Abstract
Exposure to poly and perfluoroalkyl substances (PFASs) has been associated with adverse health effects in humans and wildlife. Understanding pollution sources is essential for environmental regulation but source attribution for PFASs has been confounded by limited information on industrial releases and rapid changes in chemical production. Here we use principal component analysis (PCA), hierarchical clustering, and geospatial analysis to understand source contributions to 14 PFASs measured across 37 sites in the Northeastern United States in 2014. PFASs are significantly elevated in urban areas compared to rural sites except for perfluorobutane sulfonate (PFBS), N-methyl perfluorooctanesulfonamidoacetic acid (N-MeFOSAA), perfluoroundecanate (PFUnDA) and perfluorododecanate (PFDoDA). The highest PFAS concentrations across sites were for perfluorooctanate (PFOA, 56 ng L−1) and perfluorohexane sulfonate (PFOS, 43 ng L−1) and PFOS levels are lower than earlier measurements of U.S. surface waters. PCA and cluster analysis indicates three main statistical groupings of PFASs. Geospatial analysis of watersheds reveals the first component/cluster originates from a mixture of contemporary point sources such as airports and textile mills. Atmospheric sources from the waste sector are consistent with the second component, and the metal smelting industry plausibly explains the third component. We find this source-attribution technique is effective for better understanding PFAS sources in urban areas.
Introduction
Exposure to poly- and perfluoroalkyl substances (PFASs) has been associated with many negative health outcomes including compromised immune function, metabolic disruption, obesity, and altered liver function.1 PFASs in surface waters are an emerging concern for U.S. public water supplies and long-chain compounds bioaccumulate in aquatic food webs, posing health risks to seafood consumers.2-6 Production of PFASs and their precursors has shifted dramatically over the last two decades toward shorter-chain and polyfluorinated species.7 Diverse point sources and atmospheric deposition of some PFASs confounds understanding of the dominant contributors to contamination in the aquatic environment. Regulatory databases such as the U.S. EPA’s Facility Registry Survey (FRS)8 and the Toxic Release Inventory9 presently contain limited to no information on magnitudes of PFASs released to the environment.
Multivariate statistical analyses based on chemical composition profiles can be a powerful tool for diagnosing contamination sources, as illustrated for many other organic contaminants.10 Principal components analysis (PCA) provides information on interrelationships among various chemicals and is useful for deriving common source profiles. Two-way hierarchical clustering can be used as a confirmatory analysis of PCA by generating a flexible number of subgroups of similar sites (those affected by a common source type) without dictating the number of clusters a priori. Clustering of compounds identifies chemicals that co-occur to form a unique signature. These techniques have not been routinely applied to interpret PFAS contamination and show potential for interpreting sources in surface water and seawater.4,11
Here we combine PCA and hierarchical clustering of PFAS profiles measured in surface waters from 37 rivers, streams and estuaries in the Northeastern United States with geospatial analysis of potential sources. Few measurements are available for PFASs in U.S. surface waters over the past five years and the importance of different sources is poorly understood. Source regions for air pollution are commonly identified using back trajectories.12,13 We apply an analogous approach for identifying sources of aquatic pollution based on hydrological distances within a watershed. The main objective of this study is to identify major sources of surface water PFAS contamination in diverse watersheds using information on chemical composition and geospatial analytical tools that consider surface hydrology.
Methods
Sample collection and analysis
We collected surface water samples from rivers/creeks and estuaries at approximately 1 m depth at 28 sites in the state of Rhode Island (RI) in June, 2014 and 9 sites the New York Metropolitan Area (NY/NJ) in October, 2014 (Figure 1). A complete description of sampling sites is provided in the Supporting Information (SI Table S1). Precipitation and flow rates in rivers tend to be higher in June, potentially resulting in enhanced dilution and a low bias for some PFASs measured in RI rivers compared to NY/NJ.
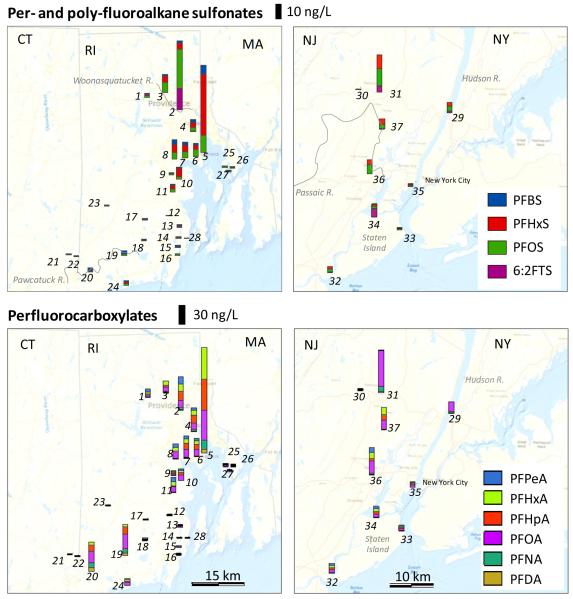
Figure 1.
Concentrations of PFASs measured in surface waters from Rhode Island and the New York Metropolitan Area. Full names of individual compounds are listed in Table S2. N-MeFOSAA and N-EtFOSAA are not shown but were detected in ~70% of the samples at concentrations <1 ng/L.
Samples were stored in one-liter pre-rinsed polypropylene bottles at −20 °C and thawed at room temperature. Each sample was shaken vigorously for homogenization before subsampling 500 ml for the analysis of 21 PFASs. Each unfiltered sample was spiked with 20 μL of a 0.1 ng μL−1 mass labeled PFAS mixture (Wellington; Guelph, Canada; individual compounds are listed in Table S2) as internal standards for quantification. PFASs were extracted using an Oasis Wax solid phase extraction (SPE) cartridge (6 mL, 150 mg sorbent) following the method of Taniyasu et. al.14 (see SI Section S1 for details). A nitrogen evaporator (ZIPVAP) was used to concentrate the extract to 1 mL (methanol: water; v:v = 1:1).
Sample detection for 21 native PFASs (Tables S2, S3) was performed using an Agilent 6460 LC-MS/MS equipped with an online-SPE system (Agilent 1290 Infinity Flex Cube) in dynamic multiple reaction mode (sample chromatogram in Figure S1). At least one negative control (field or procedural blank) and one positive control (spiked with 2 ng of the 21 PFASs in 500 ml water) were included in every extraction batch. Whole method recovery tested using the positive controls was 70-120% for all but 4 PFASs that ranged from 60-70%, which is comparable to recoveries reported by previous studies.3,14,15. The 4 PFASs are perfluoropentanate (PFPeA), perfluoroheptanate (PFHpA), N-methyl perfluorooctanesulfonamidoacetic acid (MeFOSAA) and N-ethyl perfluorooctanesulfon-amidoacetic acid (EtFOSAA). Potential analyte loss during sample preparation was corrected using internal standards spiked prior to sample extraction. The limit of detection (LOD, Figure S2) was defined as equivalent to the blank plus the concentration corresponding to a signal-to-noise ratio of three. Variability between duplicates obtained at two sites was <20%. PFASs in five field blanks (HPLC grade water) prepared following the sample preparation procedure were all below the LOD.
We quantified branched isomers for perfluorooctanate (PFOA), perfluorohexane sulfonate (PFHxS), perfluorooctane sulfonate (PFOS), N-MeFOSAA and N-EtFOSAA using calibration standards for the linear isomers, assuming the same instrumental response factor (Table S3). Seven compounds namely perfluorododecane sulfonate (PFDS), 8:2 fluorotelomer sulfonate (8:2 FTS), perfluorooctane sulfonamide (FOSA), and PFCAs with more than 12 carbon atoms) were detected in less than half of samples and were excluded from additional statistical analysis (see Table S2 for details). For the 14 PFASs that had detection frequencies of greater than 60% (Table S2), we used the Robust Regression on Order Statistics approach for censored log-normally distributed environmental data described by Helsel16 to assign values to samples with concentrations below the LOD.
Statistical and spatial analysis
We used principal components analysis (PCA) and hierarchical clustering to group sites with statistically distinct PFAS composition profiles. PCA was performed using MATLAB’s Statistics Toolbox (MathWorks, Inc.) on normalized (z-score to remove the effect of concentration difference at different sites) PFAS concentration data. The inverse of variances of the data were used as variable weights and varimax rotation was applied to interpret the meaning of extracted principal components. Hierarchical Cluster analysis was conducted using the hclust function in the R statistical computing package (version 3.1.3).
We characterized the watershed for each freshwater sampling site using the U.S. Geological Survey’s (USGS) National Elevation Dataset (3 arc-second for site 15 and 16 and 1 arc-second for others) and the Hydrologic Tool in ArcGIS Pro 1.2 and ArcGIS online. Estuarine sampling sites were excluded from the geospatial analysis due to the confounding influence of tidal waters diluting potential source profiles. Population within each watershed was based on ESRI’s U.S. Demographic Database.17 We used the USGS’s StreamStats database (version 4)18 to characterize water flow rates for each location and to compute mass flow (kg/yr) of PFASs at each site and per-capita mass flows (kg/person/yr).
For all inland sites (non-estuarine), we acquired a list and geospatial data for plausible PFAS sources from the US EPA Facility Registry Service (FRS) database on facilities and sites subject to environmental regulation (see SI for the search criteria).8 These include airports, facilities for metal plating/coating, printing, sewage treatment, waste management (including landfills), and manufacturers of semiconductor, textile, paint/coating/adhesive, ink, paper, and petroleum products. A caveat of this analysis is that not all facilities included in the FRS database necessarily release PFASs and the database may not comprehensively include all possible sources.
Hydrological distances of point sources from each sampling site were computed using the ArcGIS Trace Downstream tool. Within each watershed, we defined an indicator for the impact of potential point sources as a function of distance from sampling locations by assuming exponential decay in the source signature19 (i.e., impact = 1/ed, where d = hydrological distance, km). This approach provides additional information on plausible sources that complements multivariate statistical analysis but cannot be considered a quantitative estimate of contributions to sampling locations since magnitudes of PFAS discharges are not available.
Results and Discussion
Concentrations and spatial patterns
Figure 1 shows the compound specific composition and concentrations of PFASs measured in surface water samples as part of this work. Sampling sites in NY/NJ had much greater population density in upstream watersheds (10-43x) compared to RI but the highest concentrations of most PFASs were measured near the city of Providence, RI (Figure 1, Figure S2). The range of measured PFAS concentrations reported here are comparable or lower than U.S. surface waters from other regions collected between 2000-2009 (Table S4).2,20-24
All sites had detectable PFOA and PFNA and over 90% contained detectable PFHxS, PFOS, PFDA, and 6:2 FtS (Table S2, S3, Figure S2). The highest individual PFAS concentration across sites was PFOA (56 ng L−1) at Site 31 (Passaic River, NJ). Highest concentrations of PFHxS (43 ng L−1) and PFNA (14 ng L−1) were measured at Site 5 (Mill Cove, RI). The maximum PFOS concentration (27 ng L−1) was measured at Site 2 (Woonasquatucket River, RI) within the City of Providence, RI. This is much lower than maximum levels reported in earlier studies of US surface waters that range between 43-244 ng L−1 (Table S4) and reflects the continued decline in environmental PFOS burdens in North America following elimination of production in 2002.25,26
Measured PFAS concentrations in urban regions were significantly higher (Wilcoxin rank sum test, p<0.017) than rural sites for all compounds except PFBS, N-MeFOSAA, PFUnDA and PFDoDA (Figure S3). Sites 1-9 in RI and Sites 29-37 in NY/NJ are all urban areas, defined by population densities of greater than 1000 individuals per square mile (2590 km2), and population densities of greater than 500 individuals per square mile in surrounding census blocks.27 We did not find a statistically significant correlation between total population in each upstream watershed and PFAS concentrations measured at each sampling site (p=0.12 to 0.95 across compounds). We derived per-capita discharges (Figure S4) using a similar approach as Pistocchi and Loos.28 Highest median per-capita discharges (μg person−1 day−1) across compounds, in decreasing order, were for PFOA (27), PFHxA (14), PFHpA (10), PFOS (9), PFHxS (7), and PFNA (5) (Figure S4). These are lower than previously reported in Europe ca. 2007 (e.g., PFOA: 82 μg person−1 day−1, PFOS: 57 μg person−1 day−1).28
Source identification
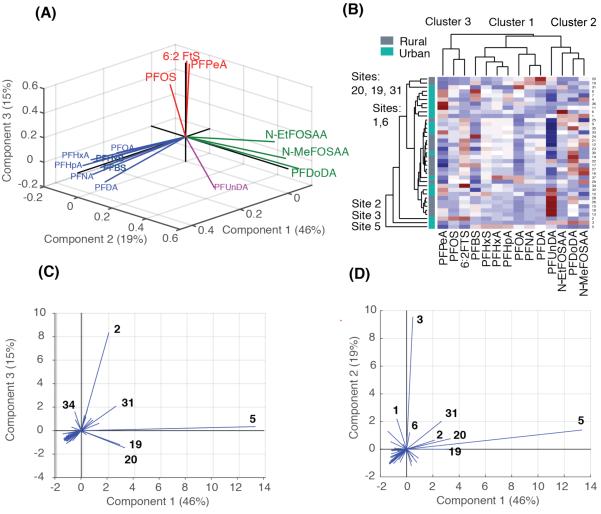
Both hierarchical clustering and PCA identified three distinct groupings of PFASs (Figure 2a, b). The first component/cluster explains 46% of variability in the PCA and includes two major end products of the fluorochemical manufacturing industry (PFOA, PFNA), and a mix of other compounds: PFBS, PFHxS, PFHxA, PFDA. Site 5 (Mill Cove, RI) contains the highest summed PFASs across all sites and is dominated by this mixture of PFASs. PCA results suggest Site 5 is statistically similar to the Pawcatuck River, RI sampling locations (Sites 20, 19) and the Passaic River, NJ (Site 31). However, these sites are grouped separately in the hierarchical clustering analysis (Figure 2b), suggesting some differences in source contributions.
Figure 2.
Multivariate statistical analysis of surface water data. Panel (A) shows loadings of principal components analysis (PCA) and Panels (C) and (D) show score plots for three components across sampling sites. Panel (B) compares PCA results to hierarchical clustering of compounds and sites. Sites with statistically distinct PFAS profiles are indicated on plots (C) and (D) and highlighted on the hierarchical clustering diagram. The three principal components together explain 80% of the variance in PFAS composition.
Geospatial analysis of the watersheds for Sites 5, 19, 20 and 31 reveals a mixture of potential sources (Figure S5). For Site 5, the greatest source impact as a function of distance within the watershed is from T.F. Green Airport, the largest public airport in Rhode Island. Prior work indicates uses of AFFF in modern airports release diverse PFASs to downstream aquatic environments, including the compounds identified as part of the first PCA/cluster.4,29-31 For Sites 19 and 20, textile mills in the upstream watersheds have the highest impact as a function of distance (Table S5). PFASs are used for water resistant coating in textiles and washing and disposal of wastewater at textile mills provides a vector for their entry to the aquatic environment. For Site 31, PCA scores suggest a mix of components 1-3 (Figure 2 c, d). This site also clusters differently than Sites 19 and 20 (Figure 2b). The FCA database indicates the watershed of Site 31 (Figure S5) contains diverse industrial sources that must account for this profile including metal plating, printing, a landfill, petroleum and coal products manufacturing. Overall, we conclude that the first PCA component and cluster of PFASs (PFOA, PFNA PFBS, PFHxS, PFHxA, PFDA) represents a mixture of contemporary sources including airports and textile mills.
The second component/cluster explains 19% of the variability in PFASs and includes two long-chain PFASs (PFUnDA and PFDoDA) and two precursors to PFOS (MeFOSAA and EtFOSAA) (Figure 2). PFUnDA and PFDoDA mainly originate from fluorotelomer alcohols or other fluototelomer based products.32 Both N-MeFOSAA and N-EtFOSAA are intermediate degradation products from the volatile parent compound N-alkyl perfluorooctane sulfamideoethanol (FOSE) with PFOS as the final degradation product. This profile is most pronounced at Site 3 along the Woonasquatucket River in RI and is also evident at Site 1 (Slack’s Tributary, RI) and Site 6 (Buckeye Brook, RI). For Site 3, the largest source impact based on distance is from a wastewater treatment plant 1 km upstream. No industrial facilities exist upstream of Sites 1 and 6. Landfill/waste management facilities are located within 2 km of all three sites but are not hydrologically connected to the sampling locations (Figure S5). Both landfills and wastewater treatment plants are known atmospheric sources of fluorotelomer alcohols and FOSE.33 Concentrations of N-MeFOSAA, PFUnDA and PFDoDA were not spatially variable at most sites and only slightly elevated at Site 3, consistent with an atmospheric input pathway. We thus infer that this component is most likely attributable to sources from the waste sector.
The third component explains 15% of the variability in PFASs and includes PFPeA, PFOS, and 6:2 FTS. This component is most pronounced at Site 2 along the Woonasquatucket River, within the City of Providence, RI. GIS analysis of the watershed at this site reveals the presence of 14 metal coating/plating industries upstream (Figure 2d, Table S5, Figure S5). PFOS was historically used as a mist/fume control agent in metal plating, in surface coatings and as the major component in AFFFs for fighting petroleum related fire.25,26,34 Some PFOS applications such as metal plating have been replaced by less stable fluorotelomer based chemicals such as 6:2 FtS,35 which will eventually degrade into PFPeA and PFHxA (yields of 1.1% and 1.5% in activated sludge).36 It is likely that PFHxA is not included in the cluster because other direct sources can contribute one order of magnitude more PFHxA than PFPeA.37,38 We conclude that the distinct PFAS profile at Site 2 is can be explained by the metal plating industry.
Implications
Multivariate statistical tools such as PCA and hierarchical clustering of PFAS profiles combined with data on hydrological proximity of potential sources are useful for identifying sources of surface water contamination. We find aquatic transport pathways (hydrological distance and river flow directions) are critical for source identification. This contrasts many other persistent organic pollutants that are primarily transported atmospherically, allowing sources within a radius surrounding the sampling sites to be linked to concentrations.39 We conclude that the approach demonstrated here for RI and NY/NJ has potential for diagnosing PFAS source contributions in urbanized regions with elevated concentrations and lacking specific information on the magnitude of PFAS discharges from diverse industries. Background PFAS concentrations at most rural sites in this study contain a mix of diverse source signatures that are not statistically distinguishable using these methods. This analysis could be refined in future applications by analyzing additional emerging short-chain PFASs and precursors to develop more unique chemical signatures for specific industries (i.e., those contributing to the first component/cluster).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge financial support for this study from the Smith Family Foundation, the Harvard John A. Paulson School of Engineering and Applied Sciences TomKat fund and the Harvard NIEHS Center Grant (P30ES000002). X.Z. was partially supported by a Postdoctoral Fellowship from the Natural Sciences and Engineering Research Council of Canada (PDF-437949-2013). We thank Pete August (URI), Wenlu Zhao, Minggang Cai (Xiamen Univ.), Kirk Barrett (Manhattan College) for assistance with sample collection and GIS data. We thank Linda Green and Elizabeth Herron, University of Rhode Island Watershed Watch program (http://web.uri.edu/watershedwatch) leaders as well as the many URI Watershed Watch volunteer water quality monitors who collected water samples.
Footnotes
Supporting Information Available: Details on analytical methods, data analyses, supporting figures and tables. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. Jama-J Am Med Assoc. 2012;307:391–397. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Post GB, Louis JB, Lippincott RL, Procopio NA. Occurrence of Perfluorinated Compounds in Raw Water from New Jersey Public Drinking Water Systems. Environ. Sci. Technol. 2013;47:13266–13275. doi: 10.1021/es402884x. [DOI] [PubMed] [Google Scholar]
- 3.Castiglioni S, Valsecchi S, Polesello S, Rusconi M, Melis M, Palmiotto M, Manenti A, Davoli E, Zuccato E. Sources and fate of perfluorinated compounds in the aqueous environment and in drinking water of a highly urbanized and industrialized area in Italy. J. Hazard. Mater. 2015;282:51–60. doi: 10.1016/j.jhazmat.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 4.de Solla SR, De Silva AO, Letcher RJ. Highly elevated levels of perfluorooctane sulfonate and other perfluorinated acids found in biota and surface water downstream of an international airport, Hamilton, Ontario, Canada. Environ. Int. 2012;39:19–26. doi: 10.1016/j.envint.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Hurley S, Houtz EF, Goldberg D, Wang M, Park J, Nelson DO, Reynolds P, Bernstein L, Anton-Culver H, Horn-Ross P. Preliminary Associations between the Detection of Perfluoroalkyl Acids (PFAAs) in Drinking Water and Serum Concentrations in a Sample of California Women. Environ. Sci. Technol. Letters. 2016 [Google Scholar]
- 6.Happonen M, Koivusalo H, Malve O, Perkola N, Juntunen J, Huttula T. Contamination risk of raw drinking water caused by PFOA sources along a river reach in south western Finland. Sci. Total Environ. 2016;541:74–82. doi: 10.1016/j.scitotenv.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Wang ZY, Cousins IT, Scheringer M, Hungerbuhler K. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int. 2013;60:242–248. doi: 10.1016/j.envint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 8.US EPA [accessed Nov 2015];Facility Registry Service (FRS) http://www.epa.gov/enviro/epa-frs-facilities-state-single-file-csv-download.
- 9.U.S. EPA [accessed May 2016];Toxics Release Inventory (TRI) Program. https://http://www.epa.gov/toxics-release-inventory-tri-program.
- 10.Johnson GW, Ehrlich R, Full W, Ramos S. Principal components analysis and receptor models in environmental forensics. Academic Press; San Diego, CA: 2002. [Google Scholar]
- 11.Benskin JP, Ahrens L, Muir DCG, Scott BF, Spencer C, Rosenberg B, Tomy G, Kylin H, Lohmann R, Martin JW. Manufacturing Origin of Perfluorooctanoate (PFOA) in Atlantic and Canadian Arctic Seawater. Environ. Sci. Technol. 2012;46:677–685. doi: 10.1021/es202958p. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Meyer T, Muir DC, Teixeira C, Wang X, Wania F. Atmospheric deposition of current use pesticides in the Arctic: snow core records from the Devon Island Ice Cap, Nunavut, Canada. Environ Sci Process Impacts. 2013;15:2304–11. doi: 10.1039/c3em00433c. [DOI] [PubMed] [Google Scholar]
- 13.Westgate JN, Wania F. On the construction, comparison, and variability of airsheds for interpreting semivolatile organic compounds in passively sampled air. Environ. Sci. Technol. 2011;45:8850–7. doi: 10.1021/es202490b. [DOI] [PubMed] [Google Scholar]
- 14.Taniyasu S, Kannan K, So MK, Gulkowska A, Sinclair E, Okazawa T, Yamashita N. Analysis of fluorotelomer alcohols, fluorotelorner acids, and short- and long-chain perfluorinated acids in water and biota. J. Chromatogr. A. 2005;1093:89–97. doi: 10.1016/j.chroma.2005.07.053. [DOI] [PubMed] [Google Scholar]
- 15.D'eon JC, Crozier PW, Furdui VI, Reiner EJ, Libelo EL, Mabury SA. Perfluorinated Phosphonic Acids in Canadian Surface Waters and Wastewater Treatment Plant Effluent: Discovery of a New Class of Perfluorinated Acids. Environ. Toxicol. Chem. 2009;28:2101–2107. doi: 10.1897/09-048.1. [DOI] [PubMed] [Google Scholar]
- 16.Helsel DR. Nondetects and data analysis : statistics for censored environmental data. Wiley-Interscience; Hoboken, N.J.: 2005. p. xv.p. 250. [Google Scholar]
- 17.ESRI Demographics [accessed May 2016];Population density in the United States. 2012 http://www.esri.com/data/esri_data.
- 18.U.S. Geological Survey StreamStats [accessed May 2016]; http://ssdev.cr.usgs.gov/streamstats/
- 19.Sakurai T, Serizawa S, Kobayashi J, Kodama K, Lee JH, Maki H, Zushi Y, Sevilla-Nastor JB, Imaizumi Y, Suzuki N, Horiguchi T, Shiraishi H. Temporal trends for inflow of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) to Tokyo Bay, Japan, estimated by a receptor-oriented approach. Sci. Total Environ. 2016;539:277–85. doi: 10.1016/j.scitotenv.2015.08.142. [DOI] [PubMed] [Google Scholar]
- 20.Hansen KJ, Johnson HO, Eldridge JS, Butenhoff JL, Dick LA. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ. Sci. Technol. 2002;36:1681–1685. doi: 10.1021/es010780r. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama S, Strynar MJ, Helfant L, Egeghy P, Ye XB, Lindstrom AB. Perfluorinated compounds in the Cape Fear Drainage Basin in North Carolina. Environ. Sci. Technol. 2007;41:5271–5276. doi: 10.1021/es070792y. [DOI] [PubMed] [Google Scholar]
- 22.Konwick BJ, Tomy GT, Ismail N, Peterson JT, Fauver RJ, Higginbotham D, Fisk AT. Concentrations and patterns of perfluoroalkyl acids in Georgia, USA surface waters near and distant to a major use source. Environ. Toxicol. Chem. 2008;27:2011–2018. doi: 10.1897/07-659.1. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama SF, Strynar MJ, Reiner JL, Delinsky AD, Lindstrom AB. Determination of Perfluorinated Compounds in the Upper Mississippi River Basin. Environ. Sci. Technol. 2010;44:4103–4109. doi: 10.1021/es100382z. [DOI] [PubMed] [Google Scholar]
- 24.Lasier PJ, Washington JW, Hassan SM, Jenkins TM. Perfluorinated chemicals in surface waters and sediments from northwest Georgia, USA, and their bioaccumulation in Lumbriculus variegatus. Environ. Toxicol. Chem. 2011;30:2194–201. doi: 10.1002/etc.622. [DOI] [PubMed] [Google Scholar]
- 25.Paul AG, Jones KC, Sweetman AJ. A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ. Sci. Technol. 2009;43:386–392. doi: 10.1021/es802216n. [DOI] [PubMed] [Google Scholar]
- 26.Armitage JM, Schenker U, Scheringer M, Martin JW, MacLeod M, Cousins IT. Modeling the Global Fate and Transport of Perfluorooctane Sulfonate (PFOS) and Precursor Compounds in Relation to Temporal Trends in Wildlife Exposure. Environ. Sci. Technol. 2009;43:9274–9280. doi: 10.1021/es901448p. [DOI] [PubMed] [Google Scholar]
- 27.US Census Bureau [accessed June 2016];Urban And Rural Definitions. 1995 http://www.census.gov/population/censusdata/urdef.txt.
- 28.Pistocchi A, Loos R. A Map of European Emissions and Concentrations of PFOS and PFOA. Environ. Sci. Technol. 2009;43:9237–9244. doi: 10.1021/es901246d. [DOI] [PubMed] [Google Scholar]
- 29.Ahrens L, Norström K, Viktor T, Cousins AP, Josefsson S. Stockholm Arlanda Airport as a source of per- and polyfluoroalkyl substances to water, sediment and fish. Chemosphere. 2015;129:33–38. doi: 10.1016/j.chemosphere.2014.03.136. [DOI] [PubMed] [Google Scholar]
- 30.Awad E, Zhang XM, Bhavsar SP, Petro S, Crozier PW, Reiner EJ, Fletcher R, Tittemier SA, Braekevelt E. Long-Term Environmental Fate of Perfluorinated Compounds after Accidental Release at Toronto Airport. Environ. Sci. Technol. 2011;45:8081–8089. doi: 10.1021/es2001985. [DOI] [PubMed] [Google Scholar]
- 31.Filipovic M, Woldegiorgis A, Norstrom K, Bibi M, Lindberg M, Osteras AH. Historical usage of aqueous film forming foam: A case study of the widespread distribution of perfluoroalkyl acids from a military airport to groundwater, lakes, soils and fish. Chemosphere. 2015;129:39–45. doi: 10.1016/j.chemosphere.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Government of Canada [accessed Nov 2015];Chemical Substances: Long-Chain (C9-C20) Perfluorocarboxylic Acids (PFCAs), Their Salts, and Their Precursors. http://www.chemicalsubstanceschimiques.gc.ca/challenge-defi/summary-sommaire/pfcapsapfcsp-eng.php.
- 33.Ahrens L, Shoeib M, Harner T, Lee SC, Guo R, Reiner EJ. Wastewater Treatment Plant and Landfills as Sources of Polyfluoroalkyl Compounds to the Atmosphere. Environ. Sci. Technol. 2011;45:8098–8105. doi: 10.1021/es1036173. [DOI] [PubMed] [Google Scholar]
- 34.Moody CA, Field JA. Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ. Sci. Technol. 2000;34:3864–3870. [Google Scholar]
- 35.Persistent Organic Pollutants Review Committee of UNEP Technical paper on the identification and assessment of alternatives to the use of perfluorooctane sulfonic acid, its salts, perfluorooctane sulfonyl fluoride and their related chemicals in open applications (UNEP/POPS/POPRC.8/INF/17/Rev.1) 2012.
- 36.Wang N, Szostek B, Buck RC, Folsom PW, Sulecki LM, Gannon JT. 8-2 Fluorotelomer alcohol aerobic soil biodegradation: Pathways, metabolites, and metabolite yields. Chemosphere. 2009;75:1089–1096. doi: 10.1016/j.chemosphere.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 37.Dauchy X, Boiteux V, Rosin C, Munoz JF. Relationship Between Industrial Discharges and Contamination of Raw Water Resources by Perfluorinated Compounds: Part II: Case Study of a Fluorotelomer Polymer Manufacturing Plant. Bull. Environ. Contam. Toxicol. 2012;89:531–536. doi: 10.1007/s00128-012-0705-9. [DOI] [PubMed] [Google Scholar]
- 38.Butenhoff JL, Kennedy GL, Jr., Frame SR, O'Connor JC, York RG. The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rat. Toxicology. 2004;196:95–116. doi: 10.1016/j.tox.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Khairy M, Muir D, Teixeira C, Lohmann R. Spatial Distribution, Air-Water Fugacity Ratios and Source Apportionment of Polychlorinated Biphenyls in the Lower Great Lakes Basin. Environ. Sci. Technol. 2015;49:13787–97. doi: 10.1021/acs.est.5b00186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.