Abstract
An audience can have a profound effect on the dynamics of communicative interactions. As a result, non-human primates often adjust their social decision-making strategies depending on the audience composition at a given time. Here we sought to test how the unique vocal behaviour of multiple audience members affected decisions to communicate. To address this issue, we developed a novel experimental paradigm in which common marmosets directly interacted with multiple ‘virtual monkeys’ (VMs), each of whom represented an individual marmoset with distinct vocal behaviour. This active social signalling paradigm provided subjects an opportunity to interact with and learn about the behaviour of each VM in the network and apply this knowledge in subsequent communicative decisions. We found that subjects' propensity to interact with particular VMs was determined by the behaviour of each VM in the audience and suggests that marmoset social decision-making strategies are highly adaptive to nuances of the immediate communication network.
Keywords: marmoset, social decision-making, communication networks, antiphonal calling
1. Background
The composition of an audience is known to affect the patterns of communication in a diversity of animal species [1–6]. Given that the individuals within a particular social scene can change over time, non-human primates must monitor these changes and adapt their social decision-making strategies accordingly [7,8]. Despite the fact that networks of multiple individuals, and not dyadic interactions, are more characteristic of primates and other animals [9], relatively little is known about how individuals change their decisions to communicate based on the unique vocal behaviours of each conspecific in a particular audience. Here we sought to test this issue by implementing a novel active social signalling paradigm designed to simulate a natural primate communication network. In these experiments, a common marmoset (Callithrix jacchus) directly engaged in vocal interactions with multiple ‘virtual monkeys’ (VMs). Each VM was a speaker that broadcast calls from an individual marmoset from our colony, thus encapsulating the vocal identity of that individual. We then used novel interactive playback software to assign specific behavioural attributes to each individual VM and test whether marmoset decisions were determined solely by the specific behavioural characteristics of a particular VM (independent of the other VM) or whether decisions were made by comparing the behaviour of both VMs in the scene.
Marmosets frequently engage in natural vocal exchanges known as antiphonal calling [10]. This behaviour involves the reciprocal exchange of the species-typical phee call between visually occluded individuals, a vocalization encoded with a rich corpus of information about the caller, such as individual identity, sex and group dialect [11]. Similar to contact calls produced by other primates, the phee call functions to alert conspecifics to a signaller's presence when visually occluded. Antiphonal interactions are governed by social rules such as when it is appropriate to vocalize and exhibiting reciprocity throughout the vocal exchange [12]. Antiphonal calling mediates various dimensions of marmoset social behaviour, such as maintaining social contact within the group and territorial interactions [10,12–15]. These interactions occur throughout the year between individuals that vary in relatedness [10,12–15]. Building on previous studies showing that response latency and reciprocity are important for maintaining these vocal exchanges in dyadic interactions [10,16], the current experiments tested whether these behavioural cues alone were the basis of marmoset decisions to communicate when multiple individuals composed an audience. To address this issue, we categorically manipulated two behavioural cues: response latency (long or short) and response probability (high or low) of each VM in a communication network. We hypothesized that if these cues alone influenced marmosets' decisions to communicate, then we would observe an increased response rate to VMs with a high response likelihood and short response latency, regardless of the behaviour of other audience members. However, if the relative behaviour of conspecifics in the audience contributed to the decision-making process, then response rates would vary based on the composition of network members.
2. Material and methods
A more detailed description of the methods is provided in the electronic supplementary material. Eight adult marmosets participated in this experiment (five females, three males) from a colony of approximately 30 monkeys. Subjects were placed in a mesh cage on one side of the experimental room where a microphone recorded their vocalizations (figure 1a). A dark curtain was placed in the centre of the room, 1.8 m in front of the subject, which acted to occlude two speakers on the opposite side of the room. Speakers were approximately 3.3 m away from the subject. Each speaker represented a VM as it simulated an individual marmoset by broadcasting phee calls from that marmoset throughout each session with distinctive vocal behaviour. All phee calls used as stimuli were prerecorded from 12 familiar animals within the home colony. Previous experiments show that marmosets recognize a caller's individual identity based on phee calls produced during antiphonal calling [16].
Figure 1.
(a) Schematic depicting the experimental set-up. (b) Schematic of the experimental logic. Phee calls produced by subjects are shown in purple. Stimuli broadcast from each VM are shown in blue and red. When subjects were silent for 20–30 s following the last stimulus presentation from a VM, a spontaneous phee call from one of the VMs was broadcast.
The logic of the experiment was to first provide subjects experience of how each VM responded to subjects' vocalizations, and then test how knowledge of each VM affected subsequent communication decisions (figure 1b). VMs served both as vocal partners to subjects and as audience members. Subjects learned about the behaviour of VMs in their audience through interactions with those individuals, and could alter their vocal behaviour accordingly. When a VM produced a call, subjects decided whether to produce a response based on what they had learned. In this design, when subjects emitted a phee call, one VM produced an antiphonal phee response. Each VM was assigned specific attributes regarding the likelihood and latency in which it responded to subjects. This allowed subjects to gain knowledge about the behaviour of the VMs. When subjects were silent for 20–30 s, one VM produced a ‘spontaneous’ phee call with equal probability (50%). We measured how likely subjects were to respond to these ‘spontaneous’ calls given what they had learned about each VM during the previous vocal interactions. Spontaneously produced phee calls function to elicit an antiphonal call from a conspecific and initiate a new bout of calling [10]. Phee calls produced in spontaneous and antiphonal contexts are known to exhibit subtle acoustic differences, but are not distinguishable in a discriminant function analysis [11]. As a result, they are classified behaviourally by when they are produced relative to other phees. Importantly, all data presented here are only from subjects' responses to ‘spontaneous’ phee calls of VMs. A phee call was considered a response if it was produced by subjects within 8 s of the offset of the VM call. Custom software was developed in MATLAB that determined the timing of each VM's vocalization stimulus broadcasts and recorded subjects' vocalizations.
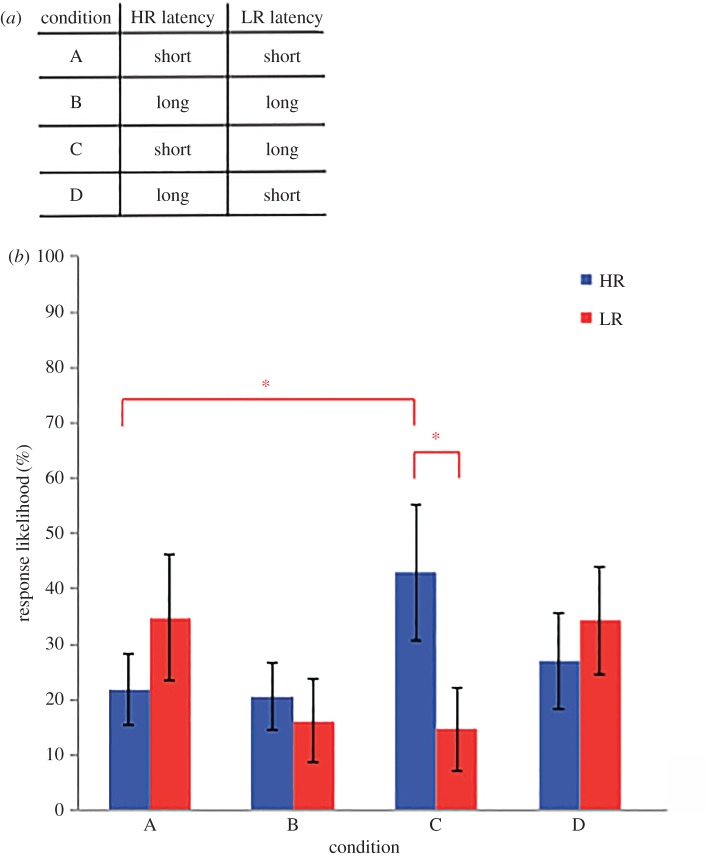
VMs were defined by the following two behavioural characteristics of their responses to subjects' phee calls. VMs responded to subjects with (i) either a short (1–3 s) or long (13–15 s) latency and (ii) a high (80%; HR = high responder) or a low (20%; LR = low responder) response likelihood. Combining these behavioural cues together gave rise to four VM types: HR-short, LR-short, HR-long and LR-long. We tested the influence of two VM types in the four following test conditions (A: HR-short/LR-short; B: HR-long/LR-long; C: HR-short/LR-long and D: HR-long/LR-short; figure 2a). Each condition was repeated three times for each subject with different marmoset calls used for the respective VMs.
Figure 2.
(a) Table depicting the latency of response for each VM in each experimental condition. (b) Average response likelihoods of subjects across all test conditions. Errors bars represent 95% confidence interval of the mean. *p < 0.05.
These behavioural characteristics were tested based on previous work suggesting that they are perceptually meaningful in marmoset antiphonal calling. First, a response latency of greater than 9 s was significantly less likely to elicit an antiphonal response, suggesting that this was the period during which marmosets perceive a subsequent call as a response to their own phee [12]. Second, the length of marmoset antiphonal calling bouts was significantly longer for cagemates than non-cagemates [10], indicating that response probabilities changed based on social context.
A total of six stimulus sets were used for each subject for a given test condition. Stimulus sets comprised phee calls produced by an opposite sex adult individual from a different family group in the colony relative to the subject. This allowed us to control for differences that may emerge in marmoset behaviour related to the sex and relatedness of the individuals [10]. Stimuli were systematically counterbalanced across experimental sessions to control for differential responding to a particular monkey's vocalizations. Each stimulus set of vocalizations from a particular caller could serve as either VM type within a test condition. As a result, although the calls from a specific marmoset may be used to construct a VM, its vocal behaviour was not constant across sessions. This design ensured that we tested subjects' decisions with respect to VM behaviour within a particular session, rather than more longitudinal experience with that caller.
Data were analysed after subjects heard at least two antiphonal responses from each VM in order to ensure that subjects had experience of the respective behavioural characteristics of each VM. All data presented here represent an average of the response likelihoods to spontaneous calls across the three experimental sessions for each condition. Data were analysed in JMP using a three-way (likelihood/latency of HR/latency of LR) univariate repeated-measures ANOVA and post hoc Tukey HSD (honest significant difference) comparisons.
3. Results
Marmosets' decisions to communicate were dependent on the relative vocal behaviour of the audience members (figure 2b). We observed a significant three-way interaction between the type of responder (HR/LR), the latency of HR (short/long) and the latency of LR (short/long) on subjects' responses (F1,7 = 7.23, p = 0.008). Post hoc Tukey HSD tests revealed that subjects responded significantly more to HR-short (M = 43, s.d. = 25.6) than to LR-long (M = 14.7, s.d. = 10.8) in condition C (Tukey HSD, p < 0.001). Notably, subjects did not show a preference for HR-short in all conditions. In condition A, responses to HR-short (M = 22, s.d. = 5.78) were not significantly different from responses to LR-short (M = 34.8, s.d. = 19) (Tukey HSD, p > 0.1). In fact, subjects were significantly more likely to respond to HR-short in condition C (when paired with LR-long) (M = 43, s.d. = 25.6) compared with HR-short in condition A (when paired with LR-short) (M = 22, s.d. = 5.78) (figure 2b; Tukey HSD, p < 0.05).
4. Discussion
Here we found that the relative behaviour of conspecifics in the audience had a significant effect on marmosets' decision to communicate in a network. At least two significant findings emerged from this experiment. First, subjects' social decision-making strategies adapted to the nuances of the immediate social scene through social learning. Although each VM represented a familiar animal from the home colony, their vocal behaviour differed in each given test session. As a result, subjects learned how the particular VM behaved in that scene and modified their decisions accordingly. The ability to modify decisions based on the current behaviour of an individual is likely critical to primate living, as group members may behave unpredictably when access to resources or social dynamics change. Second, marmosets' preference to engage in antiphonal interactions with the HR-short VM was evident only in networks comprising a LR-long VM. Other networks with HR-short VMs did not elicit a similarly robust response. This suggests that these behavioural characteristics alone do not determine the basis for decisions in marmoset communication networks. Rather, marmosets likely compare the behaviour of all conspecifics in the scene before deciding with whom to engage in a vocal exchange. An awareness of the behaviour of multiple individuals in a vicinity is likely crucial to navigating the complex social dynamics evident in our Order [17,18].
Primate social cognition and communication are intertwined and likely played complementary roles in language evolution [19]. This relationship is evident in communication networks, where social cognitive mechanisms mediate behavioural interactions through the exchange of vocal signals. However, experimental approaches are limited in effectively testing the processes that unfold in these dynamic settings (but see [20]). A key advantage of interactive playbacks [21] is that researchers function as active participants in ongoing social interactions (via the VMs) and not passive observers. Active social signalling paradigms can investigate elements of primate social cognition and communication that cannot be tested with more traditional playback approaches and, when combined with neural recordings in naturally behaving marmosets [22,23], can be used to elucidate dimensions of the primate social brain that were not previously possible.
Supplementary Material
Acknowledgement
We thank Colleen Law and Ava Foudeh for assistance with data collection.
Ethics
This work was approved by the UCSD IACUC (S09147).
Data accessibility
The data are available in the Dryad Digital Repository [24].
Authors' contributions
C.R.T. and C.T.M. conceived and designed the experiment. C.R.T. and L.W. collected and analysed the data. All authors contributed to the intellectual content and revisions of this manuscript. All authors have agreed to be held accountable for this work and have approved the final version of the manuscript for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NIH R01 DC012087.
References
- 1.Doutrelant C, McGregor PK, Oliveira RF. 2001. The effect of an audience on intrasexual communication in male Siamese fighting fish, Betta splendens. Behav. Ecol. 12, 283–286. ( 10.1093/beheco/12.3.283) [DOI] [Google Scholar]
- 2.Evans CS, Marler P. 1994. Food-calling and audience effects in male chickens, Gallus gallus: their relationships to food availability, courtship and social facilitation. Anim. Behav. 47, 1159–1170. [Google Scholar]
- 3.Zuberbuhler K. 2008. Audience effects. Curr. Biol. 18, R189–R190. ( 10.1016/j.cub.2007.12.041) [DOI] [PubMed] [Google Scholar]
- 4.Seagraves KM, Arthur BJ, Egnor SER. 2016. Evidence for an audience effect in mice: male social partners alter the male vocal response to female cues. J. Exp. Biol. 219, 1437–1448. ( 10.1242/jeb.129361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dzieweczynski TL, Earley RL, Green TM, Rowland WJ. 2005. Audience effect is context dependent in Siamese fighting fish, Betta splendens. Behav. Ecol. 16, 1025–1030. ( 10.1093/beheco/ari088) [DOI] [Google Scholar]
- 6.Gros-Louis J. 2004. The function of food-associated calls in the white faced capuchin monkeys, Cebus capucinus, from the perspective of the signaler. Anim. Behav. 67, 431–440. ( 10.1016/j.anbehav.2003.04.009) [DOI] [Google Scholar]
- 7.Seyfarth RM, Cheney DL. 2012. Knowledge of social relations. In The evolution of primate societies (eds Mitani J, Call J, Kappeler PM, Palombit R, Silk JB), pp. 629–642. Chicago, IL: University of Chicago Press. [Google Scholar]
- 8.Miller CT, Freiwald W, Leopold DA, Mitchell JF, Silva AC, Wang X. 2016. Marmosets: a neuroscientific model of human social behavior. Neuron 90, 219–233. ( 10.1016/j.neuron.2016.03.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGregor PK. 2005. Animal communication networks. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 10.Miller CT, Wang X. 2006. Sensory-motor interactions modulate a primate vocal behavior: antiphonal calling in common marmosets. J. Compar. Physiol. A 192, 27–38. ( 10.1007/s00359-005-0043-z) [DOI] [PubMed] [Google Scholar]
- 11.Miller CT, Mandel K, Wang X. 2010. The communicative content of the common marmoset phee call during antiphonal calling. Am. J. Primatol. 72, 974–980. ( 10.1002/ajp.20854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller CT, Beck K, Meade B, Wang X. 2009. Antiphonal call timing in marmosets is behaviorally significant: interactive playback experiments. J. Compar. Physiol. A 195, 783–789. ( 10.1007/s00359-009-0456-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow C, Mitchell J, Miller CT. 2015. Vocal turn-taking in a nonhuman primate is learned during ontogeny. Proc. R. Soc. B 282, 210150069 ( 10.1098/rspb.2015.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen HC, Kaplan G, Rogers LJ. 2009. Contact calls of common marmosets (Callithrix jacchus): influence of age of caller on antiphonal calling and other vocal responses. Am. J. Primatol. 71, 165–170. ( 10.1002/ajp.20636) [DOI] [PubMed] [Google Scholar]
- 15.Bezerra BM, Souto A. 2008. Structure and usage of the vocal repertoire of Callithrix jacchus. Int. J. Primatol. 29, 671–701. ( 10.1007/s10764-008-9250-0) [DOI] [Google Scholar]
- 16.Miller CT, Thomas AW. 2012. Individual recognition during bouts of antiphonal calling in common marmosets. J. Compar. Physiol. A 198, 337–346. ( 10.1007/s00359-012-0712-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheney DL, Seyfarth RM. 2007. Baboon metaphysics: the evolution of a social mind. Chicago, IL: University of Chicago Press. [Google Scholar]
- 18.Seyfarth RM, Cheney DL. 2015. Social cognition. Anim. Behav. 103, 191–202. ( 10.1016/j.anbehav.2015.01.030) [DOI] [Google Scholar]
- 19.Seyfarth RM, Cheney DL. 2014. Evolution of language from social cognition. Curr. Opin. Neurobiol. 28, 5–9. ( 10.1016/j.conb.2014.04.003) [DOI] [PubMed] [Google Scholar]
- 20.Cheney DL, Seyfarth RM, Silk J. 1995. The responses of female baboons (Papio cynocephalus ursinus) to anomalous social interactions: evidence for causal reasoning? J. Comp. Psychol. 109, 134–141. [DOI] [PubMed] [Google Scholar]
- 21.King SL. 2015. You talkin’ to me? Interactive playback is a powerful yet underused tool in animal communication research. Biol. Lett. 11, 20150403 ( 10.1098/rsbl.2015.0403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eliades SJ, Wang X. 2008. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature 453, 1102–1106. ( 10.1038/nature06910) [DOI] [PubMed] [Google Scholar]
- 23.Miller CT, Thomas AW, Nummela S, de la Mothe LA. 2015. Responses of primate frontal cortex neurons during natural vocal communication. J. Neurophysiol. 114, 1158–1171. ( 10.1152/jn.01003.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toarmino CR, Wong L, Miller CT. 2017. Data from: Audience affects decision-making in a marmoset communication network. Dryad Digital Repository. ( 10.5061/dryad.1vn0s) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Toarmino CR, Wong L, Miller CT. 2017. Data from: Audience affects decision-making in a marmoset communication network. Dryad Digital Repository. ( 10.5061/dryad.1vn0s) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data are available in the Dryad Digital Repository [24].