Abstract
Field metabolic rate (FMR) links the energy budget of an animal with the constraints of its ecosystem, but is particularly difficult to measure for small organisms. Landscape degradation exacerbates environmental adversity and reduces resource availability, imposing higher costs of living for many organisms. Here, we report a significant effect of landscape degradation on the FMR of free-flying Apis mellifera, estimated using 86Rb radio-isotopic turnover. We validated the relationship between 86Rb kb and metabolic rate for worker bees in the laboratory using flow-through respirometry. We then released radioisotopically enriched individuals into a natural woodland and a heavily degraded and deforested plantation. FMRs of worker bees in natural woodland vegetation were significantly higher than in a deforested landscape. Nectar consumption, estimated using 22Na radio-isotopic turnover, also differed significantly between natural and degraded landscapes. In the deforested landscape, we infer that the costs of foraging exceeded energetic availability, and honeybees instead foraged less and depended more on stored resources in the hive. If this is generally the case with increasing landscape degradation, this will have important implications for the provision of pollination services and the effectiveness and resilience of ecological restoration practice.
Keywords: 22Na kb, 86Rb kb, Apis mellifera, cost of living, field metabolic rate, honeybee
1. Background
Energetic expenditure is fundamental to many aspects of species biology, conservation management, and agricultural production [1–3], particularly in the provision of pollination services [2,4]. Field metabolic rate (FMR) is a crucial index of energetic expenditure that quantifies the cost of living in an ecological context. Measured in the ecosystem in which the individuals live, FMR encompasses all the constraints imposed on the animal by different ecological conditions. Furthermore, in altered ecosystems these costs can change unpredictably as the realized niche shifts in response to interacting biotic and abiotic factors [5]. Altered cost of living may have cascading influences through the ecosystem in the case where the study organism provides a critical ecological service, such as insect-mediated pollination [2]. In reinstating insect-mediated pollination in heavily altered landscapes, it is critical to understand how the cost of living has been altered by environmental degradation that may elevate the FMR and restrict food intake.
We quantified the energetic cost of environmental degradation to a globally significant hymenopteran pollinator, the honeybee (Apis mellifera L.) by measuring the FMR of free-flying workers. Although the doubly labelled water (DLW) method [6] facilitated the first FMR measurements of free-ranging vertebrates, technical limitations have made it less useful in the measurement of invertebrate FMR, but see exceptions: [7–11]. Among the alternatives to DLW for measuring FMR, Odum & Golley [12] proposed measuring the elimination rate of radioactive isotopes directly related to energy turnover. Of the many radionuclides tested to date [13–17], the elimination rate of rubidium-86 (86Rb kb) has the highest correlation with the rate of carbon dioxide production (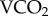 ) [16,17].
) [16,17].
Rubidium is an alkali metal that appears to be handled by the body in a similar manner to K+ [18], and recent work has shown that the Na/K ATPase that is ubiquitous to the cell membranes of all organisms, and contributes substantially to the energy budget of an organism [19], has a strong affinity for Rb+ [20]. On this basis, the theorized mechanism linking 86Rb kb to 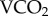 is that 86Rb+ ions are subsumed into the intracellular pool, and the remaining isotope is excreted within the first 24 h of enrichment [17], leading to a rapid loss of radioactivity in the first day [14,16,17]. Subsequent 86Rb kb is dependent on the substitution of K+ ions into the intracellular pool proportional to the metabolic activity of the Na/K ATPase. As such, increased metabolic activity in general has been shown to have predictable influences on 86Rb kb in both endotherms and ectotherms [16,17]. It is the sequestration of 86Rb into the intracellular pool that facilitates the use of 86Rb kb to measure metabolic rate, rather than food intake or elimination [13,14].
is that 86Rb+ ions are subsumed into the intracellular pool, and the remaining isotope is excreted within the first 24 h of enrichment [17], leading to a rapid loss of radioactivity in the first day [14,16,17]. Subsequent 86Rb kb is dependent on the substitution of K+ ions into the intracellular pool proportional to the metabolic activity of the Na/K ATPase. As such, increased metabolic activity in general has been shown to have predictable influences on 86Rb kb in both endotherms and ectotherms [16,17]. It is the sequestration of 86Rb into the intracellular pool that facilitates the use of 86Rb kb to measure metabolic rate, rather than food intake or elimination [13,14].
Additional to information on energy use, the food intake required to supply the energetic cost of living is also critically important [12,21,22], which can theoretically be measured for insects [23] using the biological turnover of radioactive sodium-22 (22Na kb). Unlike 86Rb, 22Na remains predominantly in the extracellular body pool and input of cold sodium from the diet can be deduced from the decline in specific activity of the isotope (22Na/23Na). This requires repetitive sampling of the haemolymph of the bees, however, which would have seriously compromised their fitness and food intake was instead estimated from the biological elimination rate of the 22Na kb. Assuming that all 23Na intake is from dietary sources (i.e. food, rather than water), then volumes of food consumed can be estimated from the 23Na content of the most common food sources available [24–26]. Hence, using the two radioisotopes in combination allows measurement of both 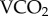 and food intake.
and food intake.
We aimed to establish a workable radio-isotopic enrichment protocol for insects as small as honeybees, and also that the relationship between 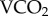 and 86Rb kb conformed to expectations established for a broader range of ectotherms [2]. By releasing honeybees enriched with 86Rb and 22Na into a natural woodland, and into adjacent cleared pine plantation, we tested whether a degraded landscape with lower resource availability per unit area provided a more substantial energetic challenge to honeybees than a naturally resourced landscape. Our data suggest that bee behaviour was modified when challenged by a less biodiverse, nutritionally depauperate landscape.
and 86Rb kb conformed to expectations established for a broader range of ectotherms [2]. By releasing honeybees enriched with 86Rb and 22Na into a natural woodland, and into adjacent cleared pine plantation, we tested whether a degraded landscape with lower resource availability per unit area provided a more substantial energetic challenge to honeybees than a naturally resourced landscape. Our data suggest that bee behaviour was modified when challenged by a less biodiverse, nutritionally depauperate landscape.
2. Methods
(a). Laboratory calibrations of radioisotopic turnover with metabolic rate
For the laboratory validations between 31 July and 16 August 2012, 80 honeybee (A. mellifera linguistica) workers were collected from two domesticated hives (40 per hive) maintained in a natural forage environment at the University of Western Australia (UWA) Shenton Park Field Station (31.9° S, 115.8° E). They were transferred to the laboratory within 1 h of collection to establish the correlation between radioisotope turnover and metabolic rate. For the duration of the calibration study, the bees were kept in JZBZ queen cages (John L. Guilfoyle Pty. Ltd., Bellevue, New South Wales, Australia) in groups of five workers from the same hive. During radio-isotopic enrichments the feeding tube of the queen cages was packed with a candy composed of honey mixed into powdered sucrose (confectioners' sugar or icing sugar; Sugar Australia (CSR), Yarraville, Victoria, Australia). Water was provided by painting water drops onto the queen cage [27]. When 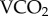 was measured by flow-through respirometry, greater volumes of water and food were provided by packing the lower half of the JZBZ queen cages with cotton wool and submerging this in a 10% honey solution.
was measured by flow-through respirometry, greater volumes of water and food were provided by packing the lower half of the JZBZ queen cages with cotton wool and submerging this in a 10% honey solution.
To enrich the bees with radioactive 86Rb, each cage of five bees was provided with the candy described above, enriched with 0.05 MBq ml−1 of 86RbCl (Perkin Elmer, Brisbane, Queensland, Australia) for 24 h. To calibrate the isotope turnovers with metabolic rate and food intake, 16 cages (80 bees) were maintained for two days in a flow-through respirometry system measuring 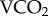 (see [28]). Low metabolic rates were imposed by measuring eight of the cages at 20°C, and higher metabolic rates with eight of the cages at 30°C [29] in a custom-built incubator. Temperature within the system was measured by DS1921H iButtons (Maxim Integrated Products, Inc. San Jose, CA, USA). The bees from two cages at the cooler temperature were excluded due to insufficient radioactive enrichment. The average enrichment, based upon disintegrations per minute, was approximately 3 000% of the background, and the two excluded cages were less than 500% which is insufficient above background for measurement reliability.
(see [28]). Low metabolic rates were imposed by measuring eight of the cages at 20°C, and higher metabolic rates with eight of the cages at 30°C [29] in a custom-built incubator. Temperature within the system was measured by DS1921H iButtons (Maxim Integrated Products, Inc. San Jose, CA, USA). The bees from two cages at the cooler temperature were excluded due to insufficient radioactive enrichment. The average enrichment, based upon disintegrations per minute, was approximately 3 000% of the background, and the two excluded cages were less than 500% which is insufficient above background for measurement reliability.
Compressed air flow through the respirometer was regulated at 100 ml min−1 (ATPD) by an Aalborg DFC 26S (Aalborg, New York, USA) mass flow controller, passed through a glass chamber of approximately 250 ml volume. Incurrent air was not dried (averaging approximately 7% RH), but CO2 was removed from the incurrent air stream using Sodasorb CO2 scrubber (calcium hydroxide granules; Sigma-Aldrich, Castle Hill, NSW, Australia). Excurrent air was dried by a Drierite column (anhydrous calcium sulfate; W.A. Hammond Drierite Co. Ltd. Xenia, OH, USA), and passed through a Qubit S151 gas analyser (Qubit Systems, Inc. Kingston, Ontario, Canada) to measure CO2 concentrations. Although Drierite has been suggested to cause errors in respirometry systems, following the guidelines of [30] mitigates these errors during steady-state measurements such as those described here. The gas analysers were calibrated to zero using a Sodasorb and 1 500 ppm CO2 calibration gas mixture (BOC Gases, Welshpool, Western Australia, Australia). All data were collected using a DI-710 data acquisition board (DATAQ Instruments Inc., Akron, OH, USA) and recorded using custom-written Visual Basic (v. 6.0) software (Microsoft). Baseline readings of background FiCO2 were established for 1 h before and after metabolic trials. Metabolic data were analysed by a custom-written Visual Basic program (P Withers 2007, personal communication) to determine the average 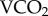 for the entire exposure period at each ambient temperature (Ta). All calculations and calibration of the metabolic system were after Withers [28]. Importantly, during respirometry trials, food and water were available ad libitum, and the bees were not post-absorptive. All results were averaged over the total respirometry trial (in hours) and so are representative of average daily metabolic rate (ADMR). Following all experimental programmes the bees were dispatched by terminal chilling and disposed of as radioactive waste.
for the entire exposure period at each ambient temperature (Ta). All calculations and calibration of the metabolic system were after Withers [28]. Importantly, during respirometry trials, food and water were available ad libitum, and the bees were not post-absorptive. All results were averaged over the total respirometry trial (in hours) and so are representative of average daily metabolic rate (ADMR). Following all experimental programmes the bees were dispatched by terminal chilling and disposed of as radioactive waste.
(b). Isotope counts
At the beginning and the end of the respirometry period at least three, 60 s whole body counts of 86Rb gamma emissions were made of each cage of five bees using multi-channel analyser (MCA) software coupled to a Gamma-Rad5 portable gamma counter (Amptek Inc. Bedford, MA., USA) with a 76 × 76 mm sodium iodide (NaI) crystal, until the coefficient of variation of the average count was less than 5%. 86Rb activity was detected and counted using the 1.0766 MeV emission peak (range 1.008–1.143 MeV). For the laboratory calibration, each individual queen cage (containing five bees) was chilled until all bees were immobilized, and the radiation present was measured within a 5 cm diameter plastic vial placed directly over the NaI crystal. Counting of radioactivity in the free-ranging bees followed an identical procedure, except that each bee was measured individually. Additional to measuring the 1.0766 MeV 86Rb gamma emission peak, 22Na activity was detected and counted using the 511 keV peak (range 463–559 keV). Typically, the bees had regained coordination by the end of the counting procedure.
Two sets of counts were made, an equilibration set following enrichment, and a recapture set following the experimental treatments. The recapture counts were corrected for isotopic decay (T½ of 86Rb = 18.66 days and 22Na = 2.60 years) by dividing all equilibration sample counts by the exponential decay constant of each isotope (e−kp×t; [15]). The biological turnover (kb) of the isotopes between equilibrium and ‘recapture’ was calculated as kb = ln(EC) − ln(RC)/t, where EC and RC are the corrected equilibrium and ‘recapture’ counts respectively and t is the elapsed time in days.
(c). Measurement of free-ranging isotope turnovers
For the field trial, six nucleus hives were populated by unrelated queens bred from captive lines maintained by the UWA Centre for Integrative Bee Research (CIBER). Each hive was established identically with two frames of brood, one frame of honey and pollen, and one foundation frame. These were established outdoors for two weeks at the UWA Shenton Park Field Station to grow to suitable colony size to tolerate experimental disturbances (T Bates 2015, personal communication). As all six nucleus hives were established at the same time, in the same manner and then allowed to mature for 14 days in the same location, we assume that their condition was standardized prior to radio-isotopic enrichment and transport to the field locations. Ten to 15 worker bees (70 in total) returning to each hive from foraging bouts were collected and enriched for 24 h prior to release with honey candy (honey mixed with confectioners' sugar) enriched with a solution of 0.05 MBq ml−1 of 86RbCl and 0.01 MBq ml−1 22NaCl (Perkin Elmer, Brisbane, Queensland, Australia). Each enriched bee was marked with a unique queen bee marker (Honeybee Australis & CB Palmer & Co., Ipswich, Queensland, Australia), and gamma emissions were counted from each individually.
Three randomly selected nucleus hives were placed in each of two fenced enclosures maintained by the Western Australian Water Corporation (Aroona Resources) on the Gnangara Mound, north of Perth. The Gnangara Mound defines a large, elevated area of sand north of Perth, Western Australia, subtended by an aquifer, which is currently the chief source of potable water for the city. Although the native vegetation is predominantly Banksia-dominated woodland [31], the area was extensively clear-felled in the late 1920s for the establishment of commercial pine plantations [32,33], and has also been exploited for commercial extraction of construction sands since the 1980s [31]; sub-urban and semi-rural residential estates were developed in the early 1990s. The two study locations represent a large, undisturbed remnant of Banksia woodland (31.58° S, 115.81° E), and a tract of long-term pine plantation monoculture that had been clear-felled, and subsequently severely burned approximately two months prior to the present study (31.63° S, 115.82° E). The combined ecological degradation of these impacts caused drastic and lasting reductions in floral diversity in this region, although data are deficient (A Ritchie 2016, personal communication). We subsequently refer to the undisturbed Banksia woodland as our natural site, and the degraded, burned area as our deforested site. Location summaries can be found in electronic supplementary material, figure S1.
Basic ecological data were collected during the field measurements, including temperature and relative humidity, measured every 5 min using data loggers (El-USB2, Lascar Electronics Whiteparish, UK) encased in black plastic canisters (Safecap picket safety caps, Hickson Industries Rylstone, New South Wales, Australia), mounted on top of each hive. The relative productivity of each landscape was determined by counting the number of Banksia menziesii and B. attenuata inflorescences within 5 m either side of five, 1 km transects laid parallel across each 1 km2 site. These two species are the most prolific and conspicuous nectar sources in the region during the austral autumn, when these measurements were made. As nectar is difficult to extract from Banksia inflorescences, two, 10 µl nectar samples were collected from B. menziesii, by manually centrifuging [34,35] four inflorescences from one tree, and five from another. Nectar from B. attenuata would also have been collected this way, had enough inflorescences been available. An additional potential nectar source, Calothamnus quadrifidus was noted in the region, and while not surveyed, six 10 µl nectar samples were collected from three plants (two samples each) by inserting glass microcapillary tubes into the nectary. The 23Na content of these nectar samples was measured by flame photometry for subsequent calculations of nectar intake by the bees.
Following their enrichment, equilibration counts of gamma emissions were recorded for each individually marked honeybee worker prior to their being returned to their natal hive. The hives were all placed at the two Gnangara locations for six days during the austral autumn (8–14 May 2015), after which the hives were collected and destroyed, and the remaining isotope in the marked bees was counted. Using the established relationship between 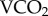 and 86Rb kb, the FMR was estimated for each individual. The total sodium content of each bee was measured by ashing each bee and suspending the ash in 1 ml of distilled water. Sodium concentrations of 100 µl aliquots were determined using an IL143 flame photometer (Instrumentation Laboratories, Bedford MA, USA) with internal lithium standardization.
and 86Rb kb, the FMR was estimated for each individual. The total sodium content of each bee was measured by ashing each bee and suspending the ash in 1 ml of distilled water. Sodium concentrations of 100 µl aliquots were determined using an IL143 flame photometer (Instrumentation Laboratories, Bedford MA, USA) with internal lithium standardization.
Nectar intake of free-ranging bees was estimated from the sodium turnover of each bee, calculated from the total 23Na content of each bee, multiplied by the 22Na kb. Assuming that the bees are in sodium balance, daily nectar intake can then be estimated by calculating the amount of nectar of known sodium concentration needed to account for the observed 22Na kb.
(d). Statistics
Metabolic rate and radioisotope kbs measured during the laboratory calibration were compared at both experimental Tas using Student t-tests. Reduced major axis (RMA) regression analyses, using the lmodel2 package [36], were used to calibrate 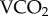 obtained from flow-through respirometry with 86Rb kb by incorporating all measurements from both experimental Tas into a single regression set. Radioisotope kbs and climatic conditions (average daily Ta and RH) were compared between field sites by analysis of variance (ANOVA) where hive identity was nested within habitat types, and blossom counts were compared between field sites using Student t-tests. Average daily metabolic rates were estimated for both sites by applying the RMA regression equations derived from laboratory trials to the 86Rb kb measurements made in the field, and food intake was calculated based on 22Na kb and nectar sodium concentrations. All statistical analyses were conducted using R v. 3.0.3 [37]. Values are given as mean ± s.e.m., and regressions were performed using metabolic rates of cages as independent data points. The number of independent samples is represented by n, while the number of individual bees is represented by N.
obtained from flow-through respirometry with 86Rb kb by incorporating all measurements from both experimental Tas into a single regression set. Radioisotope kbs and climatic conditions (average daily Ta and RH) were compared between field sites by analysis of variance (ANOVA) where hive identity was nested within habitat types, and blossom counts were compared between field sites using Student t-tests. Average daily metabolic rates were estimated for both sites by applying the RMA regression equations derived from laboratory trials to the 86Rb kb measurements made in the field, and food intake was calculated based on 22Na kb and nectar sodium concentrations. All statistical analyses were conducted using R v. 3.0.3 [37]. Values are given as mean ± s.e.m., and regressions were performed using metabolic rates of cages as independent data points. The number of independent samples is represented by n, while the number of individual bees is represented by N.
3. Results
(a). Laboratory calibrations
Metabolic rate (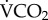 ) of the honeybees increased between 20°C (3.94 ± 0.631 mlCO2 d−1, n = 6, N = 30) and 30°C (7.43 ± 0.526 mlCO2 d−1, n = 8, N = 40; t11 = 4.2, p = 0.001). Over the two-day respirometry period, the 86Rb kb was significantly higher at 0.18 ± 0.02 per day at Ta = 30°C (n = 8 replicates, N = 40 individuals), than 0.03 ± 0.01 per day at Ta = 20°C (n = 6, N = 30; t11 = 5.13, p = 3.75 × 10−4). The calibration relationship between
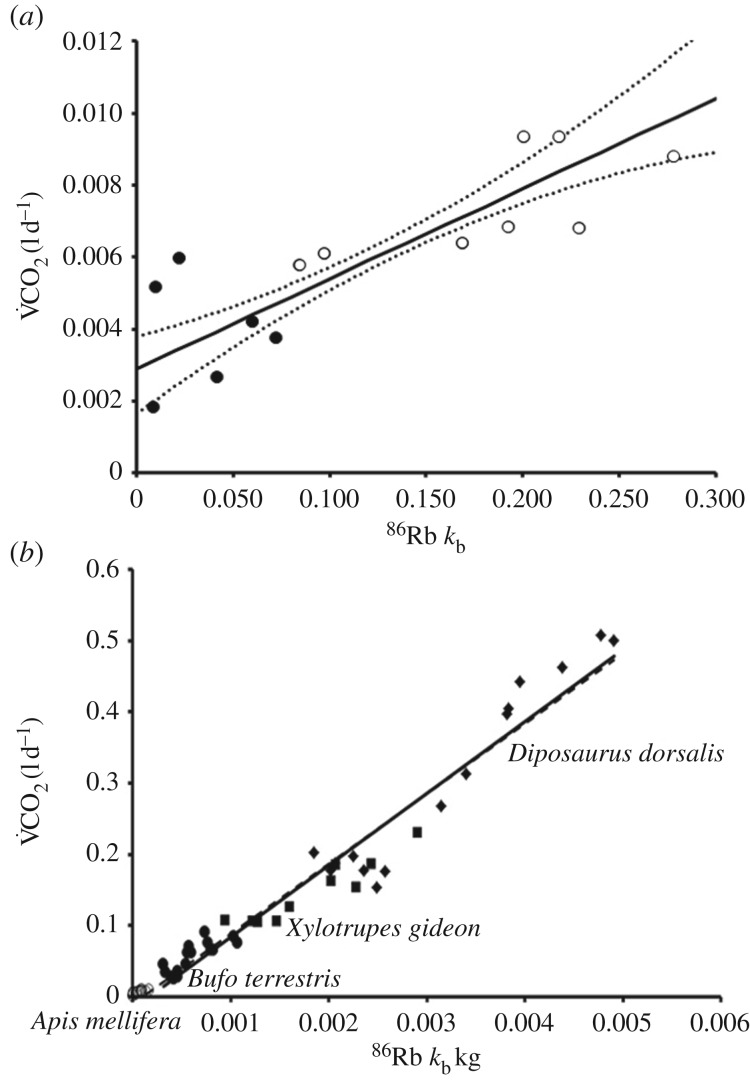
) of the honeybees increased between 20°C (3.94 ± 0.631 mlCO2 d−1, n = 6, N = 30) and 30°C (7.43 ± 0.526 mlCO2 d−1, n = 8, N = 40; t11 = 4.2, p = 0.001). Over the two-day respirometry period, the 86Rb kb was significantly higher at 0.18 ± 0.02 per day at Ta = 30°C (n = 8 replicates, N = 40 individuals), than 0.03 ± 0.01 per day at Ta = 20°C (n = 6, N = 30; t11 = 5.13, p = 3.75 × 10−4). The calibration relationship between 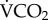 and 86Rb kb in the honeybee was positive and significant (r2 = 0.67, p = 3.30 × 10−4; figure 1a). The slope of this relationship was not significantly different from the general ectotherm relationship noted in [17] (F1,39 = 1.42 × 10−4; p = 0.991; figure 1b).
and 86Rb kb in the honeybee was positive and significant (r2 = 0.67, p = 3.30 × 10−4; figure 1a). The slope of this relationship was not significantly different from the general ectotherm relationship noted in [17] (F1,39 = 1.42 × 10−4; p = 0.991; figure 1b).
Figure 1.
(a) Reduced major axis regression relationships of radio-isotopic turnovers to V̇CO2 measured by respirometry against 2-day averaged 86Rb kb of honeybees (r2 = 0.67). Black points represent data collected at 20°C, and white points at 30°C. The line represents the average RMA regression relationship, and dashed lines represent the 95% CI of the predicted V̇CO2, n = 14, N = 70. (b) Comparisons of honeybee 86Rb kb correlation with metabolism (open circle) to the generalized relationship for ectotherms adapted from [17] for Diposaurus dorsalis (diamond, r2 = 0.74; [14]), Bufo terrestris (filled circle, r2 = 0.73; [13]), and Xylotrupes gideon (square, r2 = 0.89; [17]). The solid line represents the previous regression published for ectotherms (V̇CO2 = 101 × 86Rb kb−0.017, r2 = 0.76), and the dashed line represents the fit across all species with the inclusion of honeybees (V̇CO2 = 98.1 × 86Rb kb−0.010, r2 = 0.96, p = 4.0 × 10−37). The two lines are not significantly different in slope, (F1,39 = 1.42 × 10−4; p = 0.991), but have different intercepts (F1,41 = 4.71; p = 0.036). All published regressions used to compile this figure were significant.
(b). Field measurements
The hive locations were similar in their climate (table 1), and so would impose similar thermo-energetic demands on workers' foraging. The deforested landscape had fewer Banksia menziesii inflorescences per kilometre transect (t = 3.36, p = 0.0100; figure 2b), and no B. attenuata inflorescences. Nectar 23Na concentration was measured for only two species for which significant nectar volumes could be collected: B. menziesii (21 mmol l−1) and Calothamnus quadrifidus (18 mmol l−1).
Table 1.
Ecophysiological correlates of the natural landscape and the deforested landscape. While there was no difference in the temperature (Ta) or relative humidity (RH), there were more Banksia blossoms in the natural landscape on which the bees could forage. Very few other flowering resources were available. As a result, both rubidium (86Rb) and sodium (22Na) isotope turnovers were higher in the natural landscape, suggesting that the honeybees were more active, energetic foragers in this habitat. n.s. indicates non-significant comparisons.
| Ta (°C) | RH (%) |
Banksia blossom |
86Rb kb |
22Na kb |
|
|---|---|---|---|---|---|
| B. menzesii | B. attenuata | (ml CO2 d−1) | (µl nectar d−1) | ||
| natural | |||||
| 25.5 ± 0.15 | 40.8 ± 0.26 | 157.2 ± 39.43 | 2.4 ± 1.03 | 0.28 ± 0.008 (9.91 ± 0.94) | 0.49 ± 0.02 (164.6 ± 31.0) |
| deforested | |||||
| 19.0 ± 0.11 | 56.9 ± 0.28 | 19.8 ± 10.80 | 0 | 0.15 ± 0.013 (6.82 ± 1.08) | 0.21 ± 0.03 (65.9 ± 27.4) |
| n.s. | n.s. |
t = 3.36 p = 0.0100 |
n.s. |
F1,9 = 5.25 p = 0.048 |
F1,9 = 13.4 p = 0.005 |
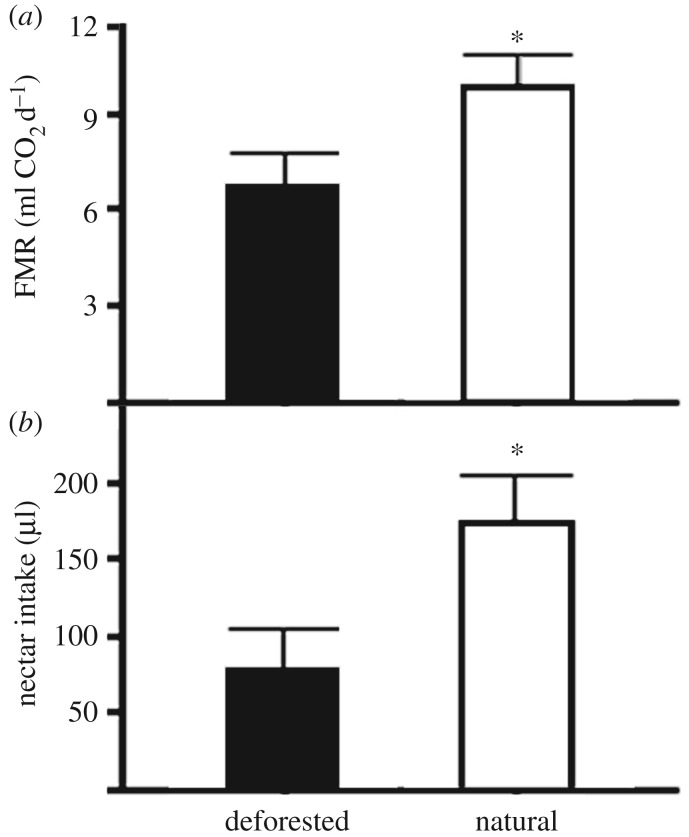
Figure 2.
Comparisons of natural (white bars) and deforested (black bars) landscapes during the field trial of (a) predicted field metabolic rate based on 86Rb kb, and (b) predicted nectar intake based on 22Na kb. Error bars are 1 s.e.m. and significant differences (p < 0.05) are represented by an asterisk.
From the 13 recaptured bees that retained their individual identification labels (20% recapture in the deforested landscape, and 13% in the natural landscape), we found a significantly higher 86Rb kb and FMR in the undisturbed landscape than in the deforested landscape (9.9 ± 0.94 ml CO2 d−1 versus 6.8 ± 1.08 ml CO2 d−1; table 1). Similarly, the nectar intake estimated from the turnover of 22Na kb was significantly higher in the undisturbed than in the disturbed landscape (164.6 ± 31.0 µl d−1 versus 65.9 ± 27.4 µl d−1 see table 1).
4. Discussion
We found that increased metabolic rates were associated with increased radioisotope turnovers in the laboratory. Given that 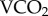 measurement by flow-through respirometry provides the most accurate quantification of metabolic rate [38], and that our respirometry data are consistent with previously published honeybee metabolic rates [29,39,40], we conclude that our laboratory calibration is accurate for honeybees. The correlation between
measurement by flow-through respirometry provides the most accurate quantification of metabolic rate [38], and that our respirometry data are consistent with previously published honeybee metabolic rates [29,39,40], we conclude that our laboratory calibration is accurate for honeybees. The correlation between 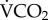 and 86Rb was statistically significant but the correlation coefficient was lower than measured in studies on larger ectotherms [13,14,17]. Enriching the bees via ingestion resulted in lower levels of 86Rb enrichment than by injection [17]. Future studies enriching their subjects this way should increase the activity of 86Rb provided in the diet to ensure higher enrichments and facilitate improved measurements, because a more powerful enrichment increases the signal to noise ratio of the gamma counter. During several pilot studies we noted substantial re-enrichment of our laboratory bees throughout the respirometry trials, and presume that this resulted from excreted isotope being re-ingested. In a respirometry chamber, where the bees were maintained on a liquid diet, this was difficult to avoid, and probably contributed substantially to the lower r2 of our data compared with previous reports, which nevertheless maintained a significant correlation between 86Rb kb and
and 86Rb was statistically significant but the correlation coefficient was lower than measured in studies on larger ectotherms [13,14,17]. Enriching the bees via ingestion resulted in lower levels of 86Rb enrichment than by injection [17]. Future studies enriching their subjects this way should increase the activity of 86Rb provided in the diet to ensure higher enrichments and facilitate improved measurements, because a more powerful enrichment increases the signal to noise ratio of the gamma counter. During several pilot studies we noted substantial re-enrichment of our laboratory bees throughout the respirometry trials, and presume that this resulted from excreted isotope being re-ingested. In a respirometry chamber, where the bees were maintained on a liquid diet, this was difficult to avoid, and probably contributed substantially to the lower r2 of our data compared with previous reports, which nevertheless maintained a significant correlation between 86Rb kb and 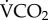 . The consensus between our data and previous reports [17] suggests that 86Rb kb is a useful and reliable method to infer FMR in invertebrate systems, and that the functional basis of the technique does not differ between vertebrates and insects.
. The consensus between our data and previous reports [17] suggests that 86Rb kb is a useful and reliable method to infer FMR in invertebrate systems, and that the functional basis of the technique does not differ between vertebrates and insects.
During the austral autumn in southwestern Australia there are few floral nectar resources available, the most obvious being two Banksia species. Assuming that honeybee foraging activity does not change with landscape context [41], lower floral abundance implies greater foraging effort (higher FMR) by individual worker bees in the deforested landscape in order to collect equivalent nectar resources to those in the natural setting. Counter to our expectations, we measured lower FMR in the deforested landscape than in the natural woodland, and lower food intakes: both suggestive of reduced levels of activity. There are two critical caveats on this interpretation that bear future investigation. While honeybee foraging activity appears consistent in different landscape contexts [41], it is well known to fluctuate in response to the quantity and quality of resources stored in the hive [42–44]. While we assumed that our hives were equivalently provisioned following their identical establishment at the Shenton Park facility, we did not quantify this (although all hives did have over three full frames of honey and brood upon return to the laboratory). While it seems unlikely, our random sampling of hives could have placed the three most well-resourced hives in the deforested landscape, and the results may have been influenced to an unknown extent in this way. Secondly, it is important to bear in mind that our data suggest reduced levels of activity by individual worker bees in the deforested landscape, whereas at the hive level it is plausible that greater numbers of workers could have been foraging at lower per capita rates. Future studies could incorporate measurement of FMR with measurements of hive activity (sensu [45,46]). While the behaviour of the colony as a ‘super-organism’ may (or may not) offset the energetic constraint imposed by the environment upon its constituent individuals in ways that offer rewarding scientific opportunites, our understanding of the ecological energetic impacts of land-use change at the level of the individual worker bee still offers some useful insights and comparisons.
Few studies exist reporting the FMR of insects, but the FMR that we measured for honeybees was higher than allometric expectations for a ‘reptile’ the same size as our bees (cf. [47]) in both the undisturbed (673 ± 45.8%) and the deforested landscapes (448 ± 63.5%). Hymenopteran FMR may, however, be substantially greater than expected for a ‘reptile’ of similar size because nectivory and flight are rare or non-existent traits in reptiles, but common in the Hymenoptera, and are typically associated with high metabolic rates [48,49]. The FMR that we measured for honeybees was much lower than that reported in [50] for the bumblebee Bombus terrestris measured using the DLW method. The FMR of the bumblebee was 16 000% of allometric expectations. Similarly, in the validation study underpinning their FMR data, [9] report metabolic rates that are far in excess of those of our honeybees and are even twice the FMR reported for hummingbirds [9,51]. Comparing our data with bumblebees [50] implies that bumblebees have very much higher energy requirements, foraging costs and costs of transport than honeybees [52], which is plausible on the basis of their wing loading being approximately four times that of honeybees, depending upon which bee populations are compared [53,54].
Sodium-22 kb has been used to measure food intake in a number of vertebrate species [21,22,24,26,55–61], but ours is only the second invertebrate reported [23]. We estimated nectar intakes ranging from 65.9 to 164.6 µl d−1 in deforested versus natural habitats, respectively, which translates to daily nectar intake ranging from 67 to 202 mg. This equates to food intakes of roughly twice the body mass of the bees each day, which is similar to the required intake of other, high-energy nectivores [21,62]. Although consistent with other findings, this intake rate requires verification with measurement of honeybee foraging activity in different landscape contexts, and with different floral resources.
The cost of living has always been quantified in terms of metabolic rate [1,3,63], but projections from laboratory measurements to ecological contexts have been based upon complex statistical models, subtended by critical assumptions [64–67]. Although rarely undertaken, measuring FMR can test some of these model projections [2]. Recent niche-envelope modelling [68] predicted an ADMR of 9.5 ± 0.003 ml CO2 d−1 in the 1 km buffer of natural vegetation surrounding the hives, and 12.9 ± 0.005 ml CO2 d−1 in the deforested landscape under similar climatic conditions to our field study. The FMR that we measured for honeybees in the natural landscape was 104.6% of model projections of ADMR in the same landscape [68]. In natural habitats, therefore, the model expectations of energetic requirement appear consistent with the actual energy expenditure of actively foraging honeybees. In the deforested habitat, however, the estimates from model projections were less consistent with measured FMR and our actual measurements were only 52.5% of the model projections of ADMR [68]. These model projections, however, did not incorporate the social behavioural adaptations that allow honeybees to accumulate stored resources and modify their foraging activity on the basis of ecological patterns of resource availability. We therefore conclude that the use of radio-isotopic turnover can be a powerful tool to test model estimations [2]. Testing model estimations with field measurements in this way provides the means to identify model uncertainties that otherwise may not be evident, even in very high-resolution mechanistic models. Where modelling approaches are used to inform conservation management, field tests measuring FMR should be explored to improve extrapolations of ecological energetics from the energetics of individual animals [69,70].
(a). Methodological considerations
With greater societal awareness of the importance of undertaking research with as little environmental and ecological impacts as possible [71], it may be difficult to procure permits to release radioactive animals into the wild. One of the great advantages noted in previous reports of this technique is that the levels of enrichment required to measure small animals constitute a fraction of the internationally recognized safe limits of exposure of 1 mGyd−1 [14,16,72]. In order to measure the FMR of free-ranging insects, the levels of enrichment required are lower again. Indeed, by the standards of the Radiation Council of Western Australia, individual bees in this study did not reach high enough levels of enrichment to be considered ‘radioactive’ under the legislation to which the Radiation Council is answerable. Furthermore, the rapid physical decay rate of the isotopes that we used specify that no significant 86Rb would remain in the dead bees after six months of storage, and 22Na levels would deplete to background within 2 years. These advantages of the technique have been discussed since the technique was first explored [12–17].
5. Conclusion
Our data suggest that the bees behaved differently when challenged by a less biodiverse, nutritionally depauperate landscape. This provides some evidence to support speculations that landscape context may have ecological energetic impacts upon honeybee pollination capacity [2]. Questions remain with regard to the landscape-level influences on the FMR of solitary insect pollinators that may be prohibitively high in heavily impacted landscapes for species unable to depend on stored resources. We foresee that the future application of radio-isotopic turnover techniques to study invertebrate systems, particularly that of pollinators, has the potential to revolutionize our current understanding of the energetics of these vital ecosystem service providers.
Supplementary Material
Acknowledgements
The authors acknowledge the Aroona Resources and the Water Corporation of Western Australia for access to field trials, and the specific help of Gary Stephenson. The advice and input of Tiffane Bates in apiculture and both field and laboratory design constraints is gratefully acknowledged. Sodium analyses of both bees and nectar was undertaken in Philip Withers' laboratory at the UWA School of Animal Biology. The authors are grateful for editorial advice and suggestions from Craig Franklin, Eric Peters, Ken Nagy, and Paul Cooper prior to submission.
Ethics
No animal ethics approvals were required to conduct this research. Use of radioisotopes was approved by the Radiation Council of Western Australia under approvals 08/08/01 and 14/05/02 to SDB.
Data accessibility
All data used in this manuscript are present in the manuscript and its electronic supplementary material.
Authors' contributions
The study was jointly conceptualized by S.T., S.D.B., K.W.D., and R.K.D. The design and execution of the laboratory validation was undertaken by S.T. The field programme was designed by S.T., S.D.B., K.W.D., and R.K.D., and executed by S.T. and S.D.B. The manuscript was initially drafted by S.T., with input and refinement by S.D.B., K.W.D., and R.K.D.
Competing interests
We have no competing interests.
Funding
This research was funded by an Australian Research Council (ARC) Industry Linkage grant no. LP110200304.
References
- 1.Bradshaw SD. 2003. Vertebrate ecophysiology: an introduction to its principles and applications. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Tomlinson S, Arnall S, Munn AJ, Bradshaw SD, Maloney SK, Dixon KW, Didham RK. 2014. Applications and implications of ecological energetics. Trends Ecol. Evol. 29, 280–290. ( 10.1016/j.tree.2014.03.003) [DOI] [PubMed] [Google Scholar]
- 3.McNab BK. 2002. The physiological ecology of vertebrates; a view from energetics. Ithaca, NY: Cornell University Press. [Google Scholar]
- 4.McCallum KP, McDougall FO, Seymour RS. 2013. A review of the energetics of pollination biology. J. Comp. Physiol. B 183, 867–876. ( 10.1007/s00360-013-0760-5) [DOI] [PubMed] [Google Scholar]
- 5.Soberón J, Nakamura M. 2009. Niches and distributional areas: concepts, methods, and assumptions. Proc. Natl Acad. Sci. USA 106, 19 644–19 650. ( 10.1073/pnas.0901637106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lifson N, McClintock R. 1966. Theory of use of the turnover rates of body water for measuring energy and material balance. J. Theor. Biol. 12, 46–74. ( 10.1016/0022-5193(66)90185-8) [DOI] [PubMed] [Google Scholar]
- 7.Cooper PD. 1983. Validation of the doubly labeled water (H3H18O) method for measuring mater flux and energy metabolism in tenebrionid beetles. Physiol. Zool. 56, 41–46. ( 10.1086/physzool.56.1.30159963) [DOI] [Google Scholar]
- 8.King WW, Hadley NF. 1979. Water flux and metabolic rates of free-roaming scorpions using the doubly labeled water technique. Physiol. Zool. 52, 176–189. ( 10.1086/physzool.52.2.30152562) [DOI] [Google Scholar]
- 9.Wolf TJ, Ellington CP, Davis S, Feltham MJ. 1996. Validation of the doubly labelled water technique for Bumblebees Bombus terrestris (L.). J. Exp. Biol. 199, 959–972. [DOI] [PubMed] [Google Scholar]
- 10.Buscarlet LA, Proux J, Gerster R. 1978. Utilisation du double marquage HT18O dans une etude de bilan metabolique chez Locusta migratoria migratorioides. J. Insect. Physiol. 24, 225–232. ( 10.1016/0022-1910(78)90039-2) [DOI] [Google Scholar]
- 11.Yokota SD. 1979. Water, energy, and nitrogen metabolism in the desert scorpion Paruroctonus mesaensis. Riverside, CA: University of California. [Google Scholar]
- 12.Odum EP, Golley FB. 1963. Radioactive tracers as an aid to the measurement of energy flow at the population level in nature. In Radioecology (eds Schultz V, Kement AL), pp. 403–410. New York, NY: Reinhold. [Google Scholar]
- 13.Peters EL. 1996. Estimating energy metabolism of goldfish (Carassius auratus) and southern toads (Bufo terrestris) from 86Rb elimination rates. Copeia 1996, 791–804. ( 10.2307/1447640) [DOI] [Google Scholar]
- 14.Peters EL, Ibrahim SA, Tracy CR, Whicker FW, Nagy KA. 1995. Estimation of the metabolic rate of the desert iguana (Dipsosaurus dorsalis) by a radionuclide technique. Physiol. Zool. 68, 316–341. ( 10.1086/physzool.68.2.30166506) [DOI] [Google Scholar]
- 15.Bradshaw SD, Bradshaw FJ. 2007. Isotopic measurements of field metabolic rate (FMR) in the Marsupial Honey possum (Tarsipes rostratus). J. Mammal. 88, 401–407. ( 10.1644/06-MAMM-A-154R1.1) [DOI] [Google Scholar]
- 16.Tomlinson S, Maloney SK, Withers PC, Voigt CG, Cruz-Neto AP. 2013. From doubly labelled water to half-life; validating radio-isotopic rubidium turnover to measure metabolism in small vertebrates. Methods Ecol. Evol. 4, 619–628. ( 10.1111/2041-210X.12056) [DOI] [Google Scholar]
- 17.Tomlinson S, Mathialagan PD, Maloney SK. 2014. Special K: testing the potassium link between radioactive rubidium (86Rb) turnover and metabolic rate. Journal of Experimental Biology 217, 1040–1045. ( 10.1242/jeb.096222) [DOI] [PubMed] [Google Scholar]
- 18.Adam WR, Craik DC. 1989. Intracellular compartmentalization of potassium. Am. J. Kidney Dis. 14, 277–280. ( 10.1016/S0272-6386(89)80202-1) [DOI] [PubMed] [Google Scholar]
- 19.Withers PC. 1992. Comparative animal physiology. Fort Worth, TX: Saunders College Publishing. [Google Scholar]
- 20.Skou JC, Esmann M. 1992. The Na,K-ATPase. J. Bioenerg. Biomembr. 24, 249–261. [DOI] [PubMed] [Google Scholar]
- 21.Bradshaw SD, Bradshaw FJ. 1999. Field energetics and the estimation of pollen and nectar intake in the marsupial honey possum, Tarsipes rostratus, in heathland habitats of South-Western Australia. J. Comp. Physiol. B 169, 569–580. ( 10.1007/s003600050257) [DOI] [PubMed] [Google Scholar]
- 22.Green B. 1978. Estimation of food consumption in the Dingo, Canis familiaris dingo, by means of 22Na turnover. Ecology 59, 207–210. ( 10.2307/1936363) [DOI] [Google Scholar]
- 23.Buscarlet LA. 1974. The use of 22Na for determining the food intake of the migratory locust. Oikos 25, 204–208. ( 10.2307/3543643) [DOI] [Google Scholar]
- 24.Green B, Libke J, Mitchell N, Newgrain K. 1999. Validation of 22Sodium turnover in estimating sodium and food intake in an amphibian (Bufo marinus). Copeia 1999, 487–490. ( 10.2307/1447496) [DOI] [Google Scholar]
- 25.Gauthier M, Thomas DW. 1990. Evaluation of the accuracy of 22Na and tritiated water for the estimation of food consumption and fat reserves in passerine birds. Can. J. Zool. 68, 1590–1594. ( 10.1139/z90-235) [DOI] [Google Scholar]
- 26.Gallagher KJ, Morrison DA, Shine R, Grigg GC. 1983. Validation and use of 22Na turnover to measure food intake in free-ranging lizards. Oecologia 60, 76–82. ( 10.1007/BF00379323) [DOI] [PubMed] [Google Scholar]
- 27.Sammataro D, Avitabile A. 2011. The beekeeper’s handbook New York, NY: Cornell University Press. [Google Scholar]
- 28.Withers PC. 2001. Design, calibration and calculation for flow-through respirometry systems. Aust. J. Zool. 49, 445–461. ( 10.1071/ZO00057) [DOI] [Google Scholar]
- 29.Tomlinson S, Dixon KW, Didham RK, Bradshaw SD. 2015. Physiological plasticity of metabolic rates in the invasive honey bee and an endemic Australian bee species. J. Comp. Physiol. B 185, 835–844. ( 10.1007/s00360-015-0930-8) [DOI] [PubMed] [Google Scholar]
- 30.White CR, Portugal SJ, Martin GR, Butler PJ. 2006. Respirometry: anhydrous Drierite equilibrates with carbon dioxide and increases washout times. Physiol. Biochem. Zool. 79, 977–980. ( 10.1086/505994) [DOI] [PubMed] [Google Scholar]
- 31.Mitchell D, Williams K, Deesmond A. 2002. Swan coastal plain 2 (SWA2—Swan Coastal Plain subregion). In A biodiversity audit of Western Australia’s 53 Biogeographical Subregions in 2002 Kensington, Western Australia: Department of Conservation and Land Management. [Google Scholar]
- 32.Finn H, Stock W, Valentine L. 2009. Pines and the ecology of Carnaby's Black-Cockatoos (Calyptorhynchus latirostris) in the Gnangara Sustainability Strategy study area. Perth, Western Australia: Gnangara Sustainability Strategy Taskforce. [Google Scholar]
- 33.Perry DH. 1948. Black cockatoos and pine plantations. West. Aust. Nat. 1, 133–135. [Google Scholar]
- 34.Armstrong DP, Patton DC. 1990. Methods for measuring amounts of energy available from banksia inflorescences. Aust. J. Ecol. 15, 291–297. ( 10.1111/j.1442-9993.1990.tb01033.x) [DOI] [Google Scholar]
- 35.Bradshaw SD, Phillips RD, Tomlinson S, Holley BJ, Jennings S, Bradshaw FJ. 2007. Ecology of the Honey possum, Tarsipes rostratus, in Scott National Park, Western Australia. Aust. Mammal. 29, 25–38. ( 10.1071/AM07003) [DOI] [Google Scholar]
- 36.Legendre P. 2014. lmodel2: Model II Regression. CRAN R-project.
- 37.Team RC. 2014. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 38.Frappell P. 2006. Respirometry, the gold standard. Physiologist 49, 12. [Google Scholar]
- 39.Chown SL, Marais E, Terblanche JS, Klok CJ, Lighton JRB, Blackburn TM. 2007. Scaling of insect metabolic rate is inconsistent with the nutrient supply network model. Funct. Ecol. 21, 282–290. ( 10.1111/j.1365-2435.2007.01245.x) [DOI] [Google Scholar]
- 40.Stabentheiner A, Vollmann J, Kovac H, Crailsheim K. 2003. Oxygen consumption and body temperature of active and resting honeybees. J. Insect. Physiol. 49, 881–889. ( 10.1016/S0022-1910(03)00148-3) [DOI] [PubMed] [Google Scholar]
- 41.Steffan-Dewenter I, Kuhn A. 2003. Honeybee foraging in differentially structured landscapes. Proc. R. Soc. Lond. B 270, 569–575. ( 10.1098/rspb.2002.2292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeley TD. 2009. The wisdom of the hive: the social physiology of honey bee colonies. Harvard, MA: Harvard University Press. [Google Scholar]
- 43.Fewell JH, Winston ML. 1992. Colony state and regulation of pollen foraging in the honey bee, Apis mellifera L. Behav. Ecol. Sociobiol. 30, 387–393. ( 10.1007/BF00176173) [DOI] [Google Scholar]
- 44.Fewell JH, Winston ML. 1996. Regulation of nectar collection in relation to honey storage levels by honey bees, Apis mellifera. Behav. Ecol. 7, 286–291. ( 10.1093/beheco/7.3.286) [DOI] [Google Scholar]
- 45.Henry M, Beguin M, Requier F, Rollin O, Odoux JF, Aupinel P, Aptel J, Tchamitchian S, Decourtye A. 2012. A common pesticide decreases foraging success and survival in honey bees. Science 336, 348–350. ( 10.1126/science.1215039) [DOI] [PubMed] [Google Scholar]
- 46.Decourtye A, Devillers J, Aupinel P, Brun F, Bagnis C, Fourrier J, Gauthier M. 2011. Honeybee tracking with microchips: a new methodology to measure the effects of pesticides. Ecotoxicology 20, 429–437. ( 10.1007/s10646-011-0594-4) [DOI] [PubMed] [Google Scholar]
- 47.Nagy KA. 2005. Field metabolic rate and body size. J. Exp. Biol. 208, 1621–1625. ( 10.1242/jeb.01553) [DOI] [PubMed] [Google Scholar]
- 48.McNab BK. 1988. Food habits and the basal rate of metabolism in birds. Oecologia 77, 343–349. ( 10.1007/BF00378040) [DOI] [PubMed] [Google Scholar]
- 49.Nagy KA, Girard IA, Brown TK. 1999. Energetics of free-ranging mammals, reptiles, and birds. Annu. Rev. Nutr. 19, 247–277. ( 10.1146/annurev.nutr.19.1.247) [DOI] [PubMed] [Google Scholar]
- 50.Wolf TJ, Ellington CP, Begley IS. 1999. Foraging costs in bumblebees: field conditions cause large individual differences. Insect. Soc. 46, 291–295. ( 10.1007/s000400050148) [DOI] [Google Scholar]
- 51.Tiebout HM, Nagy KA. 1991. Validation of the doubly labeled water method (3HH18O) for measuring water flux and CO2 production in the tropical hummingbird Amazilia saucerottei. Physiol. Zool. 64, 362–374. ( 10.1086/physzool.64.1.30158529) [DOI] [Google Scholar]
- 52.Heinrich B. 1975. Energetics of pollination. Annu. Rev. Ecol. Syst. 6, 139–170. ( 10.1146/annurev.es.06.110175.001035) [DOI] [Google Scholar]
- 53.Hepburn HR, Pirk CWW, Radloff SE. 2011. Energetic aspects of flight. In Honeybees of Asia (eds Hepburn HR, Radloff SE). New York, NY: Springer. [Google Scholar]
- 54.Dudley R, Ellington CP. 1990. Mechanics of forward flight in bumblebees: I. Kinematics and morphology. J. Exp. Biol. 148, 19–52. [Google Scholar]
- 55.Green B, Anderson J, Whateley T. 1984. Water and sodium turnover and estimated food consumption in free-living lions (Panthera leo) and spotted hyaenas (Crocuta crocuta). J. Mamm. 65, 593–599. ( 10.2307/1380842) [DOI] [Google Scholar]
- 56.Herd RM. 1985. Estimating food intake by captive emus, Dromaius novaehollandiae, by means of sodium-22 turnover. Aust. Wildl. Res. 12, 455–460. ( 10.1071/WR9850455) [DOI] [Google Scholar]
- 57.Delgiudice GD, Duquette LS, Seal US, Mech LD. 1991. Validation of estimating food intake in Gray Wolves by 22Na turnover. J. Wildl. Manage. 55, 59–71. ( 10.2307/3809241) [DOI] [Google Scholar]
- 58.Farley SD, Robbins CT. 1997. Validation of 22Sodium to estimate food intake of bears. J. Wildl. Manage. 1997, 52–56. ( 10.2307/3802413) [DOI] [Google Scholar]
- 59.Bradshaw SD. 2000. Field studies of the nutrition of Australian native animals. Proc. Nutr. Soc. Aust. 24, 155–184. [Google Scholar]
- 60.Moro D, Bradshaw SD. 2002. Diet and feeding rates of an arid-zone island population of House mice and Short-tailed mice in Western Australia. Aust. J. Zool. 50, 249–265. ( 10.1071/ZO01068) [DOI] [Google Scholar]
- 61.Nagy KA, Bradshaw SD. 1995. Energetics, osmoregulation and food consumption by free-living desert lizards, Ctenophorus (=Amphibolurus) nuchalis. Amphibia-Reptilia 16, 25–35. ( 10.1163/156853895X00163) [DOI] [Google Scholar]
- 62.Stiles FG. 1973. Food supply and the annual cycle of the Anna hummingbird. University of California Publications in Zoology 97, 1–109. [Google Scholar]
- 63.Hulbert AJ, Else PL. 2000. Mechanisms underlying the cost of living in animals. Annu. Rev. Physiol. 62, 207–235. ( 10.1146/annurev.physiol.62.1.207) [DOI] [PubMed] [Google Scholar]
- 64.Kooijman SALM. 2010. Dynamic Energy Budget Theory for Metabolic Organisation, 3rd Edition. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 65.Kearney M. 2006. Habitat, environment and niche: what are we modelling? Oikos 115, 186–191. ( 10.1111/j.2006.0030-1299.14908.x) [DOI] [Google Scholar]
- 66.Kearney M. 2012. Metabolic theory, life history and the distribution of a terrestrial ectotherm. Funct. Ecol. 26, 167–179. ( 10.1111/j.1365-2435.2011.01917.x) [DOI] [Google Scholar]
- 67.Austin MP. 2007. Species distribution models and ecological theory: a critical assessment and some new approaches. Ecol. Modell. 200, 1–19. ( 10.1016/j.ecolmodel.2006.07.005) [DOI] [Google Scholar]
- 68.Tomlinson S, Webber BL, Bradshaw SD, Dixon KW, Renton M. At review Incorporating biophysical ecology into high-resolution restoration targets: insect pollinator habitat suitability models. Ecol. Appl. [Google Scholar]
- 69.Mathot KJ, Dingemanse NJ. 2015. Energetics and behavior: unrequited needs and new directions. Trends Ecol. Evol. 30, 199–206. ( 10.1016/j.tree.2015.01.010) [DOI] [PubMed] [Google Scholar]
- 70.Halsey LG, Matthews PGD, Rezende EL, Chauvaud L, Robson AA. 2015. The interactions between temperature and activity levels in driving metabolic rate: theory, with empirical validation from contrasting ectotherms. Oecologia 177, 1117–1129. ( 10.1007/s00442-014-3190-5) [DOI] [PubMed] [Google Scholar]
- 71.Rotblat J. 1999. A Hippocratic Oath for scientists. Science 286, 1475 ( 10.1126/science.286.5444.1475) [DOI] [PubMed] [Google Scholar]
- 72.Agency IAE. 1992. Effects of ionising radiation on plants and animals at levels implied by current radiation protection standards. In Techincal Report Series No. 332 Vienna, International Atomic Energy Agency.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this manuscript are present in the manuscript and its electronic supplementary material.