Abstract
Species within a guild vary their use of time, space and resources, thereby enabling sympatry. As intra-guild competition intensifies, such behavioural adaptations may become prominent. We assessed mechanisms of facilitating sympatry among dhole (Cuon alpinus), leopard (Panthera pardus) and tiger (Panthera tigris) in tropical forests of India using camera-trap surveys. We examined population-level temporal, spatial and spatio-temporal segregation among them across four reserves representing a gradient of carnivore and prey densities. Temporal and spatial overlaps were higher at lower prey densities. Combined spatio-temporal overlap was minimal, possibly due to chance. We found fine-scale avoidance behaviours at one high-density reserve. Our results suggest that: (i) patterns of spatial, temporal and spatio-temporal segregation in sympatric carnivores do not necessarily mirror each other; (ii) carnivores are likely to adopt temporal, spatial, and spatio-temporal segregation as alternative mechanisms to facilitate sympatry; and (iii) carnivores show adaptability across a gradient of resource availability, a driver of inter-species competition. We discuss behavioural mechanisms that permit carnivores to co-occupy rather than dominate functional niches, and adaptations to varying intensities of competition that are likely to shape structure and dynamics of carnivore guilds.
Keywords: competition, inter-species interactions, copredators, south Asia, spatial aggregation, temporal activity patterns
1. Introduction
Mechanisms that allow species in carnivore guilds to coexist have long interested ecologists. At large scales, co-occurrence is generally facilitated through spatial segregation [1]. Because a number of large carnivore species now survive in remnant habitat patches [2] precluding spatial segregation, the resulting enforced sympatry may result in intense competitive responses like intimidation, kleptoparasitism, mortality and even spatial exclusion [3,4]. These competitive interactions have in fact been observed among large carnivores confined within protected reserves [3,5]. In this context, factors or behavioural adaptations that facilitate sympatry among potentially competing species within insular habitats are key to understanding community structure and dynamics in changing environments.
Sympatric carnivores may show behavioural mechanisms such as partitioning of diet, or differential use of space and/or time, to avoid competitive encounters [5,6]. Subordinate competitors may avoid activity centres, or locations of higher population density of dominant species [7]. Similarly, species may adapt their circadian activity patterns to reduce temporal activity overlap [8]. At finer scales, competing species respond through active avoidance or intensified aggression [4]. Adaptations that facilitate intra-guild sympatry may be more pronounced as competition within the guild intensifies [9] through ‘character displacement’ (sensu [10]). Limited availability, diversity or spatial clumping of resources, as well as population densities of the species, can intensify competition [3,11]. In addition, anthropogenic factors may also influence intra-guild competition directly by affecting species densities, or indirectly by modifying resource levels and distribution [12]. Behavioural mechanisms that allow species to coexist across a gradient of resource availability are not well understood but could provide insights into drivers of community structure across space and time [13,14].
Observations of marked or radio-collared individuals allow assessments of behavioural responses to competitors at fine spatial and temporal scales [6], but typically do not provide insights on population-level mechanisms that facilitate sympatry and cross-population comparisons [15]. Variations in population-level competitive interactions among carnivore species have actually seldom been empirically examined across a gradient of resource availability. Such cross-population comparisons can clarify adaptability of species to competitive intensity and mechanisms of competitor avoidance. In this context, non-invasive camera-trap survey methodology [16] is being increasingly used to address population-level assessments of carnivore ecology and behaviour because of its ability to sample even low-density populations at large spatial scales.
We assessed behavioural mechanisms facilitating co-occurrence among three large carnivore species, dhole Cuon alpinus, leopard Panthera pardus and tiger Panthera tigris, in the Western Ghats, south-western India. This carnivore guild is well suited to our study because of the high degree of dietary overlap among the sympatric predators [6,17,18] as well as empirical evidence of competition over ungulate prey species [6,18–20]. Radio-telemetry and ad libitum observations also show that diurnal dholes temporally segregate from the more crepuscular/nocturnal felids [6,21,22].
Using photographic data from surveys—which were also designed to estimate carnivore demographic parameters—we examined temporal, spatial and combined spatio-temporal behavioural mechanisms that could potentially facilitate sympatry for each species pair. We also examined how these three mechanisms may function in four study sites characterized by different population densities of these carnivores and their ungulate prey.
2. Material and methods
(a). Study area
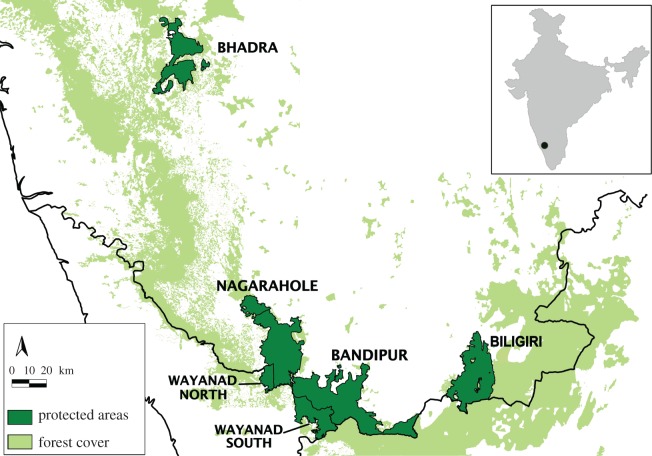
Our study was conducted at four sites, which covered five wildlife reserves representing differing carnivore and prey densities. These included two high-density reserves, Nagarahole (here, including the adjoining southern block of Wayanad reserve) and Bandipur (including southern block of Wayanad reserve), one medium-low density reserve, Biligiri Rangaswamy Temple (henceforth, Biligiri) reserve and a relatively low-density reserve, Bhadra (figure 1 and table 1). Together, these reserves represent a gradient of densities of ungulate prey as well as carnivores reliably estimated from previously conducted scientific studies (see table 1, [23]; K. U. Karanth 2013, unpublished results). Furthermore, the Malenad landscape in the Western Ghats, within which these four reserves are embedded, also supports larger, viable meta-populations of these three carnivores [25,26]. All four reserves are currently well protected from hunting and other anthropogenic impacts. However, historical duration of such protection varies, leading to differences in animal population densities. Details of reserve-specific features are in table 1.
Figure 1.
Map of study area showing four reserves and surrounding reserve forests in the Western Ghats landscape. Inset shows the location of the study area in India.
Table 1.
Habitat characteristics and carnivore (dhole, leopard and tiger) and prey relative densities in four reserves in the Western Ghats considered in our study.
| Bhadra | Biligiri | Nagaraholea | Bandipura | |
|---|---|---|---|---|
| area | 492 km2 | 540 km2 | 775 km2 | 1178 km2 |
| habitat type | evergreen, moist deciduous forests, swampy grasslands | mixed deciduous, evergreen and scrub forests, montane grasslands | moist and dry deciduous forests, with swampy hadlus (open areas) | deciduous and dry deciduous scrub forests, savannah woodland |
| precipitation | 2000–2540 mm | 500–1800 mm | 1000–1500 mm | 700–1200 mm |
| conservation status | large number of villages resettled from park in 2002 | relatively high level of anthropogenic pressures, including people residing within the park | well protected; few tribal settlements inside park | well protected with low anthropogenic threats. High presence of invasive plants |
| surrounding land-uses | reserve forests and coffee plantations | highly modified human-inhabited areas, including croplands | reserve forests, coffee plantations and crop fields | reserve forests, croplands and pasture lands, plantations |
| carnivore densitiesb | dhole: low leopard: high tiger: low (3.4 tigers km−2 [23]) |
dhole: medium leopard: low tiger: high |
dhole: medium leopard: high tiger: high (14 tigers km−2 [24]) |
dhole: high leopard: high tiger: high (12 tigers km−2 [24]) |
| prey densitiesb | large preyc: medium small-mid prey: low arboreal prey: high total ungulate density = 17 individuals km–2 [23] |
large prey: high small-mid prey: high arboreal prey: low |
large prey: high small-mid prey: high arboreal prey: high total ungulate density = 56 individuals km–2 [23] |
large prey: high small-mid prey: medium arboreal prey: high total ungulate density = 35 individuals km−2 [23] |
aWe use the names Nagarahole and Bandipur in our study to represent the adjoining Protected Areas, Nagarahole and the northern part of Wayanad, and Bandipur and the southern part of Wayanad, respectively.
bDensities of carnivores and prey based on previous studies, where specified, and long-term experience in the study sites (K. U. Karanth 2013, unpublished results).
cWe segregate prey into large (sambar Rusa unicolor and gaur Bos gaurus), small-mid sized (chital Axis axis, muntjac Muntiacus muntjac and wild pig Sus scrofa) and arboreal (langur Semnopithecus entellus and bonnet macaque Macaca radiata) prey.
(b). Field survey
Non-invasive camera-trap surveys of animals can track temporal activity and space use, providing reliable data for population-level inferences [16,27]; such inferences are impractical at a population level using methods involving capture and handling of large carnivores, such as radio-telemetry [15]. We relied on a set of extensive and rigorously collected data on the focal species. A total of 562 camera-trap stations were deployed across the five reserves during January to June 2013 to obtain these photo-capture data as a part of a long-term carnivore-monitoring programme in India [6,18,23,28,29].
We set up 122, 99, 161 and 180 camera-trap stations, in Bhadra, Biligiri, Nagarahole and Bandipur, respectively (electronic supplementary material, figure S1). Trap stations, comprising a pair of camera-traps triggered by animal movement, were separated by Euclidean distances of around 1.5 km on average (electronic supplementary material, table S1), and active for 30 consecutive days at each site. We placed camera-traps along forest trails and dirt roads commonly used by all three species as travel routes, to maximize photo-captures [25,26]. Date, time and trap location were recorded for every photo-capture (henceforth, ‘encounter’). Each photo-encounter was a detection of either a single individual or a cluster of individuals. Multiple photo-captures less than 60 s apart were collapsed into a single independent data point.
(c). Assessing temporal segregation
We tested for temporal segregation in each species pair, within each reserve, in the following manner. First, we described temporal activity patterns for each species in each reserve. We did this by fitting a von Mises probability density distribution (akin to a circular or wrapped normal distribution [30]) to data on the time of encounter for each species, separately in each reserve. For this analysis, we only considered the time of encounter, ignoring the calendar date. Thus, all encounters were collapsed into a single 24 h period. Second, we tested whether species differed in their temporal activity patterns within each reserve. We did this for each species pair in each reserve, using a Watson's U2-test [31]. Third, we calculated the proportion of time within a 24 h period that (i) each species was exclusively active, (ii) activity of any two species overlapped, and (iii) activity of all three species overlapped in each reserve. We generated confidence intervals for each of these estimated proportions through empirical bootstrapping [32,33]. We inferred behavioural character displacement by identifying differences in species pair-wise temporal overlaps, as calculated above, across the different reserves.
(d). Overlap in space-use
The occupancy modelling framework [34] is increasingly being used for measuring animal space-use patterns. Its ability to probabilistically tease apart true absence from non-detection addresses the key issue of imperfect detection, which biases traditional presence–absence surveys. For each reserve, we used single-season, two-species habitat occupancy models [35] to assess segregation (or overlap) in space-use between each species pair. In each reserve, we treated individual camera-trap stations as potential sites for occupancy. We aggregated three consecutive days of surveys into a single sample, achieving 10 temporal replicates at each reserve.
The two-species occupancy model [35] estimates the following parameters: (i) ψA and (ii) ψB, representing probabilities of space-use by species A and B, respectively, (iii) pA and (iv) pB, for the probabilities of detecting species A and B, respectively, in a replicate conditional on their presence, and absence of the other species, and (v) rA and (vi) rB, representing probabilities of detecting species A and B, respectively, in a replicate when both species are present [35]. As we assessed fine-scale space use (scale here being defined by the inter-camera-trap distances) within reserves, our interpretation of the parameter ψ represents probability of use [36]. While we tested for the presence of one predator influencing replicate-level detectability of the other, these models did not converge. Therefore, we reduced the standard two-species occupancy model to assume that the detection of the two species was independent, by setting pA = rA and pB = rB, and by fixing δAB = (rAB)/(rA × rB) = 1.
The derived parameter in the occupancy model that measures species' spatial segregation (or overlap) is ϕAB, or the species interaction factor (henceforth, SIF), estimated as (ψAB)/(ψA × ψB) [35]. Estimates of SIF < 1 can be interpreted as the two species co-occurring less frequently than expected under a hypothesis of species independence (spatial segregation), while estimates that are greater than one can be interpreted as co-occurrence at sites more frequently than expected under a hypothesis of species independence. A value of SIF = 1 implies that the two species use sites independently. We specifically tested for spatial overlap, or segregation, for all species pairs within each park by comparing (i) a full model where SIF was estimated and (ii) a reduced model, where SIF was fixed to 1 (i.e. assuming independence in site-use). Model comparisons were based on Akaike's information criterion (AIC) [37]. We compared SIF values across reserves to test for differences in population-level spatial segregation (or aggregation).
(e). Spatio-temporal overlap
We used two methods to assess spatio-temporal segregation among the three carnivore species. First, we created a matrix of species encounters per hour for each camera-trap station, or site. Hence, the rows in the matrix represented sites, and columns represented hourly intervals of the diel cycle. Each cell in the matrix contained total number of detections of that species at a particular site during a specified hour, aggregated across the entire survey. We then calculated the proportion of sites, at each hourly interval, when (i) each species was exclusively detected, in the absence of any other species, (ii) detection of activity of any two species overlapped, and (iii) all three species were active. This analysis was done in a manner similar to the one described earlier for assessing temporal overlaps. We used empirical bootstrapping to obtain confidence intervals for these observed proportions [32,33].
Next, we estimated time-to-encounter across each species pair in each reserve using multi-response permutation procedures as described by Mielke et al. [38]. To do this, for each encounter of one species at a specific trap location, we calculated the minimum time to encounter co-predators. Thus for each species pair, a set of observed times-to-encounter was obtained. We generated expected statistical distributions of times-to-encounter by randomly assigning encounter times to camera-trap locations, in 1000 simulations. We compared the median observed time-to-encounter with the simulated distribution of expected times-to-encounter. A larger observed time-to-encounter than expected (under an assumption of species independence) reflects species segregation, while a smaller observed time-to-encounter implies species aggregation. We use this method as it tests for spatio-temporal segregation, conditional on observed space-use and temporal activity patterns of the focal species.
3. Results
In total, we obtained 503, 1018 and 930 photo-encounters of dholes, leopards and tigers, respectively, across all reserves. The total camera-trap sampling effort and numbers of photo-encounters for the three carnivore species at each reserve are shown in the electronic supplementary material, table S1.
(a). Temporal activity overlap varies across a resource gradient
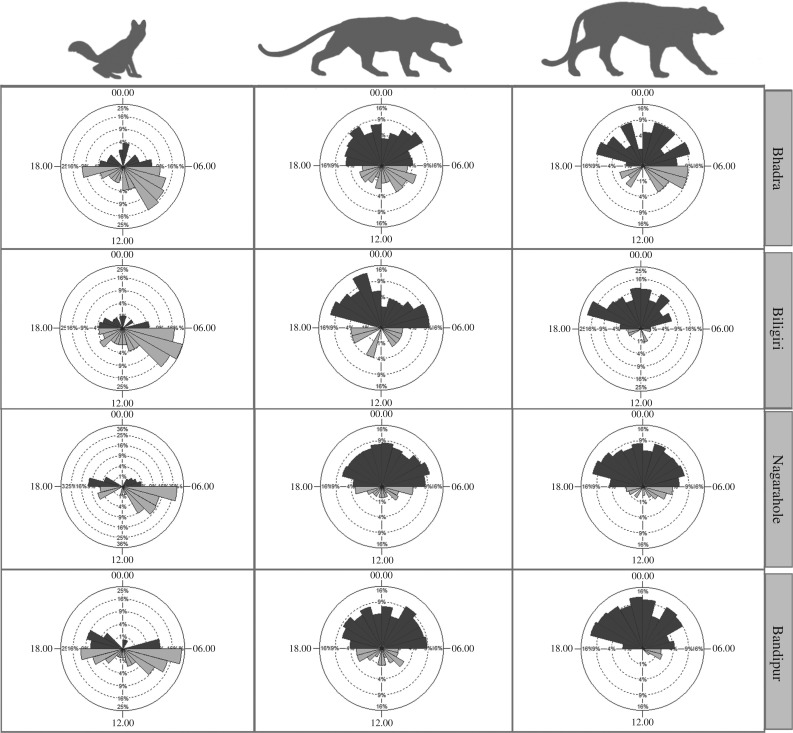
We expected dholes to be primarily diurnal, and the two felids to be nocturnal. While this pattern seemed to generally hold, all three carnivores were far more active through the diel cycle in Bhadra, the low prey density reserve, compared with the other three reserves (figure 2; electronic supplementary material, figure S2). Temporal activity patterns of tigers and leopards were significantly different in this reserve, with leopards showing relatively more diurnal activity than tigers (U2 = 0.5, p < 0.001, figure 2). Similarly, dholes were relatively more nocturnal at this reserve, and as a result, dhole temporal activity patterns did not differ significantly from the two felids (table 2). In all other reserves, however, as expected, dholes temporally segregated from tigers and leopards (figure 2 and table 2). In Biligiri, as expected, leopards and tigers had similar activity patterns (table 2 and figure 2). In the high-density reserves Nagarahole and Bandipur, however, leopards and tigers showed different temporal activity patterns, with leopards showing more crepuscular activity than tigers.
Figure 2.
Proportion of encounters of the three carnivores during daytime (06.00–17.59 h; light grey wedges) and night-time (18.00–05.59 h; dark grey wedges) in the four parks. Rose diagrams were generated using ORIANA [39]. Each plot is divided into 24 h, with percentage of detections in each hour on the response axis.
Table 2.
Differences in temporal activity between species pairs in each of the four reserves using, Watson's U2-test. Statistically significant results are denoted by asterisk.
| Bhadra |
Biligiri |
Nagarahole |
Bandipur |
|||||
|---|---|---|---|---|---|---|---|---|
| U2 | p-value | U2 | p-value | U2 | p-value | U2 | p | |
| dhole–leopard | 0.0427 | >0.1 | 1.8035 | <0.05* | 5.4353 | <0.001* | 4.4652 | <0.001a |
| dhole–tiger | 0.1475 | >0.1 | 4.5968 | <0.05* | 5.538 | <0.001* | 7.8924 | <0.001a |
| leopard–tiger | 0.5001 | <0.001* | 0.2162 | >0.05* | 0.1522 | <0.1 | 0.8371 | <0.001a |
*p < 0.05.
These differences were partially reflected in the proportion of temporal overlap across species pairs and reserves. The greatest temporal overlap was observed between tigers and leopards in all reserves, ranging from 0.76 (s.e. = 0.05) in Biligiri to 0.91 (s.e. = 0.02) in Nagarahole. Nagarahole surprisingly showed high temporal overlap of leopard and tiger, a result that is in slight contrast to the Watsons U2-test described above. As the proportional overlap approach collapses encounter times into hourly intervals, this result likely reflects that temporal activity patterns of tigers and leopards in Nagarahole overlap when measured at hourly intervals, but this overlap does not occur when measured at finer temporal scales. Mirroring results described above, however, we found relatively large temporal overlap among species in Bhadra, the reserve with the lowest prey densities (table 3).
Table 3.
Proportions of time when each species was exclusively active, activity of two species overlapped and all three species overlapped (representative diagrams illustrating each proportion are shown). Values in parentheses are bootstrapped 95% CIs.
 |
(b). Higher spatial overlap at lower prey densities
Spatial co-occurrence among species, within each reserve, was measured using the SIF for species pairs, which provides an assessment of spatial aggregation (if SIF > 1) or segregation (if SIF < 1). The values of SIF ranged between 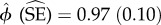 and
and 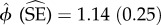 , across the four reserves (table 4), showing that by and large, the species did not show substantial patterns of spatial overlap or segregation. Nevertheless, most of the SIF estimates were more than 1, indicating some degree of aggregation rather than segregation in space-use (model comparisons in electronic supplementary material, table S2). In Bhadra, where prey densities are the lowest—and temporal overlap of species was the highest—tigers showed highest spatial aggregation with leopards. In Biligiri, which has relatively higher prey densities than Bhadra, tigers and dholes showed some degree of spatial overlap. Leopards in this reserve are found at low densities (table 1), and perhaps as a result, did not show high spatial overlap with their co-predators in this reserve.
, across the four reserves (table 4), showing that by and large, the species did not show substantial patterns of spatial overlap or segregation. Nevertheless, most of the SIF estimates were more than 1, indicating some degree of aggregation rather than segregation in space-use (model comparisons in electronic supplementary material, table S2). In Bhadra, where prey densities are the lowest—and temporal overlap of species was the highest—tigers showed highest spatial aggregation with leopards. In Biligiri, which has relatively higher prey densities than Bhadra, tigers and dholes showed some degree of spatial overlap. Leopards in this reserve are found at low densities (table 1), and perhaps as a result, did not show high spatial overlap with their co-predators in this reserve.
Table 4.
Estimated SIF for each species pair based on single-season, two-species occupancy models.
| species pair | SIF |
|||
|---|---|---|---|---|
| Bhadra | Biligiri | Nagarahole | Bandipur | |
| dhole–leopard | 1.03 (0.10) | 1.06 (0.17) | 1.07 (0.09) | 1.13 (0.08)a |
| dhole–tiger | 1.14 (0.25) | 1.13 (0.08)a | 0.99 (0.07)a | 1.03 (0.05) |
| leopard–tiger | 1.14 (0.07)a | 0.97 (0.10) | 1.08 (0.04)a | 1.10 (0.05)a |
aSpecies comparisons for which there was support (based on AIC) for SIF ≠ 1 or species dependence.
In the high-density reserve, Nagarahole, there was no evidence for spatial aggregation among dholes, leopards and tigers. In Bandipur, however, there was some evidence that leopards occurred with dholes and tigers slightly more frequently than expected under a hypothesis of independent use of space (table 4). We provide reserve-wise estimates of parameters derived from occupancy modelling for the individual species and SIFs for species pairs in table 4 and electronic supplementary material, table S3.
(c). Behavioural avoidance at higher carnivore densities
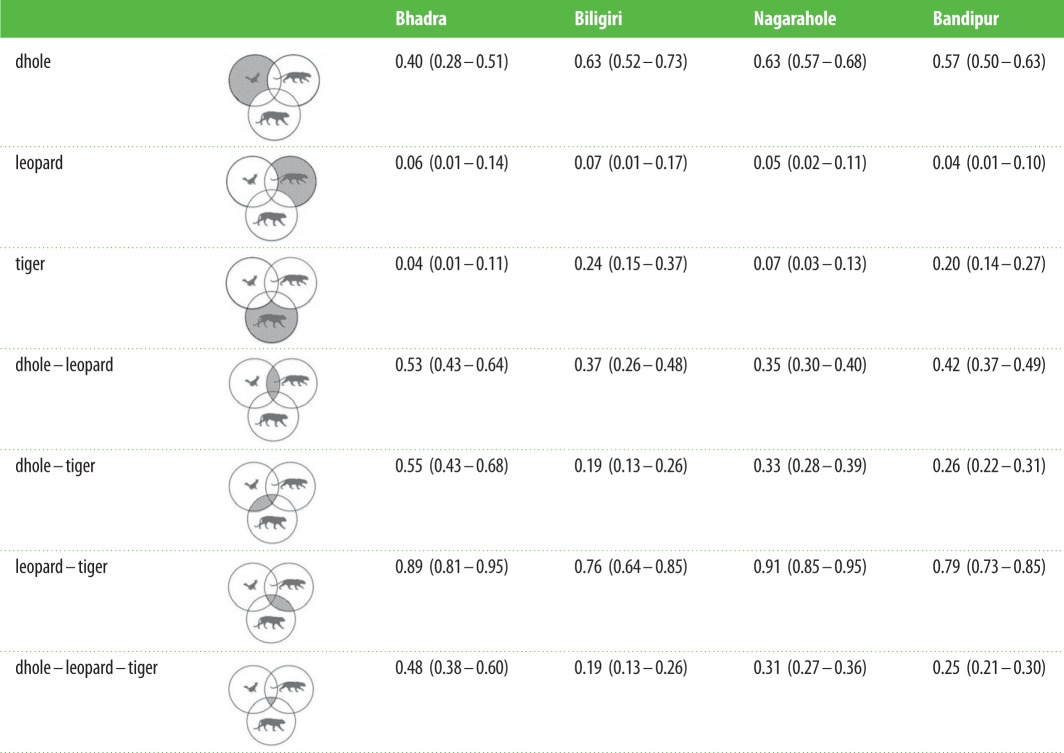
We found that spatio-temporal overlap between species pairs was much lower when compared with either purely spatial or purely temporal overlaps considered separately; all three species were photo-captured together at less than 1% of the trap sites and hourly interval categories (table 5). Furthermore, patterns of combined spatio-temporal overlap did not reflect patterns of either spatial or temporal overlaps. The reserve that had high temporal overlap, Bhadra, showed the least spatio-temporal overlap among species. In fact, the three predators show complete spatio-temporal segregation (i.e. proportion of space–time when all three species were present was estimated to be 0) in the low carnivore density reserves, Bhadra and Biligiri (table 5); this may be artefact of the lower densities of carnivores resulting in lower rates of photo-encounter in these two reserves. Leopards and tigers overlapped more in their spatio-temporal activity patterns when compared with dholes, in Nagarahole, Bandipur and Bhadra. However, this pattern was reversed in Biligiri, where leopard density was low.
Table 5.
Proportions of space and time that each species was exclusively active, activity of two species overlapped and the proportion of space–time where all three species overlapped. We provide representative diagrams to illustrate the proportions. Values in parentheses are bootstrapped 95% confidence intervals.
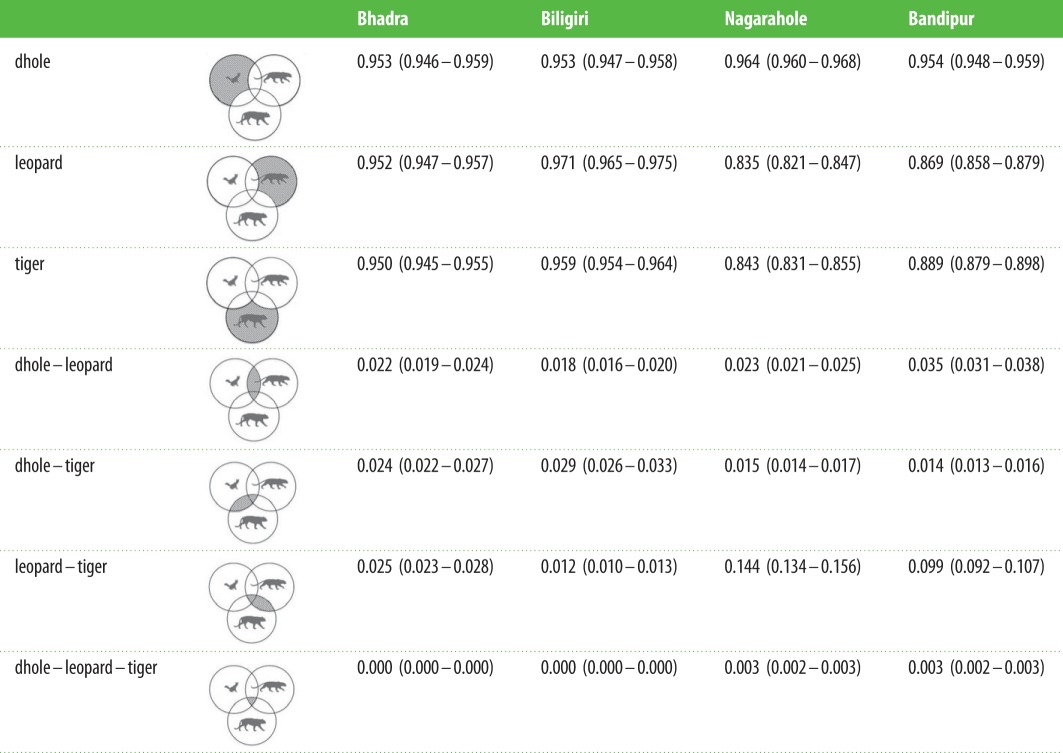 |
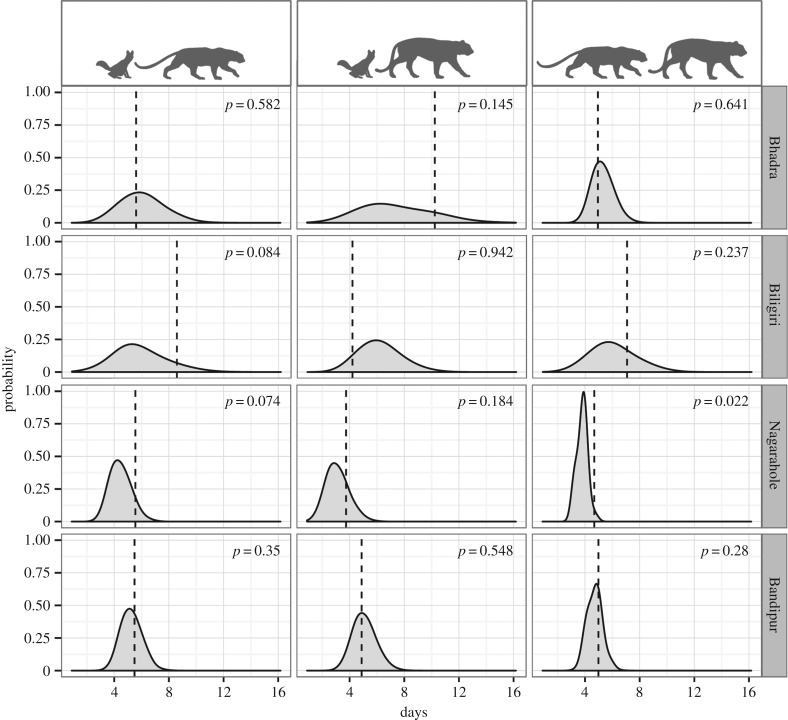
Even when spatio-temporal overlap occurred, we examined the times-to-encounter between carnivore pairs to test for behavioural avoidance. Intra-guild behavioural avoidance was most evident in Nagarahole (figure 3). However, the three carnivores did not show such avoidance behaviour in Bandipur, also a high-density reserve. Considered together, our results show that although the three carnivores segregate in space–time at relatively lower densities, this may be an artefact of their relatively low density and resulting lower photo-encounter rates (table 5). At higher densities, fine-scale behavioural avoidance is apparent in Nagarahole (figure 3), but not in Bandipur.
Figure 3.
Spatio-temporal interactions, as shown by times-to-encounter among the carnivore species pairs generated from multi-response permutation procedures. The vertical lines represent median minimum time-to-encounter between two species, while the shaded grey area show randomly simulated times-to-encounter. The p-values, representing the proportion of randomly generated times-to-encounter values that are greater than the observed time-to-encounter are given in each graph.
4. Discussion
By examining fine-scale spatio-temporal interactions among three carnivores, we show mechanisms by which intra-guild competitors can co-occur. For the carnivore species we studied, overlap in temporal, spatial or spatio-temporal activity patterns did not necessarily mirror one another. In other words, these three species use temporal activity, space-use patterns and finer scale behavioural segregation as alternative mechanisms to facilitate sympatry. Furthermore, the three carnivores show remarkable adaptability to variations in prey resource availability, by modifying their spatial and temporal activity patterns across populations in a manner similar to ‘behavioural character displacement’ (or population-level changes in species behaviour across reserves; sensu [10]).
(a). Behavioural character displacement
Sub-optimal habitats and scarcity of resources can intensify interspecific competition among carnivores [4]. For example, African wild dogs Lycaon pictus move over large distances in search of habitats where they can avoid the competitively dominant lions and hyaenas, which are known to steal their kills [40]. Under scenarios of intensive interspecific competition, a dominant competitor's presence may result in avoidance of resource-rich habitats by subordinate competitors [4]. Our results show evidence of such fine-scale avoidance behaviours in one high carnivore density reserve (Nagarahole), but not in the other (Bandipur; table 5 and figure 3). Relatively more open woodland habitat with greater visibility in Bandipur may be a structural feature that restricts fine-scale behavioural avoidance. Additionally, relatively low prey availability may compel carnivores to be more mobile through the diel cycle, investing more efforts to locate and hunt scarce prey [41]. We observed this pattern in the two lower prey density reserves (Bhadra and Biligiri), where all three species showed greater activity throughout the day. Spatial aggregation between carnivore species pairs was also the highest in Bhadra, possibly reflecting lower and more clumped prey availability rather than any specific behavioural separation.
Based on our findings, the contrasting competitive spatial displacement among these three predator species reported by Steinmetz et al. [22] from Thailand (where prey densities are even lower than Bhadra) may be an artefact of data scarcity and quality. Alternatively, there may exist a threshold of relative densities of these three predators, above which competitive spatial displacement is replaced by the behavioural adaptations we assessed in this study.
(b). Spatial scale of competitive interactions
Interactions among species are likely to vary based on the spatial scale at which they are observed [1]. In our study landscape of the Western Ghats, the three carnivores are largely concentrated within protected reserves, indicating spatial aggregation ([25,26], K. U. Karanth 2013, unpublished results). Within these reserves, however, they occur at higher densities and demonstrate behavioural segregation across time and minimal interactions across space. In one high-density area, they also exhibited spatio-temporal avoidance behaviour at even finer scales—defined by the distance between camera-trap locations within the same set of reserves.
It is clear that spatial scale and local prey resource availability mediate competitive interactions among species. The three predators show a high degree of spatial and temporal overlap in Bhadra and Biligiri (both with lower prey densities), possibly to maximize resource acquisition. By contrast, the three carnivores show temporal segregation, but relatively high spatio-temporal overlap in the high prey density Nagarahole reserve, where uniquely, segregation occurs through fine-scale spatial avoidance [42]. Our results appear to suggest that there is a trade-off among carnivore temporal activity patterns, spatial segregation and reactive behaviours, mediated by primarily by prey availability.
It is likely that we may not have detected all potential finer-scale intra-guild carnivore adaptations. For instance, we did not account for the influence of habitat refuges (e.g. presence of dens or trees) or microhabitat features (e.g. cover density), which could influence species interactions [18]. The observations that leopards climb trees to escape from tigers and dholes, and that dhole packs are seasonally localized at den sites, could be additional factors that facilitate finer scale segregation that our methods failed to detect in Bandipur. We note that it becomes increasingly difficult to detect spatial segregation or aggregation as probabilities of site-use get closer to 1. These probabilities range between 0.32 ± 0.07 (dholes in Bhadra) and 0.86 ± 0.07 (tigers in Biligiri) in our study. It is possible that the high probabilities of use for tigers in particular (0.86 ± 0.07, 0.85 ± 0.05 and 0.83 ± 0.04 in Biligiri, Nagarahole and Bandipur, respectively; electronic supplementary material, table S3) precluded any detection of spatial segregation/aggregation. But we stress that we examined interspecific mechanisms facilitating sympatry in small, insular, high carnivore density habitats, where total spatial segregation was likely not an ecological option.
(c). Population-level inferences on carnivore co-occurrence
Based on movement decisions of multiple species, Vanak et al. [4] demonstrated that intra-guild coexistence is facilitated by fine-scale behavioural mechanisms in an African large carnivore community. However, their results are based on radio-collared individuals from a closed terrestrial system. We were interested in characteristics of competing predator species across a gradient of resource availability at the population level. We relied on prior data on resource availability, non-invasive field methods and advanced analytical techniques to examine carnivore intra-guild interactions across space and time. The utility of this approach was evident in two ways. First, while patterns of spatial and temporal overlap among the same species do not differ greatly from results of earlier studies [18], combining patterns of space use, time of activity, and fine-scale spatio-temporal dimensions revealed complex and alternative mechanisms that facilitate intra-guild sympatry. Second, spatial and temporal patterns of interactions among carnivores showed clear differences across a gradient of competition, demonstrating the value of comparisons across populations.
In addition to the behavioural adaptations that we studied, antagonistic interactions among these species have been reported; tigers and leopards kill and prey on dholes [6,18], dholes, leopards and tigers steal kills from each other, and leopards can escape from tigers and dholes by climbing trees but are sometimes killed [18,20]. Such rare antagonistic interactions studied using radio-telemetry may reveal complex interplay of behaviour and ecology among these predators.
(d). Consequences of carnivore competition
Large carnivores typically occupy the highest trophic niche in their habitats. Competition among co-predators is likely to influence lower trophic levels, causing mesopredator release [43], hyperpredation [44] or cascading effects on the ecosystem [45]. Another implication of our study is the possible role of anthropogenic influences [46] on carnivore intra-guild interactions, and, consequently on large mammal communities that they are part of. Such anthropogenic impacts can include land-use changes and economic development increasing the insularity of nature reserves and carnivore communities [25,26,47], human predation on ungulate prey species [48], reduction of wild prey by competing domestic livestock, and modification of habitats through fires or manipulation of vegetation and water resources. On the other hand, exclusive management focus on flagship predators [25] could negatively impact subordinate predator populations [49]. These anthropogenic impacts may aggravate or alleviate competitive interactions among carnivores [12,50]. Such community dynamics typically play out over multiple years, with annual variations in predator and prey abundance and vital rates, co-predator suppression and anthropogenic factors all interacting to shape community structure [13]. Our study looks at a snapshot of four communities characterized by varying densities of predators and prey, and provides useful new insights into the ecological and behavioural adaptability of three globally threatened carnivore species. Such findings not only advance general scientific understanding of animal community ecology in general, but also provide basic knowledge that must necessarily underpin any effective endangered species recovery programme.
Supplementary Material
Acknowledgements
We thank the State Forest Departments of Karnataka and Kerala for providing necessary research permits and the National Tiger Conservation Authority of India, Ministry of Environment, Forests & Climate Change, Government of India for facilitating this study. We are indebted to J. D. Nichols for commenting on our methods, D. Jathanna and V. R. Goswami for help with analyses, S. Sharma, K. Yadav, J. Shankaraiah and L. Vinay on compilation of photo-capture data. We thank our research assistants and field assistants who assisted in data collection and analyses. We thank Wildlife Conservation Society, New York, USA, Centre for Wildlife Studies, Bengaluru, India and National Centre for Biological Sciences-TIFR, Bengaluru India, for administrative support.
Ethics
The study was undertaken within protected areas of Karnataka and Kerala in India, with prior permissions from the respective State Forest Departments. The study completely relied on non-invasive camera-trap surveys and therefore animal care/use committee approvals were not required.
Data accessibility
The data involve spatial locations of one threatened and two endangered species of large carnivores, restricting us from making them publicly available. Some parts of the data may be made available to interested individuals upon specific request. All code used in the analysis is provided as the electronic supplementary material.
Authors' contributions
K.U.K. conceived the study; K.U.K. and N.S.K. designed the study; N.S.K. coordinated field data collection; A.S. carried out fieldwork; A.S., D.V., M.P. and R.P. carried out statistical analyses; all authors drafted the manuscript. All authors gave final approval for publication.
Competing interests
We declare no competing interests.
Funding
We are grateful to the following grantors/donors who supported the study: Department of Biotechnology and Department of Science and Technology, Government of India; Vision Group on Science and Technology, Government of Karnataka; Liz Claiborne and Art Ortenberg Foundation, USA; Wildlife Conservation Society, New York, USA.
References
- 1.Kneitel JM, Chase JM. 2004. Trade-offs in community ecology: linking spatial scales and species coexistence. Ecol. Lett. 7, 69–80. ( 10.1046/j.1461-0248.2003.00551.x) [DOI] [Google Scholar]
- 2.Geldmann J, Joppa LN, Burgess ND. 2014. Mapping change in human pressure globally on land and within protected areas. Conserv. Biol. 28, 1604–1616. ( 10.1111/cobi.12332) [DOI] [PubMed] [Google Scholar]
- 3.Palomares F, Caro T. 1999. Interspecific killing among mammalian carnivores. Am. Nat. 153, 492–508. ( 10.1086/303189) [DOI] [PubMed] [Google Scholar]
- 4.Vanak AT, Fortin D, Thaker M, Ogden M, Owen C, Greatwood S, Slotow R. 2013. Moving to stay in place: behavioral mechanisms for coexistence of African large carnivores. Ecology 94, 2619–2631. ( 10.1890/13-0217.1) [DOI] [PubMed] [Google Scholar]
- 5.Durant SM. 1998. Competition refuges and coexistence: an example from Serengeti carnivores. J. Anim. Ecol. 67, 370–386. ( 10.1046/j.1365-2656.1998.00202.x) [DOI] [Google Scholar]
- 6.Karanth KU, Sunquist ME. 1995. Prey selection by tiger, leopard and dhole in tropical forests. J. Anim. Ecol. 64, 439–450. ( 10.2307/5647) [DOI] [Google Scholar]
- 7.Sherry TW. 1979. Competitive interactions and adaptive strategies of American redstarts and least flycatchers in a northern hardwoods forest. Auk 96, 265–283. [Google Scholar]
- 8.Carothers JH, Jaksić FM. 1984. Time as a niche difference: the role of interference competition. Oikos 42, 403–406. ( 10.2307/3544413) [DOI] [Google Scholar]
- 9.Davies TJ, Meiri S, Barraclough TG, Gittleman JL. 2007. Species co-existence and character divergence across carnivores. Ecol. Lett. 10, 146–152. ( 10.1111/j.1461-0248.2006.01005.x) [DOI] [PubMed] [Google Scholar]
- 10.Brown WLJ, Wilson EO. 1956. Character displacement. Syst. Zool. 5, 49–64. ( 10.2307/2411924) [DOI] [Google Scholar]
- 11.Sinclair A, Mduma S, Brashares JS. 2003. Patterns of predation in a diverse predator–prey system. Nature 425, 288–290. ( 10.1038/nature01934) [DOI] [PubMed] [Google Scholar]
- 12.Schuette P, Wagner AP, Wagner ME, Creel S. 2013. Occupancy patterns and niche partitioning within a diverse carnivore community exposed to anthropogenic pressures. Biol. Conserv. 158, 301–312. ( 10.1016/j.biocon.2012.08.008) [DOI] [Google Scholar]
- 13.Amarasekare P. 2008. Coexistence of intraguild predators and prey in resource-rich environments. Ecology 89, 2786–2797. ( 10.1890/07-1508.1) [DOI] [PubMed] [Google Scholar]
- 14.Peers MJ, Thornton DH, Murray DL. 2012. Reconsidering the specialist generalist paradigm in niche breadth dynamics: resource gradient selection by Canada lynx and bobcat. PLoS ONE 7, e51488 ( 10.1371/journal.pone.0051488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebblewhite M, Haydon DT. 2010. Distinguishing technology from biology: a critical review of the use of GPS telemetry data in ecology. Phil. Trans. R. Soc. B 365, 2303–2312. ( 10.1098/rstb.2010.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connell AF, Nichols JD, Karanth KU. 2010. Camera traps in animal ecology: methods and analyses. Berlin, Germany: Springer. [Google Scholar]
- 17.Andheria AP, Karanth KU, Kumar NS. 2007. Diet and prey profiles of three sympatric large carnivores in Bandipur Tiger Reserve, India. J. Zool. 273, 169–175. ( 10.1111/j.1469-7998.2007.00310.x) [DOI] [Google Scholar]
- 18.Karanth KU, Sunquist ME. 2000. Behavioural correlates of predation by tiger (Panthera tigris), leopard (Panthera pardus) and dhole (Cuon alpinus) in Nagarahole, India. J. Zool. 250, 255–265. ( 10.1111/j.1469-7998.2000.tb01076.x) [DOI] [Google Scholar]
- 19.Johnsingh A. 1992. Prey selection in three large sympatric carnivores in Bandipur. Mammalia 56, 517–526. ( 10.1515/mamm.1992.56.4.517) [DOI] [Google Scholar]
- 20.Venkataraman A. 1995. Do dholes (Cuon alpinus) live in packs in response to competition with or predation by large cats? Curr. Sci. 69, 934–936. [Google Scholar]
- 21.Ramesh T, Kalle R, Sankar K, Qureshi Q, Bennett N. 2012. Spatio-temporal partitioning among large carnivores in relation to major prey species in Western Ghats. J. Zool. 287, 269–275. ( 10.1111/j.1469-7998.2012.00908.x) [DOI] [Google Scholar]
- 22.Steinmetz R, Seuaturien N, Chutipong W. 2013. Tigers, leopards, and dholes in a half-empty forest: assessing species interactions in a guild of threatened carnivores. Biol. Conserv. 163, 68–78. ( 10.1016/j.biocon.2012.12.016) [DOI] [Google Scholar]
- 23.Karanth KU, Nichols JD, Kumar NS, Link WA, Hines JE. 2004. Tigers and their prey: predicting carnivore densities from prey abundance. Proc. Natl Acad. Sci. USA 101, 4854–4858. ( 10.1073/pnas.0306210101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Royle JA, Nichols JD, Karanth KU, Gopalaswamy AM. 2009. A hierarchical model for estimating density in camera-trap studies. J. Appl. Ecol. 46, 118–127. ( 10.1111/j.1365-2664.2008.01578.x) [DOI] [Google Scholar]
- 25.Karanth KU, Gopalaswamy AM, Kumar NS, Vaidyanathan S, Nichols JD, MacKenzie DI. 2011. Monitoring carnivore populations at the landscape scale: occupancy modelling of tigers from sign surveys. J. Appl. Ecol. 48, 1048–1056. ( 10.1111/j.1365-2664.2011.02002.x) [DOI] [Google Scholar]
- 26.Srivathsa A, Karanth KK, Jathanna D, Kumar NS, Karanth KU. 2014. On a dhole trail: examining ecological and anthropogenic correlates of dhole habitat occupancy in the Western ghats of India. PLoS ONE 9, e98803 ( 10.1371/journal.pone.0098803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton AC, Neilson E, Moreira D, Ladle A, Steenweg R, Fisher JT, Bayne E, Boutin S. 2015. Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. J. Appl. Ecol. 52, 675–685. ( 10.1111/1365-2664.12432) [DOI] [Google Scholar]
- 28.Karanth KU, Sunquist ME. 1992. Population structure, density and biomass of large herbivores in the tropical forests of Nagarahole, India. J. Trop. Ecol. 8, 21–35. ( 10.1017/S0266467400006040) [DOI] [Google Scholar]
- 29.Karanth KU, Nichols JD, Kumar NS, Hines JE. 2006. Assessing tiger population dynamics using photographic capture–recapture sampling. Ecology 87, 2925–2937. ( 10.1890/0012-9658(2006)87%5B2925:ATPDUP%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 30.Fisher NI. 1993. Statistical analysis of circular data. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 31.Watson GS. 1962. Goodness-of-fit tests on a circle. II. Biometrika 49, 57–63. ( 10.1093/biomet/49.1-2.57) [DOI] [Google Scholar]
- 32.Efron B, Tibshirani RJ. 1994. An introduction to the bootstrap. Boca Raton, FL: CRC Press. [Google Scholar]
- 33.Ridout MS, Linkie M. 2009. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 14, 322–337. ( 10.1198/jabes.2009.08038) [DOI] [Google Scholar]
- 34.MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey L, Hines JE. 2006. Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. New York, NY: Academic Press. [Google Scholar]
- 35.MacKenzie DI, Bailey LL, Nichols J. 2004. Investigating species co-occurrence patterns when species are detected imperfectly. J. Anim. Ecol. 73, 546–555. ( 10.1111/j.0021-8790.2004.00828.x) [DOI] [Google Scholar]
- 36.Mackenzie DI. 2006. Modeling the probability of resource use: the effect of, and dealing with, detecting a species imperfectly. J. Wildl. Manage. 70, 367–374. ( 10.2193/0022-541X(2006)70%5B367:MTPORU%5D2.0.CO;2) [DOI] [Google Scholar]
- 37.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference. Berlin, Germany: Springer. [Google Scholar]
- 38.Mielke PW Jr, Berry KJ, Johnson ES. 1976. Multi-response permutation procedures for a priori classifications. Commun. Stat. Theory Methods 5, 1409–1424. ( 10.1080/03610927608827451) [DOI] [Google Scholar]
- 39.Kovach WL. 2012. Oriana – Circular Statistics for Windows, ver. 4. Kovach Computing Services, Pentraeth, UK. [Google Scholar]
- 40.Creel S, Creel NM. 1995. Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim. Behav. 50, 1325–1339. ( 10.1016/0003-3472(95)80048-4) [DOI] [Google Scholar]
- 41.Valeix M, Loveridge AJ, Davidson Z, Madzikanda H, Fritz H, Macdonald DW. 2009. How key habitat features influence large terrestrial carnivore movements: waterholes and African lions in a semi-arid savanna of north-western Zimbabwe. Landsc. Ecol. 25, 337–351. ( 10.1007/s10980-009-9425-x) [DOI] [Google Scholar]
- 42.Swanson A, Caro T, Davies-Mostert H, Mills MG, Macdonald DW, Borner M, Masenga E, Packer C. 2014. Cheetahs and wild dogs show contrasting patterns of suppression by lions. J. Anim. Ecol. 83, 1418–1427. ( 10.1111/1365-2656.12231). [DOI] [PubMed] [Google Scholar]
- 43.Crooks KR, Soulé ME. 1999. Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400, 563–566. ( 10.1038/23028) [DOI] [Google Scholar]
- 44.Smith AP, Quin D. 1996. Patterns and causes of extinction and decline in Australian conilurine rodents. Biol. Conserv. 77, 243–267. ( 10.1016/0006-3207(96)00002-X) [DOI] [Google Scholar]
- 45.Terborgh J, et al. 2001. Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926. ( 10.1126/science.1064397) [DOI] [PubMed] [Google Scholar]
- 46.Miller DA, Brehme CS, Hines JE, Nichols JD, Fisher RN. 2012. Joint estimation of habitat dynamics and species interactions: disturbance reduces co-occurrence of non-native predators with an endangered toad. J. Anim. Ecol. 81, 1288–1297. ( 10.1111/j.1365-2656.2012.02001.x) [DOI] [PubMed] [Google Scholar]
- 47.DeFries R, Karanth KK, Pareeth S. 2010. Interactions between protected areas and their surroundings in human-dominated tropical landscapes. Biol. Conserv. 143, 2870–2880. ( 10.1016/j.biocon.2010.02.010) [DOI] [Google Scholar]
- 48.Velho N, Karanth KK, Laurance WF. 2012. Hunting: a serious and understudied threat in India, a globally significant conservation region. Biol. Conserv. 148, 210–215. ( 10.1016/j.biocon.2012.01.022) [DOI] [Google Scholar]
- 49.Harihar A, Pandav B, Goyal SP. 2011. Responses of leopard Panthera pardus to the recovery of a tiger Panthera tigris population. J. Appl. Ecol. 48, 806–814. ( 10.1111/j.1365-2664.2011.01981.x) [DOI] [Google Scholar]
- 50.Burton AC, Sam MK, Balangtaa C, Brashares JS. 2012. Hierarchical multi-species modeling of carnivore responses to hunting, habitat and prey in a West African protected area. PLoS ONE 7, e38007 ( 10.1371/journal.pone.0038007) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data involve spatial locations of one threatened and two endangered species of large carnivores, restricting us from making them publicly available. Some parts of the data may be made available to interested individuals upon specific request. All code used in the analysis is provided as the electronic supplementary material.