Abstract
Several studies have suggested that covert stressors can contribute to bee colony declines. Here we provide a novel case study and show using radiofrequency identification tracking technology that covert deformed wing virus (DWV) infections in adult honeybee workers seriously impact long-term foraging and survival under natural foraging conditions. In particular, our experiments show that adult workers injected with low doses of DWV experienced increased mortality rates, that DWV caused workers to start foraging at a premature age, and that the virus reduced the workers' total activity span as foragers. Altogether, these results demonstrate that covert DWV infections have strongly deleterious effects on honeybee foraging and survival. These results are consistent with previous studies that suggested DWV to be an important contributor to the ongoing bee declines in Europe and the USA. Overall, our study underlines the strong impact that covert pathogen infections can have on individual and group-level performance in bees.
Keywords: honeybees, deformed wing virus, foraging behaviour, radiofrequency identification tags, bee declines, covert pathogen infections
1. Introduction
Over the past decades, serious declines in both wild and managed bee pollinators have been recorded in many parts of the world [1–5], thereby threatening the ecosystem services they provide. The underlying cause of the recent wave of honeybee colony losses has been subject to much debate and the current consensus is that multiple stressors likely contribute to these declines, including malnutrition owing to a lack of flower diversity, exposure to agrochemicals or the spread of emerging pathogens and parasites [2,5–10]. Pinpointing these stressors, however, can be hard, especially when their effect becomes obvious only over extended periods of time, such as following exposure to sublethal doses of pesticides [9,11] or after contracting some seemingly harmless ‘covert’ pathogen infections [12,13].
Among pathogens, recent studies have suggested that deformed wing virus (DWV) represents an important long-term stressor, as it has been statistically associated with both winter mortality and colony collapse in many studies [14–24]. DWV has a near global distribution and is the most widespread of the currently described viruses that infect honeybees, often affecting between 50% and 75% of all honeybee hives [25,26]. In addition, the virus can spill over to other bees [27,28], thereby posing an additional threat. DWV is named after the characteristic wing deformities that can arise when honeybees are infected in the larval or pupal stage via the ectoparasitic mite vector Varroa destructor [29]. In this case, virus infections result in bloated abdomens, miscolouring and shortened lifespans [29]. Typically, however, infections take on a more ‘covert’ form, resulting in no visible morphological symptoms, especially when infection occurs in the adult stage or when mites carry only low virus titres. Nevertheless, the fact that colonies with covert infections can suffer from weakness, depopulation and sudden collapse [22] and that the presence of the virus has been linked with both winter mortality and colony collapse [14–24] suggests that DWV exerts a significant amount of long-term stress.
Indeed, several recent studies using controlled artificial infection of adult honeybee workers have shown a number of important effects of DWV, including the impairment of sensory responsiveness, associative olfactory learning and memory formation [30] (possibly linked to replication of the virus in the mushroom bodies and antennal lobes [31]), an accelerated pace of behavioural transition through their age-linked task allocation [32] and reduced lifespans of infected adult honeybees [25]. Furthermore, two studies in which natural variation in infection levels of DWV was combined with experimentally manipulated variation in infection levels with the microsporidian parasite Nosema showed that DWV-infected bees displayed shortened flight distances and flight durations in flight mill experiments [33], but no differences in orientation flight behaviour in a harmonic radar tracking set-up [34], whereas the reverse pattern was seen for Nosema [33,34]. As yet, however, effects of DWV on honeybee flight behaviour and foraging patterns have not yet been investigated using controlled infection set-ups.
The aim of this study, therefore, was to determine the impact of DWV as a long-term stressor in honeybees, and test experimentally if inoculation of adult bees with the virus negatively affected honeybee foraging behaviour and performance. To this end, we used passively powered radiofrequency identification (RFID) transponder tags [9,11,35–40] as a key technology that enabled us to non-invasively monitor the long-term out-hive activity of honeybee workers that were or were not experimentally infected with the virus. Tracking out-hive activity is key in studies of the impact of pathogens on honeybee health, as the worker foraging force is responsible for all resource acquisition, and the foraging range, worker activity and the magnitude of resource influx are vital to colony growth and survival [41,42]. Previously, RFID technology has been successfully used to study sublethal effects of nutritional stress and pesticides on honeybee and bumblebee foraging behaviour [9,11,35–40]. Nevertheless, applications to the study of pathogen-induced stress on honeybee foraging behaviour are still rare, and are currently limited to one study on Israeli acute paralysis virus (IAPV) [43], which documented virus-induced differences in homing ability, but without taking full advantage of the technology to study the long-term impact on foraging behaviour, and one study that showed adverse effects of a microsporidian gut parasite, Nosema apis, on honeybee foraging and survival [44]. In addition, another tracking technology—harmonic radar—was recently used by one group to show that the emerging pathogen Nosema ceranae caused impaired homing behaviour in honeybees [45] and that Nosema infection also affected honeybee orientation flight behaviour [34]. The tracking method, however, was unable to reveal any correlation between DWV infection levels and orientation flight characteristic [34].
2. Material and methods
(a). Radiofrequency identification tracking set-up
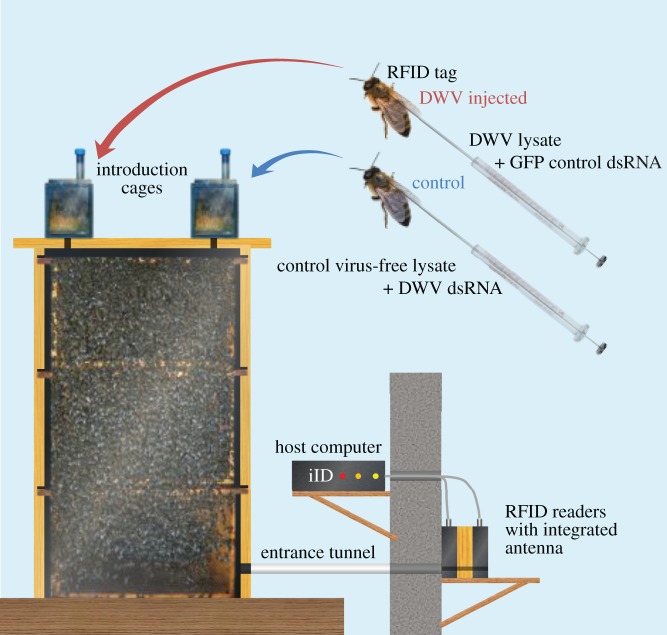
Three three-frame observation hives with Apis mellifera carnica honeybees were installed at the laboratory's apiary in Leuven, Belgium to serve as host colonies for RFID-tagged bees that were or were not experimentally infected with DWV (400 of each treatment condition per host colony, see below). Each host colony contained two frames of brood, one frame with stored pollen and honey, a queen and around 3000 host colony workers (figure 1). The colonies were placed indoors at room temperature and were connected to the outside via a single entrance tunnel to allow free foraging. The end of the tunnel was outfitted with two iID® MAJA 4.1 RFID reader modules placed in series and connected to a MAJA 4.1 host computer (Microsensys, Germany) to record and log the timing of all RFID-tagged honeybees leaving or entering the hive. By setting up the reader modules in a serial set-up, successive signals from both readers gave information regarding the direction of movement of the detected bees. The readers were separated from each other by a 4 cm wooden tunnel block to prevent interference between the readers (figure 1).
Figure 1.
Experimental set-up. Observation hives were installed indoors with two RFID readers at the hive entrance to detect and log RFID-tagged bees entering or leaving the hive. The two RFID readers modules, connected to the host computer, were placed in series to determine the walking direction of detected bees. Tagged bees which were or were not experimentally infected with deformed wing virus were introduced into the host colony via separate introduction cages shown at the top (n = 400 bees per treatment and host colony). (Online version in colour.)
(b). Introduction of control and experimentally infected bees
In each of the host colonies, we introduced 400 DWV-negative control bees and 400 DWV infection-positive honeybees. This was done by allowing bees to emerge from a single donor colony that based on a prior screen was confirmed to be free of DWV as well as of any of the major known honeybee viruses or pathogens [46], injecting newly eclosed workers with appropriate treatment solutions, and introducing these bees into one of three host colonies (see electronic supplementary material Methods for details). All colonies in our apiary, including the donor and host colonies, were treated with Thymovar for Varroa control according to the manufacturer's recommendations. The fact that only a single donor colony was used in our experiments was linked with the difficulty of finding a host colony that was free of the major known honeybee pathogens, but our experimental design partly compensated for this by incorporating replication across different host environments. Bees were allowed to emerge by placing brood frames of the donor colony in a MIR-253 incubator (Sanyo, Belgium) at 34°C and 60% humidity, after which newly eclosed workers were collected daily. Subsequently, 400 newly eclosed workers per treatment condition and host colony were injected with 3 µl of the appropriate treatment solution, using a 5 µl 26 s gauge Hamilton syringe inserted into the apical part of the thorax. Immediately afterwards, each of these bees were outfitted with a mic3® 64-bit read-only RFID transponder (Microsensys, Germany) by gluing the tag to the bee's thorax using Kombi Turbo two-component glue (Bison, The Netherlands). The tags measured 2.0 × 1.7 × 0.5 mm, weighed less than 5 mg and transmitted at 13.56 MHz. The RFID codes of all experimentally manipulated workers, together with the treatment condition, host colony and time of introduction, were added to a transponder information database by reading each code using a iID® PENmini USB pen (Microsensys, Germany). Up to 50 tagged individuals subjected to one of the two treatment conditions were kept in separate 15 × 10 × 7 cm cages kept at 34°C and 60% humidity, and contained a 10 × 8 cm piece of honey-filled comb and water, to allow the bees to settle down before introducing them into the host colonies (figure 1). Before introduction, the cages were placed on top of the observation hives, separated only by a wire mesh, for a 30 min period to increase acceptance rates [47]. Each of the 400 workers per host colony and treatment condition were introduced over the course of a period of 5 days, and foraging behaviour was monitored up to 40 days after introduction, in August–September 2012 for replicate host colonies A and B and September–October 2012 for host colony C.
(c). Controlled infection
In order to obtain two groups of adult age-matched bees that were or were not infected with DWV, we injected bees with lysate of honeybees that were either infected with DWV (and none of the other common honeybee viruses) or with that of honeybees that were confirmed to be virus-free (for details, see electronic supplementary material). To reduce the likelihood of horizontal transmission from the DWV infected to the group of uninfected bees [48], we also added a double-stranded RNA treatment to our DWV-negative lysate [49,50] to try to keep those bees DWV-free, and added a control GFP-dsRNA treatment in the DWV positive lysate to control for the possible effects of foreign dsRNA injection. The amount of DWV injected was estimated at 1.2 × 104–4.6 × 105 DWV copies per bee, and was aimed at mimicking infection loads reported for bees with covert infections (1.4 × 103–2.4 × 109 copies per bee, [51]). Based on Illumina ultra-high throughput sequencing, the DWV strain used for inoculation was determined to belong to the type B DWV master variant [52], which has recently been found to be an emergent, slightly more virulent strain of the DWV virus [25] that is currently also the most common strain in Britain [25] (for details, see the electronic supplementary material).
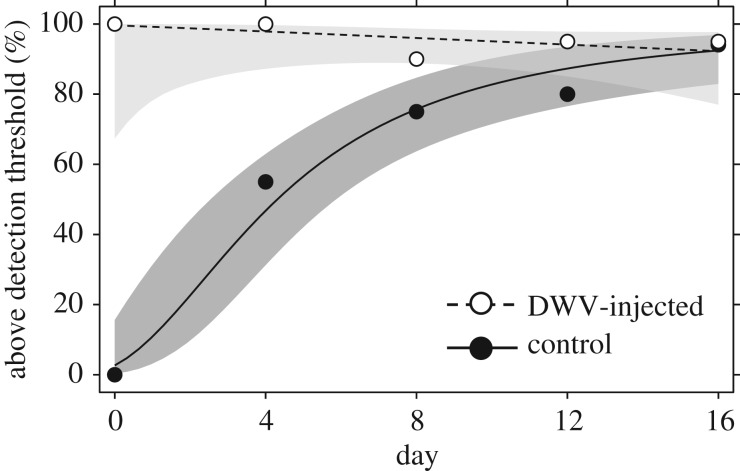
To validate the effects of the two treatment solutions, 150 additional eclosed workers were injected with each treatment solution, paint-marked and introduced into a fourth observation. Every 4 days, 20 individuals of each treatment were sampled from this colony and subjected to MLPA analysis to determine their DWV infection state [46]. These analyses confirmed the establishment of stable DWV infections in the DWV-lysate-inoculated bees, even if there was evidence that some control bees became infected during the later stages of the experiment as well (figure 2), though possibly at lower levels (see electronic supplementary material for a full discussion of the methods and results of these analyses).
Figure 2.
Treatment validation results. Evolution of the proportion of infected individuals over time in control and DWV treatment groups, based on MLPA analysis [46] of four daily sample sets of 20 individuals each from both treatments (fits and 95% CIs based on binomial GLM with treatment, log(x + 1) tranformed duration after introduction into the host colony and their interaction included as independent variables).
(d). Data analysis
Raw RFID tracking data were analysed with the Track-A-Forager Java application [53], which filters out rapid-succession scans of the same scanner, labels ingoing and outgoing flights by tagged workers, and corrects occasional errors in the data, including the possible occurrence of missed scans (for details see electronic supplementary material). To compare the foraging behaviour of DWV-infected and control bees, we quantified the number of trips performed by each individual, trip duration and the proportion of the introduced workers of each treatment group which survived up to foraging age, to gauge differential early-life mortality. In addition, we measured the age at onset of foraging, defined as the age of each individual at their first reconstructed trip, the foragers' life expectancy, measured as the age of each individual at their last scan, and forager activity span, i.e. the time difference in days between the first and last registered scan of each bee. We should note that our experiment could not distinguish between a DWV-induced reduction in direct mortality and a DWV-induced mortality owing to indirect effects, e.g. caused by a decrease in homing ability or an increase in the susceptibility to predation or other environmental stressors. Details of all statistical analyses performed are given in electronic supplemental material and the R script included on the Dryad repository.
3. Results
Visual analysis of foraging activity over the course of our experiment revealed clear disparities in the age at onset of foraging, forager life expectancy and forager activity span between the DWV-infected and control bees (figure 3). In addition, bees that survived to foraging age showed a clear deviation from the 50 : 50 ratio at which they were first introduced (figure 3), with control bees evidently having much better chances to survive to foraging age than DWV-infected ones. To thoroughly examine each of these effects as well as to look for other possible effects of DWV on foraging behaviour, we conducted a number of detailed statistical analyses. In particular, we tested for significant effects of DWV on the probability that bees would survive to foraging age, the onset of foraging, forager life expectancy and activity span as well as the number and trip duration of foraging trips carried out by individuals that survived to foraging age.
Figure 3.
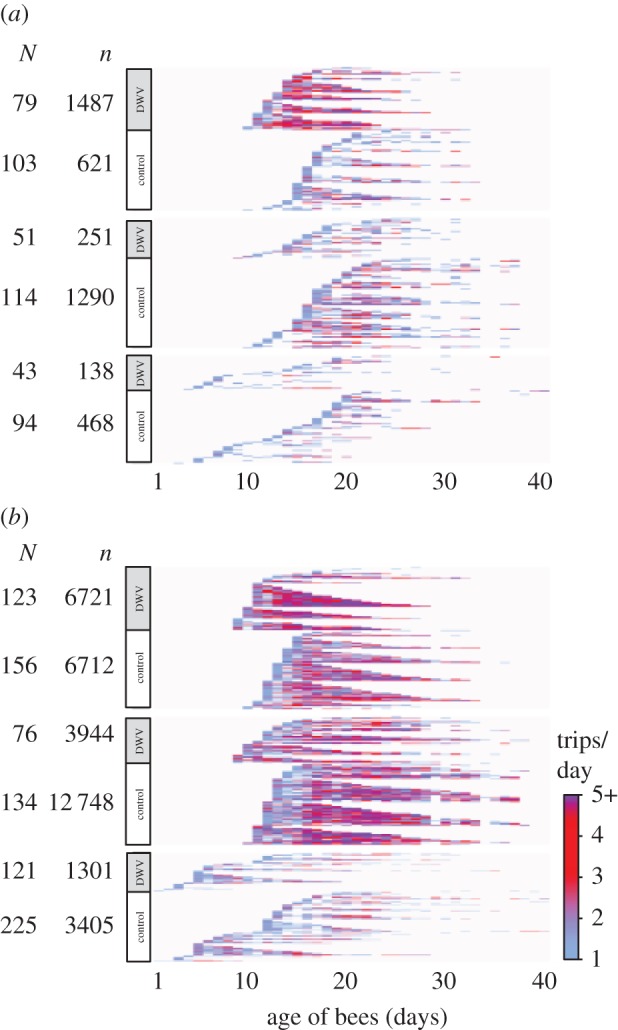
Total foraging activity of DWV-infected and control bees. The daily foraging activity over the course of the experiment is shown based on both the reconstructed foraging trips (a) and the unfiltered RFID scans (b) for colonies A (top), B (middle) and C (bottom) (N = total number of unique bees detected per treatment and host colony, n = total number of reconstructed foraging trips or scan events across all tracked bees). Individuals, represented as rows in the diagram, are sorted by treatment, age at onset of foraging and total activity span. The overrepresentation of control bees among observed foragers demonstrates that control workers had higher odds to survive to foraging age, and visual analysis of the data also indicate disparities between DWV and control bees in the age at onset of foraging, forager life expectancy and activity span (cf. figure 4). (Online version in colour.)
(a). Effect of deformed wing virus on the likelihood that bees would survive to foraging age
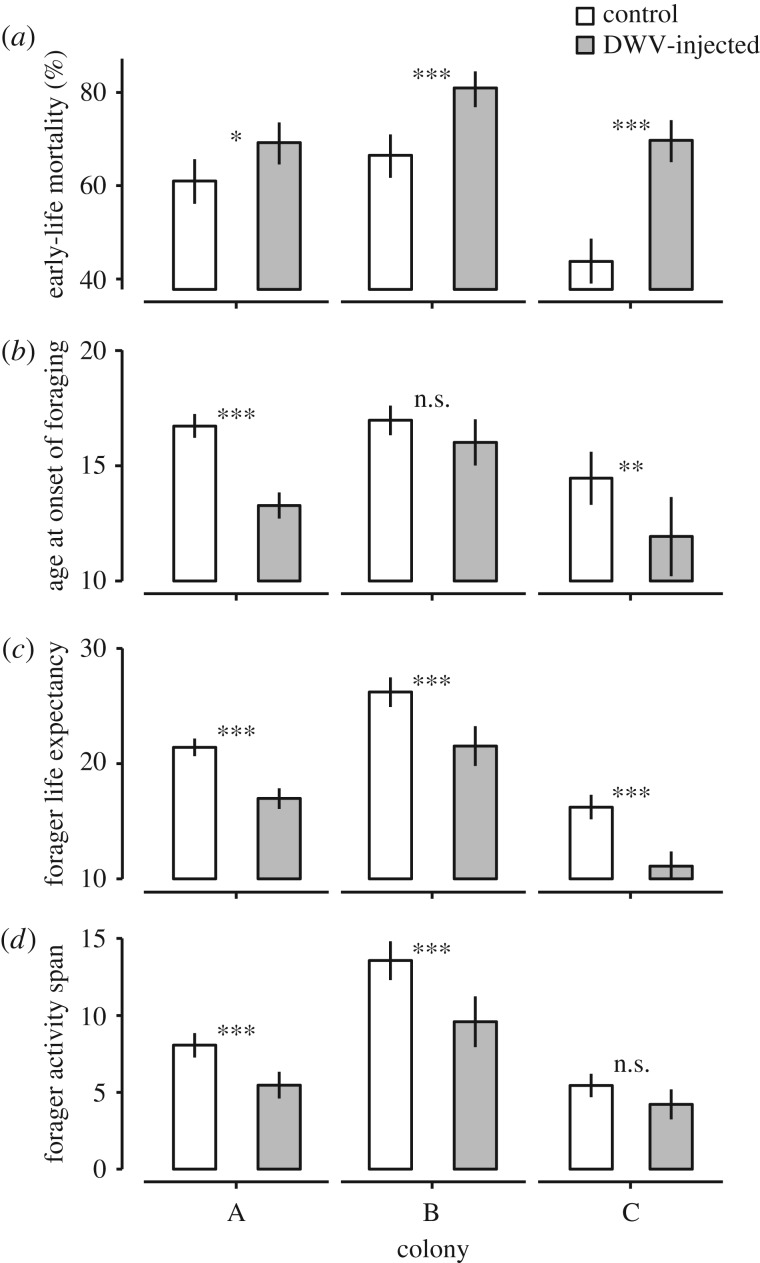
The biased representation of control bees among bees that started to forage relative to the 50 : 50 ratio at which they were first introduced (figure 3) indicates that the DWV-inoculated bees experienced greater mortality early on in their life. This is confirmed by the fact that a significantly greater proportion of the DWV-inoculated bees died before making it to foraging age than control bees in each of the three host colonies (277/400 versus 244/400, 324/400 versus 266/400 and 279/400 versus 175/400; binomial colony × treatment full factorial GLM, z = 2.44 and p = 0.015, z = 4.61 and p = 4 × 10−6, z = 7.33 and p = 2 × 10−13; figure 4a). We should note that this mortality also includes baseline mortality linked to occasional rejection of tagged bees by the host colony. To control for this baseline mortality, we also calculated the relative odds that bees would survive to foraging age. This showed that DWV-inoculated bees on average had 2.1 (s.e. 0.19) times lower odds to make it to foraging age than control bees.
Figure 4.
Effect of DWV infection on honeybee foraging and survival. Out of the 400 bees that were tagged per treatment and host colony, a significantly smaller proportion survived to foraging age (being detected at least once by the RFID scanners) in the DWV injected group than in the control group (panel a, binomial full factorial GLM, overall p < 2 × 10−16, 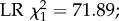 means and 95% C.L.s are shown and significance levels per colony based on Tukey post-hoc Wald z tests shown by asterisks). In addition, honeybees that were artificially infected with DWV and which survived to foraging age started to forage significantly earlier than uninfected control bees (panel b, two-way full factorial ANOVA, p = 7×10−19,
means and 95% C.L.s are shown and significance levels per colony based on Tukey post-hoc Wald z tests shown by asterisks). In addition, honeybees that were artificially infected with DWV and which survived to foraging age started to forage significantly earlier than uninfected control bees (panel b, two-way full factorial ANOVA, p = 7×10−19, 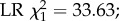 means and 95% C.L.s are shown and significance levels per colony based on Tukey post-hoc Wald z tests shown by asterisks; total number of tracked bees as in figure 3a), and DWV-infected foragers had a significantly reduced life expectancy (defined as age at last detection, panel c; ANOVA, p = 0.01,
means and 95% C.L.s are shown and significance levels per colony based on Tukey post-hoc Wald z tests shown by asterisks; total number of tracked bees as in figure 3a), and DWV-infected foragers had a significantly reduced life expectancy (defined as age at last detection, panel c; ANOVA, p = 0.01, 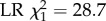 ) and activity span (defined as age at last foraging trip minus age at first foraging trip, panel d; ANOVA, p = 0.0003,
) and activity span (defined as age at last foraging trip minus age at first foraging trip, panel d; ANOVA, p = 0.0003, 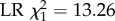 ) (means and 95% C.L.s are shown and significance levels per colony based on Tukey post-hoc Wald z tests shown by asterisks; total number of tracked bees and foraging trips as in figure 3a).
) (means and 95% C.L.s are shown and significance levels per colony based on Tukey post-hoc Wald z tests shown by asterisks; total number of tracked bees and foraging trips as in figure 3a).
(b). Effect of deformed wing virus on age at onset of foraging, life expectancy and activity span
We also found significant differences between the DWV-infected and non-infected treatments in the observed age at onset of foraging. In particular, bees that were inoculated with DWV and which survived until foraging age starting to forage 2.31 days earlier (s.e. 0.73), on average, than bees inoculated with the control solution (figure 4b; full factorial colony × treatment ANOVA, main effect of treatment: p = 7 × 10−9; 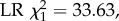 sample sizes as shown in figure 3a). In addition, infected foragers had a life expectancy that was reduced by 4.74 days on average (s.e. 0.20) compared with control bees (figure 4c; ANOVA, main effect of treatment: p = 0.01;
sample sizes as shown in figure 3a). In addition, infected foragers had a life expectancy that was reduced by 4.74 days on average (s.e. 0.20) compared with control bees (figure 4c; ANOVA, main effect of treatment: p = 0.01; 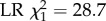 ) and a total activity span that was reduced by 2.60 days (s.e. 0.79) compared with control foragers (figure 4d; ANOVA, main effect of treatment: p = 0.0003;
) and a total activity span that was reduced by 2.60 days (s.e. 0.79) compared with control foragers (figure 4d; ANOVA, main effect of treatment: p = 0.0003; 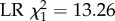 ; sample sizes for both are as given in figure 3a). Hence, DWV-infected bees had a reduced life expectancy and activity span also after they started to forage, even though the onset of foraging occurred 2.3 days earlier in the DWV-infected group than in the control group. Overall, the onset of foraging (at 12–17 days) was somewhat earlier and forager life expectancy shorter (11–26 days) than that observed in mature hives (onset at ca three weeks and forager life expectancy of ca six weeks [47,54]). Nevertheless, both figures were comparable to those found in other studies that used a comparable observation hive set-up (e.g. onset of foraging at ca 10 days and forager life expectancy of 14–40 days [55]). This discrepancy may be linked with stress induced by the RFID tags or the injection itself, as stress in honeybees is known to induce precocious foraging and shorten worker lifespan [32,56]. Even so, we expect our conclusions to be robust as our treatment effects are all measured relative to the control under identical conditions.
; sample sizes for both are as given in figure 3a). Hence, DWV-infected bees had a reduced life expectancy and activity span also after they started to forage, even though the onset of foraging occurred 2.3 days earlier in the DWV-infected group than in the control group. Overall, the onset of foraging (at 12–17 days) was somewhat earlier and forager life expectancy shorter (11–26 days) than that observed in mature hives (onset at ca three weeks and forager life expectancy of ca six weeks [47,54]). Nevertheless, both figures were comparable to those found in other studies that used a comparable observation hive set-up (e.g. onset of foraging at ca 10 days and forager life expectancy of 14–40 days [55]). This discrepancy may be linked with stress induced by the RFID tags or the injection itself, as stress in honeybees is known to induce precocious foraging and shorten worker lifespan [32,56]. Even so, we expect our conclusions to be robust as our treatment effects are all measured relative to the control under identical conditions.
(c). Effect of deformed wing virus on number of foraging trips or trip duration
Although DWV infection could in principle also have affected the number of foraging trips and the duration of the trips carried out by individuals that survived to foraging age [33], our statistical analyses revealed that there was no strong evidence for this. For example, the effect of treatment on the number of trips performed was inconsistent and different across the replicate host colonies used (quasi-Poisson GLM, effects of treatment in colonies A, B and C: A. z = 7.55 and p = 1 × 10−7, B. z = −2.49 and p = 0.04; C. z = −1.38, p = 0.42). On average, across all three colonies, workers made 5.46 trips per bee per day (s.e. 0.50) in the DWV-inoculated group versus 5.85 trips per bee per day (s.e. 0.80) in the control (sample sizes as in figure 3a), which are figures consistent with those given in other studies [47,54]. Nevertheless, given that DWV infection strongly reduced the chances for workers to survive to foraging age and that DWV infection reduced the life expectancy and total activity span of foragers (cf. results above), it is clear that DWV still had a strongly negative overall effect on the net number of trips and amount of foraging performed. Similarly, the effect on trip duration was not consistent across host colonies, as DWV-inoculated bees made significantly longer trips than control bees in colony A (gamma GLMM, Tukey post-hoc test, 1.86 h versus 1.22 h, s.e. 0.17 and 0.08, p = 0.0004), whereas there was no significant effect on trip duration in colony C (2.89 h versus 2.50, s.e. 0.41 and 0.47, p = 0.90), and an opposite trend in colony B than in colony A (1.62 h versus 1.99 h, s.e. 0.12 and 0.27, p = 0.39). Inclusion of time or worker age as explicit linear or polynomial terms, either in interaction with treatment or not, did not improve the explanatory power of any of the fitted models, and hence such analyses were not further pursued.
4. Discussion
Overall, our results demonstrate that DWV infections have strongly deleterious effects on adult honey bees, with both mortality rates, and—to a lesser extent—foraging behaviour being clearly affected. In particular, DWV-infected bees started to forage at an earlier age and showed reduced lifespans and total activity spans than control bees. Finally, next-gen sequencing demonstrated that the DWV strain we used for inoculation belonged to the type B DWB master variant [52], which has recently been found to be an emergent, more virulent strain of the DWV virus [25] that currently appears to be the most common strain in Britain [25]. The fact that our DWV lysate was prepared from a randomly selected sample of bees with overt DWV infection symptoms suggest that this strain is now also common in Continental Europe.
Our finding that DWV-inoculated workers started foraging at an earlier age and experienced so-called precocious foraging was in line with expectation, as previous studies have also found that unhealthy or stressed honeybee workers start to perform risky foraging tasks at an earlier age compared with healthy individuals. For example, Nosema [13,57,58], sacbrood virus [59] and Varroa [60,61] have all been found to induce precocious foraging in honeybees. From an ultimate perspective, diseased or health compromised workers have been suggested to benefit from starting to foraging earlier as a way to protect other individuals inside the nest from getting infected [58] or to make the most of their reduced lifetime [56]. In addition, it would be possible that the disease agent itself benefited from an earlier onset of foraging if this promoted its horizontal transmission to other host colonies [62]. Indeed, in the case of DWV, a direct influence on the behaviour of its host is not unlikely, given that DWV particles have previously been found in the mushroom bodies—a key higher brain centre of these insects [31] (but see [34] for a study where no behavioural effects were found). Irrespective of these possible adaptive causes, it is clear that precocious foraging would have a major effect on colony well-being, as premature foraging partially depletes the nurse bee population [63] and disrupts various activities inside the hive [64–66], and rapid behavioural maturation has been shown to strongly accelerate the failure of stressed honeybee colonies [63].
The strong evidence we found for a DWV-induced effect on mortality patterns and long-term survival was more unexpected. Traditionally, secondary DWV infections in adult workers are regarded as ‘covert’ and largely asymptomatic [29,67,68], but this proposition is clearly challenged by our findings, which document very clear and significant long-term effects of the virus. Although increased mortality has been documented in bees that display overt DWV infection symptoms and crippled wings [69–71], similar mortality in bees that acquired the virus in the adult stage has been demonstrated only recently in experimentally caged and non-foraging bees [25]. Our results now show that this mortality effect continues after the onset of foraging, and that the virus therefore acts as a long-term stressor on honeybee health and survival. DWV-induced mortality could have several causes. Given that DWV have been shown to occur in the honeybee brain, including in the mushroom bodies [31,62,72], which are involved in learning and memory, and that DWV infections have been shown to induce learning deficits [30], it is possible that increased mortality is caused by impaired orientation capabilities or predator avoidance or that it makes them more susceptible to other environmental stressors. Indeed, the DWV Kakugo strain has earlier been found to be associated with increased aggression and risk-taking behaviour [73,74]. Alternatively, it is possible that DWV directly results in increased mortality, e.g. owing to costly upregulation of the host's immune system [75]. However, given the well-documented effect on direct, early-life mortality, both in our study and that of McMahon et al. [25], we consider a direct mortality effect most likely. Furthermore, and regardless of the underlying causes, it is clear that the early disappearance of DWV-infected bees and their significantly shorter activity spans would have strongly deleterious effects on the total amount of pollen and nectar foraging performed by infected colonies. Additionally, a shorter activity span of workers would also cause fewer workers to engage in discovering novel food patches, thereby impacting the flow of information and causing further synergistic costs to global colony health [47].
The fact that in our RFID data, DWV inoculation did not affect trip duration or the number of trips performed by DWV-infected foragers went against the conclusions of [33], who concluded that DWV infection but not Nosema ceranae reduced average trip duration. As there was significant variation in the impact of the virus across our three replicate host environments, however, it is possible that the same effect would still have been found with a larger number of replicate donor and/or host colonies. Given that our donor bees all came from a single, rare uninfected colony, we had a priori not expected any large variation in the impact of the virus. Possible reasons for this variation could be linked with seasonal factors, variation in the genetic compatibility with the host colonies, or subtle differences in the performance or health of the host colonies, such as the possible presence of Nosema among the host workers, which we did not explicitly look at, but which is known to cause precocious foraging and affect longevity, activity and out-of-hive performance of honeybees [13,45,58,76,77]. Alternatively, it is possible that the variation in DWV impact is linked to some of the control bees having become infected during the later stages of our experiment, which our treatment validation results suggest may have been the case (though likely at lower levels, figure 2), and that the speed at which this occurred differed across host colonies. These results also suggest that a single dsRNA injection was not sufficient to fully protect bees for extended periods of time, and that continued oral administration would have to be used for effective long-term control via RNA interference [49,50,78]. Despite this variation in the effect of the virus, however, it was clear that overall, DWV had a strongly deleterious effect across all three colonies, with significant effects on early-life mortality (figure 4a), forager life expectancy (figure 4c) and forager activity span (figure 4d) in three replicate host colony environments, and significant effects on the onset of foraging in two out of three host colonies (figure 4b). These findings are consistent with studies showing that DWV is among the most important predictors implicated in honeybee colony declines in both Europe [14,16,17,24] and the USA [18] and hence an important contributory factor to the current pollination crisis.
Overall, our results highlight the impact of long-term stressors on bee health and survival, thereby reinforcing the conclusions of several recent tracking studies that have studied stress-induced changes in bees caused by either pathogens [33,43–45], nutritional stress [36] or pesticide exposure [9,11,35,37–40]. We hope that in the future, these approaches may continue to be used to further our understanding of the factors involved in the ongoing pollinator declines [1–5] and how they interact with each other in exerting long-term stress [2,5–10].
Supplementary Material
Acknowledgements
We thank Merav Gleit, Nitzan Paldi and Beeologics for providing the dsRNA and An Vandoren, Ulrich Ernst, Jurgen Huybrechts and Vicky Cranshof for technical assistance.
Data accessibility
All raw data files and code used in analyses are available in Dryad: http://dx.doi.org/10.5061/dryad.fm0r1. Illumina reads and the full genome sequence of the DWV inoculate are available from the Sequence Read Archive and GenBank (accession nos. PRJNA336281 and KX783225).
Authors' contributions
K.B., D.C., D.C.d.G., L.S. and T.W. conceived and designed the work; K.B., D.C. and L.D.S. performed the experiments; K.B., A.V.G. and T.W. analysed the data; L.D., D.C.d.G., L.D.S., L.B., S.J.M. and T.W. contributed reagents, materials, or analysis tools and K.B., M.H.D.L., L.B., S.J.M. and T.W. wrote the paper. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Financial support was provided by FWO-Vlaanderen (grant no. G.0628.11) and the IWT (PhD fellowship of K.B.).
References
- 1.Ellis JD, Evans JD, Pettis J. 2010. Colony losses, managed colony population decline, and colony collapse disorder in the United States. J. Apic. Res. 49, 134–136. ( 10.3896/ibra.1.49.1.30) [DOI] [Google Scholar]
- 2.McMenamin AJ, Genersch E. 2015. Honey bee colony losses and associated viruses. Curr. Opin. Insect Sci. 8, 121–129. ( 10.1016/j.cois.2015.01.015) [DOI] [PubMed] [Google Scholar]
- 3.Lee K, et al. 2015. A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie 46, 292–305. ( 10.1007/s13592-015-0356-z) [DOI] [Google Scholar]
- 4.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 5.Neumann P, Carreck N. 2010. Honey bee colony losses. J. Apic. Res. 49, 1–6. ( 10.3896/ibra.1.49.1.01) [DOI] [Google Scholar]
- 6.Goulson D, Nicholls E, Botías C, Rotheray EL. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
- 7.Barron AB. 2015. Death of the bee hive: understanding the failure of an insect society. Curr. Opin. Insect Sci. 10, 45–50. ( 10.1016/j.cois.2015.04.004) [DOI] [PubMed] [Google Scholar]
- 8.Manley R, Boots M, Wilfert L. 2015. Emerging viral disease risk to pollinating insects: ecological, evolutionary and anthropogenic factors. J. Appl. Ecol. 52, 331–340. ( 10.1111/1365-2664.12385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill RJ, Ramos-Rodriguez O, Raine NE. 2012. Combined pesticide exposure severely affects individual-and colony-level traits in bees. Nature 491, 105–108. ( 10.1038/nature11585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitehorn PR, O'Connor S, Wackers FL, Goulson D. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352. ( 10.1126/science.1215025) [DOI] [PubMed] [Google Scholar]
- 11.Henry M, Béguin M, Requier F, Rollin O, Odoux J-F, Aupinel P, Aptel J, Tchamitchian S, Decourtye A. 2012. A common pesticide decreases foraging success and survival in honey bees. Science 336, 348–350. ( 10.1126/science.1215039) [DOI] [PubMed] [Google Scholar]
- 12.Bryden J, Gill RJ, Mitton RAA, Raine NE, Jansen VAA. 2013. Chronic sublethal stress causes bee colony failure. Ecol. Lett. 16, 1463–1469. ( 10.1111/ele.12188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kralj J, Fuchs S. 2010. Nosema sp. influences flight behavior of infected honey bee (Apis mellifera) foragers. Apidologie 41, 21–28. ( 10.1051/apido/2009046) [DOI] [Google Scholar]
- 14.Dainat B, Evans JD, Chen YP, Gauthier L, Neumann P. 2012. Predictive markers of honey bee colony collapse. PLoS ONE 7, e32151 ( 10.1371/journal.pone.0032151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Highfield AC, El Nagar A, Mackinder LCM, Noel L, Hall MJ, Martin SJ, Schroeder DC. 2009. Deformed wing virus implicated in overwintering honeybee colony losses. Appl. Environ. Microbiol. 75, 7212–7220. ( 10.1128/aem.02227-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacandritsos N, Granato A, Budge G, Papanastasiou I, Roinioti E, Caldon M, Falcaro C, Gallina A, Mutinelli F. 2010. Sudden deaths and colony population decline in Greek honey bee colonies. J. Invert. Pathol. 105, 335–340. ( 10.1016/j.jip.2010.08.004) [DOI] [PubMed] [Google Scholar]
- 17.Genersch E, et al. 2010. The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41, 332–352. ( 10.1051/apido/2010014) [DOI] [Google Scholar]
- 18.Cornman RS, Tarpy DR, Chen Y, Jeffreys L, Lopez D, Pettis JS, Vanengelsdorp D, Evans JD. 2012. Pathogen webs in collapsing honey bee colonies. PLoS ONE 7, e43562 ( 10.1371/journal.pone.0043562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthoud H, Imdorf A, Haueter M, Radloff S, Neumann P. 2010. Virus infections and winter losses of honey bee colonies (Apis mellifera). J. Apic. Res. 49, 60–65. ( 10.3896/ibra.1.49.1.08) [DOI] [Google Scholar]
- 20.Lanzi G, De Miranda JR, Boniotti MB, Cameron CE, Lavazza A, Capucci L, Camazine SM, Rossi C. 2006. Molecular and biological characterization of deformed wing virus of honeybees (Apis mellifera L.). J. Virol. 80, 4998–5009. ( 10.1128/JVI.80.10.4998-5009.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox-Foster DL, et al. 2007. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318, 283–287. ( 10.1126/science.1146498) [DOI] [PubMed] [Google Scholar]
- 22.Berenyi O, Bakonyi T, Derakhshifar I, Koglberger H, Nowotny N. 2006. Occurrence of six honeybee viruses in diseased Austrian apiaries. Appl. Environ. Microbiol. 72, 2414–2420. ( 10.1128/aem.72.4.2414-2420.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kielmanowicz MG, Inberg A, Lerner IM, Golani Y, Brown N, Turner CL, Hayes GJ, Ballam JM. 2015. Prospective large-scale field study generates predictive model identifying major contributors to colony losses. PLoS Pathog. 11, e1004816 ( 10.1371/journal.ppat.1004816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evison SE, Roberts KE, Laurenson L, Pietravalle S, Hui J, Biesmeijer JC, Smith JE, Budge G, Hughes WO. 2012. Pervasiveness of parasites in pollinators. PLoS ONE 7, e30641 ( 10.1371/journal.pone.0030641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMahon DP, Natsopoulou ME, Doublet V, Fürst M, Weging S, Brown MJF, Gogol-Döring A, Paxton RJ. 2016. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. R. Soc. B 283, 20160811 ( 10.1098/rspb.2016.0811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravoet J, Maharramov J, Meeus I, De Smet L, Wenseleers T, Smagghe G, de Graaf DC. 2013. Comprehensive bee pathogen screening in Belgium reveals Crithidia mellificae as a new contributory factor to winter mortality. PLoS ONE 8, e72443 ( 10.1371/journal.pone.0072443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravoet J, De Smet L, Meeus I, Smagghe G, Wenseleers T, de Graaf DC. 2014. Widespread occurrence of honey bee pathogens in solitary bees. J. Invert. Pathol. 122, 55–58. ( 10.1016/j.jip.2014.08.007) [DOI] [PubMed] [Google Scholar]
- 28.Fürst MA, McMahon DP, Osborne JL, Paxton RJ, Brown MJF. 2014. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 506, 364–366. ( 10.1038/nature12977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Miranda JR, Genersch E. 2010. Deformed wing virus. J. Invert. Pathol. 103, S48–S61. ( 10.1016/j.jip.2009.06.012) [DOI] [PubMed] [Google Scholar]
- 30.Iqbal J, Mueller U. 2007. Virus infection causes specific learning deficits in honeybee foragers. Proc. R. Soc. B 274, 1517–1521. ( 10.1098/rspb.2007.0022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah KS, Evans EC, Pizzorno MC. 2009. Localization of deformed wing virus (DWV) in the brains of the honeybee, Apis mellifera Linnaeus. Virol. J. 6, 182 ( 10.1186/1743-422x-6-182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Natsopoulou ME, McMahon DP, Paxton RJ. 2016. Parasites modulate within-colony activity and accelerate the temporal polyethism schedule of a social insect, the honey bee. Behav. Ecol. Sociobiol. 70, 1019–1031. ( 10.1007/s00265-015-2019-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells T, Wolf S, Nicholls E, Groll H, Lim KS, Clark SJ, Swain J, Osborne JL, Haughton AJ. 2016. Flight performance of actively foraging honey bees is reduced by a common pathogen. Envrion. Microb. Rep. 8, 728–737. ( 10.1111/1758-2229.12434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf S, Nicholls E, Reynolds AM, Wells P, Lim KS, Paxton RJ, Osborne JL. 2016. Optimal search patterns in honeybee orientation flights are robust against emerging infectious diseases. Sci. Rep. 6, 32612 ( 10.1038/srep32612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider CW, Tautz J, Gruenewald B, Fuchs S. 2012. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS ONE 7, e30023 ( 10.1371/journal.pone.0030023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molet M, Chittka L, Stelzer RJ, Streit S, Raine NE. 2008. Colony nutritional status modulates worker responses to foraging recruitment pheromone in the bumblebee Bombus terrestris. Behav. Ecol. Sociobiol. 62, 1919–1926. ( 10.1007/s00265-008-0623-3) [DOI] [Google Scholar]
- 37.Decourtye A, Devillers J, Aupinel P, Brun F, Bagnis C, Fourrier J, Gauthier M. 2011. Honeybee tracking with microchips: a new methodology to measure the effects of pesticides. Ecotoxicology 20, 429–437. ( 10.1007/s10646-011-0594-4) [DOI] [PubMed] [Google Scholar]
- 38.Gill RJ, Raine NE. 2014. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Funct. Ecol. 28, 1459–1471. ( 10.1111/1365-2435.12292) [DOI] [Google Scholar]
- 39.Thompson H, Coulson M, Ruddle N, Wilkins S, Harkin S. 2016. Thiamethoxam: assessing flight activity of honeybees foraging on treated oilseed rape using radio frequency identification technology. Environ. Toxicol. Chem. 35, 385–393. ( 10.1002/etc.3183) [DOI] [PubMed] [Google Scholar]
- 40.Fischer J, Müller T, Spatz A-K, Greggers U, Gruenewald B, Menzel R. 2014. Neonicotinoids interfere with specific components of navigation in honeybees. PLoS ONE 9, e91364 ( 10.1371/journal.pone.0091364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naug D. 2009. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 142, 2369–2372. ( 10.1016/j.biocon.2009.04.007) [DOI] [Google Scholar]
- 42.Feigenbaum C, Naug D. 2010. The influence of social hunger on food distribution and its implications for disease transmission in a honeybee colony. Insect. Soc. 57, 217–222. ( 10.1007/s00040-010-0073-6) [DOI] [Google Scholar]
- 43.Li Z, et al. 2013. Viral infection affects sucrose responsiveness and homing ability of forager honey bees, Apis mellifera L. PLoS ONE 8, e77354 ( 10.1371/journal.pone.0077354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lach L, Kratz M, Baer B. 2015. Parasitized honey bees are less likely to forage and carry less pollen. J. Invert. Pathol. 130, 64–71. ( 10.1016/j.jip.2015.06.003) [DOI] [PubMed] [Google Scholar]
- 45.Wolf S, McMahon DP, Lim KS, Pull CD, Clark SJ, Paxton RJ, Osborne JL. 2014. So near and yet so far: harmonic radar reveals reduced homing ability of Nosema infected honeybees. PLoS ONE 9, e0103989 ( 10.1371/journal.pone.0103989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Smet L, Ravoet J, de Miranda JR, Wenseleers T, Mueller MY, Moritz RFA, de Graaf DC. 2012. BeeDoctor, a versatile MLPA-based diagnostic tool for screening bee viruses. PLoS ONE 7, e47953 ( 10.1371/journal.pone.0047953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seeley TD. 2009. The wisdom of the hive: the social physiology of honey bee colonies. Cambridge, MA: Harvard University Press. [Google Scholar]
- 48.Möckel N, Gisder S, Genersch E. 2011. Horizontal transmission of deformed wing virus: pathological consequences in adult bees (Apis mellifera) depend on the transmission route. J. Gen. Virol. 92, 370–377. ( 10.1099/vir.0.025940-0) [DOI] [PubMed] [Google Scholar]
- 49.Desai SD, Eu YJ, Whyard S, Currie RW. 2012. Reduction in deformed wing virus infection in larval and adult honey bees (Apis mellifera L.) by double-stranded RNA ingestion. Insect Mol. Biol. 21, 446–455. ( 10.1111/j.1365-2583.2012.01150.x) [DOI] [PubMed] [Google Scholar]
- 50.Maori E, Paldi N, Shafir S, Kalev H, Tsur E, Glick E, Sela I. 2009. IAPV, a bee-affecting virus associated with colony collapse disorder can be silenced by dsRNA ingestion. Insect Mol. Biol. 18, 55–60. ( 10.1111/j.1365-2583.2009.00847.x) [DOI] [PubMed] [Google Scholar]
- 51.Martin SJ, Highfield AC, Brettell L, Villalobos EM, Budge GE, Powell M, Nikaido S, Schroeder DC. 2012. Global honey bee viral landscape altered by a parasitic mite. Science 336, 1304–1306. ( 10.1126/science.1220941) [DOI] [PubMed] [Google Scholar]
- 52.Mordecai GJ, Wilfert L, Martin SJ, Jones IM, Schroeder DC. 2016. Diversity in a honey bee pathogen: first report of a third master variant of the deformed wing virus quasispecies. ISME J. 10, 1264–1273. ( 10.1038/ismej.2015.178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Geystelen A, Benaets K, de Graaf DC, Larmuseau MHD, Wenseleers T. 2015. Track-a-Forager: a program for the automated analysis of RFID tracking data to reconstruct foraging behaviour. Insect. Soc. 63, 175–183. ( 10.1007/s00040-015-0453-z) [DOI] [Google Scholar]
- 54.Winston ML. 1987. The biology of the honey bee. Cambridge, MA: Harvard University Press. [Google Scholar]
- 55.Tenczar P, Lutz CC, Rao VD, Goldenfeld N, Robinson GE. 2014. Automated monitoring reveals extreme interindividual variation and plasticity in honeybee foraging activity levels. Anim. Behav. 95, 41–48. ( 10.1016/j.anbehav.2014.06.006) [DOI] [Google Scholar]
- 56.Tofilski A. 2009. Shorter-lived workers start foraging earlier. Insect. Soc. 56, 359–366. ( 10.1007/s00040-009-0031-3) [DOI] [Google Scholar]
- 57.Goblirsch M, Huang ZY, Spivak M. 2013. Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLoS ONE 8, e0058165 ( 10.1371/journal.pone.0058165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dussaubat C, Maisonnasse A, Crauser D, Beslay D, Costagliola G, Soubeyrand S, Kretzchmar A, Le Conte Y. 2013. Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J. Invert. Pathol. 113, 42–51. ( 10.1016/j.jip.2013.01.002) [DOI] [PubMed] [Google Scholar]
- 59.Bailey L, Fernando EFW. 1972. Effects of sacbrood virus on adult honey-bees. Ann. Appl. Biol. 72, 27–35. ( 10.1111/j.1744-7348.1972.tb01268.x) [DOI] [Google Scholar]
- 60.Downey DL, Higo TT, Winston ML. 2000. Single and dual parasitic mite infestations on the honey bee, Apis mellifera L. Insect. Soc. 47, 171–176. ( 10.1007/pl00001697) [DOI] [Google Scholar]
- 61.Janmaat AF, Winston ML. 2000. The influence of pollen storage area and Varroa jacobsoni Oudemans parasitism on temporal caste structure in honey bees (Apis mellifera L.). Insect. Soc. 47, 177–182. ( 10.1007/pl00001698) [DOI] [Google Scholar]
- 62.Cardoen D, Ernst UR, Boerjan B, Bogaerts A, Formesyn E, de Graaf DC, Wenseleers T, Schoofs L, Verleyen P. 2012. Worker honeybee sterility: a proteomic analysis of suppressed ovary activation. J. Proteom Res. 11, 2838–2850. ( 10.1021/pr201222s) [DOI] [PubMed] [Google Scholar]
- 63.Perry CJ, Søvik E, Myerscough MR, Barron AB. 2015. Rapid behavioral maturation accelerates failure of stressed honey bee colonies. Proc. Natl Acad. Sci. USA 112, 3427–3432. ( 10.1073/pnas.1422089112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khoury DS, Myerscough MR, Barron AB. 2011. A quantitative model of honey bee colony population dynamics. PLoS ONE 6, e18491 ( 10.1371/journal.pone.0018491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woyciechowski M, Moroń D. 2009. Life expectancy and onset of foraging in the honeybee (Apis mellifera). Insect. Soc. 56, 193–201. ( 10.1007/s00040-009-0012-6) [DOI] [Google Scholar]
- 66.Schmickl T, Crailsheim K. 2004. Inner nest homeostasis in a changing environment with special emphasis on honey bee brood nursing and pollen supply. Apidologie 35, 249–263. ( 10.1051/apido:2004019) [DOI] [Google Scholar]
- 67.Yue C, Schroder M, Gisder S, Genersch E. 2007. Vertical-transmission routes for deformed wing virus of honeybees (Apis mellifera). J. Gen. Virol. 88, 2329–2336. ( 10.1099/vir.0.83101-0) [DOI] [PubMed] [Google Scholar]
- 68.de Miranda JR, Fries I. 2008. Venereal and vertical transmission of deformed wing virus in honeybees (Apis mellifera L.). J. Invert. Pathol. 98, 184–189. ( 10.1016/j.jip.2008.02.004) [DOI] [PubMed] [Google Scholar]
- 69.Chen YP, Higgins JA, Feldlaufer MF. 2005. Quantitative real-time reverse transcription-PCR analysis of deformed wing virus infection in the honeybee (Apis mellifera L.). Appl. Environ. Microbiol. 71, 436–441. ( 10.1128/aem.71.1.436-441.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tentcheva D, Gauthier L, Bagny L, Fievet J, Dainat B, Cousserans F, Colin ME, Bergoin M. 2006. Comparative analysis of deformed wing virus (DWV) RNA in Apis mellifera and Varroa destructor. Apidologie 37, 41–50. ( 10.1051/apido:2005057) [DOI] [Google Scholar]
- 71.Yue C, Genersch E. 2005. RT-PCR analysis of deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J. Gen. Virol. 86, 3419–3424. ( 10.1099/vir.0.81401-0) [DOI] [PubMed] [Google Scholar]
- 72.Fujiyuki T, Matsuzaka E, Nakaoka T, Takeuchi H, Wakamoto A, Ohka S, Sekimizu K, Nomoto A, Kubo T. 2009. Distribution of kakugo virus and its effects on the gene expression profile in the brain of the worker honeybee Apis mellifera L. J. Virol. 83, 11 560–11 568. ( 10.1128/jvi.00519-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujiyuki T, Takeuchi H, Ono M, Ohka S, Sasaki T, Nomoto A, Kubo T. 2004. Novel insect picorna-like virus identified in the brains of aggressive worker honeybees. J. Virol. 78, 1093–1100. ( 10.1128/jvi.78.3.1093-1100.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fujiyuki T, Takeuchi H, Ono M, Ohka S, Sasaki T, Nomoto A, Kubo T. 2005. Kakugo virus from brains of aggressive worker honeybees. Adv. Virus Res. 65, 1–27. ( 10.1016/s0065-3527(05)65001-4) [DOI] [PubMed] [Google Scholar]
- 75.Shen MQ, Yang XL, Cox-Foster D, Cui LW. 2005. The role of varroa mites in infections of Kashmir bee virus (KBV) and deformed wing virus (DWV) in honey bees. Virology 342, 141–149. ( 10.1016/j.virol.2005.07.012) [DOI] [PubMed] [Google Scholar]
- 76.Mayack C, Naug D. 2009. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invert. Pathol. 100, 185–188. ( 10.1016/j.jip.2008.12.001) [DOI] [PubMed] [Google Scholar]
- 77.Naug D, Gibbs A. 2009. Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie 40, 595–599. ( 10.1051/apido/2009039) [DOI] [Google Scholar]
- 78.Hunter W, et al. 2010. Large-scale field application of RNAi technology reducing Israeli acute paralysis virus disease in honey bees (Apis mellifera, Hymenoptera: Apidae). PLoS Pathog. 6, e1001160 ( 10.1371/journal.ppat.1001160) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data files and code used in analyses are available in Dryad: http://dx.doi.org/10.5061/dryad.fm0r1. Illumina reads and the full genome sequence of the DWV inoculate are available from the Sequence Read Archive and GenBank (accession nos. PRJNA336281 and KX783225).