Abstract
Bitterness perception in mammals is mostly directed at natural toxins that induce innate avoidance behaviours. Bitter taste is mediated by the G protein-coupled receptor TAS2R, which is located in taste cell membranes. One of the best-studied bitter taste receptors is TAS2R38, which recognizes phenylthiocarbamide (PTC). Here we investigate the sensitivities of TAS2R38 receptors to PTC in four species of leaf-eating monkeys (subfamily Colobinae). Compared with macaque monkeys (subfamily Cercopithecinae), colobines have lower sensitivities to PTC in behavioural and in vitro functional analyses. We identified four non-synonymous mutations in colobine TAS2R38 that are responsible for the decreased sensitivity of the TAS2R38 receptor to PTC observed in colobines compared with macaques. These results suggest that tolerance to bitterness in colobines evolved from an ancestor that was sensitive to bitterness as an adaptation to eating leaves.
Keywords: colobines, phenylthiocarbamide, TAS2R38 receptor, functional analysis
1. Introduction
Mammals discriminate five basic flavour qualities: bitterness, sweetness, umami, saltiness and sourness. Bitterness is mostly associated with natural toxins that induce innate avoidance behaviours in animals [1]. The ability to taste bitterness is mediated by the G protein-coupled receptor TAS2R in taste cells [2]. One of the best-studied TAS2Rs, TAS2R38 mediates the perception of the bitterness of synthetic phenylthiocarbamide (PTC), propylthiouracil (PROP) and phytotoxins, such as goitrin, limonin and sinigrin [3,4]. PTC is a prototypical agonist for TAS2R38 in humans and some primates, and allelic variants in TAS2R38 result in different intraspecific sensitivities to PTC [5–7].
Plants have evolved protective mechanisms against herbivory, for example, by synthesizing toxic secondary compounds [8]. Colobine monkeys (subfamily Colobinae) are folivorous primates with digestive systems that are specialized for digesting leaves [9]. Analysing the sensitivity of colobines to bitterness could improve our understanding of their dietary choices. Here, we report the behavioural responses of colobines to PTC and the functional characteristics of colobine TAS2R38 receptors.
2. Material and methods
(a). Animals
PTC sensitivity was analysed using 13 captive colobine monkeys of four species (Presbytis femoralis, Nasalis larvatus, Trachypithecus cristatus and T. auratus) and individual Heck's macaque (Macaca hecki) at the Ragunan Zoo, Jakarta, Indonesia (table 1).
Table 1.
Monkey performance in behavioural tests. A trial is defined as the act of a monkey putting an apple slice into its mouth followed by acceptance or rejection of the slice.
| object (code) | sex | age | PTC-treated |
control |
total | p-valuea | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| acceptance | trial | % | acceptance | trial | % | |||||
| P. femoralis (F) | female | adult | 28 | 28 | 100 | 54 | 54 | 100 | 82 | 0.2871 |
| T. auratus 1 (A1) | female | adult | 17 | 22 | 77.27 | 38 | 39 | 97.43 | 61 | 0.003751 |
| T. auratus 2 (A2) | female | adult | 2 | 4 | 50 | 6 | 6 | 100 | 10 | 0.01092 |
| T. auratus 3 (A3) | male | adult | 21 | 21 | 100 | 36 | 36 | 100 | 57 | 0.5243 |
| T. auratus 4 (A4) | male | adult | 33 | 33 | 100 | 53 | 53 | 100 | 86 | 0.2619 |
| T. auratus 5 (A5) | male | adult | 2 | 2 | 100 | 3 | 3 | 100 | 5 | 0.2803 |
| T. cristatus 1 (C1) | female | adult | 12 | 12 | 100 | 22 | 22 | 100 | 34 | 0.8344 |
| T. cristatus 2 (C2) | female | adult | 16 | 16 | 100 | 27 | 28 | 100 | 44 | 0.939 |
| T. cristatus 3 (C3) | female | adult | 41 | 41 | 100 | 80 | 80 | 100 | 121 | 0.1077 |
| T. cristatus 4 (C4) | female | adult | 44 | 44 | 100 | 77 | 77 | 100 | 121 | 0.108 |
| T. cristatus 5 (C5) | male | adult | 14 | 15 | 93.33 | 26 | 27 | 96.30 | 42 | 0.9487 |
| T. cristatus 6 (C6) | male | adult | 4 | 4 | 100 | 8 | 8 | 100 | 12 | 0.7788 |
| N. larvatus (N) | male | adult | 7 | 7 | 100 | 17 | 17 | 100 | 24 | 0.8961 |
| M. hecki | male | adult | 0 | 10 | 0 | 12 | 12 | 100 | 22 | 2.20 × 10−16 |
aProbability that the proportion of acceptance in the PTC-treated session is the same as that in the control session [10].
(b). Behavioural experiments
Animals were given apple slices that had been soaked overnight in control (water) or test (2 mM PTC in water) solution, as previously reported [11]. Details are provided in the electronic supplementary material, Methods.
(c). TAS2R38 genotyping
Genomic DNA was extracted from faecal samples collected from the cages (see electronic supplementary material, Methods for details). The TAS2R38 sequences from colobines were aligned to previously determined TAS2R38 orthologues from colobine (Colobus guereza) and taster individuals of Japanese and rhesus macaques, humans and chimpanzees (accession numbers: JQ272224.1, AB623012.1, AB623025.1, AY258597.1 and JQ272199.1, respectively) using ATGC software (Genetyx Corporation, Tokyo, Japan). A phylogenetic tree was constructed based on the TAS2R38 alignment using maximum-likelihood (ML) implemented in MEGA5.2 [12]. The Jones–Taylor–Thornton model was used to calculate pairwise distances between amino acid sequences [13]. Validation of trees was performed using bootstrap resampling (500 replicates).
(d). Functional assays of TAS2R38
Functional properties of the TAS2R38 receptor in colobines were evaluated using calcium imaging methods, as described previously [6,14,15]. To calculate responses to PTC, the amplitude of fluorescence was plotted against PTC concentration using IGOR Pro (WaveMetrics) and curves were fitted using nonlinear regression f(x) = Imin + (Imax − Imin)/(1 + (x/EC50)h), where x is the ligand concentration and h is the Hill coefficient used to calculate EC50 (half maximal effective concentration) for the ligand–receptor interaction between PTC and TAS2R38.
(e). Statistical analysis
Differences in the frequency of acceptance between PTC and the control were assessed by the proportion test [10] using the stats package in R v. 3.3.1 (R Core Team (https://www.R-project.org/)). EC50 was compared between M. fuscata receptors and mutant receptors mimicking those of colobines by nonlinear model fitting to dose–response data [16] using the drc package in R.
3. Results
Most individual colobines ate both PTC-treated (50–100%) and control (90–100%) apple slices. In contrast, the one M. hecki individual strictly rejected PTC-treated apples (0%), although it readily ate control apple slices (100%; table 1); this is similar to past studies of M. fuscata [11]. This suggests that most colobines could not discriminate between PTC-treated and control apple slices. Thus, behavioural sensitivity to PTC was lower in colobines than in the macaque. The genetic basis for this behaviour was investigated by determining the nucleotide sequences of TAS2R38, which encodes the TAS2R38 receptor in colobines. We found the sequences of TAS2R38 orthologues from each species were intact, except for that of T. cristatus-B, which encoded a premature stop codon at nucleotide position 552. The TAS2R38 sequences from colobines encoded amino acids different from those encoded by functional TAS2R38 in macaques, chimpanzees and humans (electronic supplementary material, figure S1). Cells transfected with TAS2R38 expressing a vector from P. femoralis and T. cristatus-A responded very slightly to PTC and the EC50 could not be measured (figure 1). We also found that translated TAS2R38 from N. larvatus, T. auratus and T. cristatus-B did not respond to PTC, even at the highest PTC concentration (100 µM). Taken together, lower responses were observed for colobine TAS2R38 receptors than for the M. fuscata TAS2R38 receptor, which responded to PTC in a concentration-dependent manner with EC50 0.58 ± 0.17 µM. This result was consistent with the PTC-tolerant behaviour of colobines.
Figure 1.
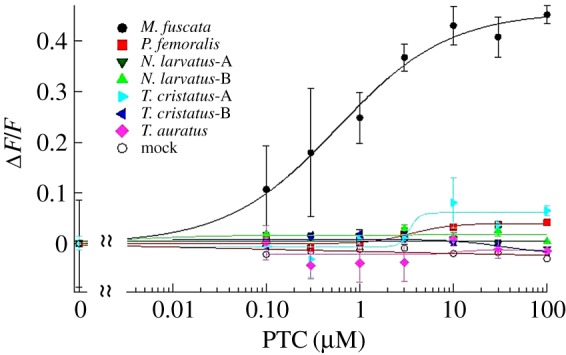
In vitro responses of TAS2R38 of M. fuscata and colobines transfected into HEK 293T cells were assayed, using an intracellular calcium indicator (Calcium4). The responses to various concentrations of PTC were calculated as the normalized peak response (F) relative to background fluorescence (F0): ΔF/F [=(F − F0)/F0]. Average responses (n = 3) for each time course are plotted against the concentration of PTC. (Online version in colour.)
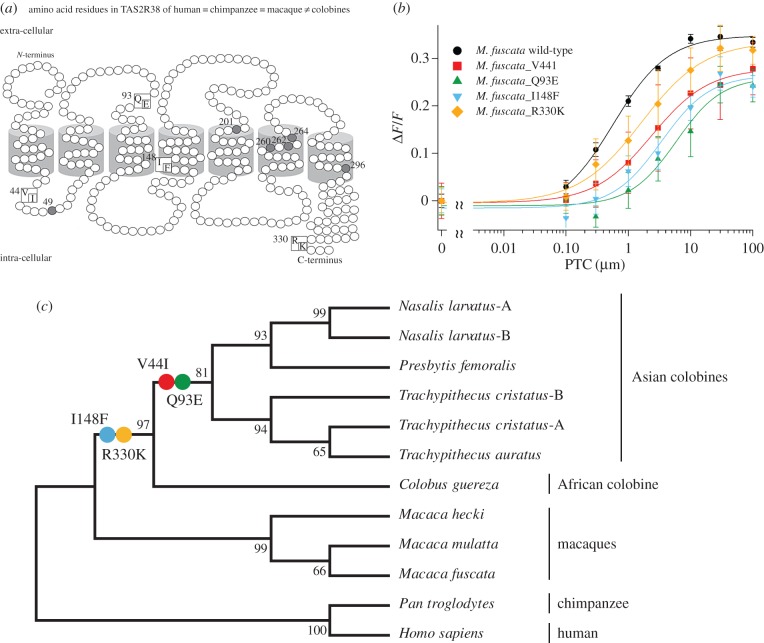
The critical amino acid positions for human TAS2R38 functionality [17] are not altered in the colobine TAS2R38 receptor. We found that all colobines differed at four amino acid positions that are conserved in humans, chimpanzees and macaques (figure 2a). Valine was mutated to isoleucine at amino acid 44 (V44I), glutamine to glutamic acid at amino acid 93 (Q93E), isoleucine to phenylalanine at amino acid 148 (I148F) and arginine to lysine at 330 (R330K). To check whether these four amino acids are responsible for the low response of TAS2R38 to PTC, we mimicked those receptors by performing site-directed mutagenesis of the vector containing TAS2R38 of M. fuscata. Single-site mutants of macaque TAS2R38 that mimic colobine TAS2R38 show remarkably decreased responses to PTC, as indicated by EC50 values. The mutations V44I, Q93E, I148F and R330K increased the EC50 values of the receptor (2.22 ± 0.44, 5.59 ± 2.34, 2.80 ± 1.31 and 1.35 ± 0.54 µM, respectively) compared with the wild-type (figure 2b). Mutations in N. larvatus-B TAS2R38 mimicking those of the wild-type macaque failed to restore the sensitivity of the receptor to PTC (electronic supplementary material, figure S2), suggesting an additional mutation is responsible for the low sensitivity in colobines.
Figure 2.
Possible amino acid substitutions associated with the decreased response to PTC. (a) Schematic transmembrane topology of the TAS2R38 receptor of colobines. The structure shown is based on the structure of bovine rhodopsin. Grey circles represent known functional sites for PTC [18]. The positions of the four amino acid substitutions observed in colobines are shown in squares: human = chimpanzee = macaque ≠ colobines. (b) The in vitro responses of M. fuscata TAS2R38 mutated to match the colobine-type receptor. Multiple M. fuscata TAS2R38 mutants were created to evaluate the effects of amino acid changes on receptor sensitivity. Average responses (n = 4) for each time course are plotted against the concentration of PTC. The results for mutants, except R330K (p = 0.070), were significantly different from those for M. fuscata (p < 0.05). (c) Phylogenetic relationships among amino acid sequences of TAS2R38 in primates. The numbers at the nodes on the phylogeny represent bootstrap values. The relationships are consistent with the species phylogeny obtained using other molecular data, Alu mobile elements, 11 nuclear loci (SRY, DBY5, SMCY7, SMCY11, UTY18, vWF11, ZFYLI, ALB3, IRBP3, TNP2 and TTR1) and mitochondrial genome [18].
4. Discussion
We found that colobines are less responsive to PTC than are macaques. The decreased sensitivity to bitterness in colobines might be an advantageous adaptation to eating leaves. The common ancestor of primates is assumed to have had high sensitivity to PTC based on TAS2R38 [18]. In non-colobine primates, amino acid positions 44, 93, 148 and 330 are conserved. Substitutions of these amino acids might be a colobine-specific mechanism to reduce their sensitivity to PTC (as evidenced by the mutated macaque TAS2R38, which mimics the colobine receptor). Based on the phylogenetic relationships among the primate predicted TAS2R38 proteins, the amino acid substitutions arose in colobines after their divergence from the lineage that led to macaques. Two substitutions (V44I and Q93E) occurred after the separation of the Asian and African colobine lineages (figure 2c). Although the TAS2R38 sequences and sensitivities of other colobine TAS2R loci to PTC should be investigated, we hypothesize that sensitivities to bitter flavours became weak specifically in the colobine lineage as an adaptation to their leaf-eating behaviour.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the staff of Ragunan Zoo, Jakarta, Indonesia, Dr T. Ueda, Dr Y. Ishimaru, Dr T. Misaka and Dr K. Abe for providing the G16/gust44 and pEAK10 expression vector, and Dr H. Matsunami for providing the HEK293T cells. We also thank Dr Y. Terai for providing the sequence of M. heki.
Ethics
Behavioural experiments were performed in accordance with the guidelines of Bogor Agricultural University. The protocol was approved by the Animal Ethic Commission of Research and Community Service Institute, Ministry of Research, Technology and Higher Education, Bogor Agricultural University to B.S. (no. 35-2016 IPB). In vitro experiments were performed in accordance with the guidelines of Kyoto University. The protocol was approved by the Genetic Research Committee of Agency for Health, Safety and Environment, Kyoto University to H.I. (no. 120197).
Data accessibility
DNA sequences: DDBJ accessions LC167091–LC167096. Original functional data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.36d35 [19].
Authors' contributions
L.H.P.S.P. conducted experiments, wrote the original draft, and analysed and interpreted data. K.A.W. conducted experiments and analysed and interpreted data. K.T. and N.S.-H. designed the experiments, conducted experiments and analysed the data. T.H. and S.N. conducted experiments. B.S. designed the experiments, wrote the paper and revised the draft. H.I. designed the experiments, wrote the paper and finalized the draft. All authors agree to be held accountable for the content in the manuscript and approve the final version.
Competing interests
We have no competing interests to report.
Funding
This work was supported by KAKENHI grants from the Japan Society for the Promotion of Science (nos 25891012 to K.T., 12J04270 to T.H., 24370096, 24405018, 26117512, 15H05242, 26304008 to H.I.) and Bilateral Research to H.I., the Cooperative Research Programme of Kyoto University (Kyodo Riyo Theme nos 2014-B-63, 2015-B-50, 2016-B-62) to L.H.P.S.P., Dana DIPA grant 2013 from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia (code MAK: 2013.089.521219) to K.A.W. and the Programme Magister Menuju Doktor untuk Sarjana Unggul (PMDSU) Grant 2015 from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia to B.S.
References
- 1.Meyerhof W. 2005. Elucidation of mammalian bitter taste. Rev. Physiol. Biochem. Pharmacol. 154, 37–72. (doi:10.1007/s10254-005-0041-0) [DOI] [PubMed] [Google Scholar]
- 2.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJP. 2000. T2Rs function as bitter taste receptors. Cell 100, 703–711. (doi:10.1016/S0092-8674(00)80706-0) [DOI] [PubMed] [Google Scholar]
- 3.Wooding S, Gunn H, Ramos P, Thalmann S, Xing C, Meyerhof W. 2010. Genetics and bitter taste responses to goitrin, a plant toxin found in vegetables. Chem. Senses 35, 685–692. (doi:10.1093/chemse/bjq061) [DOI] [PubMed] [Google Scholar]
- 4.Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. 2010. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 35, 157–170. (doi10.1093/chemse/bjp092) [DOI] [PubMed] [Google Scholar]
- 5.Behrens M, Gunn HC, Ramos PCM, Meyerhof W, Wooding SP. 2013. Genetic, functional, and phenotypic diversity in TAS2R38-mediated bitter taste perception. Chem. Senses 38, 475–484. (doi:10.1093/chemse/bjt016) [DOI] [PubMed] [Google Scholar]
- 6.Suzuki-Hashido N, et al. 2015. Rapid expansion of phenylthiocarbamide non-tasters among Japanese macaques. PLoS ONE 10, e0132016 (doi:10.1371/journal.pone.0132016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wooding S, et al. 2006. Independent evolution of bitter-taste sensitivity in humans and chimpanzees. Nature 440, 930–934. (doi:10.1038/nature04655) [DOI] [PubMed] [Google Scholar]
- 8.Drewnowski A, Gomez-Carneros C. 2000. Bitter taste, phytonutrients, and the consumer: a review. Am. J. Clin. Nutr. 72, 1424–1435. [DOI] [PubMed] [Google Scholar]
- 9.Bauchop T, Martucci RW. 1968. Ruminant-like digestion of the langur monkey. Science 161, 698–700. (doi:10.1126/science.161.3842.698) [DOI] [PubMed] [Google Scholar]
- 10.Newcombe RG. 2016. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat. Med. 17, 873–890. (doi:10.1002/(SICI)1097-0258(19980430)17:8<873::AID-SIM779>3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- 11.Suzuki N, Sugawara T, Matsui A, Go Y, Hirai H, Imai H. 2010. Identification of non-taster Japanese macaques for a specific bitter taste. Primates 51, 285–289. (doi:10.1007/s10329-010-0209-3) [DOI] [PubMed] [Google Scholar]
- 12.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. (doi:10.1093/molbev/msr121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones DT, Taylor WR, Thornton JM. 1992. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8, 275–282. (doi:10.1093/bioinformatics/8.3.275) [DOI] [PubMed] [Google Scholar]
- 14.Imai H, Suzuki N, Ishimaru Y, Sakurai T, Yin L, Pan W, Abe K, Misaka T, Hirai H. 2012. Functional diversity of bitter taste receptor TAS2R16 in primates. Biol. Lett. 8, 652–656. (doi:10.1098/rsbl.2011.1251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueda T, Ugawa S, Yamamura H, Imaizumi Y, Shimada S. 2003. Functional interaction between T2R taste receptors and G-protein alpha subunits expressed in taste receptor cells. J. Neurosci. 23, 7376–7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritz C, Baty F, Streibig JC, Gerhard D. 2015. Dose–response analysis using R. PLoS ONE 10, e0146021 (doi:10.1371/journal.pone.0146021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lossow K, Hübner S, Roudnitzky N, Slack JP, Pollastro F, Behrens M, Meyerhof W. 2016. Comprehensive analysis of mouse bitter taste receptors reveals different molecular receptive ranges for orthologous receptors in mice and humans. J. Biol. Chem. 291, 15 358–15 377. (doi:10.1074/jbc.M116.718544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roos C, et al. 2011. Nuclear versus mitochondrial DNA: evidence for hybridization in colobine monkeys. BMC Evol. Biol. 11, 77 (doi:10.1186/1471-2148-11-77) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purba LHPS, Widayati KA, Tsutsui K, Suzuki-Hashido N, Hayakawa T, Nila S, Suryobroto B, Imai H. 2017. Data from: functional characterization of TAS2R38 receptor to phenylthiocarbamide (PTC) in colobine monkeys. Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.36d35) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Purba LHPS, Widayati KA, Tsutsui K, Suzuki-Hashido N, Hayakawa T, Nila S, Suryobroto B, Imai H. 2017. Data from: functional characterization of TAS2R38 receptor to phenylthiocarbamide (PTC) in colobine monkeys. Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.36d35) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
DNA sequences: DDBJ accessions LC167091–LC167096. Original functional data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.36d35 [19].