releasing factor and serotonin brain systems in anxiety-related disorders. J Neurosci. 2009;29:13743–13745. doi: 10.1523/JNEUROSCI.4362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Lesch KP. Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry. 2011;69:513–519. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Homberg J, Mudde J, Braam B, Ellenbroek B, Cuppen E, Joles JA. Blood pressure in mutant rats lacking the 5-hydroxytryptamine transporter. Hypertension. 2006;48:e115–e116. doi: 10.1161/01.HYP.0000246306.61289.d8. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Schubert D, Gaspar P. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol Sci. 2010;31:60–65. doi: 10.1016/j.tips.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Huang Y, Xu H, Li H, Yang H, Chen Y, Shi X. Pre-gestational stress reduces the ratio of 5-HIAA to 5-HT and the expression of 5-HT1A receptor and serotonin transporter in the brain of foetal rat. BMC Neurosci. 2012;13:22. doi: 10.1186/1471-2202-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Terashima T. Expression of reelin, the gene responsible for the reeler mutation, in embryonic development and adulthood in the mouse. Dev Dyn. 1997;210:157–172. doi: 10.1002/(SICI)1097-0177(199710)210:2<157::AID-AJA8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Imoto Y, Kira T, Sukeno M, Nishitani N, Nagayasu K, Nakagawa T, Kaneko S, Kobayashi K, Segi-Nishida E. Role of the 5-HT4 receptor in chronic fluoxetine treatment-induced neurogenic activity and granule cell dematuration in the dentate gyrus. Mol Brain. 2015;8:29. doi: 10.1186/s13041-015-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata H, Shiga T, Okado N. Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress-induced brain dysfunction. Neuroscience. 2005;133:893–901. doi: 10.1016/j.neuroscience.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Janusonis S, Gluncic V, Rakic P. Early serotonergic projections to Cajal-Retzius cells: relevance for cortical development. J Neurosci. 2004;24:1652–1659. doi: 10.1523/JNEUROSCI.4651-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Olivier JD, Nonkes LJ, Homberg JR. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci Biobehav Rev. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim JM, Stewart R, Kim SY, Bae KY, Kim SW, Shin IS, Shin MG, Yoon JS. Association of SLC6A4 methylation with early adversity, characteristics and outcomes in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:23–28. doi: 10.1016/j.pnpbp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. “A gene for...”: the nature of gene action in psychiatric disorders. Am J Psychiatry. 2005;162:1243–1252. doi: 10.1176/appi.ajp.162.7.1243. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kim J, Riggs KW, Misri S, Kent N, Oberlander TF, Grunau RE, Fitzgerald C, Rurak DW. Stereoselective disposition of fluoxetine and norfluoxetine during pregnancy and breast-feeding. Br J Clin Pharmacol. 2006;61:155–163. doi: 10.1111/j.1365-2125.2005.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Tam T, Whiteman S, Schafer WR. Serotonin promotes G(o)-dependent neuronal migration in Caenorhabditis elegans. Curr Biol. 2002;12:1738–1747. doi: 10.1016/s0960-9822(02)01199-5. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Capitanio JP, Leibel R, Deng L, LeDuc C, Haghighi F, Mann JJ. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes Brain Behav. 2010;9:575–582. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryanova V, McAllister BB, Dyck RH. Long-term outcomes of developmental exposure to fluoxetine: a review of the animal literature. Dev Neurosci. 2013;35:437–439. doi: 10.1159/000355709. [DOI] [PubMed] [Google Scholar]
- Knaepen L, Rayen I, Charlier TD, Fillet M, Houbart V, van KM, Steinbusch HW, Patijn J, Tibboel D, Joosten EA, Pawluski JL. Developmental fluoxetine exposure normalizes the long-term effects of maternal stress on post-operative pain in Sprague– Dawley rat offspring. PLoS One. 2013;8:e57608. doi: 10.1371/journal.pone.0057608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Uddin M, Chang SC, Aiello AE, Wildman DE, Goldmann E, Galea S. SLC6A4 methylation modifies the effect of the number of traumatic events on risk for posttraumatic stress disorder. Depress Anxiety. 2011;28:639–647. doi: 10.1002/da.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine K, Heikkinen T, Ekblad U, Kero P. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry. 2003;60:720–726. doi: 10.1001/archpsyc.60.7.720. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann NY Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Krebs H. Effects of p-chlorophenylalanine on time of neuronal origin during embryogenesis in the rat. Brain Res. 1976;107:638–644. doi: 10.1016/0006-8993(76)90153-0. [DOI] [PubMed] [Google Scholar]
- Lesch KP. Linking emotion to the social brain. The role of the serotonin transporter in human social behaviour. EMBO Rep. 2007;8:S24–S29. doi: 10.1038/sj.embor.7401008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP. When the serotonin transporter gene meets adversity: the contribution of animal models to understanding epigenetic mechanisms in affective disorders and resilience. Curr Top Behav Neurosci. 2011;7:251–280. doi: 10.1007/7854_2010_109. [DOI] [PubMed] [Google Scholar]
- Levitt P. Serotonin and the autisms: a red flag or a red herring? Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.98. [DOI] [PubMed] [Google Scholar]
- Liao CC, Lee LJ. Neonatal fluoxetine exposure affects the action potential properties and dendritic development in cortical subplate neurons of rats. Toxicol Lett. 2011;207:314–321. doi: 10.1016/j.toxlet.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356:2675–2683. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary–adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Luddington NS, Mandadapu A, Husk M, El-Mallakh RS. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11:93–102. doi: 10.4088/pcc.08r00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RC, Paul IA. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006a;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D, Williams L, Coppinger D, Paul IA. Neonatal citalopram exposure produces lasting changes in behavior which are reversed by adult imipramine treatment. Eur J Pharmacol. 2006b;532:265–269. doi: 10.1016/j.ejphar.2005.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar I, Bambico FR, Mechawar N, Nobrega JN. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. 2014;38:173–192. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Man KK, Tong HH, Wong LY, Chan EW, Simonoff E, Wong IC. Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: a systematic review and meta-analysis of observational studies. Neurosci Biobehav Rev. 2015;49:82–89. doi: 10.1016/j.neubiorev.2014.11.020. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Perez-Garcia CG, Abraham H, Caput D. Expression of p73 and Reelin in the developing human cortex. J Neurosci. 2002;22:4973–4986. doi: 10.1523/JNEUROSCI.22-12-04973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliarini S, Pacini G, Pelosi B, Lunardi G, Pasqualetti M. Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol Psychiatry. 2013;18:1106–1118. doi: 10.1038/mp.2012.128. [DOI] [PubMed] [Google Scholar]
- Mileva-Seitz V, Kennedy J, Atkinson L, Steiner M, Levitan R, Matthews SG, Meaney MJ, Sokolowski MB, Fleming AS. Serotonin transporter allelic variation in mothers predicts maternal sensitivity, behavior and attitudes toward 6-month-old infants. Genes Brain Behav. 2011;10:325–333. doi: 10.1111/j.1601-183X.2010.00671.x. [DOI] [PubMed] [Google Scholar]
- Miyagawa K, Tsuji M, Fujimori K, Saito Y, Takeda H. Prenatal stress induces anxiety-like behavior together with the disruption of central serotonin neurons in mice. Neurosci Res. 2011;70:111–117. doi: 10.1016/j.neures.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Monk C, Fifer WP, Myers MM, Sloan RP, Trien L, Hurtado A. Maternal stress responses and anxiety during pregnancy: effects on fetal heart rate. Dev Psychobiol. 2000;36:67–77. [PubMed] [Google Scholar]
- Mooney-Leber S, Kott JM, Shoubah FA, Perrine SA, Brummelte S. Effects of in utero antidepressant (sertraline) exposure on offspring outcome in an animal model of maternal stress and depression. Washington, DC, USA. Society for Neuroscience Meeting Poster 347.14.2014. [Google Scholar]
- Morelli E, Moore H, Rebello TJ, Gray N, Steele K, Esposito E, Gingrich JA, Ansorge MS. Chronic 5-HT transporter blockade reduces DA signaling to elicit basal ganglia dysfunction. J Neurosci. 2011;31:15742–15750. doi: 10.1523/JNEUROSCI.2989-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Chien C, Gruber N, Rurak D, Riggs W. Fetal behavioural state changes following maternal fluoxetine infusion in sheep. Brain Res Dev Brain Res. 2001;131:47–56. doi: 10.1016/s0165-3806(01)00255-3. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Bogen D, Perel J, Bregar A, Uhl K, Levin B, Wisner KL. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293:2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A, Brocke B, Fries E, Lesch KP, Kirschbaum C. The role of the serotonin transporter polymorphism for the endocrine stress response in newborns. Psychoneuroendocrinology. 2010;35:289–296. doi: 10.1016/j.psyneuen.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Durrant C, Lewis G, Flint J. Gene + environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Fox MA, Timpano KR, Moya P, Ren-Patterson R, Andrews AM, Holmes A, Watts SW, Wendland JR, Lesch K-P. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy Paul A. Origins: how the nine months before birth shape the rest of our lives. New York: Free Press; 2010. [Google Scholar]
- Nordquist N, Oreland L. Serotonin, genetic variability, behaviour, and psychiatric disorders–a review. Ups J Med Sci. 2010;115:2–10. doi: 10.3109/03009730903573246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nulman I, Rovet J, Stewart DE, Wolpin J, Gardner HA, Theis JG, Kulin N, Koren G. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336:258–262. doi: 10.1056/NEJM199701233360404. [DOI] [PubMed] [Google Scholar]
- Oberlander TF. Fetal serotonin signaling: setting pathways for early childhood development and behavior. J Adolesc Health. 2012;51:S9–S16. doi: 10.1016/j.jadohealth.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Eckstein GR, Fitzgerald C, Ellwood AL, Misri S, Rurak D, Riggs KW. Prolonged prenatal psychotropic medication exposure alters neonatal acute pain response. Pediatr Res. 2002;51:443–453. doi: 10.1203/00006450-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65:230–237. doi: 10.4088/jcp.v65n0214. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Grunau RE, Fitzgerald C, Papsdorf M, Rurak D, Riggs W. Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005;115:411–425. doi: 10.1542/peds.2004-0420. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63:898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau R. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med. 2007;161:22–29. doi: 10.1001/archpedi.161.1.22. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Bonaguro RJ, Misri S, Papsdorf M, Ross CJ, Simpson EM. Infant serotonin transporter (SLC6A4) promoter genotype is associated with adverse neonatal outcomes after prenatal exposure to serotonin reuptake inhibitor medications. Mol Psychiatry. 2008a;13:65–73. doi: 10.1038/sj.mp.4002007. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Grunau R, Mayes L, Riggs W, Rurak D, Papsdorf M, Misri S, Weinberg J. Hypothalamic-pituitary–adrenal (HPA) axis function in 3-month old infants with prenatal selective serotonin reuptake inhibitor (SSRI) antidepressant exposure. Early Hum Dev. 2008b;84:689–697. doi: 10.1016/j.earlhumdev.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008c;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Papsdorf M, Brain UM, Misri S, Ross C, Grunau RE. Prenatal effects of selective serotonin reuptake inhibitor antidepressants, serotonin transporter promoter genotype (SLC6A4), and maternal mood on child behavior at 3 years of age. Arch Pediatr Adolesc Med. 2010;164:444–451. doi: 10.1001/archpediatrics.2010.51. [DOI] [PubMed] [Google Scholar]
- Olivier JD, Blom T, Arentsen T, Homberg JR. The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1400–1408. doi: 10.1016/j.pnpbp.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Olsson CA, Foley DL, Parkinson-Bates M, Byrnes G, McKenzie M, Patton GC, Morley R, Anney RJL, Craig JM, Saffery R. Prospects for epigenetic research within cohort studies of psychological disorder: a pilot investigation of a peripheral cell marker of epigenetic risk for depression. Biol Psychol. 2010;83:159–165. doi: 10.1016/j.biopsycho.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Wong CCY, Danese A, Pariante CM, Papadopoulos AS, Mill J, Arseneault L. Increased serotonin transporter gene (SERT) DNA methylation is associated with bullying victimization and blunted cortisol response to stress in childhood: a longitudinal study of discordant monozygotic twins. Psychol Med. 2013;43:1813–1823. doi: 10.1017/S0033291712002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Van Heertum RL, Arango V, Mann JJ. Altered serotonin 1A binding in major depression: a [carbonyl-C-11] WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Patil AS, Kuller JA, Rhee EH. Antidepressants in pregnancy: a review of commonly prescribed medications. Obstet Gynecol Surv. 2011;66:777–787. doi: 10.1097/OGX.0b013e31823e0cbf. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Galea LAM, Brain U, Papsdorf M, Oberlander TF. Neonatal S100B protein levels after prenatal exposure to selective serotonin reuptake inhibitors. Pediatrics. 2009;124:e662–e670. doi: 10.1542/peds.2009-0442. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, van DE, Abrams Z, Houbart V, Fillet M, Steinbusch HW, Charlier TD. Fluoxetine dose and administration method differentially affect hippocampal plasticity in adult female rats. Neural Plast. 2014;2014:123026. doi: 10.1155/2014/123026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud N, Zewdie S, Stenz L, Adouan W, Bavamian S, Prada P, Nicastro R, Hasler R, Nallet A, Piguet C, Paoloni-Giacobino A, Aubry JM, Dayer A. Methylation of serotonin receptor 3A in ADHD, borderline personality, and bipolar disorders: link with the severity of the disorders and childhood maltreatment. Depress Anxiety. 2015 doi: 10.1002/da.22406. [DOI] [PubMed]
- Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:543–549. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky DJ, Wickramaratne PJ, Rush AJ, Hughes CW, Garber J, Malloy E, King CA, Cerda G, Sood AB, Alpert JE, Wisniewski SR, Trivedi MH, Talati A, Carlson MM, Liu HH, Fava M, Weissman MM. Children of currently depressed mothers: a STAR*D ancillary study. J Clin Psychiatry. 2006;67:126–136. doi: 10.4088/jcp.v67n0119. [DOI] [PubMed] [Google Scholar]
- Pluess M, Velders FP, Belsky J, van IJ, Zendoorn MH, Bakermans-Kranenburg MJ, Jaddoe VW, Hofman A, Arp PP, Verhulst FC, Tiemeier H. Serotonin transporter polymorphism moderates effects of prenatal maternal anxiety on infant negative emotionality. Biol Psychiatry. 2011;69:520–525. doi: 10.1016/j.biopsych.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Popa D, Lena C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ. 2013;346:f2059. doi: 10.1136/bmj.f2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikkonen K, Pesonen AK, O’Reilly JR, Tuovinen S, Lahti M, Kajantie E, Villa P, Laivuori H, Hamalainen E, Seckl JR, Reynolds RM. Maternal depressive symptoms during pregnancy, placental expression of genes regulating glucocorticoid and serotonin function and infant regulatory behaviors. Psychol Med. 2015;45(15):3217–3226. doi: 10.1017/S003329171500121X. [DOI] [PubMed] [Google Scholar]
- Ramchandani S, Bhattacharya SK, Cervoni N, Szyf M. DNA methylation is a reversible biological signal. Proc Natl Acad Sci U S A. 1999;96:6107–6112. doi: 10.1073/pnas.96.11.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampono J, Simmer K, Ilett KF, Hackett LP, Doherty DA, Elliot R, Kok CH, Coenen A, Forman T. Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry. 2009;42:95–100. doi: 10.1055/s-0028-1103296. [DOI] [PubMed] [Google Scholar]
- Rayen I, Van Den Hove DL, Prickaerts J, Steinbusch HW, Pawluski JL. Fluoxetine during development reverses the effects of prenatal stress on depressive-like behavior and hippocampal neurogenesis in adolescence. PLoS One. 2011;6:e24003. doi: 10.1371/journal.pone.0024003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio O, Potter G, Walzer C, Vallet P, Szabo G, Vutskits L, Kiss JZ, Dayer AG. Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Mol Psychiatry. 2009;14:280–290. doi: 10.1038/mp.2008.89. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LE, Grigoriadis S, Mamisashvili L, VonderPorten EH, Roerecke M, Rehm J, Dennis CL, Koren G, Steiner M, Mousmanis P, Cheung A. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry. 2013;70:436–443. doi: 10.1001/jamapsychiatry.2013.684. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sweatt JD. Annual Research Review: epigenetic mechanisms and environmental shaping of the brain during sensitive periods of development. J Child Psychol Psychiatry. 2011;52:398–408. doi: 10.1111/j.1469-7610.2010.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rurak D, Lim K, Sanders A, Brain U, Riggs W, Riggs W, Oberlander TF. Third trimester fetal heart rate and Doppler middle cerebral artery blood flow velocity characteristics during prenatal selective serotonin reuptake inhibitor exposure. Pediatr Res. 2011;70:96–101. doi: 10.1203/PDR.0b013e31821ba11a. [DOI] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21(3):884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Glynn LM. Prescient human fetuses thrive. Psychol Sci. 2012;23:93–100. doi: 10.1177/0956797611422073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid programming. Ann NY Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Segman RH, Goltser T, Heresco-Levy U, Finkel B, Shalem R, Schlafman M, Yakir A, Greenberg D, Strous R, Lerner A, Shelevoy A, Lerer B. Association of dopaminergic and serotonergic genes with tardive dyskinesia in patients with chronic schizophrenia. Pharmacogenomics J. 2003;3:277–283. doi: 10.1038/sj.tpj.6500194. [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Serretti A, Calati R, Mandelli L, De RD. Serotonin transporter gene variants and behavior: a comprehensive review. Curr Drug Targets. 2006;7:1659–1669. doi: 10.2174/138945006779025419. [DOI] [PubMed] [Google Scholar]
- Shearman LP, McReynolds AM, Zhou FC, Meyer JS. Relationship between [125I]RTI-55-labeled cocaine binding sites and the serotonin transporter in rat placenta. Am J Physiol. 1998;275:C1621–C1629. doi: 10.1152/ajpcell.1998.275.6.C1621. [DOI] [PubMed] [Google Scholar]
- Simpson KL, Weaver KJ, de Villers-Sidani E, Lu JY, Cai Z, Pang Y, Rodriguez-Porcel F, Paul IA, Merzenich M, Lin RC. Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci U S A. 2011;108:18465–18470. doi: 10.1073/pnas.1109353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- Sorensen MJ, Gronborg TK, Christensen J, Parner ET, Vestergaard M, Schendel D, Pedersen LH. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–459. doi: 10.2147/CLEP.S53009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom E, Kolare S, Souverbie F, Samuelsson EB, Pschera H, Lunell NO, Seiger A. Neurochemical differentiation of human bulbospinal monoaminergic neurons during the first trimester. Brain Res Dev Brain Res. 1993;75(1):1–12. doi: 10.1016/0165-3806(93)90059-j. [DOI] [PubMed] [Google Scholar]
- Szyf M, Weaver I, Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reprod Toxicol. 2007;24:9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Nakashima S, Ohama E, Takeda S, Ikuta F. Distribution of serotonin-containing cell bodies in the brainstem of the human fetus determined with immunohistochemistry using antiserotonin serum. Brain Dev. 1986;8(4):355–365. doi: 10.1016/s0387-7604(86)80055-9. [DOI] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Tobi EW, Goeman JJ, Monajemi R, Gu H, Putter H, Zhang Y, Slieker RC, Stok AP, Thijssen PE, Muller F, van Zwet EW, Bock C, Meissner A, Lumey LH, Eline SP, Heijmans BT. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat Commun. 2014;5:5592. doi: 10.1038/ncomms6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick EZ, Weinberg MK. Depressed mothers and infants: failure to form dyadic states of consciousness. In: Murray L, Cooper PJ, editors. Postpartum depression and child development. New York: Guilford Press; 1997. pp. 54–81. [Google Scholar]
- Turecki G, Meaney MJ. Effects of the social environment and stress on glucocorticoid receptor gene methylation: a systematic review. Biol Psychiatry. 2016;79(2):87–96. doi: 10.1016/j.biopsych.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BR, Van CB, Smits T, Van HS, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33:536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- Van den Hove DLA, Lauder JM, Scheepens A, Prickaerts J, Blanco CE, Steinbusch HWM. Prenatal stress in the rat alters 5-HT1A receptor binding in the ventral hippocampus. Brain Res. 2006;1090:29–34. doi: 10.1016/j.brainres.2006.03.057. [DOI] [PubMed] [Google Scholar]
- van IJ, Zendoorn MH, Caspers K, Bakermans-Kranenburg MJ, Beach SR, Philibert R. Methylation matters: interaction between methylation density and serotonin transporter genotype predicts unresolved loss or trauma. Biol Psychiatry. 2010;68:405–407. doi: 10.1016/j.biopsych.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kleef ES, Gaspar P, Bonnin A. Insights into the complex influence of 5-HT signaling on thalamocortical axonal system development. Eur J Neurosci. 2012;35:1563–1572. doi: 10.1111/j.1460-9568.2012.8096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaagh S, Barlow DP, Zwart R. The extraneuronal monoamine transporter Slc22a3/Orct3 co-localizes with the Maoa metabolizing enzyme in mouse placenta. Mech Dev. 2001;100:127–130. doi: 10.1016/s0925-4773(00)00510-4. [DOI] [PubMed] [Google Scholar]
- Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65:805–815. doi: 10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Szyf M, Benkelfat C, Provencal N, Turecki G, Caramaschi D, Cote SM, Vitaro F, Tremblay RE, Booij L. Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PLoS One. 2012;7:e39501. doi: 10.1371/journal.pone.0039501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121:471–479. doi: 10.1111/j.1600-0447.2009.01490.x. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE. Social influences on health: is serotonin a critical mediator? Psychosom Med. 2010;72:107–112. doi: 10.1097/PSY.0b013e3181ce6a7d. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weikum WM, Oberlander TF, Hensch TK, Werker JF. Prenatal exposure to antidepressants and depressed maternal mood alter trajectory of infant speech perception. Proc Natl Acad Sci U S A. 2012;109:17221–17227. doi: 10.1073/pnas.1121263109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikum W, Brain U, Chau CM, Grunau RE, Boyce WT, Diamond A, Oberlander TF. Prenatal serotonin reuptake inhibitor (SRI) antidepressant exposure and serotonin transporter promoter genotype (SLC6A4) influence executive functions at 6 years of age. Front Cell Neurosci. 2013:7. doi: 10.3389/fncel.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56:479–485. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005;23(1):75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Whitelaw NC, Whitelaw E. How lifetimes shape epigenotype within and across generations. Hum Mol Genet. 2006;15(Spec No 2):R131–R137. doi: 10.1093/hmg/ddl200. [DOI] [PubMed] [Google Scholar]
- Willis-Owen SA, Turri MG, Munafo MR, Surtees PG, Wainwright NW, Brixey RD, Flint J. The serotonin transporter length polymorphism, neuroticism, and depression: a comprehensive assessment of association. Biol Psychiatry. 2005;58:451–456. doi: 10.1016/j.biopsych.2005.04.050. [DOI] [PubMed] [Google Scholar]
- Yadav DS. SSRIs and congenital defects. Case registers in pregnancy? BMJ. 2009;339:b4286. doi: 10.1136/bmj.b4286. [DOI] [PubMed] [Google Scholar]
- Yang H, Sampson MM, Senturk D, Andrews AM. Sex- and SERT-associated differences in stimulated serotonin revealed by fast microdialysis. ACS Chem Neurosci. 2015;6:1487–1501. doi: 10.1021/acschemneuro.5b00132. [DOI] [PubMed] [Google Scholar]
- Yavarone MS, Shuey DL, Sadler TW, Lauder JM. Serotonin uptake in the ectoplacental cone and placenta of the mouse. Placenta. 1993;14:149–161. doi: 10.1016/s0143-4004(05)80257-7. [DOI] [PubMed] [Google Scholar]
- Zeskind PS, Stephens LE. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics. 2004;113:368–375. doi: 10.1542/peds.113.2.368. [DOI] [PubMed] [Google Scholar]
- Zheng J, Xu DF, Li K, Wang HT, Shen PC, Lin M, Cao XH, Wang R. Neonatal exposure to fluoxetine and fluvoxamine alteres spine density in mouse hippocampal CA1 pyramidal neurons. Int J Clin Exp Pathol. 2011;4:162–168. [PMC free article] [PubMed] [Google Scholar]