Abstract
White-nose syndrome (WNS) is a fungal disease responsible for decimating many bat populations in North America. Pseudogymnoascus destructans (Pd), the psychrophilic fungus responsible for WNS, prospers in the winter habitat of many hibernating bat species. The immune response that Pd elicits in bats is not yet fully understood; antibodies are produced in response to infection by Pd, but they may not be protective and indeed may be harmful. To understand how bats respond to infection during hibernation, we studied the effect of Pd inoculation on the survival and gene expression of captive hibernating Myotis lucifugus with varying pre-hibernation antifungal antibody titres. We investigated gene expression through the transcription of selected cytokine genes (Il6, Il17a, Il1b, Il4 and Ifng) associated with inflammatory, Th1, Th2 and Th17 immune responses in wing tissue and lymph nodes. We found no difference in survival between bats with low and high anti-Pd titres, although anti-Pd antibody production during hibernation differed significantly between infected and uninfected bats. Transcription of Il6 and Il17a was higher in the lymph nodes of infected bats compared with uninfected bats. Increased transcription of these cytokines in the lymph node suggests that a pro-inflammatory immune response to WNS is not restricted to infected tissues and occurs during hibernation. The resulting Th17 response may be protective in euthermic bats, but because it may disrupt torpor, it could be detrimental during hibernation.
Keywords: bats, wildlife disease, fungal infection, cytokines, gene expression, Pseudogymnoascus destructans
1. Introduction
The emergence of a new disease most frequently results from a change in the ecology of the host, pathogen or environment, or an interaction of all three [1]. A classic tenet of disease ecology is that disease is not simply a factor of the host's resilience and resistance, but rather occurs at the intersection of host dynamics, properties of the pathogen and environmental conditions (i.e. the disease triangle) [2]. White-nose syndrome (WNS), the epizootic disease caused by a fungus, has killed millions of North American bats since 2007 [3]. In what is often described as a ‘perfect storm’, WNS is deadly due to the negative synergism among susceptible hosts (hibernating bats) that have limited opportunity to respond to infections during hibernation, a cold-loving fungal pathogen Pseudogymnoascus destructans (hereafter Pd) that invades and destroys the skin, and the cool, humid environment in which the host and pathogen reside [4–6]. While Palaearctic bats in Europe and Asia have probably coexisted with Pd for several thousand years [7,8], the fungus has only recently spread to North America via anthropogenic introduction, causing mass mortality. Predictions of regional or range-wide extinction of once common species highlight the need to understand the mechanisms behind survival and mortality of North American bats infected with Pd [9].
A comprehensive understanding of the physiological responses of the host is pivotal to decoding the factors contributing to WNS. In general, fungal species are unable to cause severe diseases in mammals due to the high body temperature and effective immune system of the host [10]. However, Pd is successful at invading the epidermis and adnexa of many North American bats because infection occurs during hibernation, when body temperature is significantly lowered [11] and some immune responses are presumably downregulated [12]. Infection causes cupping erosions on wing membranes, muzzles and ears [13]. The invasive cutaneous mycosis [14] causes alterations in hibernating behaviour [5,15,16], physiology [17–19] and immune function [20]. These result in increased frequency of arousals from hibernation in WNS-affected little brown myotis (Myotis lucifugus) [16,21] and cause mortality by depleting the fat reserves necessary for survival before winter is over [5,15].
Hosts must mount an appropriate immune response to pathogens to avoid reductions in fitness or survival rates. In a previous study, we found that little brown myotis affected by WNS detect fungal infection within epidermal tissue and activate an innate immune response that includes local inflammation [20]. To generate the appropriate adaptive response, antigens should then be transmitted by activated dendritic cells to the regional lymph node where inflammatory cytokines should help generate an appropriate T-cell response. One possible outcome is a humoral adaptive response involving the selection and activation of B cells to generate antibodies specific to fungal antigens. Antibodies that can bind to Pd have been detected in North American and European species of bats [22], suggesting a humoral immune response might be used during fungal infection. However, we found that although North American and European bat species in the wild vary in their levels of antibodies that recognize Pd, titres do not correlate with either protection or susceptibility to WNS [23]. We have also shown that some North American bats that had not yet been exposed to Pd have antibodies that are cross-reactive to Pd [22], presumably stimulated in response to another fungus. By contrast, some European bats appear to have relatively low levels of anti-Pd antibodies despite endemic Pd exposure, suggesting that their low antibody titres are beneficial and that a significant humoral responses to Pd may indeed be maladaptive [22].
We propose that the limited resources available to mount an immune response during hibernation represent a bottleneck to WNS survival. Antibody-mediated responses to fungal pathogens are more often associated with immune pathology instead of protection [24]. Conversely, other T-cell-mediated immune responses are more typically associated with controlling fungal infections in humans and other mammals [23]. These adaptive immune responses are regulated by the cytokines produced by different subsets of T cells. Antibody responses are driven by T helper 2 (Th2) immune responses, including the cytokine interleukin 4 (IL-4) [25], but protective antifungal immune responses are more likely to be driven by different T-cell subsets, either Th1 or Th17 cells [26,27]. The cytokines interleukin 6 (IL-6) and interleukin 17A (IL-17A), produced by Th17 cells, are key mediators of antifungal immunity, at least in most tissues in humans and mice [28]. Thus, understanding host immune response to pathogens involves both identifying the type of immune responses exhibited in an infected population, as well as experimentally evaluating the physiological consequences of those responses.
Because mounting an appropriate immune response to fungal pathogens is important to host survival, the downregulation of immune function during the hibernation period has been cited as playing a major role in WNS [13]. Suppression of certain immune responses is seen as a major physiological trade-off of torpor [12], and has been clearly demonstrated for antibody responses to T-cell-dependent antigens, but not T-cell-independent antigens, in hibernating 13-lined ground squirrels [29]. Antibody responses to the Pd cell wall are likely to be T-cell independent [30] and we wanted to test whether hibernation prevents the amplification of this response in bats. Our previous work has shown that hibernation does not prevent inflammatory responses to Pd in the infected tissue [20]. However, it is not known whether that inflammatory response can be transmitted to the local lymphoid tissue during hibernation and whether it can generate an appropriate adaptive response regionally. We wanted to test whether the cytokine expression that we had previously detected in the infected tissue (including IL-6, IL-23 and IL-17C) [20] generated the expected antifungal Th17 response in the regional lymphoid tissue.
We tested the hypothesis that anti-Pd humoral responses may result in differences in susceptibility to WNS by being either harmful or protective. Bats with higher titres may be pre-disposed to a humoral response to Pd, at the expense of a response that is more likely to be protective. We also further studied responses to Pd-infection during hibernation by looking at the expression of inflammatory and immunoregulatory cytokines associated with Th1, Th2 and Th17 responses in two relevant tissues: the wing tissue, showing responses at the site of infection, and the axillary lymph nodes that drain the wing tissue, where adaptive immune responses to the infection will be coordinated. We predicted that (i) infected bats with high pre-hibernation anti-Pd titres will exhibit lower survival compared with infected bats with low titre. We also predicted that (ii) infected bats with high titres will have a greater Th2 than Th1 or Th17 signature compared with bats with low titres and (iii) hibernation will not prevent the amplification of a humoral response or the generation of a Th1, Th2 or Th17 response in the regional lymph node.
2. Material and methods
(a). Bat capture and sampling protocols
We established a captive population of M. lucifugus naive to Pd in August 2014, with bats caught during swarming at a major hibernaculum in Greenland, MI, USA. After capture with harp traps, bats were sexed, aged (juvenile/adult), weighed and banded. Wings were swabbed with dry cotton swabs for analysis by quantitative-PCR (qPCR) to confirm negative Pd-status [31]. After swabbing, bats were placed in individual cheesecloth bags and transported to Bucknell University. After 4 days of acclimatization, we punctured a vein in the uropatagium (tail wing membrane) using a 27.5-gauge sterile needle [32] and collected blood into heparinized, microhematocrit, capillary tubes (Kimball Chase Life Science, Vineland, NJ, USA). The contents of the capillary tubes were immediately centrifuged to separate plasma from blood cells; plasma was flash frozen and stored at –80°C. Titres of anti-Pd antibody were measured in triplicate following Johnson et al. [22].
(b). Experimental hibernation procedures
Based upon measured anti-Pd antibody titres, we placed bats into one of two treatment groups—high (mean 43.0 ± 96.3) or low titre (mean 10.0 ± 0.6)—which were further subdivided into control and infected groups resulting in four groups for the experiment: (i) high infected, (ii) low infected, (iii) high control and (iv) low control. Two replicate cages were used for each treatment. To the extent possible, we randomly selected an equal number of males and females to be placed into each treatment group (n = 14–15 bats per group) with a matching distribution of body masses in each group. To assess hibernation behaviour, a temperature data logger (WeeTag, Alpha Mach Inc, Ste-Julie, QC, Canada) was attached with a bonding agent (Torbot, Cranston, RI, USA) after hair between the shoulder blades was trimmed. Loggers recorded skin temperature (Tsk) every 10 min. Each bat was then either sham inoculated (control groups) with 50 µl of phosphate-buffered saline with 0.05% Tween-20 (PBST) or inoculated (infected groups) with 5000 conidia of Pd, suspended in 50 µl PBST [31]. The solution was dispensed onto the ventral surface of one wing below the wrist, and distributed along the wing by gentle manipulation of the wing [21,22,31].
All infected bats were inoculated, temperature loggers were attached and animals were placed into low-temperature environmental chambers (Percival model no. I36VLC8) on 26 September 2014. Bats in each treatment group were housed in separate, open-air, aluminium cages (two cages per treatment; Zoo Med Laboratories, San Luis Obispo, CA, USA). Cage effect did not confound the main effects in the statistical models, and we were able to use individual bats as experimental units for our analyses. Each cage was provided with ad libitum water and placed into environmental chambers set to maintain a constant temperature of 4°C and greater than or equal to 90% relative humidity. Control and inoculated treatments were housed in separate environmental chambers, with replicates of each treatment housed in the same chamber, to ensure that no Pd transmission can occur between cages. Moreover, cages were not in contact, also preventing Pd transmission between cages [4]. Data loggers recording temperature and relative humidity (TransiTemp II, MadgeTech, Warner, NH, USA) were placed inside each environmental chamber to confirm environmental conditions. To avoid disturbance and unnatural arousals from hibernation, chambers were opened to provide freshwater and remove moribund bats only once every three weeks. Activity of bats during hibernation was monitored with infrared cameras placed inside the chambers.
(c). Post-hibernation procedures
At 16 weeks post-exposure (3 February 2015), we sampled the surviving bats. Cages were removed from environmental chambers 20 min prior to the start of euthanasia to allow all animals to rewarm from torpor and reach euthermic body temperature. For each individual, time between removal of cage from the chamber and the time that a bat was euthanized was recorded (range = 20–118 min, mean = 69 min). Individual bats were removed from their cage and immediately swabbed on the wing tissue for qPCR analysis identifying DNA from Pd, and the wings were photographed with UV transillumination to assess the intensity of infection with Pd [33]. We then recorded mass and euthanized bats using isoflurane followed by decapitation. A sample of whole blood was immediately collected into 75 µl heparinized capillary tubes. Blood samples were processed as described above. The remaining carcass was dissected immediately and the wing tissue and lymph nodes stored in RNAlater. Post-hibernation anti-Pd antibody titres were measured in triplicate as described in bat capture and sampling protocols. Infection status of individuals was confirmed with Pd-specific qPCR [31] and by analysis of UV images. Fresh samples are needed for RNA extraction and antibody titre measurements, thus bats found dead prior to the end of the 16-week hibernation period were not used for further analyses.
(d). RNA extraction and quantitative-PCR analysis
RNA from wing and lymph node tissues was extracted using RNA isolation kits (Qiagen, Redwood, CA, USA) with an on-column DNase I treatment (details provided in the electronic supplementary material, S1). PCR primers for the cytokine genes interleukin 6 (Il6) and the reference gene, ancient ubiquitous protein 1 (aup1), were designed based on the transcriptome assembly generated in [20]. Primers for interleukin 1b (Il1b), interleukin 4 (Il4), interleukin 17A (Il17a) and interferon-γ (ifng) transcripts were designed based on M. lucifugus genome sequence (Ensembl). Primer details and amplification efficiencies are reported in the electronic supplementary material, S2. qPCR assays were run on duplicates for each gene and sample on a LightCycler 96 instrument (Roche, Basel, Switzerland) with Fast Start Essential DNA Green Master (Roche) (details provided in the electronic supplementary material, S1).
(e). Data analyses
(i). Arousal frequency, scaled mass index and survival analyses
Arousals from torpor were determined for each individual using temperature logger data with runlength encoding and a custom function (ArousalFinder) in RStudio v. 0.99.879 (R Core Team, 2015) as described in [34]. The date of death for each individual was determined as the point of last arousal or, for survivors, as the day of sampling after hibernation. Arousal frequency was determined as the probability of bat arousal on a given day post Pd-exposure (number of arousals/number of days between first and last arousal) [34].
A scaled mass index (SMI; pre-hibernation = mass × (mean forearm length/forearm length)0.02029, post-hibernation = mass × (mean forearm length/forearm length)0.1462) was calculated as suggested in [35]. Differences in survival between groups were estimated with function Survfit in R based on Cox's non-parametric hazard model [36].
(f). Statistical analyses
All analyses were conducted in R v. 3.2.1 (R Core Team, 2015). The differences in anti-Pd titres between September and February within survivors were tested using paired, one-sided Wilcoxon's tests.
Differences in post-hibernation titre and mRNA level of log-transformed Il6 and Il1b in the wing tissue and Il6, Il17a, Il1b and Il4 in the lymph node tissue were analysed using analysis of variance (ANOVA) in R. The main effects in the initial model for post-hibernation titre included pre-hibernation titre (high and low group), infection status (infected or control) and their interaction. As covariates, we included the proportion of body mass lost during hibernation (δSMI = (pre-hibernation SMI − post-hibernation SMI)/pre-hibernation SMI), time to euthanasia (i.e. minutes after removing the cage from the hibernation chamber until euthanasia) and the interaction of each of the covariates with the main effects. Proportion of SMI lost during hibernation partially reflects water loss due to evaporation, which was expected to increase plasma concentration of anti-Pd antibodies. Time to euthanasia indicates the time that the bat has spent at euthermic body temperature before euthanasia, which was predicted to be reflected in the immune response. We excluded the interactions of infection status and proportion SMI lost as well as infection status and time to euthanasia from the model because they were not significant. Tukey's honestly significant differences (HSD) test from package agricolae was used for post hoc pairwise comparisons of post-hibernation anti-Pd titres. We ran the test between infected and uninfected bats from low and high pre-hibernation titre groups, grouping the individuals into four groups, because we found a significant interaction of these effects in the ANOVA. The model included the interactions of pre-hibernation titre and time to euthanasia, and pre-hibernation titre and proportion SMI lost, as well as proportion SMI lost and time to euthanasia, as in the initial ANOVA.
Because pre-hibernation titre was not associated with survival, we only included the main effect of infection group in the gene expression models. We predicted that the time to euthanasia could affect the activation of gene expression, but after we found that neither it nor the interaction between time to euthanasia and infection group was significant in the models, we excluded it from the models. Proportion SMI lost during hibernation was included in all gene expression models, because it had a significant effect on two of the genes. The interaction of proportion SMI lost and infection was not significant in any model, and was therefore excluded. We chose to use proportion of SMI lost in the final models because it is more directly related to fat consumed by the bat during the hibernation period than the SMI after hibernation. The residuals of the ANOVAs conformed to a normal distribution based on qqplots, and had non-significant heteroscedasticity based on the Fligner–Killeen test. All ANOVAs were performed using function lm and type II sums of squares were estimated using function ANOVA from package car [37]. Significance was determined based on α of 0.05. When the covariates were significant in the model, we calculated Pearson's correlation coefficients between the covariate and the dependent variable.
Alternative models for all variables were built using post-hibernation SMI instead of proportion SMI lost as the covariate. To ensure that potential cage effects did not bias our results, we additionally analysed the post-hibernation titre and the log-transformed gene expression values by maximum-likelihood linear mixed models (lmer, package lme4) [38] treating cage as the random effect. The results were visualized using R base graphics and ggplot2 [39].
3. Results
To determine if pre-existing antibodies to Pd had an effect on WNS survival, we compared mortality rates during the captive infection experiment. Infected bats had higher mortality than control bats not exposed to Pd (p = 0.001; electronic supplementary material, S3). The expected mortality of infected bats was, on average, 2.2-fold higher than in the control bats (95% confidence intervals 1.2 and 4.1). Neither pre-hibernation SMI nor arousal frequency had a significant effect on the observed mortality of infected or uninfected bats (table 1).
Table 1.
Details on the number of individuals (males /females) and mean ± standard deviation of body condition index (SMI), anti-Pd titres before and after hibernation, and arousal frequency during hibernation, grouped by treatment and titre level before hibernation.
| experimental group | N (M/F) | N survivors (M/F) | SMI pre-hib. | SMI post-hib. | arousals per day | pre-hib. titre | post-hib. titre |
|---|---|---|---|---|---|---|---|
| control low | 11/12 | 6/9 | 10.22 (±0.89) | 6.97 (±0.80) | 0.21 (±0.05) | 9.99 (±1.72) | 13.33 (±4.75) |
| control high | 13/11 | 3/5 | 10.20 (±1.03) | 6.45 (±0.63) | 0.23 (±0.07) | 44.45 (±64.15) | 21.37 (±12.20) |
| infected low | 11/12 | 2/4 | 10.36 (±0.86) | 7.04 (±1.06) | 0.22 (±0.05) | 9.81 (±2.00) | 12.88 (±4.04) |
| infected high | 13/12 | 2/6 | 10.09 (±0.87) | 6.91 (±0.86) | 0.23 (±0.06) | 26.39 (±17.15) | 67.66 (±62.11) |
Next we examined how exposure to Pd influenced anti-Pd antibody levels. Despite not affecting survival, anti-Pd antibodies post-hibernation were significantly higher than pre-hibernation in bats that survived hibernation in all four experimental groups (Wilcoxon's test, control low p < 0.001, control high p = 0.004, infected low p = 0.017, infected high p = 0.016, figure 1). The 6.7 ± 5.0-fold increase in titre for bats not infected with Pd was presumably due to physiological changes that occur during hibernation (dehydration) or exposure to other fungal antigens. Titres were highest after hibernation in infected bats that were assigned to the high-titre group pre-hibernation (table 2, figure 1) and increased 5.8 ± 2.6-fold in this group. We also found a significant interaction explaining post-hibernation antibody titres between high/low-titre assignment and post-hibernation time to euthanasia (table 2); antibody titres decreased in high bats (both infected and control) the longer they were aroused before euthanasia, but remained low in low bats. A significant interaction explaining post-hibernation antibody titres between high/low-titre assignment and proportion body mass lost during hibernation was also found (table 2), but the interaction between high/low-titre assignment and post-hibernation body condition was not significant in the alternative model (electronic supplementary material, S4).
Figure 1.
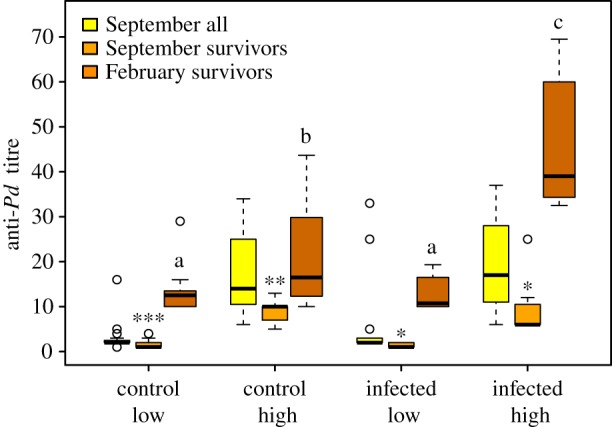
Anti-Pd titres of M. lucifugus before hibernation (September) and after hibernation (February). Asterisks indicate significant differences between September and February titres within survivor bats (paired Wilcoxon's tests), and letters indicate differences between groups in titres measured in February (Tukey's test). N = 6–14 per group (survivors) and 23–25 per group (all individuals.) *p = 0.01–0.05, **p = 0.001–0.05, ***p < 0.001. (Online version in colour.)
Table 2.
Significant effects for the post-hibernation titre and relative mRNA expression of cytokine genes with type II sums of squares. Estimates and standard errors for the main effects were obtained from linear model using function lm in R. Full models and model selection in electronic supplementary material, S4. *p = 0.01–0.05; **p = 0.001–0.05; ***p < 0.001.
| response variable | independent variables | type II sum sq. | d.f. | F | estimate ± s.e. | Pr(>F) |
|---|---|---|---|---|---|---|
| February anti-Pd titre | pre-hibernation titre (high versus low) | 1204.76 | 1 | 59.08 | 1.68 ± 14.23 | 6.36 × 10−8*** |
| infection | 772.26 | 1 | 37.87 | 28.45 ± 2.92 | 2.34 × 10−6*** | |
| time to euthanasia | 114.78 | 1 | 5.63 | −0.39 ± 0.07 | 2.60 × 10−2* | |
| pre-hibernation titre (high versus low) × Infection | 1165.77 | 1 | 57.16 | −27.79 ± 3.68 | 8.45 × 10−8*** | |
| pre-hibernation titre (high versus low) × Proportion SMI lost | 132.51 | 1 | 6.50 | −85.54 ± 33.56 | 1.76 × 10−2* | |
| pre-hibernation titre (high versus low) × Time to euthanasia | 574.26 | 1 | 28.16 | 0.41 ± 0.08 | 1.92 × 10−5*** | |
| residuals | 489.45 | 24 | ||||
| interleukin-6, wing | infection | 4.72 | 1 | 6.50 | 0.77 ± 0.3 | 1.60 × 10−2* |
| proportion SMI lost | 4.86 | 1 | 6.69 | −7.23 ± 2.8 | 1.46 × 10−2* | |
| residuals | 22.54 | 31 | ||||
| interleukin-1β, wing | proportion SMI lost | 3.58 | 1 | 9.36 | −5.73 ± 1.87 | 4.54 × 10−3** |
| residuals | 11.87 | 31 | ||||
| interleukin-6, lymph node | infection | 1.65 | 1 | 4.68 | 0.50 ± 0.24 | 4.02 × 10−2* |
| residuals | 8.81 | 25 | ||||
| interleukin 17A, lymph node | infection | 8.25 | 1 | 7.99 | 1.11 ± 0.39 | 9.10 × 10−3** |
| residuals | 25.80 | 25 |
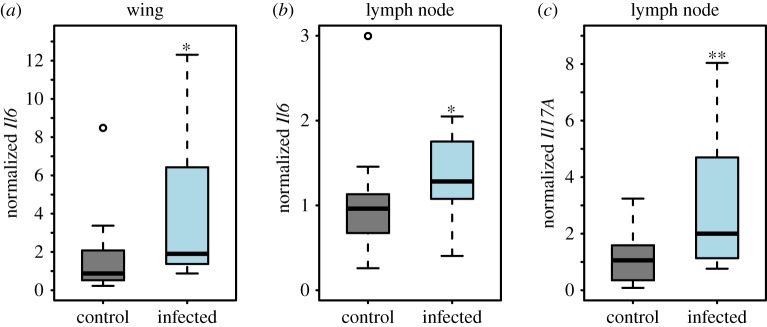
Given that an antibody-mediated immune response does not appear to be protective or pathological [22], we next wanted to examine whether the local immune response in the wing tissue is generating the expected response in the regional lymph node. Expression was compared between bats exposed to Pd and uninfected bats for five cytokines in the wing tissue and the axillary lymph nodes. Of the five cytokines (Il4, Il1b, Il17, Il6 and ifng) that we examined, we found increased expression in bats with WNS for Il6 in the wing tissue and lymph node (figure 2a,b and table 2) and for Il17a in the lymph nodes (figure 2c and table 2). We did not find significant WNS-associated increases for the other immunoregulatory cytokines that we examined, in either the lymph nodes or the wing tissue.
Figure 2.
Cytokine gene expression normalized to Aup1 and to the low-titre control group in control and infected bats. (a) Il6 expression in the wing tissue, N = 21 (control) and 12 (infected), (b) Il6 and (c) Il17a mRNA expression in the axillary lymph nodes. N = 16 (control Il6), 18 (control Il17a) and 12 (infected). *p = 0.01–0.05, **p = 0.001–0.05. (Online version in colour.)
To assess whether the elevated levels of inflammatory cytokine gene expression that we observed might influence WNS pathology, we determined whether cytokine gene expression correlated with other physiological measurements, such as hibernation behaviour. However, we found no statistically significant difference in arousal frequency between treatment and control groups, thus making the correlation with cytokine expression uninformative. Previous hibernation experiments of similar duration have also shown no differences in arousal frequency between infected and uninfected bats [21]. We used the proportion of SMI lost during hibernation as a measure of a loss of body condition during hibernation [40]. We found a significant negative correlation between wing tissue Il6 and Il1b mRNA expression and proportion SMI lost during hibernation (p = 0.011 and 0.004; electronic supplementary material, S5). In summary, a greater decline in mass during hibernation (and, presumably, lower likelihood of survival) correlated with lower Il6 and Il1b expression in the wing.
We found a strong, negative correlation between post-hibernation SMI and proportion SMI lost during hibernation (Pearson's r = −0.72, t = −5.9935, d.f. = 33, p-value = 4.9 × 10−7). Consequently, the results of models using the former instead of the latter as a covariate produced similar results for the gene expression models (electronic supplementary material, S3). Thus, bat body condition at the end of hibernation reflects the amount of body mass lost during hibernation, and both correlate with cell-mediated immune responses (table 2; electronic supplementary material, S6).
We also built alternative models including the replicate cage as random effects, and found that the main effects of linear mixed models agreed well with the linear models (table 2; electronic supplementary material, S7).
4. Discussion
Our experiment elucidates the underlying immune responses of hibernating M. lucifugus to the fungal pathogen, P. destructans. Owing to our previous observation of relatively low anti-Pd antibody levels among European bats resistant to WNS, we predicted higher mortality in bats naive to Pd with higher pre-hibernation titres of antibodies that were cross-reactive with Pd. However, we observed no difference in survival between these groups. This experimentally confirms that antibodies play no causative role in either a protection or pathology in WNS, as previously suggested by the lack of correlation found in wild bats [22]. We then further examined transcription levels of Il17a, Il4, Il6, Il1b and ifng to determine whether a T-cell response (Th17, Th2 or Th1) occurs during Pd infection in the winter. We found that Pd infection induced the expression of Il6, an inflammatory cytokine that can be associated with a Th17 response, in the wing tissue. This observation is consistent with our previous transcriptomic study [20] and indicates that a local inflammatory immune response occurs at the site of infection. We also examined cytokine gene expression in the lymph nodes that drained the infected tissue to determine if either hibernation or Pd infection prevents the subsequent generation of a regional T-cell response. We found increased expression of Il6 and Il17a in the axillary lymph nodes in infected compared to uninfected bats, demonstrating the initiation of a Th17 response. This observation reveals that hibernation (i.e. torpor and arousals during overwintering) does not prevent regional immune responses, although it remains to be determined whether this response occurs during torpor or is restricted to periodic euthermic arousals.
The results from our experiment indicate that both humoral and cell-mediated immune responses to Pd occur in M. lucifugus during hibernation, or at least during the periodic arousals. Antibodies to Pd increased during infection while the bats were hibernating, particularly in bats that had higher antibody titres entering hibernation. This demonstrates that B cells retain the ability to respond to infection in bats while hibernating, similar to T-cell-independent B-cell responses in hibernating 13-lined ground squirrels [29]. Hibernation also did not prevent the local inflammation that led to the generation of a Th17 response in the draining lymph nodes for the infected tissue. It is possible that the increased arousal frequencies observed in some bats during WNS [5,15] may be a misguided attempt to accelerate this protective response. The resulting Th17 response could even contribute to the disruption of torpor patterns observed during WNS [15]. Unfortunately, our torpor arousal data for this experiment cannot address this question because we did not find significantly increased arousal frequencies either for bats with high Il17 expression levels or Pd infection. Previous studies have found that the increased arousal frequency is most pronounced at the end of the hibernation period [15,34]. In this study, the bats were taken out of hibernation at the beginning of the last quartile of hibernation in order to have enough individuals in each group for statistical analysis, probably explaining the lack of difference in arousal rates between infected and control bats.
We found that local inflammation at the site of infection, represented by increased Il6 and Il1b expression, was probably protective as it was associated with lower decreases in body condition. WNS may represent, in part, a protective local inflammatory response that contributes to pathology when the inflammation becomes systemic. The Th17 response to Pd may also contribute to the inflammatory pathology that is present upon emergence from hibernation [14]. Our results indicate that M. lucifugus generates a Th17 response to Pd during hibernation, similar to euthermic mammals infected cutaneously with other fungi [27]. However, the response is not able to clear the infection during hibernation and its generation may lead to disruptions in thermoregulatory behaviours that contribute to WNS mortality.
Adaptive immune responses to pathogenic fungi can provide sterilizing protection, lead to a chronic infection or cause pathological inflammation [41]. The outcome is determined by the type of effector T cells that are generated and varies depending on the nature of the fungal pathogen. In our prior study [20], only a single IL-17 family member was significantly upregulated in the wing tissue in bats with WNS: Il17c, which is produced by non-immune cells. The increased expression of Il6, found in both studies, together with the increased expression of other cytokines and chemokines found in our transcriptomic study [22], is expected to initiate a Th17 response that should recruit neutrophils to the site of infection. Indeed, in the present study we found a significant increase in lymph node levels of Il17a and Il6 mRNAs between infected and uninfected bats, demonstrating that this Th17 response has been initiated. In some instances, fungal pathogens can prevent the formation of an appropriate host response, as observed during infection by the fungal pathogen causing chytridiomycosis in amphibians [42]. However, this does not appear to occur during WNS, at least by the same mechanism of suppressing the generation of an appropriate immune response. We have found that generation of a Th17 immune response in the lymph node probably occurs, and suggest the lack of neutrophils and other leukocytes at the site of infection could be due to a block, which is removed after emergence from hibernation.
The ecological effects of WNS, and whether species and populations can persist, depends on the interplay between the host, environment and pathogen. Interspecies and individual preferences for hibernacula microclimates and winter ecology dictate how effectively Pd is able to colonize bats [31,34,43]. Infection severity depends on competing microbes [44] and the initial defensive responses of the bat [20,22,45]. As shown in the current study, the type of host immune response may determine whether the infection remains commensal, as found in some European bat species, or becomes pathological, as is typical in M. lucifugus and many other North American bat species. If lacking an appropriate immune response, as described here, severe infection can cause electrolyte depletion [46], evaporative water loss [18] and other metabolic changes [19], which may lead to an increase in arousal frequency. The increase in arousal frequency caused by WNS explains 58% of the morbidity associated with Pd infection due to depletion of fat stores [15]. Even those bats that survive the full winter season are faced with recovery from infection [47] and possibly a rapid reversal of immune suppression in spring that appears to cause immune reconstitution inflammatory syndrome [14]. These may delay the parturition of female bats and result in lower body condition of both juveniles and females before the subsequent hibernation, increasing the risk of WNS-associated mortality in following years [48] and leading to an extinction vortex. The results we present here contribute to unravelling mechanisms causing WNS by describing the activation of inflammatory pathways during the hibernation period. However, the relationships between different contributing factors determine the ultimate outcome.
Here we assessed the function and importance of adaptive immune responses during hibernation as a means of persistence towards WNS in one of the most heavily impacted species, M. lucifugus. We conclude that antibody responses do not cause resistance or directly contribute to pathology, but that other types of immune responses may be more protective. We showed that hibernation does not prevent the amplification of antibody responses or the generation of a Th17 response in bats. Furthermore, the stimulation of an inappropriate inflammatory immune response during hibernation may negatively affect survival. The consequences of Pd infection on immune responses in each species should be taken into consideration in planning of conservation measures [49].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Cindy Rhone, Gretchen Long and the rest of the animal care staff at Bucknell and Lasse Ruokolainen (University of Helsinki) for the arousalFinder function.
Ethics
This study was carried out on bats from non-endangered species in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All methods were approved by the Institutional Animal Care and Use Committee at Bucknell University (protocol DMR-016). The bats were collected under a State of Michigan Scientific Collector's Permit (SC 1475).
Data accessibility
Data available from Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.8m0k4 [50].
Authors' contributions
T.M.L. sampled bats, did antibody and qPCR assays, compiled data, ran statistics and wrote the manuscript. J.M.P. sampled bats, did qPCR assays, ran statistics and contributed to writing the manuscript. J.S.J., designed the study, collected bats, did antibody assays and contributed to writing the manuscript. E.J.R. sampled bats, compiled data, designed primers and contributed to writing the manuscript. S.G. tended to bats during hibernation, downloaded data loggers and contributed to writing the manuscript. A.K. collected bats and contributed to writing the manuscript. D.M.R. designed the study, participated in the experimental work and contributed to writing the manuscript. K.A.F. designed the study, participated in the experimental work and contributed to writing the manuscript.
Competing interests
We have no competing interests.
Funding
We thank US Fish and Wildlife Service (F14AP00739) and Svenska Kulturfonden for the finance of this study.
References
- 1.Schrag SJ, Wiener P. 1995. Emerging infectious disease: what are the relative roles of ecology and evolution? Trends Ecol. Evol. 10, 319–324. ( 10.1016/S0169-5347(00)89118-1) [DOI] [PubMed] [Google Scholar]
- 2.Scholthof K-BG. 2007. The disease triangle: pathogens, the environment and society. Nat. Rev. Microbiol. 5, 152–156. ( 10.1038/nrmicro1596) [DOI] [PubMed] [Google Scholar]
- 3.Coleman JTH, Reichard JD. 2014. Bat white-nose syndrome in 2014: a brief assessment seven years after discovery of a virulent fungal pathogen in North America. Outlooks Pest Manag. 25, 374–377. ( 10.1564/v25_dec_08) [DOI] [Google Scholar]
- 4.Lorch JM, et al. 2011. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature 480, 376–378. ( 10.1038/nature10590) [DOI] [PubMed] [Google Scholar]
- 5.Warnecke L, Turner JM, Bollinger TK, Lorch JM, Misra V, Cryan PM, Wibbelt G, Blehert DS, Willis CKR. 2012. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc. Natl Acad. Sci. USA 109, 6999–7003. ( 10.1073/pnas.1200374109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeder DM, Moore MS. 2013. White-nose syndrome: a deadly emerging infectious disease of hibernating bats. In Bat evolution, ecology, and conservation (eds Adams RA, Pedersen SC), pp. 413–434. New York, NY: Springer. [Google Scholar]
- 7.Leopardi S, Blake D, Puechmaille SJ. 2015. White-nose syndrome fungus introduced from Europe to North America. Curr. Biol. 25, R217–R219. ( 10.1016/j.cub.2015.01.047) [DOI] [PubMed] [Google Scholar]
- 8.Zukal J, et al. 2016. White-nose syndrome without borders: Pseudogymnoascus destructans infection tolerated in Europe and Palearctic Asia but not in North America. Sci. Rep. 6, 19829 ( 10.1038/srep19829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frick WF, Pollock JF, Hicks AC, Langwig KE, Reynolds DS, Turner GG, Butchkoski CM, Kunz TH. 2010. An emerging disease causes regional population collapse of a common North American bat species. Science 329, 679–682. ( 10.1126/science.1188594) [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Solache MA, Casadevall A. 2010. Global warming will bring new fungal diseases for mammals. mBio 1, e00061-10 ( 10.1128/mBio.00061-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blehert DS, et al. 2009. Bat white-nose syndrome: an emerging fungal pathogen? Science 323, 227 ( 10.1126/science.1163874) [DOI] [PubMed] [Google Scholar]
- 12.Bouma HR, Carey HV, Kroese FGM. 2010. Hibernation: the immune system at rest? J. Leukoc. Biol. 88, 619–624. ( 10.1189/jlb.0310174) [DOI] [PubMed] [Google Scholar]
- 13.Wibbelt G, et al. 2013. Skin lesions in European hibernating bats associated with Geomyces destructans, the etiologic agent of white-nose syndrome. PLoS ONE 8, e74105 ( 10.1371/journal.pone.0074105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meteyer CU, Barber D, Mandl JN. 2012. Pathology in euthermic bats with white nose syndrome suggests a natural manifestation of immune reconstitution inflammatory syndrome. Virulence 3, 583–588. ( 10.4161/viru.22330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeder DM, et al. 2012. Frequent arousal from hibernation linked to severity of infection and mortality in bats with white-nose syndrome. PLoS ONE 7, e38920 ( 10.1371/journal.pone.0038920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox A, Warnecke L, Turner JM, McGuire LP, Jameson JW, Misra V, Bollinger TC, Willis CKR. 2014. Behaviour of hibernating little brown bats experimentally inoculated with the pathogen that causes white-nose syndrome. Anim. Behav. 88, 157–164. ( 10.1016/j.anbehav.2013.11.026) [DOI] [Google Scholar]
- 17.Cryan PM, Meteyer CU, Boyles JG, Blehert DS. 2010. Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biol. 8, 135 ( 10.1186/1741-7007-8-135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willis CKR, Menzies AK, Boyles JG, Wojciechowski MS. 2011. Evaporative water loss is a plausible explanation for mortality of bats from white-nose syndrome. Integr. Comp. Biol. 51, 364–373. ( 10.1093/icb/icr076) [DOI] [PubMed] [Google Scholar]
- 19.Verant ML, Meteyer CU, Speakman JR, Cryan PM, Lorch JM, Blehert DS. 2014. White-nose syndrome initiates a cascade of physiologic disturbances in the hibernating bat host. BMC Physiol. 14, 10 ( 10.1186/s12899-014-0010-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Field K, Johnson J, Lilley T, Reeder S, Rogers E, Behr M, Reeder D. 2015. The white-nose syndrome transcriptome: activation of anti-fungal host responses in wing tissue of hibernating bats. PLoS Pathog. 11, e1005168 ( 10.1371/journal.ppat.1005168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brownlee-Bouboulis SA, Reeder DM. 2013. White-nose syndrome-affected little brown myotis (Myotis lucifugus) increase grooming and other active behaviors during arousals from hibernation. J. Wildl. Dis. 49, 850–859. ( 10.7589/2012-10-242) [DOI] [PubMed] [Google Scholar]
- 22.Johnson JS, et al. 2015. Antibodies to Pseudogymnoascus destructans are not sufficient for protection against white-nose syndrome. Ecol. Evol. 5, 2203–2214. ( 10.1002/ece3.1502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco JL, Garcia ME. 2008. Immune response to fungal infections. Vet. Immunol. Immunopathol. 125, 47–70. ( 10.1016/j.vetimm.2008.04.020) [DOI] [PubMed] [Google Scholar]
- 24.Casadevall A, Pirofski L. 2012. Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell Host Microbe 11, 447–456. ( 10.1016/j.chom.2012.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerman LM, Bowden RM, Vogel LA. 2014. A vertebrate cytokine primer for eco-immunologists. Funct. Ecol. 28, 1061–1073. ( 10.1111/1365-2435.12273) [DOI] [Google Scholar]
- 26.Lin L, et al. 2009. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 5, e1000703 ( 10.1371/journal.ppat.1000703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernández-Santos N, Gaffen SL. 2012. Th17 cells in immunity to Candida albicans. Cell Host Microbe 11, 425–435. ( 10.1016/j.chom.2012.04.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vautier S, Sousa MdaG, Brown GD. 2010. C-type lectins, fungi and Th17 responses. Cytokine Growth Factor Rev. 21, 405–412. ( 10.1016/j.cytogfr.2010.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouma HR, Henning RH, Kroese FGM, Carey HV. 2013. Hibernation is associated with depression of T-cell independent humoral immune responses in the 13-lined ground squirrel. Dev. Comp. Immunol. 39, 154–160. ( 10.1016/j.dci.2012.11.004) [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature 449, 819–826. ( 10.1038/nature06246) [DOI] [PubMed] [Google Scholar]
- 31.Johnson JS, et al. 2014. Host, pathogen, and environmental characteristics predict white-nose syndrome mortality in captive little brown myotis (Myotis lucifugus). PLoS ONE 9, e112502 ( 10.1371/journal.pone.0112502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeder DM, Widmaier EP. 2009. Hormone analysis in bats. In Ecological and behavioral methods for the study of bats (eds Kunz TH, Parsons S), pp. 554–566. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 33.Turner GG, et al. 2014. Nonlethal screening of bat-wing skin with the use of ultraviolet fluorescence to detect lesions indicative of white-nose syndrome. J. Wildl. Dis. 50, 566–573. ( 10.7589/2014-03-058) [DOI] [PubMed] [Google Scholar]
- 34.Lilley TM, Johnson JS, Ruokolainen L, Rogers EJ, Wilson CA, Schell SM, Field KA, Reeder DM. 2016. White-nose syndrome survivors do not exhibit frequent arousals associated with Pseudogymnoascus destructans infection. Front. Zool. 13, 12 ( 10.1186/s12983-016-0143-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891. ( 10.1111/j.1600-0706.2009.17643.x) [DOI] [Google Scholar]
- 36.Therneau T, Lumley T. 2016. Survival analysis, including penalized likelihood. See http://crantastic.org/packages/survival/versions/17017.
- 37.Fox J, Weisberg S. 2011. An R companion to applied regression. Beverley Hills, CA: Sage Publications. [Google Scholar]
- 38.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 39.Wickham H. 2010. ggplot2: elegant graphics for data analysis, 1st edn 2009. Corr. 3rd printing 2010 edition New York, NY: Springer. [Google Scholar]
- 40.Jonasson KA, Willis CKR. 2011. Changes in body condition of hibernating bats support the thrifty female hypothesis and predict consequences for populations with white-nose syndrome. PLoS ONE 6, e21061 ( 10.1371/journal.pone.0021061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wüthrich M, Deepe GS, Klein B. 2012. Adaptive immunity to fungi. Annu. Rev. Immunol. 30, 115–148. ( 10.1146/annurev-immunol-020711-074958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fites JS, Reinert LK, Chappell TM, Rollins-Smith LA. 2014. Inhibition of local immune responses by the frog-killing fungus Batrachochytrium dendrobatidis. Infect. Immun. 82, 4698–4706. ( 10.1128/IAI.02231-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson JS, Lacki MJ, Thomas SC, Grider JF. 2012. Frequent arousals from winter torpor in Rafinesque's big-eared bat (Corynorhinus rafinesquii). PLoS ONE 7, e49754 ( 10.1371/journal.pone.0049754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornelison CT, Gabriel KT, Barlament C, Crow SA. 2014. Inhibition of Pseudogymnoascus destructans growth from conidia and mycelial extension by bacterially produced volatile organic compounds. Mycopathologia 177, 1–10. ( 10.1007/s11046-013-9716-2) [DOI] [PubMed] [Google Scholar]
- 45.Moore MS, Reichard JD, Murtha TD, Nabhan ML, Pian RE, Ferreira JS, Kunz TH. 2013. Hibernating little brown myotis (Myotis lucifugus) show variable immunological responses to white-nose syndrome. PLoS ONE 8, e58976 ( 10.1371/journal.pone.0058976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cryan PM, et al. 2013. Electrolyte depletion in white-nose syndrome bats. J. Wildl. Dis. 49, 398–402. ( 10.7589/2012-04-121) [DOI] [PubMed] [Google Scholar]
- 47.Fuller NW, Reichard JD, Nabhan ML, Fellows SR, Pepin LC, Kunz TH. 2011. Free-ranging little brown myotis (Myotis lucifugus) heal from wing damage associated with white-nose syndrome. EcoHealth 8, 154–162. ( 10.1007/s10393-011-0705-y) [DOI] [PubMed] [Google Scholar]
- 48.Frick WF, Reynolds DS, Kunz TH. 2010. Influence of climate and reproductive timing on demography of little brown myotis Myotis lucifugus. J. Anim. Ecol. 79, 128–136. ( 10.1111/j.1365-2656.2009.01615.x) [DOI] [PubMed] [Google Scholar]
- 49.Reeder DM, Field KA, Slater MH. 2016. Balancing the costs of wildlife research with the benefits of understanding a panzootic disease, white-nose syndrome. ILAR J. 56, 275–282. ( 10.1093/ilar/ilv035) [DOI] [PubMed] [Google Scholar]
- 50.Lilley TM, Prokkola JM, Johnson JS, Rogers EJ, Gronsky S, Kurta A, Reeder DM, Field KA. 2017. Data from: Immune responses in hibernating little brown myotis (Myotis lucifugus) with white-nose syndrome. Dryad Digital Repository. ( 10.5061/dryad.8m0k4) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lilley TM, Prokkola JM, Johnson JS, Rogers EJ, Gronsky S, Kurta A, Reeder DM, Field KA. 2017. Data from: Immune responses in hibernating little brown myotis (Myotis lucifugus) with white-nose syndrome. Dryad Digital Repository. ( 10.5061/dryad.8m0k4) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.8m0k4 [50].