Abstract
Marsh rice rats (Oryzomys palustris) fed a pelleted diet high in sucrose and casein have been used as a model for moderate to severe periodontitis. Here we characterize the prevalence, location, and histopathologic features of food-impaction lesions (FIL), a unique type of oral event, in rice rats fed standard pelleted rodent chow from weaning until 34 wk of age. Healthy female rats (n = 90; age, 4 wk) were weaned into groups (n = 10 to 24) and were euthanized at 4, 16, 22, 28, or 34 wk of age. At necropsy, high-resolution photographs of the 4 jaw quadrants were examined by 3 independent observers to determine the presence, number, and location of FIL. In addition, gross periodontitis was scored (scale, 0 to 4), and the hemimaxillar surface area containing FIL was measured. Serial sections of decalcified jaws were assessed histologically. The prevalence of FIL increased with age, and was 0% (baseline), 59.1%, 69.6%, 81.8% and 80.0% in rats at age 4, 16, 22, 28, and 34 wk, respectively. FIL were predominantly located (93.9%) in the maxillary palatal surfaces of the interproximal area between molars 2 and 3 and did not affect mandibular surfaces. The percentage of the hemimaxillar surface area occupied by FIL was 6.83%, 4.82%, 2.88%, and 6.52% in rats at age 16, 22, 28, and 34 wk, respectively. Histopathologic changes in FIL varied from localized gingivitis to larger, localized periodontitis-like lesions. These data indicate that FIL are common in rice rats fed standard rodent chow, are slight to mild in severity, and are localized to specific regions in the oral cavity, thus suggesting they may be a suitable model for local maxillary periodontitis when fed standard rodent chow.
Abbreviations: ARONJ, antiresorptive-related osteonecrosis of the jaw; FIL, food-impaction lesions
Dental food-impaction lesions (FIL) occur in several laboratory rodent species. These impactions typically contain hairs and vegetable fibers, suggesting that the grooming habits and physical properties of plant-based rodent chow may play a role in development of these lesions.4,32 Pelleted rodent chow has been associated with the development of calculus and FIL in rats (Rattus norvegicus), in which food particles can penetrate the gingival epithelium, causing local irritation, inflammation, and recession of gingiva sufficient to involve the root furcation.34 Similarly, hamsters (Mesocricetus auratus) fed a granular diet display marked interproximal FIL, associated with epithelial detachment from the tooth, inflammation, and resorption of the interproximal alveolar crest; these effects tend to be more severe in the periodontium of maxillary molars than in mandibular molars.22,28,35
Food and hair impaction have also been reported in the oral cavity of marsh rice rats (Oryzomys palustris), a model for studying the pathophysiology of periodontitis.4,31 In those reports, electron or light microscopy demonstrated impacted food debris and hair in the jaws of rice rats maintained on a natural-ingredient diet. Rice rats are extraordinarily susceptible to the initiation and progression of a spontaneous form of periodontitis that requires no intraoral mechanical manipulation.1,13-15 Periodontitis appears by age 10 to 18 wk in this species,12,13,25 compared with humans, in which periodontitis takes years to develop.3,4,12-14,16,31 To accelerate the development of periodontitis, most recent studies in rice rats use a diet high in sucrose and casein (H-SC).1,2 Although periodontal lesions in rice rats fed a H-SC diet typically affect both mandibles and maxillae and have distinct characteristics, including gingivitis, destruction of the periodontium, and alveolar bone loss,1 interproximal impaction of food fibers or hairs is not a usual feature of these lesions.4 Despite these observations, the overall prevalence, location, and histopathologic characteristics of FIL in animals fed standard rodent chow have not been reported.
Characterizing FIL in rice rats is important for researchers using this model, because many studies of rice rats involve oral health outcomes. This information also is important for animal husbandry and veterinary staff, given that FIL may lead to reduced food intake and therefore decreased body weight. This study is the first to systematically describe the prevalence, location, and histopathologic features of maxillary FIL in rice rats fed standard rodent chow, a different type of oral event than those previously reported.
Materials and Methods
Animal source.
We received 30 pairs of marsh rice rats from Dr Kent Edmonds (Department of Biology, Indiana University Southeast, New Albany, IN). Immediately after arrival, the colony-derived rodents were maintained in a biocontainment facility (Biosafety Level 2) for 6 mo before being transferred to a conventional rodent housing room. During this period, rice rats were euthanized and necropsied every 4 wk to monitor for the presence of clinical signs compatible with infectious diseases, pathogens, or antibodies to infectious agents. CD1 mice (Mus musculus) and CD rats (Rattus norvegicus) were exposed to soiled bedding from rice rat cages for a minimum of 6 wk before being submitted for necropsy, serology, and parasitology. Sentinels were monitored for rat and mouse viruses, bacteria, and ecto- and endoparasites, as previously described.3
Animals and experimental groups.
The data presented in this report represent part of a large-scale study into the relationship of periodontitis to antiresorptive-related osteonecrosis of the jaw (ARONJ) in rice rats. Rice rats were paired by using a monogamous continuous-breeding system. After a lactation period of approximately 4 wk, litters were weaned, and 90 female pups were selected as the study population. These clinically normal, healthy female rats were randomized into 5 experimental groups after weaning, with one group euthanized at age 4 wk (baseline; n = 24). The remaining groups were fed pelleted rodent chow (irradiated diet no. 7912, Teklad LM-485 Rodent Diet; Envigo, Tampa, FL) and then euthanized at age 16 wk (n = 22), 22 wk (n = 23), 28 wk (n = 11), or 34 wk (n = 10). Experimental animals were group-housed (2 to 5 rats per cage) in static filter-top cages (area, 143 in2) with pine-shaving bedding and continuous access to food and water. The housing room was maintained at 68 to 79 °F (20.6 to 26.1 °C), with an average humidity of 30% to 70% and a 12: 12-h light:dark cycle. Body weight was measured every 2 wk. The Animal Care Services resource at the University of Florida is an AAALAC-accredited animal care and use program. The animal protocol was approved by the University of Florida IACUC. All animal care and experimental procedures were in accordance with federal policies and guidelines of the University of Florida IACUC. Adequate measures were taken at all times to minimize pain and discomfort.
Assessment of FIL.
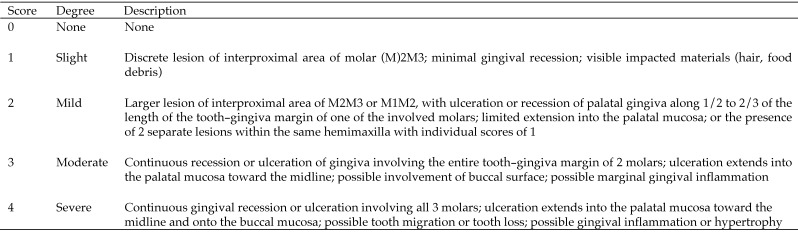
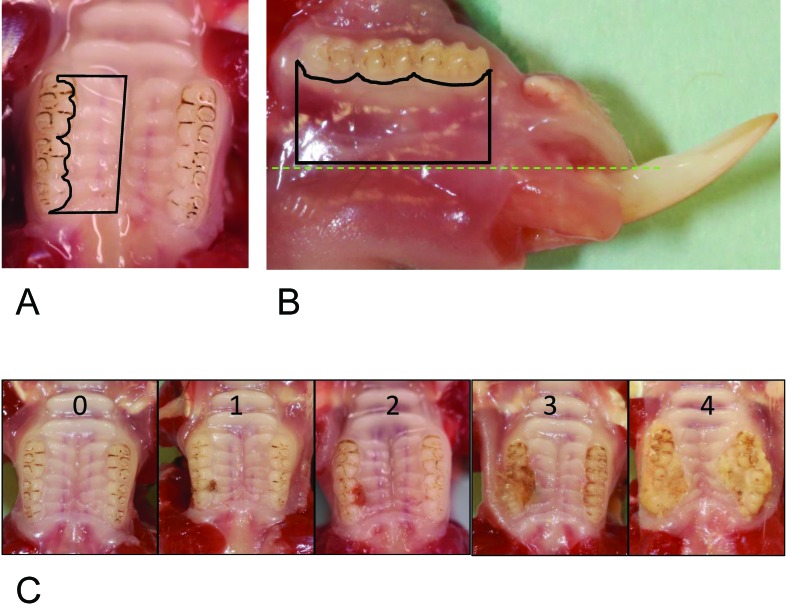
Rice rats were euthanized by CO2 inhalation followed by cervical dislocation, and maxillae and mandibles were immediately excised and dissected. High-resolution photos of all jaw quadrants were obtained by using a digital camera (EOS 6D, Canon, Tokyo, Japan) attached to a macro lens (Canon EF 100 mm 1:2.8). Photos were analyzed in a blind fashion and a random order by 3 independent observers (JGM, DBK, and JIA). Photos of each jaw quadrant were assessed for gross periodontitis lesions and assigned a severity score according to a 5-point scale (Figure 1; Figure 2 C). The number, location, size, and severity of FIL were analyzed by using AxioVision SE64 Rel software (version 4.9.1, Carl Zeiss, Oberkochen, Germany). This software allowed ready display of images at various magnifications to facilitate evaluation of the excised jaws. The outline tool was used to measure the area and size of oral lesions and structures. The FIL area was expressed as percentage of total hemimaxillar surface area according to the formula FIL area = 100% × FIL area / total area. Means for lesional area were calculated only from rats with a periodontitis score of at least 1. Figure 2 A and B depict how the rectangular total hemimaxillar and mandibular surface areas were defined, respectively. The hemimaxilla was delineated mesially (cranially), by a straight line that originated at the center of the mesial surface of the first molar (M1), extending to the midsagittal line of the hard palate; medially, by a midsagittal line through the hard palate; distally, by a straight line from the midsagittal line that extended to the distal surface of M3; and laterally, by an irregular delineation along the palatal surfaces of M1 through M3. The mandible was delineated mesially by a straight line that crossed the mesial surface of M1; ventrally, by a line parallel to the occlusal surface that followed an imaginary track with the dorsal border of the incisor; distally, by a perpendicular line that crossed the distal surface of M3; and dorsally, by an irregular delineation along the lingual surfaces of M1 through M3. Left and right maxillae and mandibles were measured and analyzed independently.
Figure 1.
Scoring system for characterizing gross periodontal lesions in the maxillae of rice rats.
Figure 2.
Modality for calculating the total hemimaxillar or hemimandiblar areas and a visual presentation of the periodontitis scoring system used to characterize maxillary food-impaction lesions (FIL) in rice rats. (A) Black lines drawn by using the outline tool of the software program (AxioVision SE64 Rel software, version 4.9.1) demarcate the total hemimaxillar area. The lesional area was expressed as a percentage of the total hemimaxillar area. (B) Black lines drawn by using the outline tool of the software program demarcate the total mandibular area. Left and right mandibles were measured and analyzed independently. (C) Representative photos of the different periodontitis scoring values (0-4) used to characterize gross periodontal lesions associated with FIL observed in maxillae. In the present study, only hemimaxillae with periodontitis scores between 0 and 3 were observed.
Histopathologic analyses.
After photos were taken, maxillae were fixed in 4% paraformaldehyde for 48 h and then moved to 70% ethanol.1,2 Maxillae were decalcified in 5% formic acid, embedded in paraffin, and serially sectioned (thickness, 5 μm) in a mesiodistal plane from the midsagittal palatal plane toward the buccal surfaces of the maxillary molars. A total of 10 adequately aligned serial sections, 250 μm apart from each other, were obtained from each rat and stained with hematoxylin and eosin for histopathologic analysis. Histologic sections were reviewed by a board-certified veterinary pathologist (MKR).
Statistical analysis.
Data are expressed as the mean ± SEM for each group. Data were evaluated by using ANOVA followed by the Holm–Sidak test for multiple comparisons. When ANOVA assumptions regarding normality of data were not met, the nonparametric Kruskal–Wallis test was used. Regardless of the tests used, a P value less than 0.05 was considered to be statistically significant.
Results
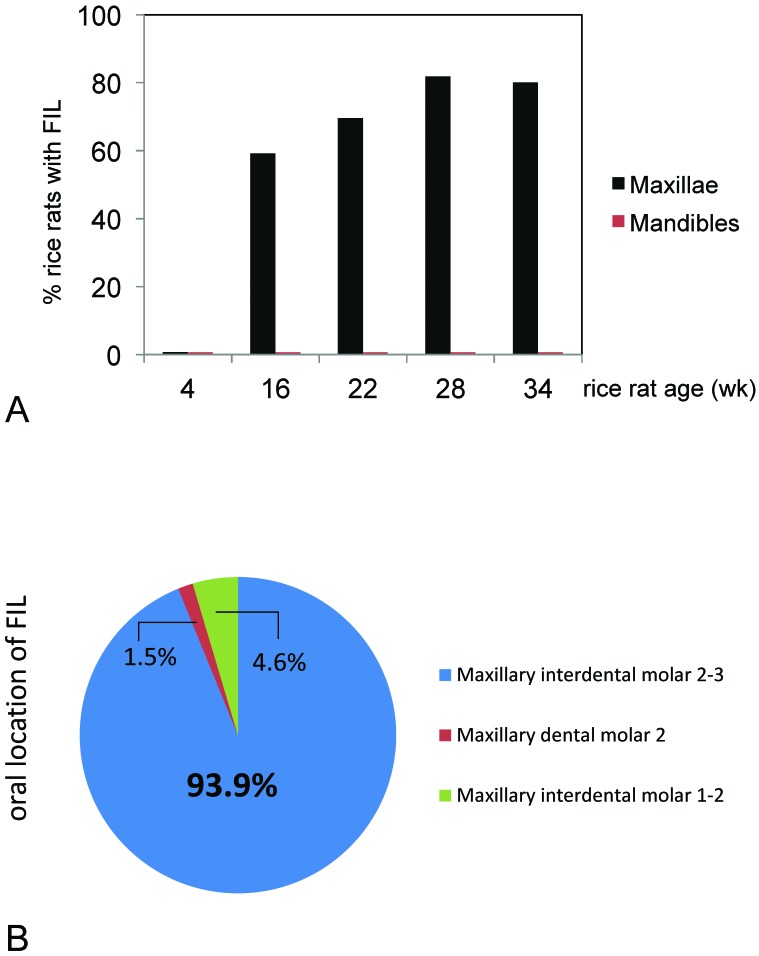
The 4-wk-old rice rats (baseline group) had healthy oral cavities, with no signs of FIL. In contrast, FIL were identified in the maxillae of older rats, with prevalences of 59.1%, 69.6%, 81.8%, and 80.0% of rats 16, 22, 28, and 34 wk in age, respectively (Figure 3 A). Of the 66 rats 16 wk and older, 46 (69.7%) had at least one maxillary FIL, whereas the remaining 20 rats (30.3%) showed no evidence of FIL. Among all FIL, 93.9% occurred at the palatal surface of the interproximal area of M2 and M3, with 4.6% at the palatal surface of the interproximal area of M1 and M2 and 1.5% at the palatal surface of M2 (Figure 3 B). No FIL affected the mandible of any rat.
Figure 3.
(A) Prevalence of food-impaction lesions (FIL) in the maxillae and mandibles of rice rats. Rats were fed standard rodent chow from 4 to 34 wk of age. In contrast to the healthy status of the oral cavity of rice rats at age 4 wk, rats at age 16 wk and older displayed unilateral or bilateral FIL that occurred only in maxillae; no rats had FIL that affected the mandible. (B) FIL predominantly (93.9%) affected the maxillary palatal surface at the interproximal space between M2 and M3; only 4.6% of FIL occurred at the maxillary palatal surface of the interproximal area between M1 and M2, and 1.5% of FIL at the maxillary palatal surface of M2.
The FIL observed in this study received periodontitis scores ranging from 0 to 3; none met the criteria for a periodontitis score of 4. Lesions corresponding to gross periodontitis scores of 2 or 3 were significantly (P < 0.05) larger than lesions with a periodontitis score of 1. The lesional area (mean ± SEM) of FIL scored 1 was 2.35% ± 0.15%, whereas FIL scored 2 and 3 had surface areas of 8.42% ± 0.68% and 17.05% ± 7.00%, respectively. A periodontitis score of 1 was most prevalent, accounting for 61.5% of all lesions, whereas 27.7% of FIL received a periodontitis score of 2 and 10.8% were scored as 3.
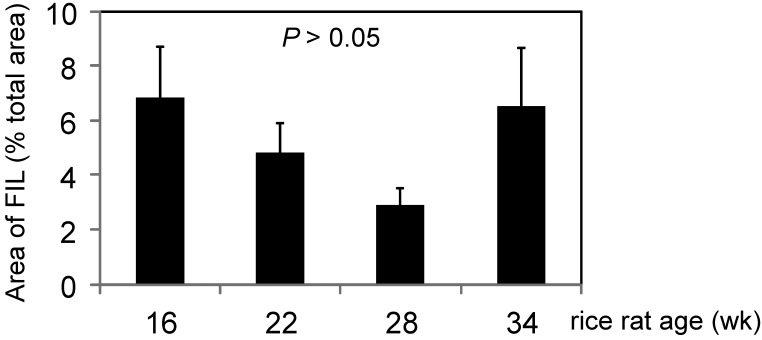
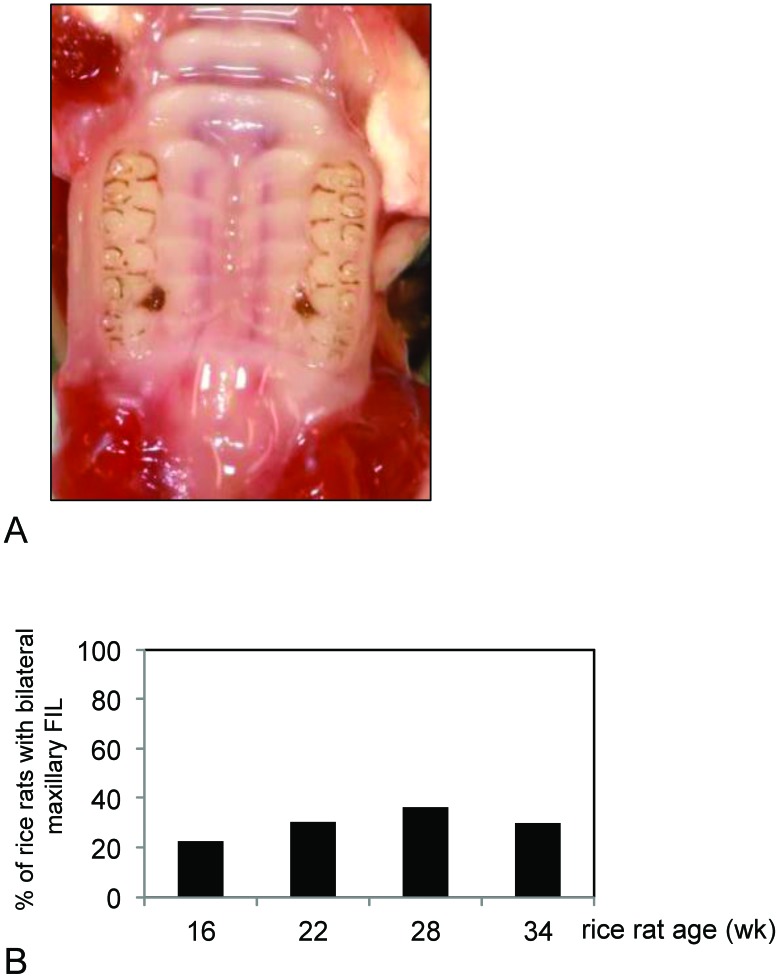
Periodontitis scores (mean ± SEM) for the hemimaxillae of all rice rats were 0.00 ± 0.00, 0.77 ± 0.16, 0.70 ± 0.12, 0.68 ± 0.14, and 1.00 ± 0.24 at 4, 16, 22, 28, and 34 wk of age, respectively. Rats 16 wk of age and older had higher (P < 0.05) mean periodontitis scores than did 4-wk-old rats. Mean lesional surface areas in affected rats were 6.83%, 4.82%, 2.88%, and 6.52% of hemimaxillar surface area at the ages of 16, 22, 28, and 34 wk, respectively (Figure 4). Five rats (3 at 16 wk; 1 each at 22 and 28 wk) had 2 noncontiguous lesions within the same hemimaxilla. In these cases, the FIL usually appeared in the right maxillae, with a larger lesion at M2M3 (5.76% ± 3.12%) and a smaller lesion at M1M2 (1.77% ± 0.85%). The 22-wk-old rat had 2 lesions in both the left and right maxillae. Some rats exhibited FIL bilaterally (in both hemimaxillae; Figure 5 A), with prevalences of 23.8%, 30.4%, 36.4%, and 30.0% in rice rats 16, 22, 28, and 34 wk of age, respectively (Figure 5 B).
Figure 4.
Area (mean ± SEM) of food impaction lesions (FIL) expressed as a percentage of total hemimaxilla area for rice rats aged 16, 22, 28, and 30 wk. FIL area with a periodontitis score of at least 1 did not differ significantly between rice rats aged 16, 22, 28, and 34 wk.
Figure 5.
Bilateral food impaction lesions (FIL) in the maxillae of rice rats. (A) Representative photo demonstrates bilateral maxillary FIL (periodontitis score of 1) involving the maxillary interproximal area between molar (M) 2 and M3. (B) Percentages of rats with bilateral FIL at 16, 22, 28, and 34 wk of age.
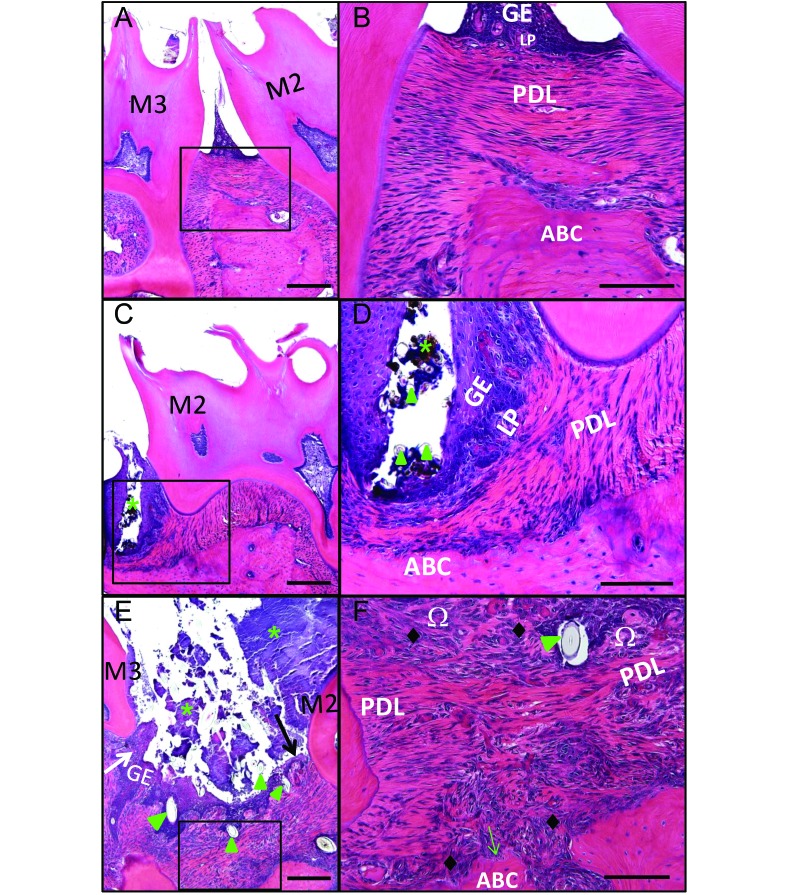
The histologic features of the periodontium of rats with periodontitis score 0 showed a normal gingival epithelium, with no changes in the lamina propria, periodontal ligament, or alveolar bone crest (Figure 6 A and B). Periodontal lesions in affected rats varied depending on the severity and extent of the oral lesions. FIL corresponding to a gross periodontitis score of 1 were histopathologically characterized by localized gingivitis, with debris, bacterial plaque, hair shafts, and vegetable fibers, generally located at and limited to the M2M3 interproximal space (Figure 6 C and D). The impacted materials were generally enclosed by interproximal gingival epithelium, which was moderately hyperplastic but not disrupted. Intraepithelial inflammatory cell infiltration or slight inflammatory infiltration in the lamina propria typically was present around the impaction, but neither the periodontal ligament nor the alveolar bone crest demonstrated any noteworthy changes (Figure 6 C and D). Lesions achieving gross periodontitis scores of 2 or 3 frequently contained similar impactions of hair shafts and vegetable fibers in the gingival epithelium, accompanied by dense bacterial plaque (Figure 6 E and F). They also included moderate hyperplasia and erosion or ulceration of the gingival epithelium, moderate inflammatory cell infiltration of the gingival epithelium or lamina propria, disruption of the periodontal ligament or transseptal fibers, empty osteocytic lacunae in the apical region of the alveolar bone crest, resorption of palatal bone or the alveolar bone crest, and fibrosis of the lamina propria (Figure 6 E and F).
Figure 6.
Histopathologic changes associated with maxillary food impaction lesions (FIL) in rice rats. Comparative photos taken at the interproximal space between maxillary molar (M)2 and M3 of representative rats fed standard diet. The photos on the right are magnified views of the inset in the corresponding left image. (A, B) The periodontium of the rat with periodontitis score 0 has a normal gingival epithelium (GE), with no changes in the lamina propria (LP), periodontal ligament (PDL), or alveolar bone crest (ABC). (C, D) Food impaction lesions corresponding to a gross periodontitis score of 1 were histopathologically defined as localized gingivitis. These oral lesions were represented by a cavity filled with organic debris, bacterial plaque (*), and hair shafts or vegetable fibers (green arrowheads), generally located at the interproximal space between M2 and M3. The size was limited to the area between these 2 molars. The cavities were generally enclosed by interproximal GE, which was moderately hyperplastic but intact (not disrupted). Intraepithelial inflammatory cell infiltration or slight inflammatory infiltration in the LP frequently were present, surrounding the cavity. However, neither the PDL nor ABC showed noteworthy changes. Food impaction lesions corresponding to gross periodontitis scores of 2 and 3 were larger than those with a score of 1. (E, F) Lesions with a gross periodontitis of score 3 were histopathologically characterized by moderate hyperplasia (white arrow) and erosion or ulceration of the GE (black arrow), moderate inflammatory cell infiltration of the GE or LP (Ω) or both, disruption of the PDL or transseptal fibers or both, ABC resorption, fibrosis of the lamina propria (♦), and the presence of hair shafts or vegetable fibers (green arrowheads). Bacterial plaques (*) were always present in lesions of this severity. Hematoxylin and eosin stain; bars: 200 μm (A, C, E), 100 μm (B, D, F).
Discussion
The current study is the first to systematically describe the features and prevalence of FIL in marsh rice rats (Oryzomys palustris) maintained on standard rodent chow. The characterization of FIL in rice rats fed standard rodent chow offers new information that supports this species as being susceptible to spontaneous localized periodontitis, which is distinct from that of the more well-established rice rat model of periodontitis that requires feeding of a H-SC diet. In addition, our findings provide insight into the oral health of rice rats and related species which is required for maintaining these animals as research models.
In the current study, FIL in rice rats fed a standard chow diet exhibited 3 attributes: 1) high prevalence that increased with age; 2) slight to mild severity; and 3) high specificity of location within the oral cavity. Of adult rice rats (age, 16 wk or greater) in this study, 70% developed at least one FIL. Within specific age groups, 59.1% of 16-wk-old rice rats had at least one FIL, whereas by 28 wk, more than 80% of rats had at least one FIL. These data indicate that FIL are a common occurrence in this species under laboratory conditions that include standard rodent chow. Most affected rats had slight to mild FIL. Specifically, 89% of lesions (58 of 65) were considered slight to mild, receiving a score of 1 or 2, whereas only 10.8% (7 of 65) were considered moderate in severity and thus earned a score of 3. Furthermore, the percentage lesional surface area did not differ between age groups, and periodontitis scores were not higher in older animals compared with younger rats, suggesting the possibility that lesions do not progress in severity with prolonged exposure to the standard chow food. A small percentage of rats had periodontitis lesions that scored 3 (that is, FIL area of 25% or less), with more than half of these cases occurring in the 16-wk group. These cases suggest that some rats may be more susceptible to early initiation of lesions. Whether lesions of increased size and severity resulted from a single lesion or were the product of 2 lesions within the same hemimaxilla that progressed and eventually merged is unclear. Longitudinal experiments in rats between 4 and 16 wk of age are required to fully characterize the initiation of the impaction and the progression of FIL.
In the current study, FIL affected maxillae only and were almost always located interproximal to M2M3. Furthermore, among rats with 2 lesions in the same maxillae, the larger lesion was typically located interproximal to M2M3, suggesting that M2M3 lesions started before the M1M2 lesions or that this location facilitated the more rapid progression of the FIL to a larger size and severity, compared with FIL at M1M2. These data, along with studies in golden hamsters 22,27,28,35, also a member of family Cricetidae, suggests that the locational specificity is associated with anatomic features of the teeth and the chewing style of these species. The proximity of the opening of a major salivary gland to M2M3 may contribute to the specificity of this location in rice rats and is a phenomenon analogous to that in ferrets, in which the initial injury of the gingivae and surrounding structures that result in periodontitis is induced by calculus formed in the openings of the ducts of these glands.22
FIL have been previously described in other laboratory animal species, including dogs, rats, mice, and hamsters.8,22,23,28, 29,34,35 Hamsters housed in wood shavings and maintained on a coarse laboratory chow diet over approximately 98 d developed mild periodontitis, usually in the form of interproximal lesions in the maxillae, associated with impaction of fibrous food and hair between teeth.27 Maxillary M2 and M3 were the most prevalent locations of gross FIL in golden hamsters.22 Macroscopically, these lesions were characterized by deposits of food particles and foreign bodies, such as sawdust and hairs, on and between the teeth, with adjacent periodontal involvement. Not surprisingly, rice rats (Oryzomys palustris), a species that belongs to the same family as hamsters (Cricetidae), develop similar maxillary lesions. Together, these findings suggest that cricetid rodents are particularly susceptible to oral lesions when maintained on natural-ingredient diets, a potentially important consideration when selecting these animals as model organisms for any condition that requires maintaining them in the laboratory.
The physical properties of food influence periodontal health and contribute to the initiation of gingival lesions in rodents and other laboratory species.22,23,28,29,34,35 The differences in the distribution and appearance between the periodontal lesions of rice rats fed H-SC diet compared with standard rodent chow likely can be attributed, at least in part, to the different physical properties of the feeds. Similar findings in previous studies indicate that a ‘mixed-grain’ natural diet resulted in the impaction of hair between the maxillary molars of rice rats, whereas these hair impactions were largely absent in animals fed semipurified diets or ground rodent chow.4 In the current study, the pelleted standard rodent chow (Teklad LM-485 Mouse/Rat Diet, no. 7912), consisted primarily of plant-based sources (ground corn, ground outs, wheat middlings, alfalfa meal), whereas H-SC pellets are a semipurified diet that is based on the AIN-93G diet formulation for rodents and consist primarily of sucrose (67%), casein (20%), corn oil (5%), and powdered cellulose (3%).
Although the use of H-SC diet to rapidly induce periodontal disease in rice rats remains a valuable model for studying periodontitis, future studies in rice rats focusing on oral health outcomes should consider these findings in animals fed standard rodent chow. Specifically, maintaining rice rats on standard chow with an expectation that they will not develop periodontitis lesions is unrealistic, given that this food produces alterations in oral health. In addition, we noted that body weights at necropsy tended to be lower in rice rats with FIL, suggesting that lesions may interfere with food intake. Therefore, results from longer-term studies that use standard chow-fed rice rats must be interpreted with caution. This consideration is also important for the husbandry and veterinary staff that monitor the health of rice rats in the laboratory, given that FIL-affected rats may exhibit decreased body weight. Our study suggests that further investigation is warranted to formulate a standard rodent chow that maintains FIL-free rice rats as improved controls for oral and dental research studies.
Our current findings suggest that this model of local periodontitis in the context of feeding a standard rat laboratory chow will benefit dental research, as well as research related to cancer and skeletal health. Food impaction is a highly prevalent clinical condition in humans that arises due to the forcing of food (particularly residues or fibers) between teeth, potentially leading to pain, gingivitis, periodontitis, halitosis, gingival abscess, alveolar bone resorption, root caries, and eventually tooth decay.9 Food impaction lesions have been reported in follow-up studies of implant prostheses,33 to induce periimplantitis,6 and to affect the embrasures between implant-supported fixed dental prostheses and adjacent teeth.19
In addition, this rice rat model may be valuable for the study of spontaneous ARONJ, which may be directly related to periodontitis or to localized dental lesions in the gingiva or mucosa. ARONJ is defined as exposed, necrotic bone in the maxillofacial region that has persisted for more than 8 wk with no history of radiation therapy to the jaws in persons who have taken potent bone antiresorptive medications.20,30 In addition, two thirds of patients with ARONJ who received oncologic doses of antiresorptive medications have had a recent discrete oral event (for example, oral lesion, tooth extraction, oral surgery),5,7,10,11,17,18,21,24,26 suggesting that injury to soft or hard tissues in the jaw is a predisposing factor for the development of ARONJ. Food impaction lesions may replicate this discrete injury and subsequent inflammation, similar to that seen in humans who have had a recent oral event.26 A reliable model of localized periodontitis is a fundamental tool for studying this disorder and enables the scientific community to improve our understanding of the pathophysiology of ARONJ.
Acknowledgments
This research was supported by NIH grant R01DE023783-01A from the National Institute of Dental and Craniofacial Research (NIDCR).
References
- 1.Aguirre JI, Akhter MP, Kimmel DB, Pingel J, Xia X, Williams A, Jorgensen M, Edmonds K, Lee JY, Reinhard MK, Battles AH, Kesavalu L, Wronski TJ. 2012. Enhanced alveolar bone loss in a model of noninvasive periodontitis in rice rats. Oral Dis 18:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre JI, Akhter MP, Kimmel DB, Pingel JE, Williams A, Jorgensen M, Kesavalu L, Wronski TJ. 2012. Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. J Bone Miner Res 27:2130–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguirre JI, Edmonds K, Zamora B, Pingel J, Thomas L, Cancel D, Schneider L, Reinhard MK, Battles AH, Akhter MP, Kimmel DB, Wronski TJ. 2015. Breeding, husbandry, veterinary care, and hematology of marsh rice rats (Oryzomys palustris), a small animal model for periodontitis. J Am Assoc Lab Anim Sci 54:51–58. [PMC free article] [PubMed] [Google Scholar]
- 4.Auskaps AM, Gupta OP, Shaw JH. 1957. Periodontal disease in the rice rat. III. Survey of dietary influences. J Nutr 63:325–343. [DOI] [PubMed] [Google Scholar]
- 5.Badros A, Weikel D, Salama A, Goloubeva O, Schneider A, Rapoport A, Fenton R, Gahres N, Sausville E, Ord R, Meiller T. 2006. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol 24:945–952. [DOI] [PubMed] [Google Scholar]
- 6.Bidra AS. 2014. Nonsurgical management of inflammatory periimplant disease caused by food impaction: a clinical report. J Prosthet Dent 111:96–100. [DOI] [PubMed] [Google Scholar]
- 7.Bilezikian JP. 2006. Osteonecrosis of the jaw—do bisphosphonates pose a risk? N Engl J Med 355:2278–2281. [DOI] [PubMed] [Google Scholar]
- 8.Burwasser P, Hill TJ. 1939. The effect of hard and soft diets on the gingival tissues of dogs. J Dent Res 18:389–393. [Google Scholar]
- 9.Du H, Gao M, Qi C, Liu S, Lin Y. 2010. Drug-induced gingival hyperplasia and scaffolds: they may be valuable for horizontal food impaction. Med Hypotheses 74:984–985. [DOI] [PubMed] [Google Scholar]
- 10.Durie BG, Katz M, Crowley J. 2005. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med 353:99–102. [DOI] [PubMed] [Google Scholar]
- 11.Filleul O, Crompot E, Saussez S. 2010. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2400 patient cases. J Cancer Res Clin Oncol 136:1117–1124. [DOI] [PubMed] [Google Scholar]
- 12.Gotcher JE, Jee WS. 1981. The progress of the periodontal syndrome in the rice rat. I. Morphometric and autoradiographic studies. J Periodontal Res 16:275–291. [DOI] [PubMed] [Google Scholar]
- 13.Gupta OP, Shaw JH. 1956. [Periodontal disease in the rice rat. I. Anatomic and histopathologic findings.] Oral Surg Oral Med Oral Pathol 9:592–603.[Article in French]. [DOI] [PubMed] [Google Scholar]
- 14.Gupta O, Shaw J. 1956. [Periodontal disease in the rice rat. II. Methods for the evaluation of the extent of periodontal disease.] Oral Surg Oral Med Oral Pathol 9:727–735.[Article in French]. [DOI] [PubMed] [Google Scholar]
- 15.Gupta OP, Shaw JH. 1956. The relation of a chelating agent to smooth-surface lesions in the white rat. J Nutr 60:311–322. [DOI] [PubMed] [Google Scholar]
- 16.Hattler AB, Snyder DE, Listgarten MA, Kemp W. 1977. The lack of pulpal pathosis in rice rats with the periodontal syndrome. Oral Surg Oral Med Oral Pathol 44:939–948. [DOI] [PubMed] [Google Scholar]
- 17.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, Nooka A, Sayegh G, Guarneri V, Desrouleaux K, Cui J, Adamus A, Gagel RF, Hortobagyi GN. 2008. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res 23:826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadu F, Lee L, Pharoah M, Reece D, Wang L. 2007. A retrospective study assessing the incidence, risk factors, and comorbidities of pamidronate-related necrosis of the jaws in multiple myeloma patients. Ann Oncol 18:2015–2019. [DOI] [PubMed] [Google Scholar]
- 19.Jeong JS, Chang M. 2015. Food impaction and periodontal, peri-implant tissue conditions in relation to the embrasure dimensions between implant-supported fixed dental prostheses and adjacent teeth: a cross-sectional study. J Periodontol 86:1314–1320. [DOI] [PubMed] [Google Scholar]
- 20.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E. 2007. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 22:1479–1491. [DOI] [PubMed] [Google Scholar]
- 21.King AE, Umland EM. 2008. Osteonecrosis of the jaw in patients receiving intravenous or oral bisphosphonates. Pharmacotherapy 28:667–677. [DOI] [PubMed] [Google Scholar]
- 22.King JD. 1949. Histologic observations on parodontal disease in the golden hamster (Cricetus auratus): calculus, food particles, and other foreign bodies as aetiological factors. J Pathol Bacteriol 61:413–425. [Google Scholar]
- 23.Klingsberg J, Butcher EO. 1959. Aging, diet, and periodontal lesions in the hamster. J Dent Res 38:421. [DOI] [PubMed] [Google Scholar]
- 24.Kyrgidis A, Vahtsevanos K, Koloutsos G, Andreadis C, Boukovinas I, Teleioudis Z, Patrikidou A, Triaridis S. 2008. Bisphosphonate-related osteonecrosis of the jaws: a case-control study of risk factors in breast cancer patients. J Clin Oncol 26:4634–4638. [DOI] [PubMed] [Google Scholar]
- 25.Leonard EP. 1979. Periodontitis. Animal model: periodontitis in the rice rat (Oryzomys palustris). Am J Pathol 96:643–646. [PMC free article] [PubMed] [Google Scholar]
- 26.Marx RE, Sawatari Y, Fortin M, Broumand V. 2005. Bisphosphonate-induced exposed bone (osteonecrosis,osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 63:1567–1575. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell DF. 1950. The production of periodontal disease in the hamster as related to diet, coprophagy, and maintenance factors. J Dent Res 29:732–739. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell DF, Chernausek DS. 1951. Two studies of periodontal disease in the Syrian hamster. J Dent Res 30:802–805. [DOI] [PubMed] [Google Scholar]
- 29.Person P. 1961. Diet consistency and periodontal disease in old albino rats. J Periodontol 32:308–311. [Google Scholar]
- 30.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. 2009. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg 67 5 Suppl:2–12. [DOI] [PubMed] [Google Scholar]
- 31.Ryder MI. 1980. Histologic and ultrastructural characteristics of the periodontal syndrome in the rice rat. I. General light microscopic observations and ultrastructural observations of initial inflammatory changes. J Periodontal Res 15:502–515. [DOI] [PubMed] [Google Scholar]
- 32.Ryder MI. 1980. Histologic and ultrastructural characteristics of the periodontal syndrome in the rice rat. II. Ultrastructural observations on changes in the gingival sulcus, gingival epithelium, and lamina propria. J Periodontal Res 15:574–584. [DOI] [PubMed] [Google Scholar]
- 33.Song YL. 2016. [The causes and treatment strategies of molar food impaction after implant restoration] Zhonghua Kou Qiang Yi Xue Za Zhi 51:7–9. [Article in Chinese]. [DOI] [PubMed] [Google Scholar]
- 34.Stahl SS, Dreizen S. 1964. The adaptation of the rat periodontium to prolonged feeding of pellet, powder, and liquid diets. J Periodontol 35:312–319. [Google Scholar]
- 35.Stahl SS, Miller SC, Goldsmith ED. 1958. Effects of various diets on the periodontal structure of hamsters. J Periodontol 29:7–14. [Google Scholar]