Abstract
The thalamus is the heavily interconnected partner of the neocortex. All areas of the neocortex receive afferent input from and send efferent projections to specific thalamic nuclei. Through these connections, the thalamus serves to provide the cortex with sensory input, and to facilitate interareal cortical communication and motor and cognitive functions. In the visual system, the lateral geniculate nucleus (LGN) of the dorsal thalamus is the gateway through which visual information reaches the cerebral cortex. Visual processing in the LGN includes spatial and temporal influences on visual signals that serve to adjust response gain, transform the temporal structure of retinal activity patterns, and increase the signal-to-noise ratio of the retinal signal while preserving its basic content. This review examines recent advances in our understanding of LGN function and circuit organization and places these findings in a historical context.
Keywords: retina, LGN, vision, primate, cat, mouse
INTRODUCTION
With the exception of the olfactory system, sensory information from the periphery is first processed in the thalamus before reaching the cerebral cortex. Although precortical thalamic processing is a fundamental feature of sensory systems, its role in sensory processing is often underappreciated. In the visual system, retinal ganglion cells (RGCs) project directly to the lateral geniculate nucleus (LGN) of the dorsal thalamus, which in turn projects to the primary visual cortex (V1). The LGN is considered a first-order thalamic nucleus on the basis of its close relationship with the retina. In contrast, the pulvinar nucleus—another thalamic nucleus involved in vision—is considered a second-order nucleus, as it primarily receives afferent input from the cortex. Although both thalamic nuclei play fundamental roles in visual processing, this review focuses on the LGN.
Historically, studies comparing visual responses in the retina and LGN have emphasized the high degree of similarity between the receptive fields of cells in these two structures. This similarity, along with the strong driving force that the retina has on the LGN, has led to a general view that the LGN does little in terms of processing or refining visual signals en route to cortex. Although the spatial organization of neuronal receptive fields in the retina and LGN are indeed quite similar, a closer examination of the activity patterns of RGCs and LGN neurons reveals major differences in the temporal structure of neuronal spike trains. These differences arise via a variety of mechanisms and serve to decrease noise in the signals transmitted to cortex and to adjust the efficacy of geniculocortical communication under different behavioral and cognitive states. In this review, we examine recent and past work on the organization of inputs to the LGN, the powerful influence of retinal inputs on evoking LGN responses, how retinal activity is transformed temporally within the LGN, and how nonretinal inputs influence these transformations. We also consider the powerful influence of behavioral state and network oscillations on the transmission of retinal information to V1. Taken together, these results extend our understanding of visual circuits in the thalamus, underscore the prevalence of dynamic processing of visual signals en route from retina to cortex, and emphasize the central role of the LGN in visual processing.
ANATOMICAL OVERVIEW
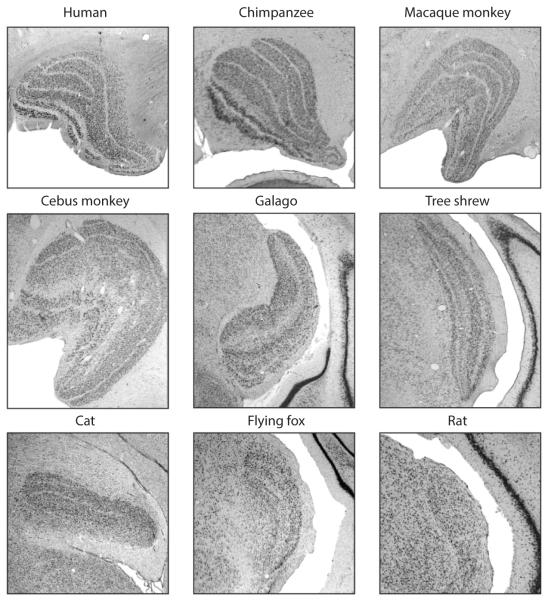
Convergence and divergence are present in the pathway from the retina to the LGN. Individual LGN neurons are estimated to receive synaptic input from one to five RGCs, and individual retinal axons provide synaptic input to multiple LGN neurons (Cleland 1986, Hamos et al. 1987, Reid & Usrey 2004). Although this convergence and divergence has functional implications for the activity profiles of LGN neurons (described in more detail below in the subsection titled “Remapping the Retinal Mosaic Increases the Efficacy and Resolution of the Visual Signal”) the tight retinotopic specificity of RGC–LGN connections ensures that presynaptic and postsynaptic receptive fields have similar sizes and shapes (Hubel & Wiesel 1961; Cleland et al. 1971a,b; Kaplan et al. 1987; Mastronarde 1987; Usrey et al. 1998, 1999; Levine & Cleland 2001; Rowe & Fischer 2001; Sincich et al. 2007, 2009; Weyand 2007; Rathbun et al. 2010). The position of the LGN between the retina and V1 is conserved across species, but its structural arrangement, including the number of layers and the organization of parallel processing streams with respect to these layers, can vary tremendously (Figure 1).
Figure 1.
Nissl-stained sections of the lateral geniculate nucleus (LGN) from nine different species: (top row) human, chimpanzee, rhesus macaque; (middle row) cebus monkey, galago, tree shrew; (bottom row) domestic cat, flying fox, rat. Adapted with permission from Usrey (2002a).
In Old World monkeys and in humans, the LGN is composed of two magnocellular layers, four parvocellular layers, and six koniocellular layers. As we discuss below (see the section titled “Cell Classes and Visual Physiology”), neurons in magnocellular, parvocellular, and koniocellular layers differ dramatically in terms of their visual physiology, a distinction evident in the responses of their afferent input. These layers also differ in their projections to V1: Magnocellular neurons project to layer 4Cα, parvocellular neurons project to layer 4Cβ, and koniocellular neurons project to layer 2/3.
Lamination in the feline LGN follows a different plan. The feline LGN has two principal layers, A and A1, which receive input from the contralateral and ipsilateral eyes, respectively. Both of these layers contain X- and Y-type neurons, and project axons to layer 4 of V1 (reviewed in Jones 2006). In addition to these layers, the feline LGN contains four C layers (Hickey & Guillery 1974) that contain Y- and/or W-type neurons: the Y-cells project to layer 4 of the cortex, and the W-cells project to the superficial layers.
Lamination is less evident in rodents; input from the contralateral eye extends across the entire LGN, and ipsilateral eye input overlaps within the binocular zone (Godement et al. 1984, Reese 1988; see also Howarth et al. 2014). Interest in rodent vision has increased rapidly over the past decade, and recent studies have identified a structural organization and segregation of cell types that is more complex than originally thought. For instance, RGCs that respond well to stimulus motion in the anterior and posterior directions provide input to a thin region along the edge of the rodent LGN, which contains neurons that provide input to direction-selective neurons in cortex (Huberman et al. 2009, Marshel et al. 2012, Piscopo et al. 2013, Cruz-Martín et al. 2014).
Independent of species and laminar classification, LGN neurons belong to one of two major classes, thalamocortical relay neurons and inhibitory interneurons, both of which receive monosynaptic excitation from the retina. Thalamocortical relay neurons are excitatory and provide glutamatergic excitation to target neurons in V1. Although historically thought to make no direct contribution to local processing, recent work in the cat has identified a sparse collateral projection from thalamocortical relay axons that provides synaptic input to other neurons in the LGN (Bickford et al. 2008). In contrast, LGN inhibitory interneurons provide only local, GABAergic inhibition within the LGN; they do not project to the primary visual cortex.
GENERAL PROPERTIES OF LATERAL GENICULATE NUCLEUS RECEPTIVE FIELDS
The prototypical, classical receptive fields of RGCs and LGN neurons have a circularly symmetric, spatially antagonistic, center–surround organization. On-center LGN neurons have a center subunit that is excited by luminance increments and suppressed by luminance decrements. In contrast, the surround subunit of on-center cells is excited by luminance decrements and suppressed by luminance increments. Off-center cells have the opposite polarity; the center is excited by dark and suppressed by light, and the surround is excited by light and suppressed by dark. This receptive field structure makes neurons selective for spatial position and phase, as well as for spatial frequency. In general, LGN neurons and their presynaptic retinal partners are not selective for stimulus orientation or direction of motion. As we discuss below (see the section titled “Cell Classes and Visual Physiology”), however, certain classes of LGN neurons in the rodent are selective for both orientation and direction of motion.
The center–surround organization of visual receptive fields was recognized more than 50 years ago (Kuffler 1952, 1953; Barlow et al. 1954). Regarding his recordings from cat RGCs, Steven Kuffler noted (1953; page 62), “In all fields there exists a central region giving a discharge pattern which is the opposite from that obtained in the periphery. The center may be predominately ‘off’, the surround ‘on’ or vice versa. In view of the fixed nature of the of the center discharge, it may be convenient to classify receptive fields into ‘on’ center and ‘off’ center.” The center–surround filter of early receptive fields appears to be fundamental for visual processing, as this receptive field organization has been observed in a wide range of species, including frogs (Barlow 1953), monkeys (De Monasterio & Gouras 1975), tree shrews (Conway & Schiller 1983), and mice (Stone & Pinto 1993). The center–surround receptive field can be modeled accurately using a difference of Gaussians (DOG) equation (Rodieck 1965, Rodieck & Stone 1965). In this model, receptive fields comprise two antagonistic linear subregions: The center and surround subunits (Gaussians) are spatially overlapping, and the center subunit is smaller in spatial extent but higher in peak amplitude than the surround subunit. Importantly, the surround subunit is not an annulus around the center subunit; instead, its presence in the center of the receptive field is overwhelmed by the strength of center subunit.
NONLINEAR RECEPTIVE FIELD PROPERTIES
Although the receptive fields of RGCs and LGN neurons can be approximated as linear filters and are indeed highly linear relative to those of cortical neurons, important nonlinear response properties, such as gain control (described in the following subsection), were identified early on from subcortical recordings. These nonlinearities are now recognized as standard features found in the retina, LGN, and visual cortex. Since their identification, the nonlinear features of the early visual system have been described in much greater detail (Bonin et al. 2005, Mante et al. 2008, Carandini & Heeger 2011).
Gain Control
The linear receptive field organization described by the DOG model provides specific predictions about the behavior of neurons that can be compared with measured responses. First, the linear DOG model predicts that the gain of a linear neuron should be constant, regardless of the strength of the visual stimulus. However, many RGCs and LGN neurons have markedly nonlinear contrast response functions that saturate at high contrasts (Enroth-Cugell & Robson 1966, Kaplan & Shapley 1986, Benardete et al. 1992, Usrey & Reid 2000, Alitto et al. 2011). The gains of these neurons are high at low contrast and low at high contrast. Second, the linear DOG model predicts that the temporal response properties of these neurons, such as response latency and duration, should be invariant as stimulus strength changes. Instead, the temporal response properties of RGCs and LGN neurons change dramatically as stimulus luminance and contrast increase (Shapley & Victor 1980, Benardete et al. 1992, Usrey & Reid 2000, Alitto & Usrey 2004, Mante et al. 2008). Responses to high-contrast stimuli have shorter latencies and durations relative to responses to low-contrast stimuli. Functionally, this difference makes visual neurons more sensitive to high temporal frequencies at high contrast and more sensitive to low temporal frequencies at low contrast.
Extraclassical Surround
Beyond the region of space defined as the classical receptive field is the extraclassical receptive field. In contrast to the classical receptive field, in which a stimulus of the preferred polarity excites the neuron (e.g., a black stimulus in the classical surround of an on-center receptive field excites an on-center neuron), in the extraclassical surround, visual stimulation of either polarity suppresses the activity of the neuron. Functionally, this behavior leads to a phenomenon known as extraclassical suppression that can be demonstrated by the area summation response function of a neuron (Jones et al. 2000, Solomon et al. 2002, Bonin et al. 2005, Alitto & Usrey 2008, Mante et al. 2008). An area summation function reveals the response of a neuron to visual stimuli of varying spatial diameters. If the visual stimulus is selected carefully, the magnitude and spatial extent of the extraclassical receptive field can be estimated from the area summation function. If the wrong stimulus is chosen, disambiguating suppression from the classical surround versus that from the extraclassical surround is difficult (Bonin et al. 2005, Alitto & Usrey 2008).
CELL CLASSES AND VISUAL PHYSIOLOGY
Primates
The primate LGN contains three types of layers—magnocellular, parvocellular, and koniocellular—that represent distinct parallel streams for visual processing. Years of investigation have demonstrated that the visual physiology, sources of retinal input, and synaptic targets within V1 differ among neurons in these layers (reviewed in Schiller & Logothetis 1990, Merigan & Maunsell 1993, Casagrande 1994, Casagrande & Kaas 1994, Lee 1996, Hendry & Reid 2000, Nassi & Callaway 2009). Briefly, magnocellular neurons respond faster, more transiently, and with greater response gain than parvocellular neurons do, whereas parvocellular neurons respond better to stimuli with high spatial frequencies and to stimuli with chromatic structure than do magnocellular neurons. These differences in the visual physiology of magnocellular and parvocellular neurons are established in the retina and are evident in the visual physiology of the RGCs that innervate them. The segregation of signals through the LGN is maintained in projections to V1; magnocellular and parvocellular neurons provide nonoverlapping input to cortical layers 4Cα and 4Cβ, respectively.
Beneath each magnocellular and parvocellular layer is a koniocellular layer containing neurons whose projections bypass cortical layers 4Cα and 4Cβ and terminate directly on neurons in the superficial layers of V1 (Fitzpatrick et al. 1983). Neurons in the koniocellular layers also differ from those in the magnocellular and parvocellular layers in many other respects. Most notably, in contrast to neurons in the magnocellular and parvocellular layers, which receive selective input from only one type of RGC (parasol or midget, respectively), the koniocellular layers receive input from a diverse array of RGCs, including the small and large bistratified cells, the recursive monostratified and bistratified cells, the narrow and broad thorny cells, and the smooth and sparse inner cells (reviewed in Dacey & Packer 2003; Wässle 2004; Masland 2011, 2012; Crook et al. 2014). Moreover, in contrast to neurons in the magnocellular and parvocellular layers, which have monocular responses, some neurons in the koniocellular layers have binocular responses (Cheong et al. 2013), suggesting that individual koniocellular neurons combine retinal inputs either directly or through polysynaptic circuits. Finally, as evidence of the diversity of circuits that establish koniocellular responses, recent results have identified the full circuitry of a retinal pathway that supplies target neurons in the koniocellular layers with orientation and directional information, similar to that found in the mouse LGN (Cheong et al. 2013, Percival et al. 2014).
Felines
The feline LGN contains X cells, Y cells, and W cells. X cells are the most common cell type in the feline LGN and have highly linear center–surround receptive fields that are nonselective for either stimulus orientation or direction of motion (Enroth-Cugell & Robson 1966, Scholl et al. 2013). Although X cells are likely the first to have been fit to the linear DOG model, these cells are not completely linear: They often display a degree of contrast gain control and extraclassical suppression (Jones et al. 2000; see also Alitto & Usrey 2004). By comparison, Y cells exhibit stronger nonlinear responses, greater contrast gain control, and more robust extraclassical suppression. Perhaps the single greatest defining feature of Y cells is that they have high-frequency nonlinear receptive-field subunits that are made evident with a so-called null test. In contrast to X cells, which have a phase-dependent null point in their responses to counterphasing sinusoidal stimuli (as predicted for cells with linear responses), the responses of Y cells display a phase-dependent frequency doubling. This response property, first observed by Enroth-Cugell & Robson (1966), is believed to result from nonlinear subunits (Hochstein & Shapley 1976, Derrington et al. 1979) contributed by rectifying amacrine cells and bipolar cells (Stafford & Dacey 1997, Demb et al. 2001). Several other key aspects of the response dynamics of Y cells also differ from those of X cells. In particular, Y cells have shorter visual response latencies, respond more transiently to visual stimuli, and can follow stimuli at higher temporal frequencies in comparison with X cells (Cleland et al. 1971a,b; Fukada 1971; Ikeda & Wright 1972; Usrey et al. 1999).
Rodents
Research on the mouse visual system has experienced tremendous growth in recent years, largely owing to the advent of molecular tools for identifying cell types, dissecting neural circuits, and manipulating activity. Although mice generally are not considered to be highly visual when compared with more traditional animal models used in vision research, dismissing results obtained from the mouse would be a mistake. Indeed, recent results from studies in mice, including those conducted in the retina and LGN, have provided valuable information that would be difficult or impossible to obtain in other model systems.
Importantly, electrophysiological and optical recordings from the mouse LGN have revealed greater diversity among cell types and response properties than has been reported for either cats or primates. This diversity includes cells that have receptive field properties generally considered more typical of V1 neurons in monkeys and cats, such as orientation selectivity and direction selectivity, and cells that have response properties not commonly found in the retinogeniculocortical pathway in more traditional animal models, such as neurons that encode the absence of contrast (Huberman et al. 2009, Marshel et al. 2012, Piscopo et al. 2013, Scholl et al. 2013, Cruz-Martín et al. 2014; see also Barlow et al. 1964). Although cells that have these more complex receptive field properties that are absent or rare in the principal layers of the LGN (those that project to cortical layer 4 in V1) in cats (A layers) and monkeys (magnocellular and parvocellular layers), they may exist in the C layers or koniocellular layers, which have received considerably less attention. In support of this possibility, anatomical and physiological studies have revealed a much greater diversity of RGCs with projections to these layers compared to the principal layers (reviewed in Dacey & Packer 2003; Wässle 2004; Masland 2011, 2012; Crook et al. 2014). Thus, results from the mouse may provide insight into the broader range of possible processing strategies utilized across mammals.
GENERAL PROPERTIES OF RETINOGENICULATE SYNAPSES
Although retinal axons provide a minority of synapses onto LGN neurons, RGCs are the primary drivers of LGN activity (Hubel & Wiesel 1961; Cleland et al. 1971a,b; Kaplan & Shapley 1984; Mastronarde 1987; Kaplan et al. 1987; Usrey et al. 1999; Rathbun et al. 2010; for review, see Sherman & Guillery 2009). Results indicate that more than 95% of LGN spikes are directly evoked by retinal inputs (Kaplan & Shapley 1984, Sincich et al. 2007). RGCs provide strong glutamatergic input onto the proximal dendrites of both LGN relay neurons and LGN local inhibitory neurons. Retinal excitatory postsynaptic potentials (EPSPs) are, relatively speaking, enormous; indeed, they are often sufficiently large that they can be measured using extracellular recording methods. These events, called S-potentials (Bishop et al. 1962), can be measured simultaneously with LGN action potentials and have therefore been useful for studies examining the responses of synaptically connected RGCs and LGN neurons.
The specificity and strength of retinal synaptic inputs onto LGN neurons can also be determined from simultaneous recordings from inside the eye and from the LGN (Cleland et al. 1971a,b). One advantage of this dual recording technique over S-potential recordings is its ability to study retinogeniculate communication for cell pairs with either strong or weak connections (Mastronarde 1987, Usrey et al. 1999). This feature is useful because the ensemble of RGCs that provide convergent input onto an LGN neuron usually includes one cell with strong connectivity and the remainder with weaker connectivity (Cleland 1986, Usrey et al. 1999). From these types of recordings, the strength of connectivity can be quantified using measures of efficacy (the percentage of retinal spikes that evoke an LGN response) and contribution (the percentage of LGN spikes evoked by a particular RGC) (Cleland et al. 1971a,b). Using S-potential and dual recording methods, the range of contribution values calculated for weakly and strongly connected cell pairs is 1–100% (Kaplan & Shapley 1984, Kaplan et al. 1987, Mastronarde 1987, Usrey et al. 1999, Levine & Cleland 2001, Rowe & Fischer 2001, Sincich et al. 2007, Weyand 2007, Rathbun et al. 2010). As might be expected, the similarity between presynaptic and postsynaptic receptive fields increases as the strength of connection increases (Usrey et al. 1999, Rathbun et al. 2010).
NONRETINAL SYNAPTIC INPUT TO THE LATERAL GENICULATE NUCLEUS
Although retinal inputs are the primary driver of spiking responses in the LGN, most synaptic inputs onto LGN neurons come from nonretinal sources (reviewed in Sherman & Guillery 2009). Nonretinal inputs onto LGN neurons include various forms of thalamic inhibition, feedback from the cortex, and diffuse modulatory systems.
Thalamic Inhibition
LGN relay neurons receive inhibitory input from two classes of neurons: local inhibitory interneurons within the LGN and inhibitory neurons within the thalamic reticular nucleus (TRN). Within the LGN, 20–30% of neurons are inhibitory interneurons that provide local GABAergic input to neighboring relay neurons. Similar to relay neurons, LGN interneurons receive direct monosynaptic excitation from the retina; unlike relay neurons, however, this input is received by both ionotropic and metabotropic glutamate receptors (Cox & Sherman 2000, Acuna-Goycolea et al. 2008). LGN inhibitory neurons form two types of synaptic connections with relay neurons, commonly referred to as F1 and F2 terminals, based on their flat anatomical shape (Gray 1969, Sherman & Guillery 2009, Cox 2014).
F2 terminals typically belong to synaptic triads, unique structures involving elements from three cells: a retinal cell, an LGN relay cell, and a local inhibitory neuron. These triads comprise a retinal (or cholinergic) synapse onto the dendrite of an LGN relay cell and an F2 terminal on the dendrite of a local inhibitory neuron that is postsynaptic to the retinal (or cholinergic) axon and presynaptic to the relay cell dendrite. Importantly, F2 terminals are found on the distal dendrites of LGN interneurons. As LGN interneurons are not electrotonically compact, synaptic activity at the F2 terminal may occur in isolation from both the integration of other synaptic inputs and the generation of action potentials that underlie transmission at F1 terminals. F2 terminals in distal dendrites are not completely isolated, however: Under certain conditions, somatically activated action potentials can back propagate through the distal dendrites and stimulate neurotransmitter release from F2 terminals (Casale & McCormick 2011, Cox 2014).
Another source of inhibitory input to LGN neurons is the TRN, a thin nucleus that forms a sheath around the dorsal thalamus (Jones 2006). Although the TRN lacks anatomically well-defined boundaries, TRN connectivity with the LGN is retinotopically organized and is distinct from TRN regions that are interconnected with other thalamic nuclei. Neurons in the TRN do not receive direct input from the retina; instead, they receive visual signals from collaterals of geniculocortical axons and corticogeniculate axons (reviewed in Jones 2006). Given this organization of input, TRN neurons appear well poised to monitor activity levels in the LGN and cortex and to influence geniculocortical communication. This influence is likely complex, as TRN synapses involve both GABAA and GABAB receptors on relay neurons and are often located in close proximity to those of corticogeniculate axons (Sherman & Guillery 2009, Ulrich et al. 2007). Moreover, TRN neurons receive cholinergic input from the brain stem and basal forebrain (Parent et al. 1988, McCormick 1992, Guillery et al. 1998), thereby providing a route for arousal mechanisms to influence geniculocortical transmission.
Cortical Feedback
The single greatest source of synaptic input to the LGN comes from corticogeniculate feedback axons (Guillery 1969; Erişir et al. 1997a,b). In contrast to retinal excitation, the influence of cortical feedback on visual processing in the LGN is poorly understood. Both LGN relay neurons and interneurons receive monosynaptic excitation from corticothalamic axons. As mentioned in the previous subsection, corticothalamic axons also target inhibitory neurons in the TRN, which in turn provide inhibition to LGN neurons. Thus, cortical feedback has both a direct excitatory and disynaptic inhibitory influence on LGN relay neurons. Relative to retinal axons, corticothalamic axons have a much larger arborization, approximately 2–3 times the spread of retinal axons (Murphy & Sillito 1996). Consequently, individual corticogeniculate axons influence activity across a larger pool of LGN neurons than do individual axons from the retina. Although corticogeniculate synapses outnumber retinogeniculate synapses, unitary EPSPs from corticogeniculate axons have significantly smaller amplitudes than those from retinal axons do (reviewed in Sherman & Guillery 2009). In addition, corticogeniculate synapses are located more distally on the dendrites of relay cells than retinogeniculate synapses. For these reasons, and because the receptive fields of LGN neurons more closely resemble those of RGCs and not cortical cells, the corticogeniculate pathway is generally considered a modulatory pathway rather than a driving pathway (Sherman & Guillery 2009).
In primates, corticogeniculate feedback respects the segregation of feedforward parallel pathways traversing the LGN. In particular, parvocellular LGN neurons receive cortical feedback from V1 pyramidal neurons whose cell bodies are located in the upper half of layer 6, whereas magnocellular neurons receive input from the pyramidal neurons in the lower half of layer 6 (Conley & Raczkowski 1990, Fitzpatrick et al. 1994). Corticothalamic feedback inputs are not only anatomically separated but also physiologically distinct; feedback neurons supplying the parvocellular and magnocellular layers of the LGN have response properties that parallel those of their target neurons (Briggs & Usrey 2009). These results indicate that corticogeniculate feedback is able to exert a stream-specific influence on the feedforward transmission of visual signals to the cortex on the basis of its filtering properties.
Diffuse Neuromodulators
LGN neurons receive a variety of inputs from nonvisual neuromodulatory areas in the brain stem, including noradrenergic input from the reticular formation, serotoninergic input from the dorsal raphe nucleus, and cholinergic input from the parabrachial nucleus. Although these inputs do not drive LGN responses directly, they play an important role in adjusting LGN responses as a function of alertness and the sleep–wake cycle. As we discuss below (see the section titled “Behavioral State and the Gating of Sensory Information in the Lateral Geniculate Nucleus”), they also play an important role in modulating network interaction between the LGN and cortex.
TRANSFORMATION OF RETINAL INFORMATION IN THE LATERAL GENICULATE NUCLEUS
Although the receptive fields of LGN neurons generally resemble those of their retinal afferents, some important differences between the spatial domains of these receptive fields accompany more dramatic differences in the temporal properties of presynaptic and postsynaptic spike trains.
Stronger Classical Surrounds in the Lateral Geniculate Nucleus
Although the receptive fields of most LGN cells resemble those of their retinal afferents in terms of spatial location and center–surround organization, one consistent difference between the receptive fields of presynaptic and postsynaptic neurons is the strength of the classical surround. Originally reported by Hubel & Wiesel (1961), the stronger classical surrounds of LGN receptive fields are believed to play an important role in shifting the spatial frequency response functions of LGN neurons toward higher frequencies (Moore et al. 2014). Moreover, because the onset of responses in the classical surround is delayed relative to the onset of responses in the center, there is a temporal cascade in coarse- to fine-tuning for preferred spatial frequencies between the retina and LGN (Moore et al. 2014). Cellular mechanisms underlying the increased classical surround likely include feedforward disynaptic inhibition via LGN interneurons. Indeed, in an elegant series of experiments, Hirsch and colleagues (Wang et al. 2011a,b) used whole-cell recording methods to study the subthreshold response properties of LGN neurons and identified clear push–pull antagonism in the LGN, whereby nonpreferred stimuli presented in the classical surround evoked synaptic inhibition.
Stronger Extraclassical Suppression in the Lateral Geniculate Nucleus
As described above (see the subsection titled “Nonlinear Receptive Field Properties”), extraclassical suppression is a form of nonlinear suppression that overlaps and extends beyond the classical receptive field of LGN neurons. This form of suppression contributes to gain control in the LGN and can be quantified from area summation response functions using patches of sinusoidal stimuli that match the preferred spatial frequency of the neuron but differ in diameter. Circuit mechanisms that contribute to extraclassical suppression in the LGN have been the subject of intense investigation, and the results of these investigations thus far paint a complex picture. On the one hand, the results of studies of surround suppression and response gain in the cat and the mouse have indicated a prominent role for corticogeniculate feedback circuits in strengthening extraclassical suppression and in modulating neuronal responsiveness in the LGN (Sillito & Jones 2002, Olsen et al. 2012). On the other hand, the results of studies performed in monkeys have suggested that the strength of surround suppression in the retina is comparable to that in the LGN and, moreover, the onset of surround suppression in the LGN is too fast for circuits involving the cortex (Alitto & Usrey 2008). Further, extraclassical suppression in the LGN has been successfully modeled as an expression of retinal contrast gain control (Bonin et al. 2005). Although future work is needed to untangle these results, the identification of multiple mechanisms for extraclassical suppression in the LGNs of different species underscores the important role extraclassical suppression plays in visual processing.
The Role of Spike Timing in Enhancing Communication and Information Processing Between the Retina and the Lateral Geniculate Nucleus
Although the percentage of LGN spikes directly evoked from the ensemble of RGCs that provide monosynaptic input may exceed 95%, not all retinal spikes generate LGN action potentials (reviewed in Usrey 2002a,b). Indeed, individual RGCs typically fire two to three times more spikes than their target neurons in the LGN do (Rathbun et al. 2010). Thus, postsynaptic potentials from some retinal spikes cross the spike threshold, and others do not. By comparing the spike trains of synaptically connected RGCs and LGN neurons, researchers have determined the time course of interaction between retinal spikes in driving LGN responses. Results show that retinal spikes that follow the shortest interspike interval (ISI), set by the refractory period of a cell, have the highest efficacy in driving LGN spikes, and the enhanced efficacy of second spikes in a pair persists for interspike intervals of up to 20–30 msec (Mastronarde 1987; Usrey et al. 1998; Levine & Cleland 2001; Rowe & Fischer 2001; Sincich et al. 2007, 2009; Weyand 2007; Rathbun et al. 2010). Moreover, retinal spikes that occur at ISIs of more than 40 msec rarely generate suprathreshold spiking responses (Sincich et al. 2007). Although retinogeniculate synapses studied in vitro display prominent synaptic depression, this depression appears to be saturated at activity levels associated with visual processing in vivo. Relatedly, modeling efforts indicate that the ISI-dependent enhancement of the second spike in a pair can be accounted for entirely by postsynaptic temporal summation (Carandini et al. 2007).
An important consequence of the enhanced efficacy of retinal spikes following short ISIs is a high-pass filtering of the retinal spike train—an effect that serves to increase the signal-to-noise ratio of LGN spikes relayed to the cortex relative to those supplied from the retina (Sincich et al. 2009, Rathbun et al. 2010, Wang et al. 2010). Similarly, retinal spikes that occur following short ISIs generate stronger response maps than do those that occur following longer ISIs (Rathbun et al. 2007, 2010). Relayed retinal spikes are also more reliable and have less temporal jitter than nonrelayed spikes do. Taken together, these results indicate that relayed retinal spikes are much more likely to be driven by visual stimulation of the retina than by random fluctuations in the membrane potentials of retinal cells that result from non-stimulus-related processes.
Remapping the Retinal Mosaic Increases the Efficacy and Resolution of the Visual Signal
As mentioned above (see the section titled “General Properties of Retinogeniculate Synapses”), the retinogeniculate projection does not maintain a 1:1 relationship between presynaptic and postsynaptic neurons. Individual RGCs often innervate multiple LGN neurons (anatomical divergence), and individual LGN neurons often receive input from multiple RGCs (anatomical convergence). Together, this divergence and this convergence result in remapping the representation of visual space by individual neurons across the LGN. This remapping appears to serve a practical role; computational analyses of the retinogeniculate projection indicate that the representation of visual space in the LGN is improved beyond the computational limits of the retinal mosaic (Martinez et al. 2014). In other words, convergence and divergence generate an interpolated map of visual space in the LGN that can facilitate the detection of stimulus position in the presence of sensor noise. Note that too much divergence and convergence in the retinogeniculate pathway would be problematic, as extreme levels would cause every LGN neuron to have the exact same receptive field. Interestingly, estimates of the optimal amount of divergence to maximize both the coverage of visual space and receptive field diversity (Martinez et al. 2014) match the amount of divergence reported by in vivo studies (reviewed in Reid & Usrey 2004).
Anatomical Divergence Produces Synchronous Activity in the Lateral Geniculate Nucleus and Strengthens Geniculocortical Communication
Divergence from a single RGC to multiple LGN neurons promotes a tight form of synchrony (<1 msec) in the responses of postsynaptic neurons (Alonso et al. 1996, Usrey et al. 1998). Multielectrode recording studies demonstrate that this form of synchrony is present only for LGN neurons with highly overlapping receptive fields and is greatest for LGN neurons of the same class (e.g., two X cells). When these criteria are met, pairs of LGN neurons that receive common input from the retina can fire up to 40% of their spikes synchronously. Functionally, synchronous firing between LGN neurons facilitates feedforward communication to target neurons in layer 4 of the visual cortex, as evidenced by studies demonstrating that the time course for interaction between spikes arriving from converging LGN axons onto a cortical target neuron is ~7 msec (Usrey et al. 2000). Thus, synchrony may serve to strengthen the relatively spare geniculate input (~5% of synapses) onto cortical target neurons. Synchrony could also serve to increase the amount of information conveyed to the cortex; information theoretic analysis shows that more information can be extracted from the spiking activity of pairs of cells when temporal correlations are taken into account (Dan et al. 1998).
BEHAVIORAL STATE AND THE GATING OF SENSORY INFORMATION IN THE LATERAL GENICULATE NUCLEUS
When an animal is alert and actively processing sensory information, the primary job of first-order thalamic nuclei, such as the LGN, is to transmit sensory information from the periphery to the cortex. The preceding sections have described how visual information from the retina is transformed in the LGN before reaching the cortex. Although these transformations have important implications for visual processing, perhaps the most significant transformation of the retinal signal in the LGN is that which takes place as a function of behavioral states such as arousal, sleep, and attention.
Thalamic Processing Shifts with Arousal
The importance of behavioral state in determining the response properties of LGN neurons was made readily apparent from early experiments by Livingstone & Hubel (1981). These scientists recorded from individual LGN neurons in cats that drifted between periods of sleep and wakefulness and documented both an increase in firing rate and a decrease in high-frequency bursts that accompanied arousal from slow-wave sleep. We now recognize that these differences in the LGN neuron response profiles represent shifts between two modes of thalamic activity: burst mode and tonic mode.
Whether or not an LGN neuron is in burst mode or tonic mode depends on the recent membrane potential history of the neuron and on the state of its low-threshold, T-type Ca2+ channels (Jahnsen & Llinás 1984a,b; Guido et al. 1992; Lu et al. 1992). When the resting membrane potential of an LGN neuron is relatively depolarized (approximately −70 mV), as is the case during arousal and wakefulness, LGN neurons generate a train of tonic spikes when the excitatory drive reaches the threshold for opening voltage-gated Na+ channels. In contrast, when the resting potential of an LGN neuron is hyperpolarized (approximately −85 mV), for example, when animals are asleep, drowsy, or anesthetized, T-type Ca2+ channels that were otherwise in an inactivated state become deinactivated. Upon deinactivation, these channels open in response to excitatory input and generate a Ca2+ plateau potential that can trigger a burst of Na+ spikes.
Although the frequency of burst activity decreases dramatically with alertness (Weyand et al. 2001, Ruiz et al. 2006, Alitto et al. 2011), the occurrence of bursts during active vision has been suggested to play an important role in ensuring the transmission of visual signals between the LGN and the cortex (Sherman 2001). Along these lines, a recent study indicates that stimulus novelty may increase the frequency of LGN bursts during a visual detection task (Ortuño et al. 2014). Although not examined in alert animals, further support for a possible role for bursts in visual processing comes from physiological recordings in anesthetized animals. These recordings demonstrate that (a) LGN burst spikes have greater efficacy in driving cortical responses than tonic spikes do (Swadlow & Gusev 2001), (b) the recent history of visual stimulation can prime LGN neurons to generate burst spikes (Alitto et al. 2005), (c) naturalistic stimuli evoke a greater percentage of burst spikes than unnatural stimuli evoke (Lesica & Stanley 2004, Denning & Reinagel 2005), and (d ) cortical feedback can modulate the occurrence of LGN burst activity (Andolina et al. 2013).
In addition to shifting LGN neurons between burst and tonic activity modes, alertness also affects other features of visual responses in the LGN. This topic has been studied extensively in the awake rabbit, in which frequent transitions between alert sensory processing and quiescence can be monitored in real time using electroencephalogram (EEG) recordings in conjunction with extracellular recording from neurons in the LGN and/or cortex (Zhuang et al. 2014). When the cortical EEG becomes desynchronized, burst frequency decreases, the strength of visual responses in the LGN increases, and basic receptive field properties such as direction selectivity are enhanced, indicating an increase in behavioral vigilance (Bereshpolova et al. 2011, Hei et al. 2014). Similarly, the enhancement of activity in mouse V1 that occurs with locomotion (Niell & Stryker 2010, Ayaz et al. 2013, Polack et al. 2013) has been extended to include enhancement of activity in the LGN (Erisken et al. 2014). Thus, alertness and physical activity play a prominent role in visual processing in the LGN.
Thalamocortical Oscillations During Quiescence and Sleep Disrupt Geniculocortical Transmission
One of the primary functions served by thalamic bursts is their participation in thalamocortical network oscillations that dominate during sleep and, to a lesser extent, periods of low arousal (Steriade 2003). During wakefulness, the cortical EEG is dominated by small-amplitude, high-frequency activity that is associated with cognition and active sensory processing. In contrast, during sleep, the cortical EEG is transformed and becomes dominated by high-amplitude, low-frequency activity. This low-frequency activity represents oscillations that are generated locally in the thalamus (in relay nuclei and in the TRN) and in the cortex and that interact to form highly structured and interdependent oscillations (Steriade 2006). The transformation from wakefulness to sleep is complex and involves a variety of subcortical structures (Brown et al. 2012). In the thalamocortical pathway, this transformation begins with the withdrawal of cholinergic input from the brain stem and basal forebrain (Steriade 2004, Jones 2004, Brown et al. 2012). This withdrawal results in the hyperpolarization of LGN relay neurons as local muscarinic receptors are silenced and inhibition supplied from the TRN increases. Cortical feedback to the LGN is also affected as cortical neurons become hyperpolarized and begin to display slow-wave oscillations. Consequently, large populations of thalamic relay neurons burst en masse, disconnecting thalamic neurons from their peripheral drivers.
Although a detailed discussion of the utility of these oscillations, and of sleep more generally, is beyond the scope of this review (for more information, see Steriade 2004, Brown et al. 2012, McCormick et al. 2015), these oscillations are important to consider when discussing the functional implications of anatomical connections in sensory systems. Thalamocortical network oscillations that occur during quiescence and sleep do not play a role in active visual processing. Rather, they serve to disrupt the thalamus from transmitting sensory information and to essentially disconnect the cortex from the periphery. Thus, when one asks how nonretinal inputs influence sensory processing in the LGN, a significant part of the answer is that these inputs play a role in the generation of thalamocortical oscillations that disrupt the transmission of sensory information to the cortex. This is true not only for neuromodulatory inputs from the brain stem, but also for nonretinal inputs to the LGN that typically carry visual signals, including corticogeniculate feedback projections and projections from the TRN.
Thalamocortical Oscillations During Active Visual Processing
Although some forms of oscillatory activity serve to disrupt the transmission of visual information between the thalamus and the cortex during periods of quiescence and sleep, other forms of this activity may actually aid the transmission of information. For instance, gamma oscillations (30–100 Hz) in the cortex of alert monkeys have been proposed to facilitate the encoding of information within local subnetworks of neurons and to enhance the communication of signals between cortical areas (Bastos et al. 2015b). Given the robust organization of feedback projections from V1 to the LGN, it has been tempting to speculate that gamma activity in V1 might entrain similar activity in the LGN, perhaps providing a mechanism to adjust dynamically the functional connectivity between the LGN and V1. In support of this possibility, strong gamma oscillations have been observed in the local field potential (LFP) and single-unit activity of LGN neurons in anesthetized animals (Neuenschwander & Singer 1996, Castelo-Branco et al. 1998, Koepsell et al. 2009); however, these oscillations are largely absent in similar recordings from alert animals (Bastos et al. 2014). Instead, oscillations in nongamma frequency bands seem to contribute to feedforward and feedback communication between the LGN and V1: Feedforward coherence is seen in the beta band (15–30 Hz), and feedback coherence is seen in the alpha band (8–14 Hz) (Bastos et al. 2014). Interestingly, although a functional role for these rhythmic interactions has yet to be demonstrated, interareal cortical recordings from alert animals have identified comparatively high-frequency oscillations associated with feedforward communication and low-frequency oscillations associated with feedback communication (van Kerkoerle et al. 2014, Bastos et al. 2015a).
Influence of Attention on Visual Response Properties in the Lateral Geniculate Nucleus
Covert spatial attention, the ability to direct visual attention to specified retinotopic locations, can improve the ability to detect and discriminate visual stimuli at attended locations compared with unattended locations. Within the cortex, spatial attention typically (a) increases neuronal responses to visual stimuli at the attended location (e.g., McAdams & Maunsell 1999) and (b) increases coherence between single-unit activity and the LFP, often in the gamma band (30–100 Hz) (e.g., Fries et al. 2008).
Although the effects of spatial attention are generally stronger in extrastriate cortical regions (e.g., V4, MT, VIP), attention also influences neuronal activity in V1 and in subcortical structures including the LGN. For instance, both single-unit recordings of LGN neurons in macaque monkeys and fMRI measures of the blood-oxygenation-level-dependent (BOLD) response in the LGN in humans revealed increased activity with spatial attention (O’Connor et al. 2002, McAlonan et al. 2008, Schneider & Kastner 2009). In monkeys, the influence of attention on LGN activity points to a pathway that involves the TRN and the GABAergic projections from the TRN to the LGN. In particular, whereas spatial attention directed toward the receptive fields of TRN neurons suppresses activity (McAlonan et al. 2006), attention directed toward the receptive fields of LGN neurons enhances activity (McAlonan et al. 2008), presumably by releasing the suppressive influence of the TRN on the LGN. Interestingly, the enhancement of LGN activity occurs throughout the entire response profile, beginning at onset and continuing through later stages of the analyzed time period, whereas attentional modulation of the TRN activity is seen only in the initial period of the visual response. This temporal relationship has implications for the circuitry involved in the modulation of LGN activity, and it suggests that other sources of input, likely corticogeniculate feedback, contribute to later stages of attentional modulation in the LGN.
Attention Enhances Geniculocortical Communication
Spatial attention also modulates the efficacy of geniculocortical communication. A recent investigation in which electrical stimulation of LGN relay neurons was paired with simultaneous recordings from postsynaptic neurons in layer 4 of the macaque V1 demonstrated that spatial attention increases the transfer of electrically evoked presynaptic spikes to suprathreshold postsynaptic responses (Briggs et al. 2013). Although the mechanisms underlying this enhanced communication are unknown, analyses suggested involvement beyond changes in postsynaptic resting potential, including a possible role for cholinergic input onto the presynaptic terminals of LGN axons. In addition to the attention-dependent enhancement of geniculocortical communication, attention was also found to increase a fast form of polysynaptic inhibition within cortical layer 4, an effect that appears to play a role in decreasing the temporal jitter of postsynaptic responses among geniculocortical target neurons (Briggs et al. 2013).
In summary, the LGN serves an essential function, transmitting visual information from the periphery to the cortex. Although the visual signal sent to the cortex largely reflects the complex processing that occurs within the retina, the LGN transforms and selectively transmits visual information based on the statistics of the visual stimulus and on the behavioral state of the animal. Given the relatively simple anatomical organization of the LGN, one main source of synaptic drive (RGCs) and one main postsynaptic target (V1), it is not surprising that the general function of the LGN is fairly well understood. However, many issues clearly remain open to investigation. For instance, despite extensive investigation into the role(s) played by corticothalamic projections, their function is still being revealed (Crandall et al. 2015). We are also just beginning to understand the role of the TRN in regulating LGN activity dynamically across behavioral states (Halassa et al. 2014). Finally, LGN processing is strongly affected by neuromodulators such as vasopressin, and the functions of these neuromodulators are largely unclear (Freeman et al. 2014).
SUMMARY POINTS.
The LGN is located between the retina and primary visual cortex and is the gateway through which visual information reaches the cerebral cortex.
Divergence and convergence in the pathway from the retina to the LGN increase the resolution of visual space beyond the limits of the retinal mosaic and establish coordinated activity among ensembles of LGN neurons, thereby facilitating geniculocortical communication.
The LGN transforms the temporal structure of retinal spike trains to increase the signal-to-noise ratios of visual signals.
Center–surround interactions, first established in the retina, are strengthened by local thalamic inhibition involving polysynaptic circuits.
Geniculocortical functional connectivity is strongly modulated by various behavioral states, including sleep, arousal, and spatial attention.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (EY013588 and EY016182) and the National Science Foundation (BCS-1228535).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Acuna-Goycolea C, Brenowitz SD, Regehr WG. Active dendritic conductances dynamically regulate GABA release from thalamic interneurons. Neuron. 2008;57:420–31. doi: 10.1016/j.neuron.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Alitto HJ, Moore BD, 4th, Rathbun DL, Usrey WM. A comparison of visual responses in the lateral geniculate nucleus of alert and anaesthetized macaque monkeys. J. Physiol. 2011;589:87–99. doi: 10.1113/jphysiol.2010.190538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitto HJ, Usrey WM. Influence of contrast on orientation and temporal frequency tuning in ferret primary visual cortex. J. Neurophysiol. 2004;91:2797–808. doi: 10.1152/jn.00943.2003. [DOI] [PubMed] [Google Scholar]
- Alitto HJ, Usrey WM. Origin and dynamics of extraclassical suppression in the lateral geniculate nucleus of the macaque monkey. Neuron. 2008;57:135–46. doi: 10.1016/j.neuron.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitto HJ, Weyand TG, Usrey WM. Distinct properties of stimulus-evoked bursts in the lateral geniculate nucleus. J. Neurosci. 2005;25:514–23. doi: 10.1523/JNEUROSCI.3369-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J-M, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–19. doi: 10.1038/383815a0. [DOI] [PubMed] [Google Scholar]
- Andolina IM, Jones HE, Sillito AM. Effects of cortical feedback on the spatial properties of relay cells in the lateral geniculate nucleus. J. Neurophysiol. 2013;109:889–99. doi: 10.1152/jn.00194.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz A, Saleem AB, Schölvinck ML, Carandini M. Locomotion controls spatial integration in mouse visual cortex. Curr. Biol. 2013;23:890–94. doi: 10.1016/j.cub.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB. Summation and inhibition in the frog’s retina. J. Physiol. 1953;119:69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Fitzhugh R, Kuffler SW. Resting discharge and dark adaptation in the cat. J. Physiol. 1954;125:28–9P. [PubMed] [Google Scholar]
- Barlow HB, Hill RM, Levick WR. Retinal ganglion cells responding selectively to direction and speed of image motion in the rabbit. J. Physiol. 1964;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Briggs F, Alitto HJ, Mangun GR, Usrey WM. Simultaneous recordings from the primary visual cortex and lateral geniculate nucleus reveal rhythmic interactions and a cortical source for γ-band oscillations. J. Neurosci. 2014;34:7639–44. doi: 10.1523/JNEUROSCI.4216-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J, Bosman CA, Schoffelen J-M, Oostenveld R, et al. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron. 2015a;85:390–401. doi: 10.1016/j.neuron.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J, Fries P. Communication through coherence with inter-areal delays. Curr. Opin. Neurobiol. 2015b;31:173–80. doi: 10.1016/j.conb.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Benardete EA, Kaplan E, Knight BW. Contrast gain control in the primate retina: P cells are not X-like, some M cells are. Vis. Neurosci. 1992;8:483–86. doi: 10.1017/s0952523800004995. [DOI] [PubMed] [Google Scholar]
- Bereshpolova Y, Stoelzel CR, Zhuang J, Amitai Y, Alonso J-M, Swadlow HA. Getting drowsy? Alert/nonalert transitions and visual thalamocortical network dynamics. J. Neurosci. 2011;31:17480–87. doi: 10.1523/JNEUROSCI.2262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME, Wei H, Eisenback MA, Chomsung RD, Slusarczyk AS, Dankowsi AB. Synaptic organization of thalamocortical axon collaterals in the perigeniculate nucleus and dorsal lateral geniculate nucleus. J. Comp. Neurol. 2008;508:264–85. doi: 10.1002/cne.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop PO, Burke W, Davis R. The interpretation of the extracellular response of single lateral geniculate cells. J. Physiol. 1962;162:451–72. doi: 10.1113/jphysiol.1962.sp006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin V, Mante V, Carandini M. The suppressive field of neurons in lateral geniculate nucleus. J. Neurosci. 2005;25:10844–56. doi: 10.1523/JNEUROSCI.3562-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Mangun GR, Usrey WM. Attention enhances synaptic efficacy and signal-to-noise in neural circuits. Nature. 2013;499:476–80. doi: 10.1038/nature12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Parallel processing in the corticogeniculate pathway of the macaque monkey. Neuron. 2009;62:135–46. doi: 10.1016/j.neuron.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol. Rev. 2012;92:1087–187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat. Rev. Neurosci. 2011;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Horton JC, Sincich LC. Thalamic filtering of retinal spike trains by postsynaptic summation. J. Vis. 2007;7(14):20. doi: 10.1167/7.14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande VA. A third parallel pathway to primate area V1. Trends Neurosci. 1994;17:305–10. doi: 10.1016/0166-2236(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Kaas JH. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In: Peters A, Rockland KS, editors. Cerebral Cortex. Primary Visual Cortex in Primates. Vol. 10. Plenum; New York: 1994. pp. 201–59. [Google Scholar]
- Casale AE, McCormick DA. Active action potential propagation but not initiation in thalamic interneuron dendrites. J. Neurosci. 2011;31:18289–302. doi: 10.1523/JNEUROSCI.4417-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco M, Neuenschwander S, Singer W. Synchronization of visual responses between the cortex, lateral geniculate nucleus, and retina in the anesthetized cat. J. Neurosci. 1998;18:6395–410. doi: 10.1523/JNEUROSCI.18-16-06395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong SK, Tailby C, Solomon SG, Martin PR. Cortical-like receptive fields in the lateral geniculate nucleus of marmoset monkeys. J. Neurosci. 2013;33:6864–76. doi: 10.1523/JNEUROSCI.5208-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG. The dorsal lateral geniculate nucleus of the cat. In: Pettigrew JD, Sanderson KJ, Levick WR, editors. Visual Neuroscience. Cambridge Univ. Press; London: 1986. pp. 111–20. [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Simultaneous recording of input and output of lateral geniculate neurones. Nat. N. Biol. 1971a;231:191–92. doi: 10.1038/newbio231191a0. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Sustained and transient neurones in the cat’s retina and lateral geniculate nucleus. J. Physiol. 1971b;217:473–96. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M, Raczkowski D. Sublaminar organization within layer VI of the striate cortex in Galago. J. Comp. Neurol. 1990;302:425–36. doi: 10.1002/cne.903020218. [DOI] [PubMed] [Google Scholar]
- Conway JL, Schiller PH. Laminar organization of tree shrew dorsal lateral geniculate nucleus. J. Neurophysiol. 1983;50:1330–42. doi: 10.1152/jn.1983.50.6.1330. [DOI] [PubMed] [Google Scholar]
- Cox CL, Sherman SM. Control of dendritic outputs of inhibitory interneurons in the lateral geniculate nucleus. Neuron. 2000;27:597–610. doi: 10.1016/s0896-6273(00)00069-6. [DOI] [PubMed] [Google Scholar]
- Cox CL. Complex regulation of dendritic transmitter release from thalamic interneurons. Curr. Opin. Neurobiol. 2014;29:126–32. doi: 10.1016/j.conb.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Packer OS, Troy JB, Dacey DM, Chalupa LM, Werner JS. The New Visual Neurosciences. MIT Press; Cambridge, MA: 2014. Synaptic mechanisms of color and luminance coding: rediscovering the X–Y-cell dichotomy in primate retinal ganglion cells; pp. 123–43. [Google Scholar]
- Crandall SR, Cruikshank SJ, Connors BW. A corticothalamic switch: controlling the thalamus with dynamic synapses. Neuron. 2015;86:768–82. doi: 10.1016/j.neuron.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martín A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, et al. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature. 2014;507:358–61. doi: 10.1038/nature12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Packer OS. Colour coding in the primate retina: diverse cell types and cone-specific circuitry. Curr. Opin. Neurobiol. 2003;13:421–27. doi: 10.1016/s0959-4388(03)00103-x. [DOI] [PubMed] [Google Scholar]
- Dan Y, Alonso J-M, Usrey WM, Reid RC. Coding of visual information by precisely correlated spikes in the LGN. Nat. Neurosci. 1998;1:501–7. doi: 10.1038/2217. [DOI] [PubMed] [Google Scholar]
- Demb JB, Zaghloul K, Haarsma L, Sterling P. Bipolar cells contribute to nonlinear spatial summation in the brisk-transient (Y) ganglion cell in mammalian retina. J. Neurosci. 2001;21:7447–54. doi: 10.1523/JNEUROSCI.21-19-07447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio FM, Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J. Physiol. 1975;25:167–95. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning KS, Reinagel P. Visual control of burst priming in the anesthetized lateral geniculate nucleus. J. Neurosci. 2005;25:3531–38. doi: 10.1523/JNEUROSCI.4417-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Lennie PM, Wright J. The mechanism of peripherally evoked responses in retinal ganglion cells. J. Physiol. 1979;289:299–310. doi: 10.1113/jphysiol.1979.sp012738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson JG. The contrast sensitivity of retinal ganglion cells of the cat. J. Physiol. 1966;187:517–52. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erişir A, Van Horn SC, Bickford ME, Sherman SM. Immunocytochemistry and distribution of parabrachial terminals in the lateral geniculate nucleus of the cat: a comparison with corticogeniculate terminals. J. Comp. Neurol. 1997a;377:535–49. [PubMed] [Google Scholar]
- Erişir A, Van Horn SC, Sherman SM. Relative numbers of cortical and brainstem inputs to the lateral geniculate nucleus. PNAS. 1997b;94:1517–20. doi: 10.1073/pnas.94.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisken S, Vaiceliunaite A, Jurjut O, Fiorini M, Katzner S, Busse L. Effects of locomotion extend throughout the mouse early visual system. Curr. Biol. 2014;24:2899–907. doi: 10.1016/j.cub.2014.10.045. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D, Itoh K, Diamond IT. The laminar organization of the lateral geniculate body and the striate cortex in the squirrel monkey Saimiri sciureus. J. Neurosci. 1983;3:673–702. doi: 10.1523/JNEUROSCI.03-04-00673.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D, Usrey WM, Schofield BR, Einstein G. The sublaminar organization of neurons in layer 6 of macaque striate cortex. Vis. Neurosci. 1994;11:307–15. doi: 10.1017/s0952523800001656. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, et al. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J. Neurosci. 2008;28:4823–35. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vis. Res. 1971;11:209–26. doi: 10.1016/0042-6989(71)90186-6. [DOI] [PubMed] [Google Scholar]
- Godement P, Salaün J, Imbert M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J. Comp. Neurol. 1984;230:552–75. doi: 10.1002/cne.902300406. [DOI] [PubMed] [Google Scholar]
- Gray EG. Electron microscopy of excitatory and inhibitory synapses: a brief review. Prog. Brain Res. 1969;31:141–55. doi: 10.1016/S0079-6123(08)63235-5. [DOI] [PubMed] [Google Scholar]
- Guido W, Lu SM, Sherman SM. Relative contributions of burst and tonic responses to the receptive field properties of lateral geniculate neurons in the cat. J. Neurophysiol. 1992;68:2199–211. doi: 10.1152/jn.1992.68.6.2199. [DOI] [PubMed] [Google Scholar]
- Guillery RW. A quantitative study of synaptic interconnections in the dorsal lateral geniculate nucleus of the cat. Z. Zellforsch. 1969;96:39–48. doi: 10.1007/BF00321474. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Feig SL, Lozsádi DA. Paying attention to the thalamic reticular nucleus. Trends Neurosci. 1998;21:28–32. doi: 10.1016/s0166-2236(97)01157-0. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, et al. State-dependent architecture of thalamic reticular subnetworks. Cell. 2014;158:808–21. doi: 10.1016/j.cell.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, Sherman SM. Synaptic circuits involving an individual retinogeniculate axon in the cat. J. Comp. Neurol. 1987;259:165–92. doi: 10.1002/cne.902590202. [DOI] [PubMed] [Google Scholar]
- Hei X, Stoelzel CR, Zhuang J, Bereshpolova Y, Huff JM, et al. Directional selective neurons in the awake LGN: response properties and modulation by brain state. J. Neurophysiol. 2014;112:362–73. doi: 10.1152/jn.00121.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annu. Rev. Neurosci. 2000;23:127–53. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- Hickey TL, Guillery RW. An autoradiographic study of retinogeniculate pathways in the cat and fox. J. Comp. Neurol. 1974;156:239–53. doi: 10.1002/cne.901560207. [DOI] [PubMed] [Google Scholar]
- Hochstein S, Shapley RM. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J. Physiol. 1976;262:265–84. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth M, Walmsley L, Brown TM. Binocular integration in the mouse lateral geniculate nuclei. Curr. Biol. 2014;24:1241–47. doi: 10.1016/j.cub.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Integrative action in the cat’s lateral geniculate body. J. Physiol. 1961;155:385–98. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–34. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Wright MJ. Receptive field organization of ‘sustained’ and ‘transient’ retinal ganglion cells which subserve different function roles. J. Physiol. 1972;227:769–800. doi: 10.1113/jphysiol.1972.sp010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H, Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J. Physiol. 1984a;349:205–26. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Ionic basis for the electro-responseiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J. Physiol. 1984b;349:227–47. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog. Brain Res. 2004;145:157–69. doi: 10.1016/S0079-6123(03)45011-5. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus Revisited. Cambridge Univ. Press; Cambridge, UK: 2006. [Google Scholar]
- Jones HE, Andolina IM, Oakely NM, Murphy PC, Sillito AM. Spatial summation in lateral geniculate nucleus and visual cortex. Exp. Brain Res. 2000;135:279–84. doi: 10.1007/s002210000574. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Purpura K, Shapley RM. Contrast affects the transmission of visual information through the mammalian lateral geniculate nucleus. J. Physiol. 1987;391:267–88. doi: 10.1113/jphysiol.1987.sp016737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Shapley R. The origin of the S (slow) potential in the mammalian lateral geniculate nucleus. Exp. Brain Res. 1984;55:111–16. doi: 10.1007/BF00240504. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. PNAS. 1986;83:2755–57. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell K, Wang X, Vaingankar V, Wei Y, Wang Q, et al. Retinal oscillations carry visual information to cortex. Front. Syst. Neurosci. 2009;3:4. doi: 10.3389/neuro.06.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J. Neurophysiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kuffler SW. Neurons in the retina; organization, inhibition and excitation problems. Cold Spring Harb. Symp. Quant. Biol. 1952;17:281–92. doi: 10.1101/sqb.1952.017.01.026. [DOI] [PubMed] [Google Scholar]
- Lee BB. Receptive field structure in the primate retina. J. Comp. Neurol. 1996;36:631–44. doi: 10.1016/0042-6989(95)00167-0. [DOI] [PubMed] [Google Scholar]
- Lesica NA, Stanley GB. Encoding of natural scene movies by tonic and burst spikes in the lateral geniculate nucleus. J. Neurosci. 2004;24:10731–40. doi: 10.1523/JNEUROSCI.3059-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MW, Cleland BG. An analysis of the effect of retinal ganglion cell impulses upon the firing probability of neurons in the dorsal lateral geniculate nucleus of the cat. Brain Res. 2001;902:244–54. doi: 10.1016/s0006-8993(01)02411-8. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981;291:554–61. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- Lu SM, Guido W, Sherman SM. Effects of membrane voltage on receptive field properties of lateral geniculate neurons in the cat: contributions of the low-threshold Ca2+ conductance. J. Neurophysiol. 1992;68:1285–98. doi: 10.1152/jn.1992.68.6.2185. [DOI] [PubMed] [Google Scholar]
- Mante V, Bonin V, Carandini M. Functional mechanisms shaping lateral geniculate responses to artificial and natural stimuli. Neuron. 2008;58:625–38. doi: 10.1016/j.neuron.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Marshel JH, Kaye AP, Nauhaus I, Callaway EM. Anterior-posterior direction opponency in the superficial mouse lateral geniculate nucleus. Neuron. 2012;76:713–20. doi: 10.1016/j.neuron.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LM, Molano-Mazón M, Wang X, Sommer FT, Hirsch JA. Statistical wiring of thalamic receptive fields optimizes spatial sampling of the retinal image. Neuron. 2014;81:943–56. doi: 10.1016/j.neuron.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. Cell populations of the retina: the Proctor lecture. Investig. Ophthalmol. Vis. Sci. 2011;52:4581–91. doi: 10.1167/iovs.10-7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–80. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN. Two classes of single-input X-cells in cat lateral geniculate nucleus. II. Retinal inputs and the generation of receptive-field properties. J. Neurophysiol. 1987;57:381–413. doi: 10.1152/jn.1987.57.2.381. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J. Neurosci. 1999;19:431–41. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J. Neurosci. 2006;26:4444–50. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–94. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, McGinley MJ, Salkoff DB. Brain state dependent activity in the cortex and thalamus. Curr. Opin. Neurobiol. 2015;31:133–40. doi: 10.1016/j.conb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuro-modulation of thalamocortical activity. Prog. Neurobiol. 1992;39:337–88. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Ann. Rev. Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Moore BD, 3rd, Rathbun DL, Usrey WM, Freeman RD. Spatiotemporal flow of information in the early visual pathway. Eur. J. Neurosci. 2014;39:593–601. doi: 10.1111/ejn.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM. Functional morphology of the feedback pathway from area 17 of the cat visual cortex to the lateral geniculate nucleus. J. Neurosci. 1996;16:1180–92. doi: 10.1523/JNEUROSCI.16-03-01180.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat. Rev. Neurosci. 2009;10:360–72. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenschwander S, Singer W. Long-range synchronization of oscillatory light responses in the cat retina and lateral geniculate nucleus. Nature. 1996;379:728–32. doi: 10.1038/379728a0. [DOI] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–79. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat. Neurosci. 2002;5:1203–9. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature. 2012;483:47–52. doi: 10.1038/nature10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortuño T, Grieve KL, Cao R, Cudeiro J, Rivadulla C. Bursting thalamic responses in awake monkey contribute to visual detection and are modulated by corticofugal feedback. Front. Behav. Neurosci. 2014;8:198. doi: 10.3389/fnbeh.2014.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Paré D, Smith Y, Steriade M. Basal forebrain cholinergic and noncholinergic projections to the thalamus and brainstem in cats and monkeys. J. Comp. Neurol. 1988;277:281–301. doi: 10.1002/cne.902770209. [DOI] [PubMed] [Google Scholar]
- Percival KA, Koizumi A, Masri RA, Buzás P, Martin PR, Grünert U. Identification of a pathway from the retina to koniocellular layer K1 in the lateral geniculate nucleus of marmoset. J. Neurosci. 2014;34:3821–25. doi: 10.1523/JNEUROSCI.4491-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscopo DM, El-Danaf RN, Huberman AD, Niell CM. Diverse visual features encoded in mouse lateral geniculate nucleus. J. Neurosci. 2013;33:4642–56. doi: 10.1523/JNEUROSCI.5187-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack P-O, Friedman J, Golshani P. Cellular mechanisms of brain state–dependent gain modulation in visual cortex. Nat. Neurosci. 2013;16:1331–39. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun DL, Alitto HJ, Weyand TG, Usrey WM. Interspike interval analysis of retinal ganglion cell receptive fields. J. Neurophysiol. 2007;98:911–19. doi: 10.1152/jn.00802.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun DL, Warland DK, Usrey WM. Spike timing and information transmission at retinogeniculate synapses. J. Neurosci. 2010;30:13558–66. doi: 10.1523/JNEUROSCI.0909-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE. ‘Hidden lamination’ in the dorsal lateral geniculate nucleus: The functional organization of this thalamic region in the rat. Brain Res. 1988;472:119–37. doi: 10.1016/0165-0173(88)90017-3. [DOI] [PubMed] [Google Scholar]
- Reid RC, Usrey WM. Functional connectivity in the pathway from retina to visual cortex. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. MIT Press; Cambridge, MA: 2004. pp. 673–79. [Google Scholar]
- Rodieck RW. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vis. Res. 1965;5:583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
- Rodieck RW, Stone J. Analysis of receptive fields of cat retinal ganglion cells. J. Neurophysiol. 1965;28:833–49. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Rowe MH, Fischer Q. Dynamic properties of retino-geniculate synapses in the cat. Vis. Neurosci. 2001;18:219–31. doi: 10.1017/s0952523801182076. [DOI] [PubMed] [Google Scholar]
- Ruiz O, Royal D, Sáry G, Chen X, Schall JD, Casagrande VA. Low-threshold Ca2+-associated bursts are rare events in the LGN of the awake behaving monkey. J. Neurophysiol. 2006;95:3401–13. doi: 10.1152/jn.00008.2006. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Logothetis NK. The color-opponent and broad-band channels of the primate visual system. Trends Neurosci. 1990;13:392–98. doi: 10.1016/0166-2236(90)90117-s. [DOI] [PubMed] [Google Scholar]
- Schneider KA, Kastner S. Effects of sustained spatial attention in the human lateral geniculate nucleus and superior colliculus. J. Neurosci. 2009;29:1784–95. doi: 10.1523/JNEUROSCI.4452-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Tan AYY, Corey J, Priebe NJ. Emergence of orientation selectivity in the mammalian visual pathway. J. Neurosci. 2013;33:10616–24. doi: 10.1523/JNEUROSCI.0404-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley RM, Victor JD. The effect of contrast on the non-linear response of the Y cell. J. Physiol. 1980;302:535–47. doi: 10.1113/jphysiol.1980.sp013259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. Thalamic relay functions. Prog. Brain Res. 2001;134:51–69. doi: 10.1016/s0079-6123(01)34005-0. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the Thalamus and Its Role in Cortical Function. 2nd ed MIT Press; Cambridge, MA: 2009. [Google Scholar]
- Sillito AM, Jones HE. Corticothalamic interactions in the transfer of visual information. Philos. Trans. R. Soc. Lond. B. 2002;357:1739–52. doi: 10.1098/rstb.2002.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincich LC, Adams DL, Economides JR, Horton JC. Transmission of spike trains at the retinogeniculate synapse. J. Neurosci. 2007;27:2683–92. doi: 10.1523/JNEUROSCI.5077-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincich LC, Horton JC, Sharpee TO. Preserving information in neural transmission. J. Neurosci. 2009;29:6207–16. doi: 10.1523/JNEUROSCI.3701-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SG, White AJ, Martin PR. Extraclassical receptive field properties of parvocellular, magno-cellular, and koniocellular cells in the primate lateral geniculate nucleus. J. Neurosci. 2002;22:338–49. doi: 10.1523/JNEUROSCI.22-01-00338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford DK, Dacey D. Physiology of the A1 amacrine: a spiking, axon-bearing interneuron of the macaque monkey retina. Vis. Neurosci. 1997;14:507–22. doi: 10.1017/s0952523800012165. [DOI] [PubMed] [Google Scholar]
- Steriade M. The corticothalamic system in sleep. Front. Biosci. 2003;8:878–99. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- Steriade M. Acetylcholine systems and rhythmic activities during the waking–sleep cycle. Prog. Brain Res. 2004;145:179–96. doi: 10.1016/S0079-6123(03)45013-9. [DOI] [PubMed] [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Stone C, Pinto LH. Response properties of ganglion cells in the isolated mouse retina. Vis. Neurosci. 1993;10:31–39. doi: 10.1017/s0952523800003205. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nat. Neurosci. 2001;4:402–8. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Besseyrias V, Bettler B. Functional mapping of GABAB-receptor subtypes in the thalamus. J. Neurophysiol. 2007;98:3791–95. doi: 10.1152/jn.00756.2007. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Alonso J-M, Reid RC. Synaptic interactions between thalamic inputs to simple cells in cat visual cortex. J. Neurosci. 2000;20:5461–67. doi: 10.1523/JNEUROSCI.20-14-05461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Reid RC. Visual physiology of the lateral geniculate nucleus in two species of new world monkey: Saimiri sciureus and Aotus trivirgatis. J. Physiol. 2000;523:755–69. doi: 10.1111/j.1469-7793.2000.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB, Reid RC. Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus. Nature. 1998;395:384–7. doi: 10.1038/26487. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB, Reid RC. Specificity and strength of retinogeniculate connections. J. Neurophysiol. 1999;82:3527–40. doi: 10.1152/jn.1999.82.6.3527. [DOI] [PubMed] [Google Scholar]
- Usrey WM. Spike timing and visual processing in the retinogeniculocortical pathway. Philos. Trans. R. Soc. Lond. B. 2002a;357:1729–37. doi: 10.1098/rstb.2002.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM. The role of spike timing for thalamocortical processing. Curr. Opin. Neurobiol. 2002b;12:411–17. doi: 10.1016/s0959-4388(02)00339-2. [DOI] [PubMed] [Google Scholar]
- van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, Poort J, et al. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. PNAS. 2014;111:14332–41. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hirsch JA, Sommer FT. Recoding of sensory information across the retinothalamic synapse. J. Neurosci. 2010;30:13567–77. doi: 10.1523/JNEUROSCI.0910-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sommer FT, Hirsch JA. Inhibitory circuits for visual processing in thalamus. Curr. Opin. Neurobiol. 2011a;21:726–33. doi: 10.1016/j.conb.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Vaingankar V, Soto Sanchez C, Sommer FT, Hirsch JA. Thalamic interneurons and relay cells use complementary synaptic mechanisms for visual processing. Nat. Neurosci. 2011b;14:224–31. doi: 10.1038/nn.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 2004;5:747–57. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Weyand TG, Boudreaux M, Guido W. Burst and tonic response modes in thalamic neurons during sleep and wakefulness. J. Neurophysiol. 2001;85:1107–18. doi: 10.1152/jn.2001.85.3.1107. [DOI] [PubMed] [Google Scholar]
- Weyand TG. Retinogeniculate transmission in wakefulness. J. Neurophysiol. 2007;98:769–85. doi: 10.1152/jn.00929.2006. [DOI] [PubMed] [Google Scholar]
- Zhuang J, Bereshpolova Y, Stoelzel CR, Huff JM, Hei X, et al. Brain state effects on layer 4 of the awake visual cortex. J. Neurosci. 2014;34:3888–900. doi: 10.1523/JNEUROSCI.4969-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]