Abstract
The recent increase in emerging fungal diseases is causing unprecedented threats to biodiversity. The origin of spread of the frog-killing fungus Batrachochytrium dendrobatidis (Bd) is a matter of continued debate. To date, the historical amphibian declines in Brazil could not be attributed to chytridiomycosis; the high diversity of hosts coupled with the presence of several Bd lineages predating the reported declines raised the hypothesis that a hypervirulent Bd genotype spread from Brazil to other continents causing the recent global amphibian crisis. We tested for a spatio-temporal overlap between Bd and areas of historical amphibian population declines and extinctions in Brazil. A spatio-temporal convergence between Bd and declines would support the hypothesis that Brazilian amphibians were not adapted to Bd prior to the reported declines, thus weakening the hypothesis that Brazil was the global origin of Bd emergence. Alternatively, a lack of spatio-temporal association between Bd and frog declines would indicate an evolution of host resistance in Brazilian frogs predating Bd's global emergence, further supporting Brazil as the potential origin of the Bd panzootic. Here, we Bd-screened over 30 000 museum-preserved tadpoles collected in Brazil between 1930 and 2015 and overlaid spatio-temporal Bd data with areas of historical amphibian declines. We detected an increase in the proportion of Bd-infected tadpoles during the peak of amphibian declines (1979–1987). We also found that clusters of Bd-positive samples spatio-temporally overlapped with most records of amphibian declines in Brazil's Atlantic Forest. Our findings indicate that Brazil is post epizootic for chytridiomycosis and provide another piece to the puzzle to explain the origin of Bd globally.
Keywords: Batrachochytrium dendrobatidis, spatial epidemiology, host–pathogen dynamics, disease distribution, spatio-temporal analysis
1. Introduction
The anthropogenic movement of parasites outside their natural ranges (pathogen pollution) is one of the largest threats to biodiversity [1,2]. Because diversity of hosts (i.e. species, populations) is positively associated with diversity of pathogens (i.e. species, genotypes) around the globe [3], many Anthropocene panzootics have a tropical origin [2]. In humans, this hypothesis is supported by an out-of-Africa emergence for many epidemics [2]. For most wildlife, the tropics are the areas of highest host diversity [4], and therefore, areas of high likelihood of pathogen emergence [2,5]. For instance, South America is a hotspot of amphibian diversity; Brazil alone harbours 1080 of the 7546 described species globally [6,7]. As the most diverse amphibian place in the world, Brazil is also a likely hotspot for amphibian pathogens, and a candidate point of origin of the wildlife disease that has caused the largest number of population declines and extinctions in the recorded history [8].
Chytridiomycosis, a disease caused by the chytrid fungus Batrachochytrium dendrobatidis (Bd), has been linked to declines of hundreds of amphibian species globally [4]. Although many studies indicate that amphibian declines are linked to the introduction of Bd to several areas of naive host populations (i.e. the novel spreading pathogen hypothesis; [9]), Bd is known to have been present in several continents before frog die-offs were reported (i.e. endemic pathogen hypothesis; [10–15]). Despite studies supporting both regional spread and endemicity in different regions, there is still substantial debate about the origin of the Bd panzootic [16–18]. To date, several regions have been suggested as the point of origin of hypervirulent Bd based on historical records of the pathogen from museum-preserved specimens [10], absence of population declines in the wild [18] and diversity of Bd lineages [19,20]. The absence of amphibian declines in Brazil attributed to chytridiomycosis, coupled with the high diversity of hosts and Bd lineages [14,18,21], raised the hypothesis that a hypervirulent Bd genotype might have spread from Brazil to other continents causing the recent global amphibian crisis [14,21].
Amphibian population declines and extinctions were reported for seemingly pristine forests of Brazil, but to date, these declines were mostly enigmatic and occurred before the discovery of chytridiomycosis [22]. Bd infection loads in Brazil are higher in pristine closed-canopy forests [23], where dozens of amphibian species experienced drastic reductions in population sizes, with a number of species going locally extinct after the late 1970s [22,24,25]. Amphibians declined in several regions, but the extinctions reported in montane protected areas in the States of São Paulo, Rio de Janeiro, Minas Gerais and Espírito Santo were among the more severe ones. Specifically, population declines were reported for over 13 species at Estação Biológica de Boraceia, state of São Paulo, after 1979 [24]. Declines were also observed during the same period of time at Parque Nacional da Serra dos Órgãos and Parque Nacional do Itatiaia (Brazil's first national park), in the state of Rio de Janeiro and Minas Gerais [24–26]. Peter Weygoldt not only reported amphibian declines at Reserva Ecológica Santa Lúcia, state of Espírito Santo, after 1981, but also speculated about a potential disease-causing agent [25]. The majority of these declines (i) occurred in pristine montane sites of the Atlantic Forest; (ii) disproportionately affected amphibian species with aquatic larvae (mostly stream breeders), and (iii) took place within a narrow time period (between 1979 and 1987).
In other Neotropical regions, many population declines attributed to Bd coincided temporally with declines in Brazil [9,27,28]. Furthermore, montane stream-breeding frogs were also at higher risk of local extinctions in Central America and Australia [9,28,29]. For instance, dozens of amphibians from the genus Atelopus declined or disappeared along high-elevation streams after outbreaks of chytridiomycosis in Central America and the tropical Andes [9]. Similarly, several frogs with aquatic larval development went extinct in the wild in eastern Australia after the emergence of Bd in natural forests [27,28,30,31]. Relict amphibian communities affected by Bd epizootics are expected to show the same signs of the ‘Ghost of Epizootics Past’ (e.g. disproportionate loss of highly aquatic species) [18]. The declines caused by Bd in Central America, the Tropical Andes and eastern Australia are similar to the enigmatic declines in Brazil's Atlantic Forest, supporting the hypothesis that historical declines observed in the Atlantic Forest were also caused by chytridiomycosis.
Here, we Bd-screened over 30 000 museum-preserved tadpoles collected in Brazil between 1930 and 2015 and quantified spatio-temporal aggregations of Bd-positive samples. We tested whether spatio-temporal aggregations of Bd overlapped with areas of historical amphibian population declines and extinctions. A spatio-temporal convergence between Bd and historical declines would support that amphibian declines in Brazil were caused by chytridiomycosis, indicating that Brazilian amphibians were not adapted to Bd, and thus weakening the hypothesis that Brazil was the global origin of Bd emergence. Alternatively, a lack of spatio-temporal association between Bd and the historical declines would further contest Bd as the cause of the enigmatic declines in Brazil and would give additional support for Brazil as the potential origin of a hypervirulent Bd genotype. We used a combination of Satscan cluster analysis and spatio-temporal randomizations to test whether areas of declines showed higher proportions of Bd-infected tadpoles. We also tested whether the incidence of Bd-infected tadpoles was higher during the peak of declines along Brazil's Atlantic Forest. Finally, we used Akaike information criterion (AIC) model averaging to test whether Bd in tadpoles responds to the impact of macro-environmental variables in the same manner as Bd in post-metamorphic anurans. Our results reveal the potential role of chytridiomycosis as the leading cause of catastrophic amphibian declines and extinctions observed in Brazil after the late 1970s, and provide another piece to the puzzle of the origin of the Bd panzootic.
2. Material and methods
(a). Sampling
We analysed 32 551 tadpoles (stored among 5597 individual vials) collected between the years of 1930 and 2015. We screened tadpoles from 13 families across 923 localities spanning the six Brazilian ecoregions; see the electronic supplementary material for the list of collections and museums from which samples were obtained. We extracted precise geographical coordinates of collection locality for the majority of analysed specimens. For those specimens without precise locality information, we extracted the municipality's geographical centroid using Geonames (http://www.geonames.org/).
(b). Disease assessment
Retrospective surveys of museum-preserved specimens have been widely used to describe historical Bd dynamics in several regions [10,14,15,32]. Thus far, retrospective studies have focused on adult frogs, despite the fact that tadpoles could be constantly exposed to waterborne Bd [33]. Bd attacks keratinized tissue, which in tadpoles is concentrated in the mouthparts [27]. Infection in tadpoles consequently causes depigmentation in both the jaw sheath and tooth rows [34–39]. Although depigmentation may also result from exposure to environmental contaminants [40] or to very low temperatures [41], the depigmentation pattern due to Bd infection is unequivocal; Bd causes patchy depigmentation with complete loss of keratin in localized areas compared with fully keratinized surrounding areas [36]. Recent studies found an overwhelming proportion of tadpoles with highly depigmented mouthparts attributed to Bd; infection prevalence was estimated at 95% in the Atlantic Forest torrent frog Hylodes japi and in the American bullfrog Lithobates catesbeianus [39], 100% in the mountain yellow-legged frog Rana muscosa from California [37] and 96% in several amphibian species from Australia [42]. In addition, more than 100 Bd genotypes were isolated from tadpoles with depigmented mouthparts across Brazil's Atlantic Forest [21]. Therefore, patterns of mouthpart depigmentation can be effectively used as a proxy for Bd infections in Brazil.
We screened individual tadpoles for Bd by visually inspecting their buccal apparatus using a dissecting microscope [43]. We included specimens ranging from Gosner stages 25 to 40 in the analyses [44]. All visualizations were performed by the same person (T.C.) for standardization purposes. We considered specimens Bd-undetected when they exhibited fully pigmented tooth rows and jaw sheaths. We considered specimens Bd-positive if they exhibited patterns of full or partial depigmentation of the buccal apparatus compatible with depigmentation due to chytridiomycosis [36,39,42]. We did not include specimens that exhibited jaw sheaths or tooth rows with slight depigmentation or with tooth rows that were easily detached by gentle manipulation.
To validate our screening method, we (i) performed both histological and qPCR screening [45] of the buccal apparatus of a subset of tadpoles with normal and depigmented mouthparts (electronic supplementary material, figure S1) and (ii) compared data of Bd isolation success from tadpoles with normal and depigmented mouthparts. Because histological screening of Bd in museum-preserved tadpoles damages specimens, we only received permission to screen 20 tadpoles from Museu de Zoologia ‘Prof. Adão José Cardoso’, Universidade Estadual de Campinas (ZUEC): 10 tadpoles with mouthparts partially or fully depigmented, and 10 tadpoles with normally pigmented mouthparts (electronic supplementary material, table S1). We sectioned each specimen's buccal apparatus, fractioned tissue (approx. 2 mm2 fragments) using a scalpel, placed tissue fragments on a glass slide with a drop of distilled water, and screened for Bd zoosporangia using a microscope at 400× magnification for 30 min according to [46]. Using a two-way contingency analysis, we confirmed that the probability of finding Bd zoosporangia is greater in tadpoles showing signs of mouthpart depigmentation (χ2 = 21.024; N = 20; p < 0.0001; electronic supplementary material, table S2).
Additionally, we collected 14 live tadpoles with fully pigmented and 10 tadpoles with fully depigmented mouthparts. We sectioned each specimen's buccal apparatus, fractioned tissue (approx. 4 mm2 fragments) using a scalpel, and extracted DNA using Prepman Ultra®. We tested samples for Bd in singlicate using Taqman qPCR [40], with standards ranging from 0.1 to 1000 zoospore genomic equivalents (g.e.) to determine Bd infection loads of each specimen; specimens with g.e. ≥ 1 were considered Bd-positive. We confirmed that the probability of detecting Bd using qPCR is greater in tadpoles showing signs of mouthpart depigmentation (χ2 = 17.143; N = 24; p < 0.0001; electronic supplementary material, table S2).
We also compared in vitro cultivation success of Bd from live tadpoles collected by LFT's laboratory in Brazil during the last 5 years. We confirmed that the probability of isolating Bd from amphibians is greater using tadpoles showing signs of mouthpart depigmentation (χ2 = 167.299; N = 174; p < 0.0001; electronic supplementary material, table S2).
(c). Spatio-temporal analyses
We compared the proportion of Bd-infected tadpoles among the six Brazilian ecoregions using a generalized linear model (GLM) with binomial distribution (logit link) and conducted detailed downstream analyses for the best-sampled ecoregion (Atlantic Forest; n = 15 981 screened tadpoles). We performed a spatio-temporal analysis to test whether Bd is randomly distributed in a space–time setting across the Atlantic Forest using Satscan v. 9.4 [47]. Satscan space–time statistic is defined by a cylindrical window with a circular geographical base and with height corresponding to time. The base reflects a purely spatial screening, while the height reproduces the time period of possible clusters. The cylindrical window moves in both space and time in a way that it screens each possible geographical location and size and visits each predefined time period, evaluating all possible overlapping cylinders of different sizes and shapes throughout the study area. We built a model by applying Kulldorff's clustering algorithm [48] under a Bernoulli probability model with 0/1 event data of Bd-positives and negative control tadpoles, screening for geographical areas with higher- or lower-than-expected spatio-temporal trends. We set the maximum spatial cluster size at 50% of the population (software default) and lumped our temporal database into four equal intervals of 21 years because our dataset does not meet a satisfactory spatial coverage at higher temporal resolutions [15]; yet we reported on datasets with time-aggregations of 20, 18, 16, 14, 12 and 10 years. We compared clusters to the entire study area using the maximum-likelihood ratio statistic to infer statistical significance for the most likely clusters. A p-value was assigned to the clusters comparing the log-likelihood ratio between the most likely clusters and a randomized dataset. For more details, see [49].
We performed spatial randomizations to test whether our detected Satscan clusters of Bd-positive samples overlapped spatio-temporally with sites of reported amphibian population declines and extinctions (electronic supplementary material, table S3). Our automation randomized the centroid location of each of our significant Satscan clusters within the Atlantic Forest 100 times, and recorded the number of reported amphibian population declines and extinctions that overlapped spatio-temporally. We then statistically compared the average number of amphibian population declines obtained with our randomizations to the observed overlap with our Satscan Bd-clusters at 95% CI. Our spatio-temporal database included 26 out of 64 (40.62%) amphibian species with reported population declines and extinctions.
We extracted the Studentized deviance residuals of a logistic regression of time (years) against Bd (positive/total observed) to use as a temporally detrended variable of Bd infection. We then used a one-tailed t-test to test whether Bd-infections (temporally detrended) were higher during the peak of the reported amphibian population declines and extinctions (i.e. 1979–1987) than during years of low amphibian population declines.
(d). Environmental analyses
We used model averaging based on the AIC, including Bd as the response variable (temporally detrended) and the following environmental factors as explanatory variables: human footprint, vegetation density, precipitation, temperature, topographic complexity and elevation; all these variables are shown to impact macroclimate associated with Bd persistence in the wild [23,50]. Model averaging allows us to make inferences based on a set of candidate models, not just the best-fit model. This GLM approach ranks parameter estimates from each possible model using a cut-off AIC weight quantile of 0.95, and thus allows us to detect the strength and the direction of explanatory variables that will most probably influence disease [51]. We extracted data on 11 temperature variables (bio1–bio11) and eight precipitation variables (bio12–bio19) from worldclim/bioclim [52]. These metrics were calculated based on a dense network of climatic stations throughout the world (i.e. precipitation data from 47 554 localities and temperature data from 24 542 localities). We also extracted data on human footprint [53], topographic complexity [54], elevation and vegetation density [55] for each sampling location using Arc Map v. 10.1 [56]. All rasters were generated at a scale of 1 km.
We performed two principal component analyses to consolidate climatic variables owing to their high cross-correlation. We used the scores of the first principal component depicting temperature and the first principal component depicting precipitation as explanatory variables in the analyses. To account for potential Bd-screening errors, we also conducted an independent AIC model averaging where we randomly selected 10% of the tadpoles with depigmented mouthparts (Bd-positive) and treated them as normal tadpoles (Bd-undetected). The influence of taxonomy was not considered in the analyses because the proportion of Bd-infected tadpoles increased with sample size similarly among sampled families (Pearson correlation r = 0.827, p = 0.0005; electronic supplementary material, figure S2).
3. Results
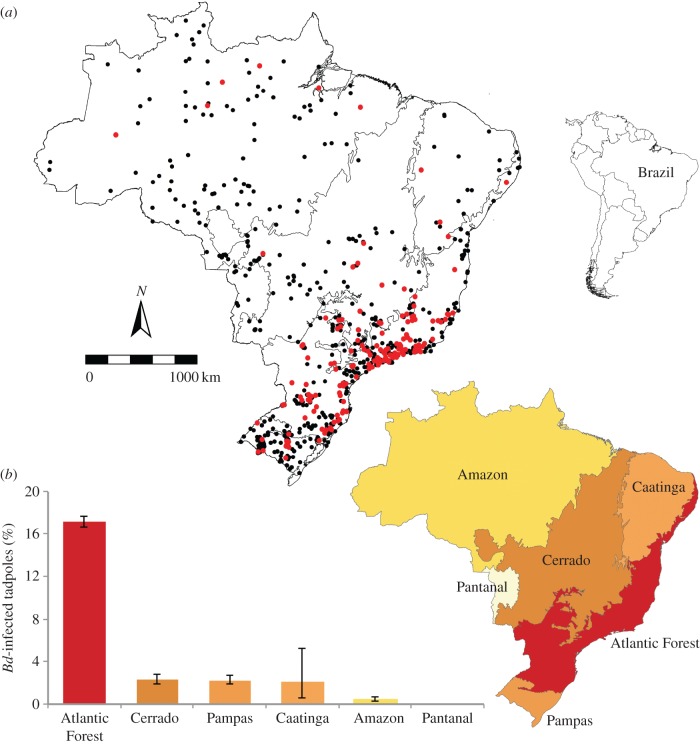
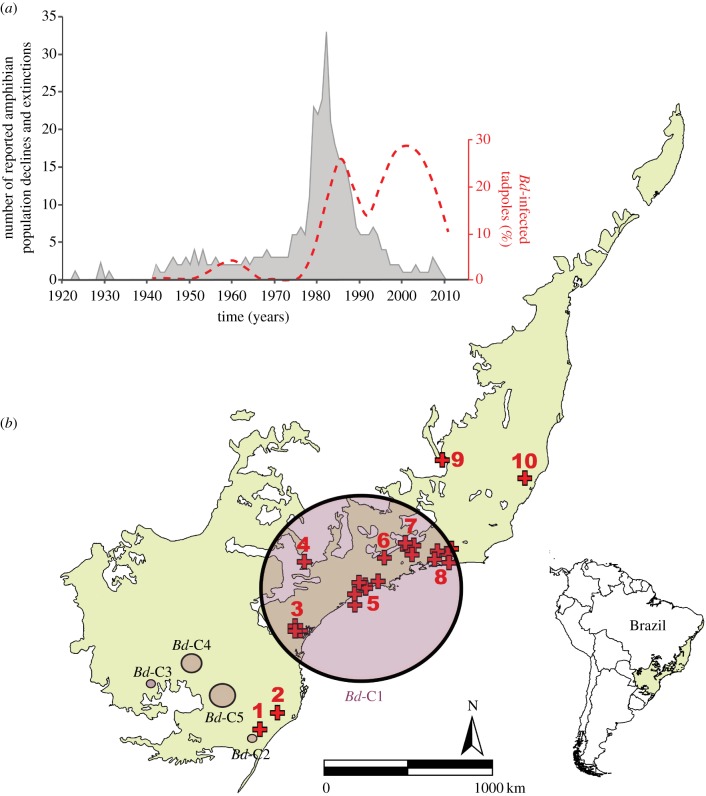
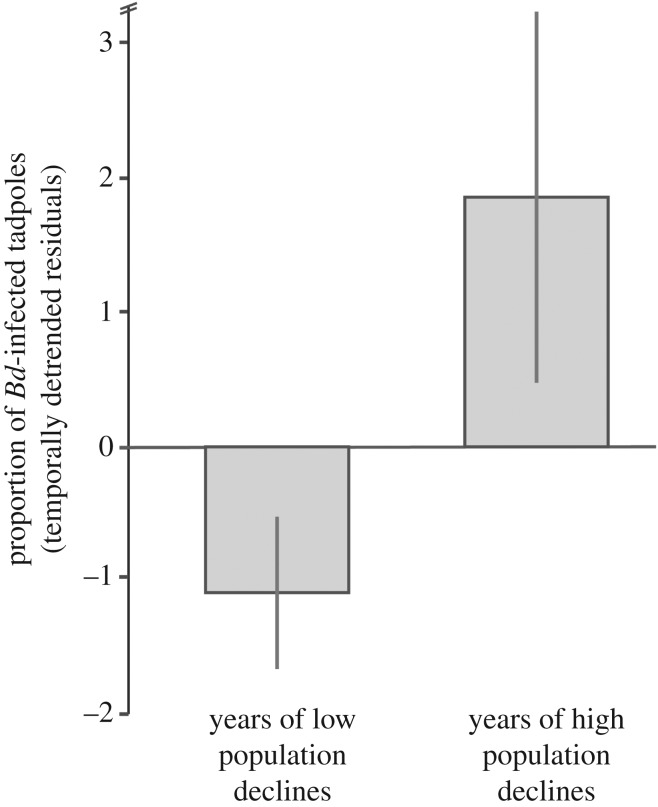
We found that Brazil's Atlantic Forest showed the highest proportion of Bd-infected tadpoles when compared with the remaining Brazilian ecoregions (Cerrado, Pampas, Caatinga, Amazon and Pantanal; χ2 = 2469.65; N = 32 551; p < 0.0001; figure 1). Our Satscan spatio-temporal analysis detected five clusters of Bd-positive samples (electronic supplementary material, table S4): one large and highly significant cluster (Bd-C1) in the southeastern Atlantic Forest with temporal aggregation from 1974 to 2015, and four smaller clusters in southern Brazil (figure 2). We repeated this analysis at six alternative time-aggregations of samples and results remained consistent for Bd-C1 (electronic supplementary material, table S5). Our null model indicated that the combined area of these five significant Bd-clusters had a higher-than-expected spatio-temporal overlap with most records of amphibian population declines and extinctions in Brazil's Atlantic forest (sites with reported declines: p = 0.002; number of populations with reported declines: p = 0.011; electronic supplementary material, figure S3). Furthermore, we found a higher proportion of Bd-positive samples (14.5%) during the peak of amphibian declines (1979–1987) than during years of low population declines (8.6%; t = 1.992; N = 73; p = 0.035; figure 3).
Figure 1.
Geographical distribution of Bd-infected (red dots) and Bd-undetected tadpoles (black dots) collected between 1930 and 2015 (a). Proportion of Bd-infected tadpoles across Brazilian ecoregions; warmer colours indicate higher proportion of Bd-infected individuals (b); averages and 95% binomial confidence intervals are shown. (Online version in colour.)
Figure 2.
Amphibian population declines and extinctions reported in Brazil's Atlantic Forest during the last 95 years and historical proportion of Bd-infected tadpoles (a). Satscan clusters of Bd-positive samples (C1–C5 circles) and sites of amphibian population declines and extinctions (red symbols) (b). Crosses represent decline sites; numbers of corresponding sites can be found in electronic supplementary material, table S3. (Online version in colour.)
Figure 3.
Proportion of Bd-infected tadpoles (temporally detrended residuals) during years of low (1930–1978; 1988–2015) and high amphibian population declines (1979–1987); averages and standard errors are shown.
Our AIC model averaging found a positive effect of elevation (std. β = 0.323; s.e. = 0.031) topographic complexity (std. β = 0.224; s.e. = 0.023), and rainfall (std. β = 0.083; s.e. = 0.022), and a negative effect of temperature (std. β = −0.125; s.e. = 0.030) on the likelihood of Bd occurrence in Brazil. Our results remained unaltered after including a conservative margin of error of 10% for potential inaccurately detecting Bd from depigmented mouthparts (electronic supplementary material, table S6).
4. Discussion
Our results provide strong evidence that chytridiomycosis caused most historical amphibian declines observed in Brazil. Specifically, the significant increase in Bd prevalence during the peak of die-offs, combined with a spatio-temporal overlap of Bd-infected tadpoles and areas of historical declines, support the hypothesis that Bd played an important role in amphibian declines across the Atlantic Forest. The most significant spatio-temporal cluster of Bd-infected tadpoles included the majority of sites with reported amphibian declines after the late 1970s. Because the time frame of these die-offs largely overlapped with declines observed in other regions [9,27,28], our results challenge the hypothesis of Brazil as the global origin of a hypervirulent Bd genotype. Our findings support The Ghost of Epizootics Past hypothesis; communities affected by chytridiomycosis in other tropical regions show the same signatures of decline as Brazil's amphibian communities. Specifically, amphibian communities in Brazil's Atlantic Forest showed a similarly high proportion of population declines among montane stream-breeding frogs (i.e. Cycloramphus, Crossodactylus, Hylodes, Phrynomedusa) when compared with communities affected by Bd in Central America and Australia [9,28,29].
There are at least three hypotheses to explain the link between Bd and amphibian population declines in Brazil after the late 1970s, including: (i) the arrival of a novel genotype of the Global Panzootic Lineage (Bd-GPL) [16,17] (the most virulent clade associated with declines on different continents) [9,18,27,57]; (ii) a sudden increase in virulence of a local Bd genotype, through genetic mutation/recombination [17,58,59] or phenotypic adaptations [60]; and (iii) a synergetic effect of multiple impacts, such as climate change, habitat alteration and pollution [61–64], which may contribute to an increase in disease incidence following the long-term presence of mainly enzootic Bd. These three hypotheses are not mutually exclusive and further studies are needed to investigate mechanisms responsible for the observed shift in disease dynamics in Brazil.
The arrival of a novel genotype within the Bd-GPL clade, or an increase in virulence of a local genotype with subsequent spread, are plausible explanations for the observed population declines of Atlantic Forest frogs. Genomic resequencing of Bd isolates revealed that a recently diversified and hypervirulent lineage (i.e. GPL) is associated with the observed amphibian population declines in Central America, Australia and North America [16,17]. Bd-GPL spread throughout Central America covering over 1500 km in 13 years [9,65]. A similar Bd spreading pattern in Brazil would cover enough ground to reach the major decline sites in the Atlantic Forest from 1979 to 1987 (i.e. Boraceia, Itatiaia, Serra dos Órgãos and Santa Tereza). Furthermore, the intensified bullfrog trade in southeastern Brazil after the 1970s may have also played an important role increasing the pace of the spread of Bd in that region [66]. The additional four narrow clusters we detected in the southern range of the Atlantic Forest also coincided with areas of bullfrog farming, which were higher in southern Brazil in the early 1990s [67]. This recent increase in bullfrog farming in the south may help explain the second peak of prevalence. Furthermore, this second peak may be also linked to local frogs evolving tolerance after the first peak and leading to enzooticity. Although Bd-GPL has been endemic in Brazil for over a century [14,15], our results cannot rule out the possibility of an introduction of a novel hypervirulent Bd-GPL genotype occurring in the late 1970s. Furthermore, the most significant Bd cluster overlaps with an area where Bd-GPL and the narrowly distributed Bd-Brazil overlap and hybridize [14,21]. These findings underscore the need for experimental work testing the virulence of hybrid Bd genotypes on the local anurofauna.
Climate change triggering independent Bd outbreaks in several pristine regions where Bd is enzootic (e.g. Atlantic Forest) would give support to the Endemic Pathogen hypothesis, which posits that Bd has globally coexisted with amphibians for a long time but emerged as a virulent pathogen due to environmental or other exogenous factors [61,68]. Global El Niño climatic events could cause amphibian population declines by increased temperature variability at regional scales, reducing frog defences against Bd [68]. Furthermore, montane frogs tend to be cold-adapted, which could make them more susceptible to the synergistic effects of disease and global climate change [69]. Although comparative genetic studies of Bd isolates found low genetic diversity consistent with a rapid expansion of Bd-GPL [70–72], climate change may well be facilitating Bd spread or outbreaks via reducing host defences. Deforestation is another obvious hypothesis to explain the historical amphibian declines in Brazil's heavily fragmented Atlantic Forest. Nevertheless, most declines were observed in protected parks, and Bd infection loads are usually higher in these pristine closed-canopy forests [23]. Furthermore, most of Atlantic Forest had been deforested before the 1960s [73], thus it is unlikely that deforestation is a confounding effect in our analyses.
Contrary to our findings linking Bd to the historical amphibian declines in Brazil, previous work on the historical distribution of Bd failed to detect an increase in pathogen prevalence during the years of decline [14,15]. Our study differs from previous research in that we focused on tadpoles while past studies focused on post-metamorphic amphibians from the Atlantic Forest [14] and the Amazon basin [15]. All anuran life stages can be infected with Bd, and although tadpoles rarely die [36,38], recently metamorphosed froglets are the most vulnerable life stage to Bd-induced mortality [38]. Thus, many tadpoles may not survive to the adult stage, and studies focused solely on adult frogs may miss important host–pathogen dynamics and may not properly detect the disease-causing agent. Bd infection in tadpoles could lead to decreases in population fitness, because infected tadpoles forage less, grow more slowly, and have reduced size at metamorphosis [38,74,75]. Decreases in tadpole fitness may thus cause downstream effects on population persistence, leading to slow-paced or silent population declines.
The strength of our results is supported by published environmental niche models and regression analyses. We detected higher proportions of Bd-infected tadpoles in areas where previous environmental niche models estimated high likelihood of Bd persistence, including the Atlantic Forest [15,18,50,63,76,77]. Furthermore, our interpretations are backed up by our multi-model inference. Our AIC model averaging showed that Bd infection likelihood in tadpoles was positively associated with elevation, rainfall and topographic complexity, and negatively associated with temperature across Brazil. These results are in agreement with previous studies that found comparable effects of the same macro-environmental variables on Bd in adult anurans [15,23,50], supporting our interpretations that Bd is the culprit behind historical amphibians population declines in Brazil.
Our study presents novel information about the global emergence of Bd and provides strong evidence for another catastrophic case of chytridiomycosis impacting dozens of amphibian species. Our results indicate that Brazil's amphibian diversity is not buffered from epizootics, and that the reported declines in the Atlantic Forest are not fundamentally different than those caused by Bd elsewhere. What sparked this panzootic is still an open question, and recent findings support that global climate change could trigger epizootics in regions of endemic Bd-GPL [69]. Therefore, further studies focused on the synergistic impacts of climate change and pathogen spread will be key to solving this problem. Studies focused on the signatures of amphibian declines in natural communities, genotype diversification, and Bd spatial turnover in areas of observed extinctions could also help elucidate patterns of host resistance and susceptibility to Bd. Understanding the history of the emergence of chytridiomycosis may not only guide efforts to prevent future amphibian die-offs, but will also contribute to our understanding of the epizootiology of other emerging fungal diseases.
Supplementary Material
Acknowledgements
Célio F. B. Haddad, Denise de Cerqueira Rossa-Feres, Richard Vogt, José Pombal Jr., Ana Prudente, Taran Grant, Gláucia Maria Funk Pontes, Felipe Franco Curcio and Sonia Cechin allowed access to museum specimens. Leandro Tacioli and David Rodriguez helped with data handling. Carlos H. L. Nunes-de-Almeida with figure editing. Carolina Lambertini with museum sampling. Luisa de Pontes Ribeiro helped with PCR analysis. Sergio Potsch de Carvalho e Silva, Itamar Alves Martins and João Luiz R. Gasparini provided data on declining populations from the states of Rio de Janeiro, São Paulo and Espírito Santo, respectively. Fernanda O. de Souza, Timothy James and Anat M. Belasen reviewed earlier versions of the manuscript.
Ethics
All museum-preserved specimens were used with the appraisal of the curators. No live specimens were used in the analyses.
Data accessibility
Database available from the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.4t53n [78].
Authors' contributions
T.C., C.G.B. and L.F.T. conceived the idea of the study. T.C. collected field data. T.C., L.F.T. and C.G.B. carried the statistical analyses, data analyses interpretation and wrote the manuscript. All authors gave final approval for publication.
Competing interests
We declare no competing interests.
Funding
Our study was funded by grants and fellowships from Fundo de Apoio ao Ensino, à Pesquisa e Extensão (FAEPEX: #1105/13 to L.F.T.), São Paulo Research Foundation (FAPESP #2014/23388-7 to L.F.T.), Coordination for the Improvement of Higher Education Personnel (PROEX-CAPES to T.C.), and National Council for Scientific and Technological Development (CNPq #302589/2013-9; #405285/2013-2; #312895/2014-3 to L.F.T. and C.G.B.).
References
- 1.Cunningham AA, Daszak P, Rodriguez JP. 2003. Pathogen pollution: defining a parasitological threat to biodiversity conservation. J. Parasitol. 89, 78–83. [Google Scholar]
- 2.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostfeld RS, Keesing F. 2012. Effects of host diversity on infectious disease. Annu. Rev. Ecol. Evol. Syst. 43, 157–182. ( 10.1146/annurev-ecolsys-102710-145022) [DOI] [Google Scholar]
- 4.IUCN. 2016. The IUCN Red List of Threatened Species. Version 2016-2. See http://www.iucnredlist.org. Downloaded on 23 February 2016.
- 5.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287, 443–449. ( 10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 6.Frost DR. 2016. Amphibian species of the world: an online reference. Version 6.0 (accessed 16 August 2016) New York, NY: American Museum of Natural History; See http://research.amnh.org/herpetology/amphibia/index.html. [Google Scholar]
- 7.Segalla MV, Caramaschi U, Cruz CAG, Grant T, Haddad CFB, Garcia PCA, Berneck BVM, Langone JA. 2016. Brazilian amphibians: list of species. Herpetol. Brazil. 5, 34–46. [Google Scholar]
- 8.Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, Hines HB, Kenyon N. 2007. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4, 125–134. ( 10.1007/s10393-007-0093-5) [DOI] [Google Scholar]
- 9.Lips KR, Diffendorfer J, Mendelson JR, Sears MW. 2008. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 6, e72 ( 10.1371/journal.pbio.0060072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weldon C, Du Preez LH, Hyatt AD, Muller R, Speare R. 2004. Origin of amphibian chytrid fungus. Emerg. Infect. Dis. 10, 2100–2105. ( 10.3201/eid1012.030804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouellet M, Mikaelian I, Pauli BD, Rodrigue J, Green DM. 2005. Historical evidence of widespread chytrid infection in North American amphibian populations. Conserv. Biol. 19, 1431–1440. ( 10.1111/j.1523-1739.2005.00108.x) [DOI] [Google Scholar]
- 12.Goka K, et al. 2009. Amphibian chytridiomycosis in Japan: distribution, haplotypes and possible route of entry into Japan. Mol. Ecol. 18, 4757–4774. ( 10.1111/j.1365-294X.2009.04384.x) [DOI] [PubMed] [Google Scholar]
- 13.Vredenburg VT, Felt SA, Morgan EC, McNally SV, Wilson S, Green SL. 2013. Prevalence of Batrachochytrium dendrobatidis in Xenopus collected in Africa (1871–2000) and in California (2001–2010). PLoS ONE 8, e63791 ( 10.1371/journal.pone.0063791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez D, Becker CG, Pupin NC, Haddad CFB, Zamudio KR. 2014. Long-term endemism of two highly divergent lineages of the amphibian-killing fungus in the Atlantic Forest of Brazil. Mol. Ecol. 23, 774–787. ( 10.1111/mec.12615) [DOI] [PubMed] [Google Scholar]
- 15.Becker CG, Rodriguez D, Lambertini C, Toledo LF, Haddad CF. 2016. Historical dynamics of Batrachochytrium dendrobatidis in Amazonia. Ecography 39, 954–960. ( 10.1111/ecog.02055) [DOI] [Google Scholar]
- 16.Farrer RA, et al. 2011. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc. Natl Acad. Sci. USA 108, 18 732–18 736. ( 10.1073/pnas.1111915108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenblum EB. 2013. Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. Proc. Natl Acad. Sci. USA 110, 9385–9390. ( 10.1073/pnas.1300130110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James TY, et al. 2015. Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: lessons from the first 15 years of amphibian chytridiomycosis research. Ecol. Evol. 518, 4079–4097. ( 10.1002/ece3.1672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schloegel LM, et al. 2012. Novel, panzootic and hybrid genotypes of amphibian chytridiomycosis associated with the bullfrog trade. Mol. Ecol. 21, 5162–5177. ( 10.1111/j.1365-294X.2012.05710.x) [DOI] [PubMed] [Google Scholar]
- 20.Bataille A, Fong JJ, Cha M, Wogan GO, Baek HJ, Lee H, Min MS, Waldman B. 2013. Genetic evidence for a high diversity and wide distribution of endemic strains of the pathogenic chytrid fungus Batrachochytrium dendrobatidis in wild Asian amphibians. Mol. Ecol. 22, 4196–4209. ( 10.1111/mec.12385) [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson TS, et al. 2016. Amphibian-killing chytrid in Brazil comprises both locally endemic and globally expanding populations. Mol. Ecol. 25, 2978–2996. ( 10.1111/mec.13599) [DOI] [PubMed] [Google Scholar]
- 22.Eterovick PC, de Queiroz Carnaval ACO, Borges-Nojosa DM, Silvano DL, Segalla MV, Sazima I. 2005. Amphibian declines in Brazil: an overview. Biotropica 37, 166–179. ( 10.1111/j.1744-7429.2005.00024.x) [DOI] [Google Scholar]
- 23.Becker CG, Zamudio KR. 2011. Tropical amphibian populations experience higher disease risk in natural habitats. Proc. Natl Acad. Sci. USA 108, 9893–9898. ( 10.1073/pnas.1014497108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heyer WR, Rand AS, da Cruz CAG, Peixoto OL. 1988. Decimations, extinctions, and colonizations of frog populations in southeast Brazil and their evolutionary implications. Biotropica 20, 230–235. ( 10.2307/2388238) [DOI] [Google Scholar]
- 25.Weygoldt P. 1989. Changes in the composition of mountain stream frog communities in the Atlantic mountains of Brazil: frogs as indicators of environmental deteriorations? Stud. Neotrop. Fauna Environ. 24, 249–255. ( 10.1080/01650528909360795) [DOI] [Google Scholar]
- 26.Guix JC, Montori A, Llorente GA, Carretero MA, Santos X. 1998. Natural history and conservation of bufonides in four Atlantic rainforest areas of southeastern Brazil. Herpetol. Natl Hist. 6, 1–12. [Google Scholar]
- 27.Berger L, et al. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl Acad. Sci. USA 95, 9031–9036. ( 10.1073/pnas.95.15.9031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hero JM, Morrison C. 2004. Frog declines in Australia: global implications. J. Herpetol. 14, 175–186. [Google Scholar]
- 29.Lips KR, Reeve JD, Witters LR. 2003. Ecological traits predicting amphibian population declines in Central America. Conserv. Biol. 17, 1078–1088. ( 10.1046/j.1523-1739.2003.01623.x) [DOI] [Google Scholar]
- 30.Hero JM, Gillespie GR. 1997. Epidemic disease and amphibian declines in Australia. Conserv. Biol. 11, 1023–1025. ( 10.1046/j.1523-1739.1997.96291.x) [DOI] [Google Scholar]
- 31.Schloegel LM, Hero JM, Berger L, Speare R, McDonald K, Daszak P. 2006. The decline of the sharp-snouted day frog (Taudactylus acutirostris): the first documented case of extinction by infection in a free-ranging wildlife species? Ecohealth 3, 35–40. ( 10.1007/s10393-005-0012-6) [DOI] [Google Scholar]
- 32.Talley BL, Muletz CR, Vredenburg VT, Fleischer RC, Lips KR. 2015. A century of Batrachochytrium dendrobatidis in Illinois amphibians (1888–1989). Biol. Conserv. 182, 254–261. ( 10.1016/j.biocon.2014.12.007) [DOI] [Google Scholar]
- 33.Longcore JE, Pessier AP, Nichols DK. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91, 219–227. ( 10.2307/3761366) [DOI] [Google Scholar]
- 34.Lips KR. 1999. Mass mortality and population declines of anurans at an upland site in western Panama. Conserv. Biol. 13, 117–125. ( 10.1046/j.1523-1739.1999.97185.x) [DOI] [Google Scholar]
- 35.Fellers GM, Green DE, Longcore JE. 2001. Oral chytridiomycosis in the mountain yellow-legged frog (Rana muscosa). Copeia 2001, 945–953. ( 10.1643/0045-8511(2001)001%5B0945:OCITMY%5D2.0.CO;2) [DOI] [Google Scholar]
- 36.Rachowicz LJ, Vredenburg VT. 2004. Transmission of Batrachochytrium dendrobatidis within and between amphibian life stages. Dis. Aquat. Organ. 61, 75–83. ( 10.3354/dao061075) [DOI] [PubMed] [Google Scholar]
- 37.Knapp RA, Morgan JAT. 2006. Tadpole mouthpart depigmentation as an accurate indicator of chytridiomycosis, an emerging disease of amphibians. Copeia 2, 188–197. ( 10.1643/0045-8511(2006)6%5B188:TMDAAA%5D2.0.CO;2) [DOI] [Google Scholar]
- 38.Garner TWJ, et al. 2009. Life history tradeoffs influence mortality associated with the amphibian pathogen Batrachochytrium dendrobatidis. Oikos 118, 783–791. ( 10.1111/j.1600-0706.2008.17202.x) [DOI] [Google Scholar]
- 39.Vieira CA, Toledo LF, Longcore JE, Longcore JR. 2013. Body length of Hylodes cf. ornatus and Lithobates catesbeianus tadpoles, depigmentation of mouthparts, and presence of Batrachochytrium dendrobatidis are related. Brazil. J. Biol. 73, 195–199. [DOI] [PubMed] [Google Scholar]
- 40.Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. 2004. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis. Aquat. Organ. 60, 141–148. ( 10.3354/dao060141) [DOI] [PubMed] [Google Scholar]
- 41.Rachowicz LJ. 2002. Mouthpart pigmentation in Rana muscosa tadpoles: seasonal changes without chytridiomycosis. Herpetol. Rev. 33, 263–264. [Google Scholar]
- 42.Obendorf DL, Dalton A. 2006. A survey for the presence of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) in Tasmania. Pap. Proc. R. Soc. Tasmania 140, 25–30. [Google Scholar]
- 43.Lambertini C, Rodrigues D, Brito FB, Leite DS, Toledo LF. 2013. Diagnóstico do fungo quitrídio: Batrachochytrium dendrobatidis. Herpetol. Brasil. 2, 12–17. [Google Scholar]
- 44.Gosner KL. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 16, 183–190. [Google Scholar]
- 45.Adams AJ, LaBonte JP, Ball ML, Richards-Hrdlicka KL, Toothman MH, Briggs CJ. 2015. DNA extraction method affects the detection of a fungal pathogen in formalin-fixed specimens using qPCR. PLoS ONE 10, e0135389 ( 10.1371/journal.pone.0135389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vieira CA, Toledo LF. 2012. Isolamento, cultivo e armazenamento do fungo quitrídio: Batrachochytrium dendrobatidis. Herpetol. Brasil. 1, 18–19. [Google Scholar]
- 47.Kulldorff M. 2015. Information management services, Inc. SaTScan TM v9.4: software for the spatial and space-time scan statistics. See www.satscan.org.
- 48.Kulldorff M. 1997. A spatial scan statistic. Commun. Stat. Theory Methods 26, 1481–1496. ( 10.1080/03610929708831995) [DOI] [Google Scholar]
- 49.Kulldorff M. 2015. SaTScan TM user guide for version 9.4.
- 50.Liu X, Rohr JR, Li Y. 2013. Climate, vegetation, introduced hosts and trade shape a global wildlife pandemic. Proc. R. Soc. B 280, 2012–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.SAS. 2016. JMP, Version 12. Cary, NC: SAS Institute Inc. [Google Scholar]
- 52.Hijmans RJ, et al. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 53.Sanderson EW, Jaiteh M, Levy MA, Redford KH, Wannebo AV, Woolmer G. 2002. The human footprint and the last of the wild. Bioscience 52, 891–904. ( 10.1641/0006-3568(2002)052%5B0891:THFATL%5D2.0.CO;2) [DOI] [Google Scholar]
- 54.Jarvis A, Reuter HI, Nelson A, Guevara E. 2009. Hole-filled seamless SRTM data V4. See http://srtm.csi.cgiar.org (accessed on July 2015).
- 55.USGS, FAO. 2000. Global forest resources assessment (FRA 2000), global forest canopy density image. See http://edc2.usgs.gov/glcc/fao/forest_canopy_image.php.
- 56.ESRI. 2012. Arcview 10.1. Redlands, CA.
- 57.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. 2010. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc. Natl Acad. Sci. USA 107, 9689–9694. ( 10.1073/pnas.0914111107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fisher MC, et al. 2009. Proteomic and phenotypic profiling of the amphibian pathogen Batrachochytrium dendrobatidis shows that genotype is linked to virulence. Mol. Ecol. 18, 415–429. ( 10.1111/j.1365-294X.2008.04041.x) [DOI] [PubMed] [Google Scholar]
- 59.Phillips BL, Puschendorf R. 2013. Do pathogens become more virulent as they spread? Evidence from the amphibian declines in Central America. Proc. R. Soc. B 280, 20131290 ( 10.1098/rspb.2013.1290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lambertini C, Becker CG, Jenkinson TS, Rodriguez D, da Silva Leite D, James TY, Zamudio KR, Toledo LF. 2016. Local phenotypic variation in amphibian-killing fungus predicts infection dynamics. Fungal Ecol. 20, 15–21. ( 10.1016/j.funeco.2015.09.014) [DOI] [Google Scholar]
- 61.Pounds JA, et al. 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161–167. ( 10.1038/nature04246) [DOI] [PubMed] [Google Scholar]
- 62.Hayes TB, Falso P, Gallipeau S, Stice M. 2010. The cause of global amphibian declines: a developmental endocrinologist's perspective. J. Exp. Biol. 213, 921–933. ( 10.1242/jeb.040865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hof C, Araújo MB, Jetz W, Rahbek C. 2011. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 480, 516–519. ( 10.1038/nature10650) [DOI] [PubMed] [Google Scholar]
- 64.Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD. 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science 341, 514–519. ( 10.1126/science.1239401) [DOI] [PubMed] [Google Scholar]
- 65.Cheng TL, Rovito SM, Wake DB, Vredenburg VT. 2011. Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proc. Natl Acad. Sci. USA 108, 9502–9507. ( 10.1073/pnas.1105538108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schloegel LM, Picco AM, Kilpatrick AM, Davies AJ, Hyatt AD, Daszak P. 2009. Magnitude of the US trade in amphibians and presence of Batrachochytrium dendrobatidis and ranavirus infection in imported North American bullfrogs (Rana catesbeiana). Biol. Conserv. 142, 1420–1426. ( 10.1016/j.biocon.2009.02.007) [DOI] [Google Scholar]
- 67.Both C, Lingnau R, Santos A Jr, Madalozzo B, Lima LP, Grant T. 2011. Widespread occurrence of the American bullfrog, Lithobates catesbeianus (Shaw, 1802) (Anura: Ranidae), in Brazil. South Am. J. Herpetol. 6, 127–134. ( 10.2994/057.006.0203) [DOI] [Google Scholar]
- 68.Rohr JR, Raffel TR. 2010. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc. Natl Acad. Sci. USA 107, 8269–8274. ( 10.1073/pnas.0912883107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, Rohr JR. 2013. Disease and thermal acclimation in a more variable and unpredictable climate. Nat. Clim. Change 3, 146–151. ( 10.1038/nclimate1659) [DOI] [Google Scholar]
- 70.Morehouse EA, James TY, Ganley AR, Vilgalys R, Berger L, Murphy PJ, Longcore JE. 2003. Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol. Ecol. 12, 395–403. ( 10.1046/j.1365-294X.2003.01732.x) [DOI] [PubMed] [Google Scholar]
- 71.James TY, Litvintseva AP, Vilgalys R, Morgan JA, Taylor JW, Fisher MC, Longcore JE. 2009. Rapid global expansion of the fungal disease chytridiomycosis into declining and healthy amphibian populations. PLoS Pathog. 5, e1000458 ( 10.1371/journal.ppat.1000458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Velo-Anton G, Rodriıguez D, Savage AE, Parra-Olea G, Lips KR, Zamudio KR. 2012. Amphibian-killing fungus loses genetic diversity as it spreads across the New World. Biol. Conserv. 146, 213–218. ( 10.1016/j.biocon.2011.12.003) [DOI] [Google Scholar]
- 73.Victor MDM, Cavalli AC, Guillaumon JR, Serra Filho R. 2005. Cem anos de devastação: revisitada 30 anos depois. Ministério do Meio Ambiente, Secretaria de Biodiversidade e Florestas, Brasília.
- 74.Venesky MD, Parris MJ, Storfer A. 2009. Impacts of Batrachochytrium dendrobatidis infection on tadpole foraging performance. Ecohealth 6, 565–575. ( 10.1007/s10393-009-0272-7) [DOI] [PubMed] [Google Scholar]
- 75.DeMarchi JA, Gaston JR, Spadaro AN, Porterfield CA, Venesky MD. 2015. Tadpole food consumption decreases with increasing Batrachochytrium dendrobatidis infection intensity. J. Herpetol. 49, 395–398. ( 10.1670/14-095) [DOI] [Google Scholar]
- 76.Ron SR. 2005. Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the new world. Biotropica 37, 209–221. ( 10.1111/j.1744-7429.2005.00028.x) [DOI] [Google Scholar]
- 77.Rödder D., et al. 2009. Global amphibian extinction risk assessment for the panzootic chytrid fungus. Diversity 1, 52–66. ( 10.3390/d1010052) [DOI] [Google Scholar]
- 78.Carvalho T, Becker CG, Toledo LF. 2017. Data from: Historical amphibian declines and extinctions in Brazil linked to chytridiomycosis. Dryad Digital Repository. ( 10.5061/dryad.4t53n) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Carvalho T, Becker CG, Toledo LF. 2017. Data from: Historical amphibian declines and extinctions in Brazil linked to chytridiomycosis. Dryad Digital Repository. ( 10.5061/dryad.4t53n) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Database available from the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.4t53n [78].