Abstract
Gas-bubble disease occurs in aquatic species that are exposed to water that is supersaturated with gases. In February 2007, municipal water supersaturated with gas was inadvertently pumped into the vivarium's aquatic housing systems and affected approximately 450 adult female Xenopus laevis. The inflow of supersaturated water was stopped immediately, the holding tanks aggressively aerated, and all experimental manipulations and feeding ceased. Within the first 6 h after the event, morbidity approached 90%, and mortality reached 3.5%. Acutely affected frogs showed clinical signs of gas-bubble disease: buoyancy problems, micro- and macroscopic bubbles in the foot webbing, hyperemia in foot webbing and leg skin, and loss of the mucous slime coat. All of the frogs that died or were euthanized had areas of mesenteric infarction, which resulted in intestinal epithelial necrosis and degeneration of the muscular tunic. Over the subsequent 2 wk, as gas saturation levels returned to normal, the clinical symptoms resolved completely in the remaining frogs. However, 3 mo later, 85% of them failed to lay eggs or produce oocytes, and the remaining 15% produced oocytes of low number and poor quality, yielding cytosolic extracts with poor to no enzymatic activity. Histology of the egg mass from a single 2- to 3-y-old frog at 3 mo after disease resolution revealed irregularly shaped oocytes, few large mature oocytes, and numerous small, degenerating oocytes. At 6 mo after the incident, the remaining frogs continued to fail to produce eggs of sufficient quantity or quality after hormonal priming. The researchers consequently opted to cull the remainder of the colony and repopulate with new frogs.
Abbreviations: GBD, gas-bubble disease; DO, dissolved oxygen; TDG, total dissolved gas
Gas-bubble disease (GBD) is a noninfectious condition that arises in aquatic species when the aqueous gas concentration, particularly of nitrogen and, to a lesser degree, argon, in the habitat water rises above normal atmospheric pressure.3,9,10,17 The water thus becomes ‘supersaturated’ with dissolved gases. The amount of gas in the supersaturated water depends on the water's temperature, the pressure and solubility of the gas, and the atmospheric pressure. Under normal saturation conditions, water exposed to the atmosphere absorbs atmospheric gases until equilibrium is reached. However, if the water temperature rapidly increases or the pressure of the water increases, the gas concentration in the water rises above atmospheric pressure levels, and the water becomes supersaturated. Total dissolved gas (TDG) pressure in supersaturated water usually exceeds 100%, and an increase as small as 1% to 2% can result in GBD, a condition that has been reported in both natural and laboratory settings.9,10,17 In aquaculture systems2 of the most common causes of gas supersaturation are 1) an increase in water temperature without reequilibration of the dissolved gases, and 2) the entrainment of air into closed, pressurized water piping systems.17 A third cause of supersaturationcan occur when the solubility of gases changes as water mixes with air as it is pumped from great depths, such as in plunge basins in hydroelectric dam projects, or deep, fresh-water wells. Water pumped from great depths can be naturally supersaturated when it arrives at the ground surface.5-7
Aquatic animals exposed to water supersaturated with gases often exhibit clinical signs related to the accumulation of gas bubbles in the vascular system and the formation of subcutaneous emphysema and gas emboli.3,5-7,9,12,14 Morbidity associated with GBD in laboratory Xenopus laevis and in Rana catesbeiana has been reported to range from 90% to 100%, with mortality of 40% to 50%.5,6 Gas-bubble–specific lesions in the described X. laevis included gas bubbles in the interdigital webbings in the hindfeet, subcutaneous bubbles in the hindlegs and lower body (and eventually in the forelegs), petechial and ecchymotic hemorrhages scattered along the skin of the legs and ventrum, sloughing of the mucous coat, and skin erosions.5 In addition, the frogs developed red-leg syndrome, a secondary infection with opportunistic bacterial pathogens.5
A hardy, semiaquatic species, laboratory X. laevis have been used extensively in biomedical research. Healthy adult female frogs can be hormonally primed and regularly produce a steady supply of hundreds of oocytes (fully grown egg precursor cells), which are quite large and can reach 1.2 mm in diameter. The oocytes are used by researchers to prepare cell-free cytoplasmic extracts to study a variety of biochemical and cellular processes, including mitosis, meiosis, signal transduction pathways, transcription, translation, nuclear transport, and mRNA expression. Here we describe the clinical history and findings, case management, and acute and chronic outcomes after GBD in a large colony of adult female laboratory X. laevisthat were housed to produce oocytes for use in cellular and biochemical research.
Case History
The approximately 450 X. laevis described in this report were healthy, sexually mature (age, 2 to 3 y), adult female frogs from Nasco (Fort Atkinson, WI). This colony was monitored for parasitic and infectious diseases through the university's facility aquatic disease surveillance program, which included testing for Batrachochytrium dendrobatidis and for potentially zoonotic infectious agents such as Mycobacterium, Salmonella, and Cryptosporidiumspp. The colony had not had any endemic pathogens in the previous 10 y. The frogs were fed a commercial diet (1 g per frog 3 times weekly; Frog Brittle, Nasco) and were housed in 4 opaque (volume, approximately 300 gal), self-flushing, pond-style holding tanks with approximately 110 frogs per tank (Figure 1), as previously described.11 Tanks were programmed to drain completely and refill over a period of 1 h with conditioned, dechloraminated tap water once in the afternoon, every 3 d, at 3 h after feeding. Water for the frog holding tanks was supplied from the Hetch Hetchy Reservoir by the San Francisco Public Utilities Commission and pumped to the local municipality that supplies water to the campus vivarium. Water temperature in the frog holding tanks was maintained at 16 to 21 °C. Water quality was tested monthly according to spectrophotometric and colorimetric methods and managed as described previously.11 Water-quality parameters were maintained within standard range for aquatic amphibians: pH, 7.00 to 8.00; conductivity, 400 to 700 μΩ; water hardness, 82 to 102 mg/L; alkalinity, 76 to 106 mg/L; ammonia, 0 to 1.00 mg/L; nitrite, 0 to 0.20 mg/L; nitrate, 0 to 50.0 mg/L; chlorine or chloramine, 0 to 0.20 mg/L; copper, 0 to 2.00 mg/L; and dissolved oxygen (DO), 8.00 to 9.00 mg/L. The room was on a 12:12-h light:dark cycle with an ambient room temperature of 23 to 25 °C.11 The frogs housed in these tanks were used for egg and oocyte collection (nonsurgical harvest) by researchers who studied signal transduction pathways and the cell cycle. The frogs were housed in Stanford's AAALAC-accredited facility, and the husbandry, hormonal priming, and oocyte collection followed the study protocol as approved by Stanford University's IACUC.
Figure 1.

X. laevis housing in pond-style, self-flushing (100% water changes) holding tanks at the time of the incident.
On 16 February 2007, animal husbandry staff reported 8 dead frogs in tank 1, and 2 dead frogs in tank 2. Frogs in all 4 tanks were observed paddling at the water's surface attempting to dive, swimming with their snouts pointed downward, or floating motionless at the water surface with their heads down. At the time the frogs were showing these signs, the tanks were in the process of refilling after draining. The incoming water was observed to be ‘milky white’ and contained bubbles (Figure 2). The water inflow was turned off immediately, and water samples were collected for analysis according to previously described spectrophotometric methods.11 All water-quality parameters were within normal limits, except for DO, which was greater than 12 mg/L (the upper limit of measurement) and thus exceeded the historical values by 3 to 4 mg/L. A saturometer (P4 Tracker, Point Four Systems, Quitlam, BC, Canada) was obtained, and the TDG was found to exceed 112% (normal is 100%). Given that water across the entire campus was supersaturated and because no alternative housing sources were available, frogs were left in their current housing, and pond aerators were obtained and placed in each tank.
Figure 2.

Milky-white, turbid water with visible gas bubbles entering the X. laevis pond-style holding tank. As described in the case history, this water was supersaturated with entrained air (total dissolved gas concentration exceeded 112%; less than 100% is normal).
Six hours later, mortality in the frog colony had reached approximately 3.5%. Of the 450 frogs in the colony, 16 had died or were euthanized by immersion for 20 min in 0.05% buffered MS222 (tricaine methanesulfonate, Western Chemical, Ferndale, WA) and immediately necropsied. All of the remaining frogs showed buoyancy problems and clinical signs previously described in X. laevis with GBD: floating in a ‘head down’ vertical position, with the hindlegs extended and feet paddling at the water's surface, and an inability to dive. Physical examination of affected frogs revealed petechial and ecchymotic cutaneous hemorrhages that were initially limited to the hindlegs and ventrum but that progressed to cover much of the body (Figure 3). In addition, loss of the mucous slime coat was apparent, evidenced by sloughed mucous coat floating in the water (Figure 4). By using a dissecting microscope to enhance visibility of the hyperemic feet, gas bubbles were observed in the interdigital webbing (Figure 5).
Figure 3.
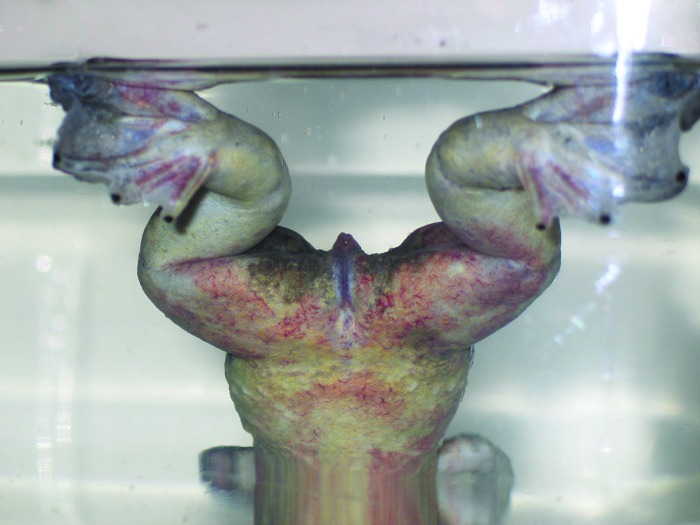
Petechia and ecchymotic hemorrhages were present on the hindlegs and extended over much of the body. In addition, note the floating in a head-down position, with hindlegs and feet at the water's surface. X. laevis normally float at the water's surface, with the head and snout higher than the hindfeet.
Figure 4.
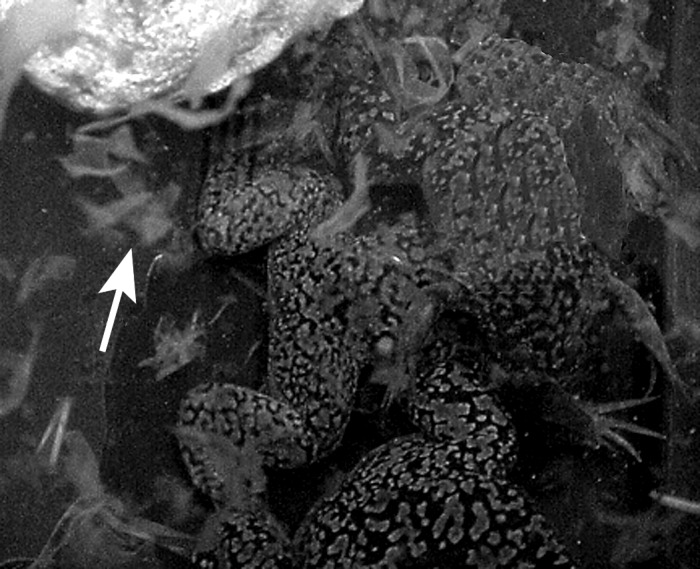
Extensive sloughing of mucous coat (arrow) within 6 h of exposure to supersaturated water.
Figure 5.
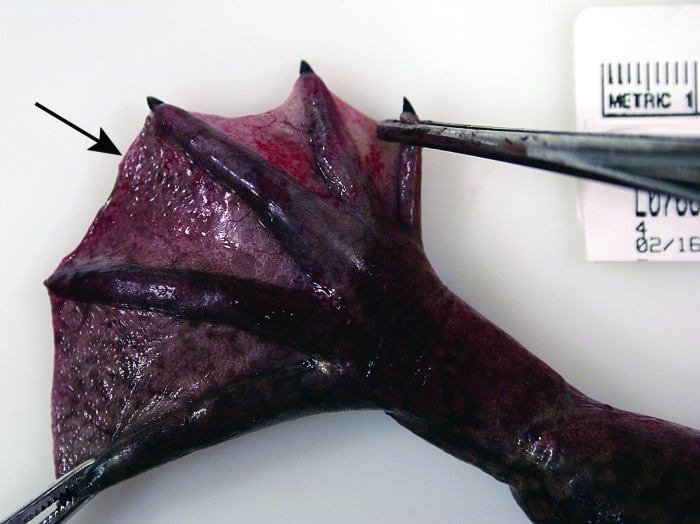
Macroscopic gas bubbles (arrow) in the interdigital webbing. Note the marked hyperemia of the webbing and lower leg.
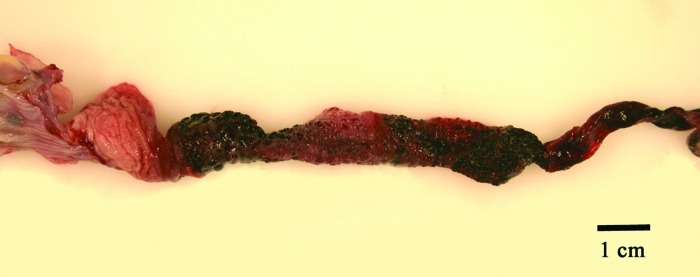
A gross necropsy was performed on all 16 frogs, and tissues were collected for histopathology. Grossly, gas bubbles were observed beneath the skin of the hindlegs and ventrum in all 16 frogs (Figure 6). Hyperemia and gas bubbles persisted in the interdigital webbing, often for several hours after euthanasia. The cutaneous petechial and ecchymotic hemorrhages noted during physical exam in all frogs were concentrated in the skin of the ventrum and the hindlegs, although hemorrhage was widely distributed across most of the body, excluding the head, in severely affected frogs. Most frogs that died or were euthanized had sloughed part or all of their mucous slime coat. Skin ulcers and lesions consistent with sepsis or red-leg syndrome as reported in an earlier report were not observed.5 In addition, other clinical signs reported in fish and previously described in X. laevis affected by GBD, such as anasarca and exophthalmia, were not noted.2,14,15 Gross examination of the viscera revealed blackened intestines with gas bubbles on the serosal surfaces (Figure 7). Frank blood and blood clots were present in the intestinal lumina.
Figure 6.
Numerous gas bubbles are present beneath the skin on the ventrum of a laboratory X. laevis affected by gas-bubble disease.
Figure 7.
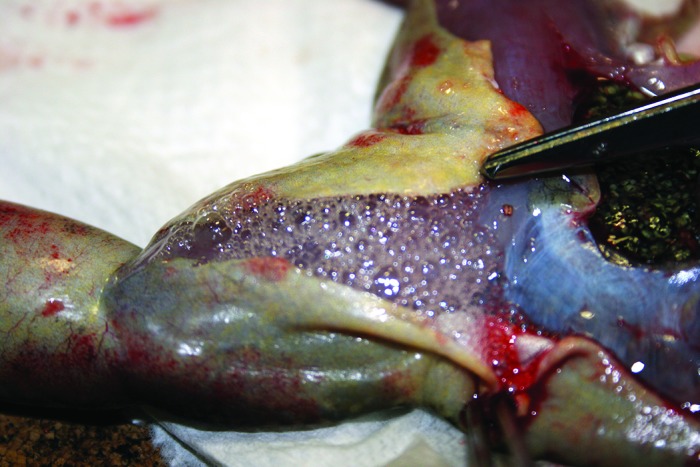
A gross image of an opened intestine demonstrates mucosal hemorrhages and superficial black material suggestive of digested blood.
For histopathology, tissues were immersion-fixed in 10% buffered neutral formalin and were immediately processed for paraffin embedding and hematoxylin and eosin staining of 4-μm sections. Histologically, the intestinal mucosa and submucosa were markedly congested. There were areas of mesenteric infarction that resulted in epithelial necrosis and early degeneration of the muscular tunic (Figure 8). In addition, mucosal ulceration, hemorrhage, and vascular dilation and congestion were apparent in the colonic mucosa (Figure 9).
Figure 8.
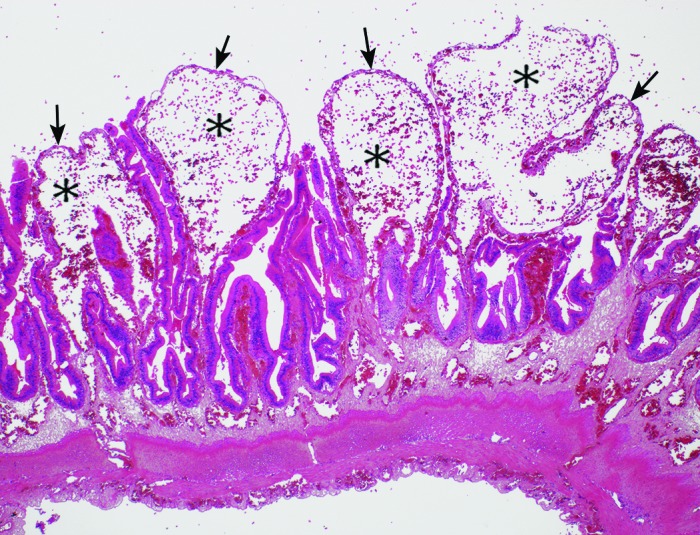
The mucosa of the small intestine is severely distorted due to vascular dilation, congestion, and hemorrhage. Villous-like structures are devoid of epithelium (arrows) and contain extremely dilated lamina proprial lymphatics (clear spaces, stippled with RBC [*]). The submucosal and adventitial vessels are dilated also. Hematoxylin and eosin stain; magnification, 10×.
Figure 9.

The normal colonic histoarchitecture is markedly altered due to mucosal ulceration (arrows) and extensive submucosal hemorrhage. Note the gas bubbles in the submucosal vessel (*). The muscular tunics are rarefied and fragmented, indicating acute degeneration (arrow heads). Hematoxylin and eosin stain; magnification, 10×.
Case Management of Surviving Frogs
The diagnosis of GBD was apparent, and the first and immediate management step was to turn off the incoming water flow.12 The municipal water works office confirmed that construction upstream from the campus water supply had created microcracks in the campus mainline water pipes, allowing air entrainment and water supersaturation. Further management strategies therefore were complicated by the citywide circulation of water containing entrained air and the lack of immediately available alternative water sources for the frog holding tanks. Pond aerators (Little Giant Fountain Head; Little Giant, Oklahoma City, OK; Figure 10) immediately were placed in the frog holding tanks. However, degassing supersaturated water requires several hours to days to accomplish, depending on the degree of saturation, the rate at which the water can be warmed to room temperature (increasing the water temperature enhances the movement of gases from the water into the air), and the composition of the entrained gas nitrogen, the primary culprit in GBD, is slower to escape from the water's surface, relative to other gases).3,5,17 Water in the holding tanks was tested every 2 to 4 h for entrained gases, nitrogen and argon included, by using a commercially available saturometer (P4 Tracker, Point Four Systems, Quitlam, BC, Canada) until the water saturation returned to normal (7 d). In addition, incoming water was tested after the county repaired the mainline microcracks and was determined to be of normal saturation before the water filling and draining cycle was fully reinstituted, at d 10 after the initial GBD event.
Figure 10.

The supersaturated water was aerated by installing fountain heads (Little Giant, Oklahoma City, OK), which increased the air–water interface to enhance the transfer of dissolved gases in the water to the atmosphere.
Water-quality tests of samples collected from the holding tanks just after the water inflow was discontinued indicated that the nitrogenous waste levels were within normal range for all holding tanks (the tanks had been fully drained just prior to the GBD event). To assist with nitrogenous waste management and to minimize the need to change the water, an empirical dose of 2 cups of AmQuel (Kordon, San Francisco, CA), a commercially available water conditioner that effectively binds to and eliminates nitrates, nitrites, ammonia and chloramines, was added to each 300-gal tank. Over the next week, the water was not changed in the tanks, and the fountains remained turned on and in place. All research stopped. The frogs were handled minimally and were not fed to minimize water pollution with bodily waste and the negative effects of feeding on potentially compromised intestines. AmQuel was added every other day at the dose described, until the normal water filling and draining cycle was fully instituted 10 d after GBD.
All of the remaining frogs appeared to be fully recovered within 14 d after the initial event and after the TDG returned to normal. However, the frogs remained off-study for a minimum of 3 mo thereafter and were not experimentally manipulated. At 3 mo after the event, 20 surviving, randomly selected frogs were hormonally primed to ovulate, according to the researcher's laboratory standard protocol.1 The researchers reported that 17 of the 20 (85%) frogs failed to lay eggs, and the remaining 3 frogs produced few (10 to 20 per frog; 20 to 100 expected) stage IV oocytes. Stage IV oocytes were collected from the 20 randomly selected frogs, and cytosolic extracts showed poor to no enzymatic activity. Histologic examination of the ovarian mass from one of these GBD-affected, but apparently fully recovered, 2- to 3-y-old frogs revealed irregularly shaped oocytes and numerous degenerating oocytes (3 or 4 per high-power field), with dispersed protein globules of albumin and a thickened outer pigmented layer (vitelline layer) and plasma membrane (theca) compared with those expected from a normal, healthy older frog (4 to 5 y; Figure 11).
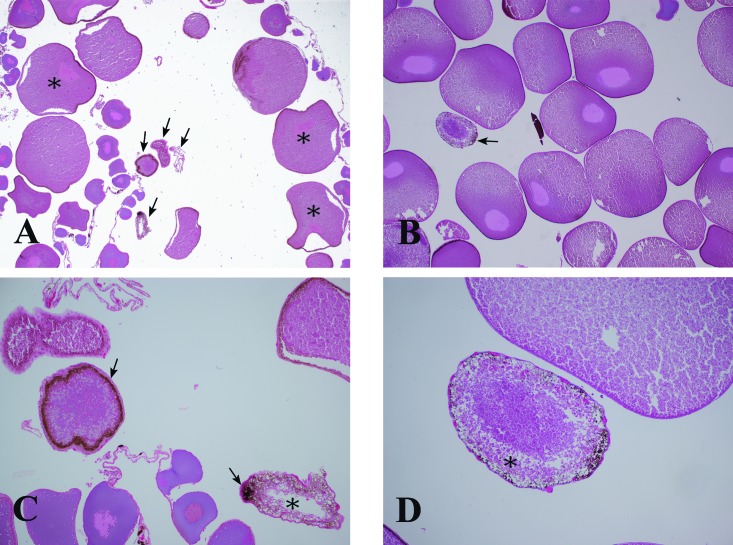
Figure 11.
(A) Cross-section through the ovary from a 2- to 3-y-old X. laevis. This frog was euthanized due to poor oocyte production at 3 mo after GBD. Note that when compared with (B) the ovaries of a healthy, older (4- to 5-y-old) frog, the affected frog has fewer large oocytes, and the many of the oocytes are irregularly shaped (*). Numerous degenerate oocytes (arrows) are present in the 2- to 3-y-old frog with GBD, compared with the older healthy frog. Hematoxylin and eosin stain; original magnification, 2.5×. At higher magnification (10×), the (C) degenerate oocytes from a 2- to 3-y old X. laeviswith GBD have a thickened, heavily pigmented vitelline envelope (arrows) and a thickened theca (the pink layer outside of the pigmented layer), compared with (D) those in an older, healthy frog. In addition, the oocytes from the frog with GBD tend to have severely diminished protein globules (asterisks) presumed to be albumin, whereas the protein globules in the degenerating oocyte of a 4- to 5-y-old, healthy X. laevis are only mildly decreased in density.
Six months after the GBD event, researchers primed and collected eggs from another 20 randomly selected, apparently healthy frogs. The eggs were insufficient in quantity or quality. Given the widespread, profound effects on the colony and importance of egg production to the lab's research, the researchers opted to cull the remainder of the colony and repopulate with new frogs. At necropsy (gross examination only), all 20 of the frogs that were examined (by DB and SG) at this time point appeared to be in good health, in good body condition and with good egg masses; however none of the egg masses from this cohort were examined histologically.
Discussion
Water pumped under pressure into housing systems for laboratory X. laevis can become supersaturated with gases due to human error or to defects in the water-delivery or mechanical systems.5,12,16 In the current report, water was pumped to campus under high pressures through the municipal water supply pipelines, where undetected microleaks in the piping allowed the entrance of air into the otherwise closed system. This situation resulted in supersaturation of the water supplied to the vivarium. The microcracks in the closed system created a situation in which air was ‘sucked’ into the water mainlines, and gases were forced under high pressure into solution.
Supersaturation is determined by measuring the sum of the saturation percentages of all of the gases dissolved in the water; the TDG percentage of the water under normal conditions is 100% or less.4 For X. laevis housing systems, the TDG percentage should not exceed 102%.12 The total dissolved gas percentage in the water described here was at least 112%.16 The severity of GBD in X. laevis appears to be related to the increase in partial pressures of argon and nitrogen n (ΔPAr+N2), and the higher the partial pressures, the more rapid the development and greater severity of the clinical signs of GBD.5 The high TDG reported here and the sudden exposure to supersaturated water may explain the severity of the clinical signs, particularly the fatal mesenteric infarcts.
The first step for degassing supersaturated water requires stopping the flow of incoming gas-saturated water.12 Aerating the water by using air stones, water diffusers, weirs, or waterfall-type water cascades also is critical to the treatment plan. The surface area of the aerated water bubbles provides a large air–water interface, thus enhancing the ability of entrained gases to escape directly to the atmosphere. In our case, the use of the fountain-head aerators in the holding tanks increased the air–water interface, increasing the transfer of dissolved gases from the water into the air.
The appearance of gas bubbles in the holding tanks and the resulting milky-looking turbidity were indications of supersaturation, but it is important to note that water can be supersaturated without displaying obvious turbidity, associated with water bubbles and a color change (for example, milky-looking water). To definitively monitor and avoid supersaturated water, aquaculture operations use saturometers, expensive and specialized instruments that measure the total pressure of gas in the water. The regular use of a saturometer is time-consuming and requires training. Not all research vivariums, especially those with small aquatics populations, have access to this equipment, particularly to saturometers that measure more than just TDG, including argon and nitrogen, specifically. Alternatively, measuring the DO by using membrane sensors in a probe (Yellow Stone Instrument Company) or by spectrophotometer or colorimetric tests (Hach Water Test Kits) are more affordable and readily available methods, although less direct indicators of entrained air in the water. Although not specific regarding which gases are entrained, DO measurements can provide laboratory animal staff with a rough indicator that the water is supersaturated, especially in the absence of visible bubbles in the water.18
After this incident, a direct line of communication was established with the municipal water company such that the animal facility receives alerts regarding construction or changes in the sourcing or treatment of the city's water supply. Since this GBD event, contemporary rack-style frog-housing modules have replaced the large, pond-style holding tanks formerly used across the campus. These low-flow, recirculating systems allow more control and monitoring of the water and include built-in mechanisms that enhance aeration and alarms that signal when entrained air, usually indicated by an increasing DO, is detected. Although gas bubbles can still occur in modular systems,16 usually due to mechanical failure or human error, the monitoring systems, when in place, alert staff quickly.
The acute deaths as a result of intestinal infarction have not been reported previously in laboratory X. laevis with GBD and, we believe, were related to the sudden exposure to very high TDG (exceeding 112%) in the water. The acute mortality rates (at 3.5%) initially were lower than what has been reported elsewhere5 (40% to 50%) and were probably mitigated by immediate suspension of the flow of incoming water, our aggressive aeration of the holding tanks, the water-conditioning treatments, and cessation of feeding and experiments. However, we did not anticipate the eventual euthanasia of the remaining colony due to decreased or failed production of eggs and oocytes after hormonal priming or of the production of oocytes that did not provide high-quality experimental materials (cytosolic extract preparations) as late as 6 mo after the event.
The time required for these frogs to eventually produce good-quality oocytes is unknown. The development of the oocyte and stages of egg maturation are described in detail13 and reviewed8 elsewhere. The X. laevis oocyte spends several months in the G2-like growth phase, starting as a stage I oocyte and maturing over several months, ultimately progressing to the large stage VI oocyte that is commonly used for extract preparation. The stage VI oocyte then enters what is essentially a G2-arrest state and can stay in that state indefinitely.8 The timeline for further maturation, which produces a ripe stage VI oocyte ready to be released for fertilization, is triggered by the steroid hormone progesterone, which is synthesized and released from follicle cells in response to pituitary hormones, is also variable. In the wild, the release of progesterone from the follicle coincides with warming water and environmental temperatures at the onset of spring.8 In the research laboratory, exogenous progesterone is administered to the frogs to induce maturation of the stage VI oocyte and egg laying.
Although the egg mass of mature X. laevis generally contains hundreds of oocytes at various stages of maturity, the maturation timeline for oocytes is often unpredictable, even when exogenous progesterone is administered under laboratory conditions. Oocyte maturation is dependent on various macro- and microenvironmental factors and is asynchronous, in that all of the oocytes in the egg mass do not progress to the next stage of development at the same time.8 Some oocytes do not progress at all, and others rapidly age and simply degenerate. The percentages of the egg mass in the various stages of oocyte development (or degeneration, for that matter) at a given point in time have not, to our knowledge, been described for X. laevis. The unpredictable oocyte maturation process, even in healthy, disease-free frogs, thus complicates estimating how long a frog needs to recover after illness and to respond to hormonal priming and how many (if any) of the remaining oocytes within the ovary are usable for research. Given the researcher's immediate need for oocytes and the uncertainties associated with frogs that might or might not ever produce sufficient numbers of good-quality oocytes, the colony was culled and replaced with new frogs.
Acknowledgments
We thank our staff veterinary pathologist, Corrine Davis, for her clinical contributions and for the histophotographs of the frogs in this report.
References
- 1.Bagowski CP, Xiong W, Ferrell JE., Jr 2000. C-jun N-terminal kinase activation in Xenopus laevis eggs and embryos. A possible nongenomic role for the JNK signaling pathway. J Biol Chem 276: 1459–1465. [DOI] [PubMed] [Google Scholar]
- 2.Belding DL, Merrill B. 1935. A preliminary report upon a hatchery disease of the salmonidae. Trans Am Fish Soc 65:76–84. [Google Scholar]
- 3.Bouck GR. 1980. Etiology of gas bubble disease. Trans Am Fish Soc 109:703–707. [Google Scholar]
- 4.Colt JE. 1983. The computation and reporting of dissolved gas levels. Water Res 17:841–849. [Google Scholar]
- 5.Colt J, Orwicz K, Brooks D. 1984. Gas bubble disease in the African clawed frog, Xenopus laevis. J Herpetol 18:131–137. [Google Scholar]
- 6.Colt J, Orwicz K, Brooks DL. 1987. Gas bubble trauma in the bullfrog Rana catesbeiana. J World Aquac Soc 18:229–236. [Google Scholar]
- 7.Ebel WJ. 1969. Supersaturation of nitrogen in the Columbia River and its effect on salmon and steelhead trout. Fish bulletin (Washington, D.C.: 1971) 68:1–11. [Google Scholar]
- 8.Ferrell JE., Jr 1999. Xenopus oocyte maturation: new lessons from a good egg. Bioessays 21:833–842. [DOI] [PubMed] [Google Scholar]
- 9.Fickeisen DH, Schneider MJ, Wedemeyer GA. 1980. Gas bubble disease introduction. Trans Am Fish Soc 109:657–658. [Google Scholar]
- 10.Gorham FP. 1901. The gas-bubble disease of fish and its cause. Bul US Fish Comm 19:33–37. [Google Scholar]
- 11.Green SL, Bouley DM, Tolwani RJ, Waggie KS, Lifland BD, Otto GM, Ferrell JE., Jr 1999. Identification and management of an outbreak of Flavobacterium meningosepticum infection in a colony of South African clawed frogs (Xenopus laevis). J Am Vet Med Assoc 214:1833–1838, 1792–1793. [PubMed] [Google Scholar]
- 12.Green SL. 2009. Gas bubble disease, p 95– 98. In: The laboratory Xenopus sp. Boca Raton (FL): CRC Press. [Google Scholar]
- 13.Hausen P, Riebesell M. 1991. The early development of Xenopus laevis: an atlas of the histology. New York:Springer-Verlag. [Google Scholar]
- 14.Marsh MC, Gorham FP. 1905. The gas disease in fishes. p 343–376. In: Report of US Bureau of Fisheries. Washington (DC): Government Printing Office. [Google Scholar]
- 15.McLeod GC. 1978. The gas bubble disease of fish, Chapter 8. p 319–339. In: Mostofsky DL. The behavior of fish and other aquatic animals. New York: Academic Press. [Google Scholar]
- 16.Roble GS, White J, Lieggi C, Lipman NS. 2010. Pathologic changes in Xenopus laevis exposed to an acute reduction in conductivity. J Am Assoc Lab Anim Sci 49:743–744. [Google Scholar]
- 17.Weitkamp DE, Katz M. 1980. A review of dissolved gas supersaturation literature. Trans Am Fish Soc 109:659–702. [Google Scholar]
- 18.YSI Environmental. [Internet] 2005Environmental dissolved oxygen values above 100% air saturation. [Cited 27 June 2016]. Available at: https://www.ysi.com/File%20Library/Documents/Technical%20Notes/T602-Environmental-Dissolved-Oxygen-Values-Above-100-percent-Air-Saturation.pdf