Abstract
The ability to allocate resources, even when limited, is essential for survival and fitness. We examine how nutrients that occur in minute amounts are allocated among reproductive, somatic, and metabolic demands. In addition to sugar, flower nectars contain two macronutrients—amino acids and fatty acids. We created artificial nectars spiked with 13C-labelled amino acids and fatty acids and fed these to adult moths (Manduca sexta: Sphingidae) to understand how they allocate these nutrients among competing sinks (reproduction, somatic tissue, and metabolic fuel). We found that both essential and non-essential amino acids were allocated to eggs and flight muscles and were still detectable in early-instar larvae. Parental-derived essential amino acids were more conserved in the early-instars than non-essential amino acids. All amino acids were used as metabolic fuel, but the non-essential amino acids were oxidized at higher rates than essential amino acids. Surprisingly, the nectar fatty acids were not vertically transferred to offspring, but were readily used as a metabolic fuel by the moth, minimizing losses of endogenous nutrient stores. We conclude that the non-carbohydrate components of nectar may play important roles in both reproductive success and survival of these nectar-feeding animals.
Keywords: nectar, Lepidoptera, allocation, amino acids, fatty acids, stable isotope
1. Background
An organism's life history and fitness is dependent on how it allocates a finite pool of resources to maintenance, growth, survival, and reproduction [1,2]. Ever-changing physiological priorities govern the relative allocation of resources to organismal processes as a function of nutrient input [2,3]. A prominent example is the oogenesis-flight syndrome when, during active migration, the reproductive system is often atrophied and flight muscles enlarged. Once migration is over, the reproductive system grows and muscles atrophy [4,5]. Under stressful conditions, allocation to storage or maintenance can take precedence over allocation to reproduction [6,7] or resources can be reallocated from existing structures, as in the case of flight muscle histolysis and the subsequent reallocation of these resources to reproduction [8,9]. Examples such as these patterns involve the assumption that the organism has access to the full spectrum of required nutrients that can be differently allocated according to need. How, in contrast, do organisms that have a very limited amount of nutrients allocate their limited resources? We address this question by examining how a nectarivorous moth uses the limited amounts of amino acids and fatty acids found in nectar.
Most nectarivores (e.g. butterflies and moths, hummingbirds, sunbirds, and some bats) feed almost exclusively on nectar. Flower nectar is mostly comprised of water and sugar, but some nectars also contain limited amounts of other macronutrients including amino acids and sometimes fatty acids [10,11]. Baker & Baker [12,13] suggested that the nectar of Lepidoptera-pollinated plants contains significantly higher concentrations of amino acids than in the nectar of plants pollinated by other taxa. It has, therefore, been suggested that these amino acids are an important resource for these insects [12,13].
The role of nectar amino acids in the fitness and life histories of lepidopterans has long been an area of research interest. Experimental findings regarding the effect of adult-derived amino acids on lepidopterans, however, are often contradictory. In some species of butterflies and moths, nectar amino acids had no marked positive effect on longevity or fecundity [14–16], whereas in others, adult feeding of amino acids enhanced longevity [17]. Mevi-Schutz et al. [18] found that female butterflies reared on low nitrogen diets preferred nectar with amino acids and showed that butterflies reared as caterpillars in natural larval conditions and fed as adults with amino acid nectar, laid more eggs than females fed only sugar nectar [19]. In the small heath butterfly (Coenonympha pamphilus), adult females receiving nectar amino acids produced heavier larvae and increased hatching success, irrespective of larval food quality [20]. In the males of this species, nectar amino acids improved reproduction [21]. Considering these are mostly behavioural and life-history studies, the metabolic use and allocation of amino acids by butterflies and moths has never been satisfactorily resolved.
O'Brien et al. [15] created nectars from sugars with naturally occurring differences in δ13C of C3 and C4 plants with the addition of free amino acids from casein hydrolysate, to determine whether nectar amino acids were incorporated into the eggs of the hawkmoth Amphion floridensis. They found no difference in the δ13C of eggs from moths fed nectar diets with and without amino acid supplements and concluded that nectar amino acids do not contribute to egg provisioning.
In a series of elegant follow-up studies, O'Brien et al. [22,23] traced the allocation of carbon from nectar sugar into eggs of A. floridensis and four species of Nymphalid butterflies. They showed that Lepidoptera females can synthesize non-essential amino acids de novo from the carbon skeletons of nectar sugars, but that essential amino acids are derived only from the larval diet, thereby placing an upper limit on the use of adult dietary resources to enhance reproductive success [22,23]. This conclusion by O'Brien et al. [22] has become the current paradigm for the roles of essential amino acids and non-essential amino acids in nectar. Using a new technology, we show that this paradigm needs to be revisited.
A second goal of this study is to understand how lepidopterans use nectar fatty acids. Lipids have the highest energy density among the three classes of macronutrients (lipids, proteins, and carbohydrates). Lipids (as fatty acids) usually occur only in trace amounts in nectar [24]. Occasionally, however, lipids can be found in high concentrations in the nectar or in specific parts of flowers; the amount of fatty acids in such flowers can be so high that the nectar appears milky to the human eye [25]. To the best of our knowledge, the absorption, allocation, and oxidation of nectar fatty acids by nectarivorous insects has never been studied. Here, we examine for the first time, to the best of our knowledge, the allocation and use of nectar lipids by a nectar-feeding insect.
Recently, a new, non-destructive, experimental approach of real-time stable isotope analysis combined with improved ability to isotopically label nutritionally important molecules has opened a whole new field for the study of metabolism and fuel use in living animals (see [26,27] and Levin et al. in press [28]). Here, we use this new methodology to compare the allocation of dietary amino acids and fatty acids between competing resource sinks: reproduction, adult flight muscle, and resting metabolism in a female nectarivorous hawk moth, the Carolina sphinx moth (Manduca sexta, Sphingidae).
2. Methods
(a). The study organism
We used the nectarivorous hawkmoth Manduca sexta (Sphingidae) from a colony maintained at the University of Arizona [29,30]. Larvae were reared under a 16 : 8 light : dark photo cycle in an environmentally controlled room (27 ± 1°C and 50% RH). All larvae were fed ad libitum with a standard artificial diet (made from wheat-germ, casein, yeasts, sucrose, agarose, and vitamins (see [31])) until pupation. Pupae that were ready to eclose (19–25 days after pupation and 1 day before eclosion) were removed daily from the colony and set aside in a tray. Pupae to be used in the feeding experiments described below were taken at random from this tray. Adult females in this species eclose from the pupa with sufficient resources to provision and lay approximately 200 eggs without any additional feeding. Nectar meals (as sugar in water) are known to increase lifespan and fecundity of adults in this species [32], so that M. sexta can be considered a partial income breeder where additional ingested nutritional input by the female is allocated to reproduction [33,34].
(b). Preparation of artificial nectars
Nectar amino acids: we created artificial nectar made of 25% (by weight) beet sugar (beet sugar δ13C ≈ −26.5‰), that was isotopically enriched (0.2 g l−1) with one of three 13C-labelled amino acids (i.e. 2.6 mM 1-13C-glycine, a non-essential amino acid; 1.5 mM 1-13C-leucine, an essential amino acid; 1.2 mM 1-13C-phenylalanine, an essential amino acid; Cambridge Isotope Laboratories, Tewksbury, MA). These amino acid concentrations are equal to, or less than, those found in natural nectars and those used in previous studies [22,35].
Nectar fatty acids: we used an artificial nectar made of 25% (by weight) beet sugar solution that was enriched with 1.0 g l−1 of 1-13C-palmitic acid (Cambridge Isotope Laboratories). Palmitic acid comprises approximately 30% (21.8–46.5%) of the fatty acids in insects used for human consumption [36] and has proven to be an effective tracer in insects [26,37]. We mixed 1 mg of palmitic acid with 40 µl of industrial food emulsifier (TWIN® 20, Sigma Aldrich, USA) in a 1.5 ml screw-top microcentrifuge tube and homogenized it for 1 min at 3000 r.p.m. using a bead homogenizer (Minilys, Bertin Technologies).
(c). Experimental procedure for measuring allocation to reproduction
Females ready to eclose were placed into individual cages (30 × 30 × 30 cm, BugDorm, UK). On the day of eclosion and after sclerotization of the wings, moths were fed daily with 250 µl of artificial nectar containing one of three amino acid tracers (see above) for five consecutive days. We used five females for each experimental treatment (n = 15 in total). For feeding, the artificial nectar was placed on a piece of Parafilm™ (Pechiney Plastic Packaging Company, USA) in five droplets of 50 µl each. Each moth's proboscis was unfurled using a long needle and its tip placed in a droplet. The moths were observed to consume the entire tracer meal during each experimental feeding. We used colony females fed 25% beet sugar ad libitum as controls. After feeding, females were returned to their cage, and a single virgin male was placed in each cage. These cages were placed in the same rearing conditions as above with the addition of an artificial nightlight (‘moonlight’) during the 8 h scotophase to promote mating.
At the end of the scotophase, males were removed from the cages, and females were again fed 250 µl of the artificial nectar (as above) and provided with a 4 × 6 cm standing oviposition platform. The platform was made of cardboard wrapped with a paper towel. Two to four drops of host plant Datura wrightii extract were placed on the upper side of the platform to stimulate oviposition [38] and smooth masking tape was placed on the underside of the platform to prevent fibres from the paper towel sticking to the eggs (which would affect the measured δ13C values).
For the next 5 days, at the end of each 8 h scotophase, the eggs of each female were collected from the underside of the platform and the female was fed again as described above (total of 1 250 µl). The eggs were dried for 48 h at 50°C with the exception of eggs collected on day five. Eggs from day five of each female were randomly separated into two groups. One group was dried (as above) to quantify maternal transfer into the eggs, and the second group was incubated and allowed to hatch to measure tracer levels in developing larvae (see below). On day six, the adult females were killed by freezing at −20°C for 2 h. Flight muscles were dissected using surgical scissors, and dried for 48 h at 50°C.
After hatching (about 5 days from oviposition), five larvae from each clutch were moved into a separate cage containing a 1 cm3 cube of unlabelled artificial standard diet (see above). The rest of the larvae were killed by freezing at −20°C for 2 h, and dried for 48 h at 50°C. The five live larvae from each clutch were reared to second instar, then starved for 6 h (to ensure an empty gut), killed by freezing in −20°C for 2 h, and then dried for 48 h at 50°C.
(d). δ13C analysis in eggs, larva, and muscles
To ensure sufficient sample masses for individual isotopic analyses, we pooled the eggs (n = 5) and pooled the first-instar larvae (n = 6) from each female. We also analysed second-instar larvae (n = 2) and flight muscle (1 mg) of adults. These samples were loaded into tin capsules, and the δ13C was measured using a Picarro (Santa Clara, CA) G2121-i cavity ring-down spectroscopy (CRDS) 13C stable isotope analyser with an A0502 ambient CO2 interface, an A0201 combustion module, and an A0301 gas interface (CM-CRDS, (as previously described [38])). All 13C concentrations are expressed in δ13CVPDB [39]. Data from the CRDS were recorded at 0.5 Hz using Picarro software.
(e). δ13C analysis in the breath
Just prior to respirometry trials, moths (n = 4 for each nectar) were fed with 200 µl of the artificial AA nectar (or 150 µl of the fatty acids nectar) as described above. Moths were then individually placed into 200 ml sealed plastic chambers. Bottled, dry, CO2-free air was passed through the chambers at a flow rate of 150 ml min−1 using a mass flow controller (Alicat, Tucson, AZ, USA). A subsample of the excurrent gas (30 ml min−1) was pulled from a manifold directly into a G212-i CRDS stable carbon isotope analyser (Picarro, Santa Clara, CA). Data from the CRDS were recorded at 0.5 Hz using Picarro software.
(f). Statistical analysis
The allocation of amino acids to eggs harvested over the first 5 days was tested using linear regression. Because of the small sample sizes, most other statistics were non-parametric. We used Kruskal–Wallace rank-sum test to compare among amino acids, and when significant differences were detected, we used a Wilcoxon test for non-parametric multiple comparisons to identify which specific pairs differed. To determine how the δ13C label in the amino acid differed from control larvae-fed unlabelled diet, we used a Dunnett's non-parametric multiple comparison with controls. We used a Tukey–Kramer honest significant difference (HSD), which accounts for multiple comparisons, to compare peak δ13C as metabolic fuel. All analyses were done with JMP 11.0, and significance was determined using α = 0.05.
3. Results
(a). Allocation of amino acids
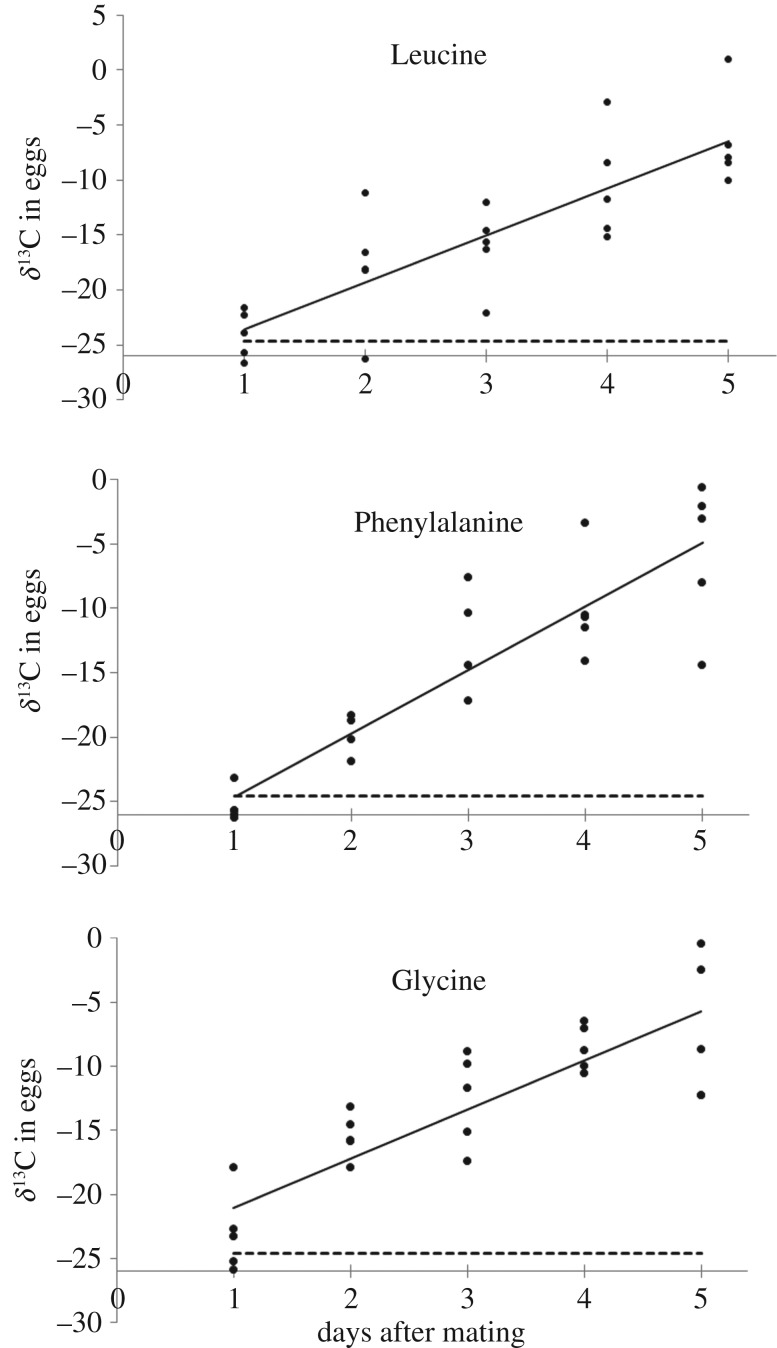
There was a linear increase in the δ13C of eggs over time in all three amino acid diet groups: δ13CLeu = −27.86 + 4.276 × day, n = 25, R2 = 0.71, p < 0.0001, δ13CPhe = −29.493 + 4.919 × day, n = 23, R2 = 0.81, p < 0.0001, δ13CGly = −24.892 + 3.836 × day, n = 25, R2 = 0.72, p < 0.0001 (figure 1).
Figure 1.
The δ13C values in the eggs of Manduca sexta consuming 13C-labelled amino acids: leucine (essential amino acid), phenylalanine (essential amino acid), and glycine (non-essential amino acid). The horizontal dashed lines represent the average δ13C in the eggs of control moths fed 25% beet sugar as adults. Note that less negative δ13C values indicate higher tracer content.
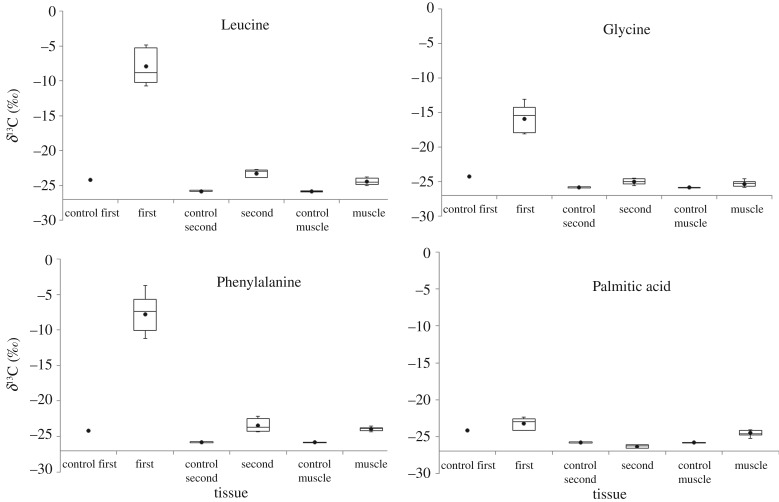
The three amino acid groups differed significantly in the δ13C values in the first larval instar offspring (Kruskal–Wallis rank-sum test, H = 9.42, p = 0.009; figure 2). The δ13C values of the two essential amino acids (leucine and phenylalanine) did not differ from each other (Wilcoxon test for non-parametric multiple comparisons, Z = 0.209, p = 0.835), but both essential amino acids had significantly higher values than glycine (phenylalanine versus glycine and leucine versus glycine, Z = 2.51, p = 0.012). All three amino acid groups of the tracer-fed first-instar offspring differed significantly from the control first-instar offspring group fed only the standard diet (Dunnett's non-parametric multiple comparison with control, glycine versus control, p = 0.003; phenylalanine versus control and leucine versus control, p < 0.0001).
Figure 2.
Box and whisker plots of the δ13C values of first- and second-instar larvae offspring and adult flight muscles (muscle) of female parent moths that consumed 13C-labelled amino acid or fatty acid tracers and their respective control (control) diets. The boxes represent the first and third quartiles and the median. The whiskers represent the maximum and minimum values. The mean is represented by a black circle. Note that less negative δ13C values indicate higher tracer content.
The δ13C of the second-instar larvae offspring also differed significantly among the three amino acid groups (Kruskal–Wallis rank-sums test, H = 8.64, p = 0.013, figure 2). While the two essential amino acids did not differ from one another (Wilcoxon test for non-parametric multiple comparisons, Z = 0.0, p = 1.0) both essential amino acids groups had δ13C values that differed significantly from the glycine group (leucine versus glycine, Z = 2.51, p = 0.012; phenylalanine versus glycine, Z = 2.29, p = 0.022). Moreover, both phenylalanine (Dunnett's non-parametric multiple comparison with control, p = 0.0003) and leucine (p = 0.0001) larvae significantly differed from the second-instar offspring fed only the standard diet (control). The glycine group did not differ (p = 0.148) from the control larvae.
The δ13C values in the flight muscle of the adult female parents differed significantly among the three amino acid groups (Kruskal–Wallis rank-sum test, H = 9.94, p = 0.007). The phenylalanine and leucine groups did not differ significantly from each other (Wilcoxon test for non-parametric multiple comparisons, Z = 1.47, p = 0.143); however, the glycine group differed significantly from the phenylalanine (Z = 2.51, p = 0.012) and leucine (Z = 2.29, p = 0.022) groups. The glycine group did not significantly differ from control moths (Dunnett's non-parametric multiple comparison with control, p = 0.238), whereas both essential amino acid groups differed from the control moths (phenylalanine, p = 0.0002, leucine p = 0.002).
(b). Amino acids as metabolic fuel
During all measurements, the moths were at complete rest. For those moths fed leucine and glycine, the δ13C in the exhaled breath began to increase minutes after feeding and reached a maximum value 1.5–2.5 h after feeding (figure 3). The maximal 13C-enrichment (5 min moving average) significantly differed between moths fed glycine and leucine (Tukey–Kramer HSD, p = 0.019) and between the moths fed glycine and phenylalanine (p = 0.007). There was no difference between the moths fed the two essential amino acids (phenylalanine and leucine; p = 0.59). Glycine-fed moths had higher maximal δ13C than the leucine-fed moths, and both of these treatment groups had higher δ13C than phenylalanine-fed moths (average δ13C ± standard error (s.e.): glycine 26.9 ± 11.4‰ leucine −9.2 ± 2.8‰ phenylalanine −20.4 ± 0.4‰).
Figure 3.
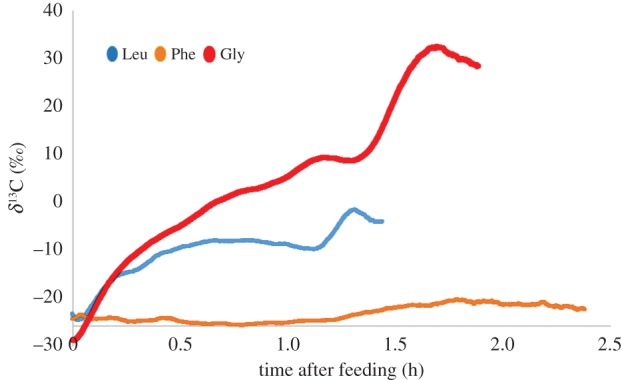
Changes in the δ13C of exhaled breath of moths consuming 13C-labelled amino acids in their first nectar meals. Leu, leucine; Phe, phenylalanine; Gly, glycine. (Online version in colour.)
(c). Allocation of fatty acids
Homogenized, but not emulsified, fatty acid left no trace of the labelled fatty acid in either the eggs, adult flight muscle, or exhaled breath—even after increasing the concentration from 1 to 5 g l−1.
For the moths that consumed the homogenized and emulsified fatty-acid-labelled nectar, there was no significant isotopic enrichment in the eggs over time (Kruskal–Wallis rank-sum test, p = 0.515), nor did the δ13C value of eggs of any day differ from that of control eggs (Dunnett's non-parametric multiple comparisons, p = 0.204).
There were no significant differences between the tissue δ13C value of the first-instar fatty acid larvae and the first-instar control group (Z = −0.72, p = 0.475), the labelled second-instar fatty acid larvae and the second-instar controls (Z = −1.77, p = 0.077), or the fatty-acid-labelled adult flight muscle and the control moths (Z = −1.74, p = 0.081; figure 2), raising the possibility that the moths were unable to assimilate the fatty acid tracer. However, in contrast to the tissues, we did observe that the δ13C values in the exhaled breath of moths increased immediately after feeding (figure 4), confirming that the emulsified fatty acid was absorbed into the body but were used exclusively as a metabolic fuel.
Figure 4.
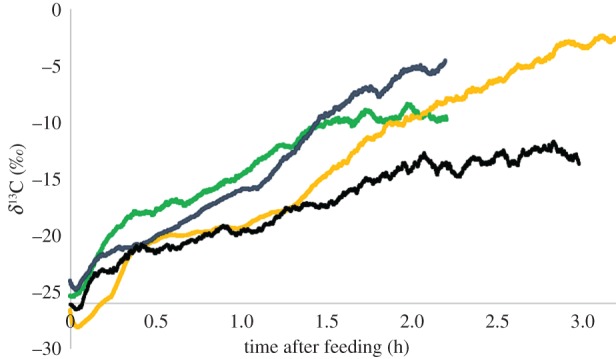
Representative traces of the δ13C in the exhaled breath of four moths fed nectar meal containing emulsified fatty acids. (Online version in colour.)
4. Discussion
Previous studies that examined allocation of nectar amino acids relied on the naturally occurring difference in δ13C between C3 and C4 plants as a tracer [15,22]. Based on that method, it was argued that in Lepidoptera, essential amino acids are derived only from the larval diet, placing an upper limit on the use of adult dietary resources to enhance reproductive success [15,22]. In their study on allocation of amino acids from pollen in Heliconius charitonia, O'Brien et al. [40] showed that essential amino acids from pollen are allocated into eggs, whereas all non-essential amino acids originated from nectar sugars. Our ability to label specific macronutrients and detect nectar dietary tracers even under low, but still biologically realistic concentrations, shows that this is a more powerful method of identifying amino acid and fatty acid use than has been previously possible. Specifically, we confirmed that both essential amino acids and non-essential amino acids are used as a source of metabolic fuel for the moth. It is likely that the relatively small isotopic differences inherent in the C3/C4 labelling method in those former studies precluded these observations. It is also important to note that the studies of O'Brien et al. [22,23] traced the fate of nectar sugar carbon into egg amino acids, whereas in this study, we traced the fate of specific amino acids that were added to the nectar.
We found that both essential and non-essential amino acids were allocated to eggs and were still present even in the early larval stages. Mevi-Schütz & Erhardt [19] suggest that utilization of amino acids by butterflies is affected by the quality of larval food and appears to be a compensatory mechanism to compensate for poor larval nutrition. In a recent study, it was found that for butterflies reared under a natural larval diet, nectar-derived amino acids affected the quality of their offspring [21]. Our findings support this observation, and suggest that even in species were no difference in number of eggs was recorded under amino acid adult-derived diet, the effect can be on the quality of the offspring and not egg number. We also showed that both non-essential amino acids and essential amino acids in nectar are allocated to reproduction demonstrating that branched and aromatic essential amino acids are important for the larvae in their early stages of life. While essential amino acids remained highly detectable in the first two instars of the larvae the non-essential amino acids were recycled and replaced (figure 2). From this, we conclude that (i) all nectar amino acids are allocated into reproduction as all are found in the eggs and early-instar larvae, (ii) non-essential amino acids are used as nitrogen and energy resources as they diminish during larval development, and (iii) essential amino acids are reserved preferentially for protein synthesis since they are maintained at high levels in the larvae. A similar observation was made in house sparrows consuming both essential and non-essential amino acids [41].
To the best of our knowledge, this is the first evidence that nectar amino acids are metabolized by lepidopterans, even under a carbohydrate-rich diet. The oxidation of amino acids was only detected during rest and thus used by moths to support resting metabolism. M. sexta moths do not fly during daylight hours and their bouts of flight can be relatively short at night (Davidowitz et al. 2015–2016, unpublished data); therefore, amino acids that are oxidized during rest provide a substantial source of metabolic fuel for these moths.
We found that nectar fatty acids are evidently not allocated to somatic tissues or reproduction by M. sexta; rather they are absorbed and readily used as metabolic fuel by the adult moth. Nectar fatty acids need to be emulsified for them to be biologically available to the moths, potentially because they lack digestive emulsifiers. We measured the 13C in the breath in one individual after 24 h and found that it was −11‰—well above the background levels of ≈ −26.5‰. This sustained 13C enrichment in the breath suggests that nectar lipids provide a longer-term source of metabolic fuel than the amino acids. Similar differences in oxidative kinetics were observed in pythons consuming laboratory mice whose amino acids or fatty acids were labelled with 13C [42].
The advanced technology that employs labelling of specific macronutrients on specific carbon atoms has enhanced our ability to track the fate of these macronutrients. It is clear that nectar amino acids, both essential and non-essential, can be incorporated into reproduction and adult tissue and allocated as fuel for resting metabolism. In contrast, nectar fatty acids are preferentially used as metabolic fuel for the resting moths and are not allocated to reproduction. Future studies can reveal the fate of additional amino and fatty acids in other nectarivores such as monarch butterflies and hummingbirds.
An organism's ability to allocate resources as metabolic demands change is essential for survival and fitness. In vertebrates, a well-supported paradigm is that first carbohydrates are metabolized, followed by lipids, with amino acid metabolism occurring only after the former two have been exhausted [41,43–46]. Recent work by McCue et al. [37] shows that insects do not adhere to this paradigm: amino acids are not universally allocated last as metabolic fuel and the order and timing of allocation of the macronutrients differs with developmental stage and species. This study supports such a paradigm shift for insects: amino acids and fatty acids are used immediately and extensively in resting metabolism. The ability to label specific nutrients on specific carbon atoms and track them in real time, advances our ability to resolve the priorities of resource allocation even when the amounts of amino acids and fatty acids are as minute as those found in nectar.
Acknowledgements
We thank Heather Costa for assistance in rearing the experimental animals and Zeev Tene for advice.
Data accessibility
The data for this study are available at http://arizona.openrepository.com/arizona/handle/10150/621812.
Authors' contributions
All authors contributed to the experimental design and manuscript preparation. E.L. conducted the experiments and G.D. performed the statistical analyses.
Funding
This study was supported by NSF (USA) grant no. IOS-1053318 to G.D.
Competing interests
We declare we have no competing interests.
References
- 1.Stearns SC. 1989. Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268. ( 10.2307/2389364) [DOI] [Google Scholar]
- 2.Boggs CL. 2009. Understanding insect life histories and senescence through a resource allocation lens. Funct. Ecol. 23, 27–37. ( 10.1111/j.1365-2435.2009.01527.x) [DOI] [Google Scholar]
- 3.Zera AJ, Harshman LG. 2001. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95–126. ( 10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 4.Johnson C. 1963. Physiological factors in insect migration by flight. Nature 198, 423–427. ( 10.1038/198423a0) [DOI] [Google Scholar]
- 5.Johnson CG. 1969. Migration and dispersal of insects by flight. London, UK: Methuen. [Google Scholar]
- 6.Perrin N, Bradley M, Calow P. 1990. Plasticity of storage allocation in Daphnia magna. Oikos 59, 70–74. ( 10.2307/3545124) [DOI] [Google Scholar]
- 7.Rogowitz GL. 1996. Trade-offs in energy allocation during lactation. Am. Zool. 36, 197–204. ( 10.1093/icb/36.2.197) [DOI] [Google Scholar]
- 8.Marden JH. 2000. Variability in the size, composition, and function of insect flight muscles. Annu. Rev. Physiol. 62, 157–178. ( 10.1146/annurev.physiol.62.1.157) [DOI] [PubMed] [Google Scholar]
- 9.Stjernholm F, Karlsson B, Boggs CL. 2005. Age-related changes in thoracic mass: possible reallocation of resources to reproduction in butterflies. Biol. J. Linn. Soc. 86, 363–380. ( 10.1111/j.1095-8312.2005.00542.x) [DOI] [Google Scholar]
- 10.Nicolson SW, Nepi M, Pacini E. 2007. Nectaries and nectar. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 11.Nepi M. 2014. Beyond nectar sweetness: the hidden ecological role of non-protein amino acids in nectar. J. Ecol. 102, 108–115. ( 10.1111/1365-2745.12170) [DOI] [Google Scholar]
- 12.Baker H, Baker I. 1986. The occurrence and significance of amino acids in floral nectar. Plant Syst. Evol. 151, 175–186. ( 10.1007/BF02430273) [DOI] [Google Scholar]
- 13.Baker H, Baker I. 1973. Amino-acids in nectar and their evolutionary significance. Nature 241, 543–545. ( 10.1038/241543b0)4693956 [DOI] [Google Scholar]
- 14.Hill C. 1989. The effect of adult diet on the biology of butterflies. Oecologia 81, 258–266. ( 10.1007/BF00379813) [DOI] [PubMed] [Google Scholar]
- 15.O'Brien DM, Schrag DP, Del Rio CM. 2000. Allocation to reproduction in a hawkmoth: a quantitative analysis using stable carbon isotopes. Ecology 81, 2822–2831. ( 10.1890/0012-9658(2000)081%5B2822:ATRIAH%5D2.0.CO;2) [DOI] [Google Scholar]
- 16.Romeis J, Wäckers F. 2002. Nutritional suitability of individual carbohydrates and amino acids for adult Pieris brassicae. Physiol. Entomol. 27, 148–156. ( 10.1046/j.1365-3032.2002.00281.x) [DOI] [Google Scholar]
- 17.Beck J. 2007. The importance of amino acids in the adult diet of male tropical rainforest butterflies. Oecologia 151, 741–747. ( 10.1007/s00442-006-0613-y) [DOI] [PubMed] [Google Scholar]
- 18.Mevi-Schütz J, Goverde M, Erhardt A. 2003. Effects of fertilization and elevated CO2 on larval food and butterfly nectar amino acid preference in Coenonympha pamphilus L. Behav. Ecol. Sociobiol. 54, 36–43. ( 10.1007/s00265-003-0601-8) [DOI] [Google Scholar]
- 19.Mevi-Schütz J, Erhardt A. 2005. Amino acids in nectar enhance butterfly fecundity: a long-awaited link. Am. Nat. 165, 411–419. [DOI] [PubMed] [Google Scholar]
- 20.Cahenzli F, Erhardt A. 2012. Enhancing offspring quality or quantity? Different ways for using nectar amino acids in female butterflies. Oecologia 169, 1005–1014. ( 10.1007/s00442-012-2254-7) [DOI] [PubMed] [Google Scholar]
- 21.Cahenzli F, Erhardt A. 2013. Nectar amino acids enhance reproduction in male butterflies. Oecologia 171, 197–205. ( 10.1007/s00442-012-2395-8) [DOI] [PubMed] [Google Scholar]
- 22.O'Brien DM, Fogel ML, Boggs CL. 2002. Renewable and nonrenewable resources: amino acid turnover and allocation to reproduction in Lepidoptera. Proc. Natl Acad. Sci. USA 99, 4413–4418. ( 10.1073/pnas.072346699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien DM, Boggs CL, Fogel ML. 2004. Making eggs from nectar: the role of life history and dietary carbon turnover in butterfly reproductive resource allocation. Oikos 105, 279–291. ( 10.1111/j.0030-1299.2004.13012.x) [DOI] [Google Scholar]
- 24.Nicolson SW, Thornburg RW. 2007. Nectar chemistry. In Nectaries and nectar, pp. 215–264. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 25.Baker HG, Baker I. 1975. Studies of nectar-constitution and pollinator–plant coevolution. In Coevolution of animals and plants (eds LE Gilbert, PH Raven), pp. 100–140. Austin, TX: University of Texas Press. [Google Scholar]
- 26.Welch KC, Péronnet F, Hatch KA, Voigt CC, McCue MD. 2015. Carbon stable-isotope tracking in breath for comparative studies of fuel use. Ann. NY Acad. Sci. USA 1365, 15–32. ( 10.1111/nyas.12737) [DOI] [PubMed] [Google Scholar]
- 27.McCue MD, Welch KC Jr. 2016. 13C-Breath testing in animals: theory, applications, and future directions. J. Comp. Physiol. B 186, 265–285. ( 10.1007/s00360-015-0950-4) [DOI] [PubMed] [Google Scholar]
- 28.Levin E, Lopez-Martinez G, Fane B, Davidowitz G. In press. Hawkmoths use nectar sugar to reduce oxidative damage from flight. Science. [DOI] [PubMed]
- 29.Davidowitz G, Nijhout HF. 2004. The effects of environmental variation on a mechanism that controls insect body size. Evol. Ecol. Res. 6, 49–62. [Google Scholar]
- 30.Davidowitz G, Nijhout HF, Roff DA. 2012. Predicting the response to simultaneous selection: genetic architecture and physiological contrains. Evolution 66, 2916–2928. ( 10.1111/j.1558-5646.2012.01644.x) [DOI] [PubMed] [Google Scholar]
- 31.Davidowitz G, D'Amico LJ, Nijhout HF. 2003. Critical weight in the development of insect body size. Evol. Dev. 5, 188–197. ( 10.1046/j.1525-142X.2003.03026.x) [DOI] [PubMed] [Google Scholar]
- 32.Sasaki M, Riddiford LM. 1984. Regulation of reproductive behaviour and egg maturation in the tobacco hawk moth, Manduca sexta. Physiol. Entomol. 9, 315–327. ( 10.1111/j.1365-3032.1984.tb00713.x) [DOI] [Google Scholar]
- 33.Papaj DR. 2000. Ovarian dynamics and host use. Annu. Rev. Entomol. 45, 423–448. ( 10.1146/annurev.ento.45.1.423) [DOI] [PubMed] [Google Scholar]
- 34.Wheeler D. 1996. The role of nourishment in oogenesis. Annu. Rev. Entomol. 41, 407–431. ( 10.1146/annurev.en.41.010196.002203) [DOI] [PubMed] [Google Scholar]
- 35.Gardener MC, Rowe RJ, Gillman MP. 2003. Tropical Bees (Trigona hockingsi) show no preference for nectar with amino acids 1. Biotropica 35, 119–125. ( 10.1111/j.1744-7429.2003.tb00269.x) [DOI] [Google Scholar]
- 36.Bukkens SG. 1997. The nutritional value of edible insects. Ecol. Food Nutr. 36, 287–319. ( 10.1080/03670244.1997.9991521) [DOI] [Google Scholar]
- 37.McCue MD, Guzman RM, Passement CA, Davidowitz G. 2015. How and when do insects rely on endogenous protein and lipid resources during lethal bouts of starvation? A new application for 13C-breath testing. PLoS ONE 10, e0140053 ( 10.1371/journal.pone.0140053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin E, Mitra C, Davidowitz G. 2016. Fed males increase oviposition in female hawkmoths via non-nutritive direct benefits. Anim. Behav. 112, 111–118. ( 10.1016/j.anbehav.2015.11.019) [DOI] [Google Scholar]
- 39.Werner RA, Brand WA. 2001. Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun. Mass Spectrosc. 15, 501–519. ( 10.1002/rcm.258) [DOI] [PubMed] [Google Scholar]
- 40.O'Brien DM, Boggs CL, Fogel ML. 2003. Pollen feeding in the butterfly Heliconius charitonia: isotopic evidence for essential amino acid transfer from pollen to eggs. Proc. R. Soc. Lond. B 270, 2631–2636. ( 10.1098/rspb.2003.2552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCue MD, Sivan O, McWilliams SR, Pinshow B. 2010. Tracking the oxidative kinetics of carbohydrates, amino acids and fatty acids in the house sparrow using exhaled 13CO2. J. Exp. Biol. 213, 782–789. ( 10.1242/jeb.039842) [DOI] [PubMed] [Google Scholar]
- 42.McCue MD, Guzman RM, Passement CA. 2015. Digesting pythons quickly oxidize the proteins in their meals and save the lipids for later. J. Exp. Biol. 218, 2089–2096. ( 10.1242/jeb.118349) [DOI] [PubMed] [Google Scholar]
- 43.Castellini M, Rea L. 1992. The biochemistry of natural fasting at its limits. Experientia 48, 575–582. ( 10.1007/BF01920242) [DOI] [PubMed] [Google Scholar]
- 44.McCue MD. 2012. Comparative physiology of fasting, starvation, and food limitation. Berlin, Germany: Springer. [Google Scholar]
- 45.Wang T, Hung CC, Randall DJ. 2006. The comparative physiology of food deprivation: from feast to famine. Annu. Rev. Physiol. 68, 223–251. ( 10.1146/annurev.physiol.68.040104.105739). [DOI] [PubMed] [Google Scholar]
- 46.Secor SM, Carey HV. 2016. Integrative physiology of fasting. Compr. Physiol. 6, 773–825. ( 10.1002/cphy.c150013) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study are available at http://arizona.openrepository.com/arizona/handle/10150/621812.