Summary
Neurosteroids are key endogenous molecules in the brain that affect many neural functions. Here, we describe recent advances in NIH-sponsored and other clinical studies of neurosteroids for CNS disorders. The neuronal GABA-A receptor chloride channel is one of the prime molecular targets of neurosteroids. Allopregnanolone-like neurosteroids are potent allosteric agonists as well as direct activators of both synaptic and extrasynaptic GABA-A receptors. Hence, neurosteroids can maximally enhance synaptic phasic and extrasynaptic tonic inhibition. The resulting chloride current conductance generates a form of shunting inhibition that controls network excitability, seizures, and behavior. Such mechanisms of neurosteroids are providing innovative therapies for epilepsy, status epilepticus, brain injury, Fragile X syndrome, and chemical neurotoxicity. The neurosteroid field has entered a new era, as many compounds have reached advanced clinical trials. Synthetic analogs have several advantages over natural neurosteroids for clinical use because of their superior bioavailability and safety trends.
Keywords: Brain injury, epilepsy, GABA-A receptor, neurosteroid, status epilepticus, tonic inhibition
Neurosteroids
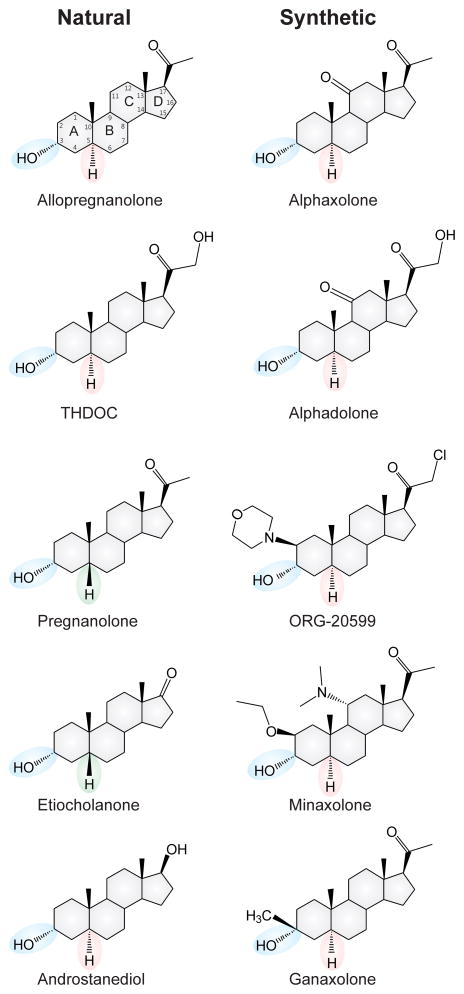
Neurosteroids are endogenous steroids synthesized within the central nervous system (CNS) and that have rapid effects on neuronal excitability (Box 1). A variety of neurosteroids are present in the brain, including pregnane, androstane and estrogen classes. The three prototype neurosteroids that are most widely studied are allopregnanolone (AP, 5α-pregnane-3α-ol-20-one), allotetrahydrodeoxycorticosterone (THDOC, 5α-pregnane-3α,21-diol-20-one), and androstanediol (5α-androstan-3α-ol-20-diol) (Figure 1). Neurosteroids are synthesized in the brain from cholesterol or steroid hormone precursors via progressive A-ring reductions [1]. All necessary enzymes for the biosynthesis of neurosteroids are present in the brain. Neurosteroid synthesis occurs in glia and neurons in many brain regions, including the hippocampus and neocortex [1,2]. The biosynthesis of neurosteroids is controlled by the translocator protein TSPO. Neurosteroids are highly lipophilic, and therefore can easily cross the blood-brain barrier.
Figure 1. Chemical structures of natural and synthetic neurosteroids.
The key stereo specific features of C3-OH and C5-H groups are highlighted for both naturally occurring neurosteroids and their synthetic analogs.
It has been known since the 1940s, from the pioneering work of Hans Selye, that steroid hormones progesterone and deoxycorticosterone can exert anesthetic and anticonvulsant actions [3–6]. In the early 1980s, the synthetic neurosteroid, alphaxolone, was found to enhance synaptic inhibition via an action on GABA-A receptors in the brain [7]. A major advance occurred when neurosteroids were found to enhance GABA-A receptor function [8]. Consequently, neurosteroids have been shown to possess robust activity in animal models and may have advantages over benzodiazepines, including lack of tolerance development with extended use [1,9–11]. The growing understanding of the mechanisms of neurosteroid modulation of GABA-A receptor function has created new opportunities for improved therapies for treating CNS disorders.
Mechanism of Action of Neurosteroids
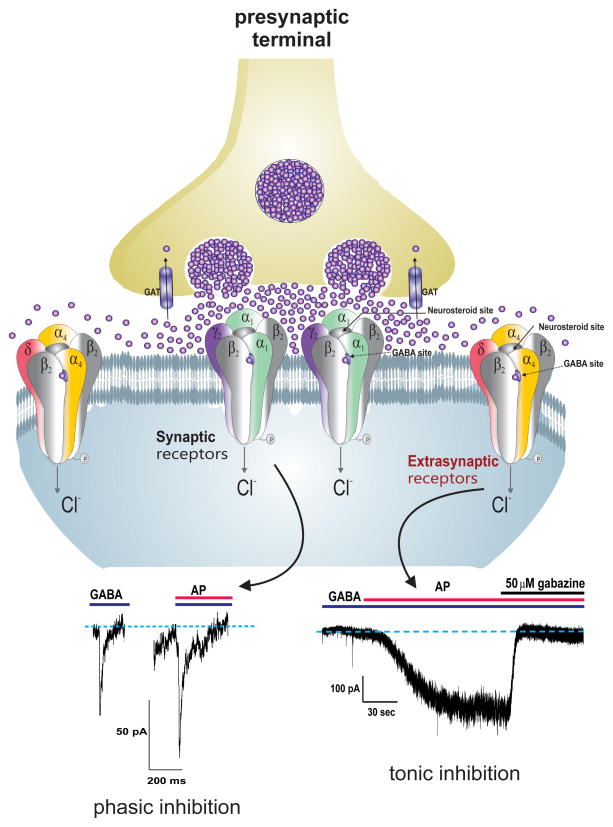
Neurosteroids rapidly alter neuronal excitability through direct interaction with GABA-A receptors (Figure 2). Hence, neurosteroids are often referred as endogenous modulators of GABA-A receptors in the brain [8, 12, 13]. GABA-A receptors are responsible for the majority of inhibitory currents in the brain. Structurally, these receptors are pentameric channels made from various subunits (α1–6, β1–4, γ1–3, δ, ε, θ, ρ1–3). GABA-A receptors are ligand-gated chloride channels which, when activated by GABA, hyperpolarize the neurons through influx of chloride ions. Based on location, GABA-A receptors are categorized into synaptic and extrasynaptic receptors (Figure 2). Synaptic (γ-containing) receptors, which are present ubiquitously within the brain, produce phasic currents in response to vesicular release of GABA. Extrasynaptic (δ-containing) receptors, which are expressed in specific brain regions including hippocampus, thalamus, amygdala, hypothalamus, and cerebellum [14], generate non-desensitizing tonic currents that are continuously gated by extracellular GABA. Tonic current sets the baseline and shunting inhibition via continuous chloride conductance in specific neurons such as granule cells within the dentate gyrus, a critical region for controlling inputs from afferent neuronal pathways [15–17].
Figure 2. Neurosteroid mechanism of action at synaptic and extrasynaptic GABA-A receptors.
Allopregnanolone and related neurosteroids enhance the function of GABA-A receptors, which are pentameric chloride channels. The “neurosteroid binding” sites are distinct from sites for GABA, benzodiazepines and barbiturates. There are two subtypes of GABA-A receptors with different desensitization kinetics. Synaptic GABA-A receptors, which are composed of 2α2βγ subunits, mediate the phasic portion of inhibition in response to action potential-dependent vesicular release of high levels of GABA, while extrasynaptic GABA-A receptors, which are composed of 2α2βδ subunits, primarily contribute to tonic inhibition when exposed to low, ambient levels of GABA. Neurosteroids activate both synaptic and extrasynaptic receptors and enhance the phasic and tonic inhibition, and thereby promote maximal inhibition in the brain. GABA transporters (GAT) in neurons and glia remove synaptically released GABA. Traces illustrate phasic, as IPSCs (left, 3 μM GABA ± 300 nM AP), and tonic currents (right, 1 μM GABA ± 3 μM AP) in dentate gyrus granule cells during neurosteroid allopregnanolone (AP) perfusion in the hippocampus slice recording. The competitive GABA-A receptor antagonist gabazine (SR-95531, 50 μM) was used to confirm the specificity of GABAergic currents.
Neurosteroids are potent positive allosteric agonists of synaptic and extrasynaptic GABA-A receptors [18–21] (Figure 2). The mode of action for neurosteroids depends on the concentration: at high concentrations, neurosteroids directly activate receptors, whereas at low concentrations, they allosterically potentiate GABA-A receptor currents. This is achieved by binding to “neurosteroid binding sites” on the receptor channel [6, 22]. Although the exact interaction site is unclear, there are two discrete binding sites for neurosteroids including an allosteric site within the α-subunit transmembrane domain and a site of direct activation at the α-β subunit interface [19, 23, 24]. It is also likely that neurosteroids function in an autocrine-like fashion in which they reach their targets by lateral membrane diffusion [18]. Structure-activity studies confirmed that the C3α-OH steroid structure is essential for the binding affinity and the receptor-enhancing function of neurosteroids [4, 21]. Apart from 5α-H stereo-selectivity, the C17 or C20-ketone group is important for high-potency positive allosteric modulation [25–29]. So far, over 500 analogs have been tested for CNS activity [29].
At higher concentrations, neurosteroids directly activate GABA-A receptors [25, 30, 31]. This is a unique mechanistic feature of neurosteroids. The concentration of neurosteroid able to directly gate GABA-A receptors (≥1 μM) is greater than the observed physiological range of endogenous neurosteroids in the brain (0.1 – 0.3 μM) [5, 17, 30]. Nevertheless, exogenous administration of neurosteroids can reach sufficiently high concentrations within the brain to achieve direct activation of GABA-A receptors [27, 32]. Hence, the mechanism of action of neurosteroids is complex. At nanomolar levels they follow allosteric agonism, meaning that the resulting maximal efficacy of GABA potentiation reaches a limiting value when the allosteric site is fully occupied. At higher (micromolar) levels that exceed allosteric-saturating levels, neurosteroids follow direct agonism, defined when the resulting efficacy of chloride conductance attains ceiling value per available receptor sites within the neurons. Thus, it is thought that the overall net inhibition during neurosteroid therapy occurs via allosteric binding and direct activation mechanisms.
Neurosteroids bind to all GABAA receptor isoforms, but δ-containing receptors exhibit preferential sensitivity at extracellular GABA concentrations [33–35] (Figure 2). Unlike benzodiazepines, neurosteroids are able to modulate all isoforms of GABA-A receptors, including those that contain benzodiazepine-insensitive α4 and α6 subunits or lack the obligatory γ2 subunit required for benzodiazepine-sensitivity [36]. The allosteric binding of neurosteroids to low-efficacy δGABA-A receptors induces a pronounced conformational change, greater channel opening, and non-desensitizing tonic inhibition [37]. Increased δGABA-A receptor expression enhances neurosteroid sensitivity through greater potentiation of tonic current [38–40]. Conversely, deficient δGABA-A receptor expression reduces the sensitivity to neurosteroids [35, 41–44]. In the dentate gyrus, ~95% tonic currents are generated by extrasynaptic δGABA-A receptors [17]. Mutations to the Gabrd gene result in dysfunctional GABA-A receptor currents, and these mutations have been linked with clinical cases of generalized epilepsies [45, 46]. Thus, diminished δGABA-A receptor-mediated tonic inhibition exerts significant impact on network excitability, behavior, and seizure susceptibility.
Despite growing evidence that many neurosteroids and clinically used drugs can modulate tonic inhibition, the structure-activity relationship of ligands at δGABA-A receptors neurons remains unclear. A recent report crafted a consensus pharmacophore for the neurosteroid modulation of extrasynaptic GABA-A receptor based upon functional screening of a vast library of neurosteroids in dentate gyrus granule neurons (35). The study identified four salient features of the pharmacophore model: (i) The C3α-OH group remains obligatory for extrasynaptic receptor functional activity; (ii) The C5-H region shows a stereoselectivity for interaction with receptors; (iii) The C17 or C20-moiety of the steroid provides coordination of the hydrophobic pocket affinity and thus alterations at the C17 or C20 region of the molecule (e.g. androstane analogs) can drastically reduce transduction kinetics of the channel for tonic currents; and (iv) additional attachments including C11 appears detrimental to promote tonic currents. In essence, there is strong evidence that neurosteroids exhibit greater sensitivity at extrasynaptic δGABA-A receptors and this allows therapeutic targeting of tonic inhibition for many brain disorders.
Clinical Use of Neurosteroids
Several synthetic neurosteroids have been prepared for therapeutic use in the past few decades (Figure 1). The best-known of these are alphaxolone, alphadolone, minaxolone, and ganaxolone [6, 9]. The first drug in this family to be commercially marketed was a mixture of alphaxolone and alphadolone, known as Althesin in Europe, in the 1970s [6]. Due to a rare but serious toxic reaction in humans to the vehicle used to formulate Althesin, it has been withdrawn from clinical studies; however, alfaxolone (Alfaxan® or Saffan®) is still used in veterinary medicine. The development of minaxolone (hypnotic) and ORG-20599 (anesthetic) was abandoned due to a lack of acceptable features. These compounds have influenced the development of newer synthetic neurosteroids.
There is renewed interest in synthetic neurosteroid analogs as promising therapeutic agents as evident from several ongoing therapeutic development programs. The neurosteroid field has advanced so that currently at least four compounds are in clinical trials for epilepsy, traumatic brain injury, status epilepticus, and Fragile X syndrome. One compound, alfaxolone, is available for veterinary use. Drug formulations of natural progesterone and AP have been created for clinical development. Ganaxolone, another synthetic neurosteroid, has been investigated extensively and is currently in clinical trials for epilepsy [11]. Currently, there is high level of interest in neurosteroid therapeutics and a number of clinical trials are in progress for unmet neuropsychiatric disorders. Here, we describe recent advances in NIH-sponsored and other clinical studies of neurosteroids for the treatment of CNS disorders.
Epilepsy
Epilepsy is one of the leading causes of chronic neurological morbidity worldwide. It affects nearly 3 million people in the USA, and every year 150,000 people are newly diagnosed with epilepsy. There is no cure for epilepsy, and antiepileptic drugs are the mainstay for controlling seizures. Presently, there is a large unmet medical need for new therapies for patients with refractory seizures, who do not respond to current medications. 5α-Reduced pregnane and androstane neurosteroids are powerful anticonvulsants (Table 1). Neurosteroids exhibit broad-spectrum anticonvulsant effects in diverse rodent seizure models [7, 8, 47]. Neurosteroids protect against seizures induced by GABA-A receptor antagonists, including pentylenetetrazol and bicuculline, and are effective against pilocarpine-induced limbic seizures as well as seizures in kindled animals [7]. The potencies of neurosteroids vary in different seizure models [48–50]. Neurosteroids are highly active in the 6-Hz model [35, 51]. Neurosteroid’s ability to suppress seizures is stereoselective [27]. Neurosteroids are highly effective in suppressing seizures due to withdrawal of GABA-A receptor modulators including neurosteroids and benzodiazepines, as well as other types of agents such as ethanol and cocaine [52–55]. However, they are less active in generalized seizure models of maximal electroshock and excitatory agents such as NMDA, kainic acid and 4-aminopyridine. In contrast to benzodiazepines, where utility in the chronic treatment of epilepsy is limited by tolerance, anticonvulsant tolerance is not evident with neurosteroids [56, 57]. Recent studies suggest that neurosteroids play a role in epileptogenesis [58, 59]. Thus, there is considerable interest in leveraging the growing understanding of neurosteroid modulation of GABA-A receptor function to mitigate epilepsy symptomatology.
Table 1.
Anticonvulsant profile of neurosteroids in epilepsy models.
| Model | Allopregnanolone | THDOC | Androstanediol | Ganaxolone | Refs |
|---|---|---|---|---|---|
| Kindling models | |||||
| Hippocampus kindling | 3.5 | 4.5 | 50 (36–64) | 3.5 | [27, 40, 58, 77, 119, 120] |
| Amygdala kindling | 14 (8–23) | 15 (10–30) | ND | 6.6 (5.1–9.7) | [119, 121] |
| Cocaine kindling | 4.77 (2.27–10.0) | ND | ND | 17.0 (ND) | [122, 123] |
| Pentylenetetrazol kindling | ND | ND | ND | 4.1 (2.7–6.4) | [124] |
| Chemoconvulsant models | |||||
| Pentylenetetrazol (mice) | 12 (10–15) | 19 (77–122) | 40 (27–60) | 3.5 (2.1–5.8) | [25, 30, 119] |
| Pentylenetetrazol (rats) | 2.14 (1.10–4.15) | 6.98 (4–12) | [53] | ||
| Bicuculline | 12 (10–15) | 12 (10–15) | 44 (24–81) | 4.6 (3.2–6.8) | [124] |
| Picrotoxin | 10 (5–19) | 10 (5–19) | 39 (21–74) | ND | [125, 126] |
| t-Butylbicycloorthobenzoate | ND | ND | ND | 11.7 (8.8–15.7) | [124] |
| Flurothyl | ND | ND | ND | 5.0 (ND) | [127] |
| N-Methyl-D-aspartate | >40 | >40 | >200 | >30 | [120, 124] |
| Kainic acid | >40 | >40 | >200 | >30 | [120, 124] |
| 4-Aminopyridine | >40 | >40 | >200 | 11.5 (8.1–16.3) | [124] |
| Strychnine | >40 | >40 | >200 | >40 | [124] |
| Electroshock models | |||||
| Maximal electroshock | 29 (19–44) | 48 (35–66) | ND | 29.7 (25.3–34.8) | [124] |
| 6-Hz stimulation | 14 (10–19) | ND | ND | 6.3 (4.0–9.8) | [51] |
| Status epilepticus models | |||||
| Pilocarpine | 7 (4–13) | 7 (4–13) | 81 (45–133) | ~6 | [70, 125, 128, 129] |
| Kainic acid | ~20 | ND | ND | ND | [81] |
The profile of neurosteroids is expressed in terms of ED50, which is the dose in mg/kg producing seizure protection in 50% of animals (mostly in mice). Values in parentheses are 95% confidence limits. ND, not determined.
AP and its synthetic analogues have been evaluated in a multitude of clinical trials to assess their utility in epilepsy patients [11, 60]. To date, more than 1000 subjects have received ganaxolone (GX) in various forms. Two pilot studies were conducted in 2006 to ascertain the safety, tolerability, dose range, and the efficacy of GX in pediatric and adolescent patients suffering from refractory epilepsy (uncontrolled by at least two conventional antiepileptic drugs). These were open-label pilot studies conducted in the inpatient [61, 62] and outpatient [63] settings, with GX incorporated as a flexible dose-escalation add-on therapy. The inpatient study administered a GX-cyclodextrin suspension starting from a dose of 1 mg/kg BID, escalated over a 16 day inpatient period up to a maximum maintenance dose of 12 mg/kg TID. 5 out of 15 drug-refractory subjects experienced at least a 25% decrease in seizure frequency at 4 and 8 week maintenance time points, and out of 17 reported adverse events, all of which were mild or moderate, with somnolence the most frequently reported adverse effect. The outpatient study used a similar dosing titration and maintenance protocol, and disclosed at least 50% seizure reduction in 12 out of 45 study participants (27%). Though most adverse events were again mild to moderate, 9 serious adverse events were reported, mainly involving an adverse change in seizure frequency.
GX was also assessed in adult patients with uncontrolled partial onset seizures. A 10 week double-blind placebo-controlled study in which 1500 mg/day of GX were administered as adjunctive therapy and showed promising results [64] which led to a 2-year open label extension with the intent to evaluate the impacts of long-term administration on safety, efficacy, and tolerability [65]. 124 subjects from the experimental arm of the previous double-blind trial (out of 131 that completed) were continued at 1500 mg/day GX (500 mg TID), with 88% of subjects reaching and 71% of subjects finishing at this dose. Adverse events were primarily minor or moderate, with just 10% discontinuing due to an adverse event. Overall, these results indicate that GX is a promising avenue for further clinical study in treatment-refractory pediatric and adult epilepsy patients, with adequate controls in place to monitor efficacy as well as the extent and severity of adverse events. Given that long-term pharmacologic intervention is often required for patients suffering severe, intractable forms of epilepsy, these data offer some preliminary reassurance that GX may satisfy relevant safety and tolerability criteria. GX is currently in Phase III randomized multinational trials as an adjunctive, or add-on, therapy for the treatment of partial onset seizures in adults with epilepsy (ClinicalTrials.gov Identifier NCT01963208). The outcome of this trial will either support or reject the overall premise of neurosteroid therapy for refractory epilepsy.
In a small pilot study, GX has been tested in female children with epilepsy caused by a mutation of the protocadherin 19 gene (PCDH19). PCDH19 female pediatric epilepsy is a rare disease that is estimated to affect approximately 15,000–30,000 females in the USA and is characterized by onset of cluster seizures before age 5, cognitive and sensory impairment of varying degrees, and behavioral disturbances. The abnormal expression of protocadherin 19 is associated with occurrence of seizures beginning in the early years of life. GX was administered as either oral liquid suspension or capsules for up to 26 weeks for controlling seizures (ClinicalTrials.gov, identifier NCT02358538).
Catamenial Epilepsy
Catamenial epilepsy, the cyclical occurrence of seizure exacerbations during particular phases of the menstrual cycle in women with preexisting epilepsy, is a specific form of pharmacoresistant epilepsy. Catamenial patients often experience more seizures at the periovulatory or perimenstrual period. There are currently no specific treatments for catamenial seizures. Mechanistically, catamenial epilepsy stems from changes in reproductive steroids and their neurosteroids, such as AP, across the menstrual cycle [66–70]. This model suggests that reproductive steroid supplementation would be of clinical benefit in at least some subset of catamenial epilepsy patients. To test this hypothesis, a randomized, double-blind, placebo-controlled, phase III clinical trial was performed to assess the therapeutic benefit of progesterone therapy in the second half of the menstrual cycle in women with intractable catamenial epilepsy [71]. A total of 294 women were assigned to either the catamenial or non-catamenial strata based on seizure pattern and frequency recorded over a baseline period, and subsequently were randomized into placebo control and progesterone arms (four total groups), and administered 200 mg progesterone (or placebo) lozenges TID on days 14–28 of treatment cycles. The trial disclosed no significant advantage of progesterone vs placebo in reduction of seizure frequency. However, post-hoc analysis that revealed that this stratum could be broken down into 3 subsets based on the periodicity of the seizure exacerbation: perimenstrual, periovulatory, and entire luteal phase in anovulatory cycles. This secondary analysis revealed that women who experienced perimenstrual catamenial exacerbation, consistent with the point in the menstrual cycle at which progesterone levels are reduced, were indeed significantly responsive to progesterone supplementation therapy. Thus, the failure of the primary outcome measure may have stemmed from the fact that subtypes of catamenial epilepsy as defined by exacerbation periodicity have distinct pathophysiologic mechanisms, demanding unique therapeutic approaches. Furthermore, marked elevation in AP levels over baseline was observed in progesterone but not placebo-treated women, with a striking inverse correlation between AP level and seizure frequency in responsive women (in the perimenstrual exacerbation category) [72, 73]. Thus, these data suggest a mechanistic role for AP in mediating the ameliorative effects of progesterone therapy in this subset of catamenial epilepsy patients.
This notion is consistent with the outcome of a recent pilot study of ganaxolone in women with catamenial epilepsy. In this open-label pilot study, ganaxolone was evaluated for the safety, tolerability, and antiseizure efficacy in two women with catamenial epilepsy [74]. Patients received oral ganaxolone (300 mg/day, twice daily) starting on day 21 of the menstrual cycle and continuing through the third full day following the beginning of menstruation. Side effects were mild. During the 4 months of this ganaxolone “pulse” therapy, both patients, whose seizures were incompletely controlled with valproate and phenytoin, had an improvement in their catamenial seizures. Further controlled clinical studies are clearly warranted to confirm the efficacy of neurosteroids in catamenial epilepsy.
Mounting preclinical evidence indicate that neurosteroids play a key role in the pathophysiology of catamenial epilepsy [67]. Perimenstrual catamenial epilepsy is believed to occur due to the withdrawal of neurosteroids as a result of the fall in progesterone at the time of menstruation. The neurosteroid withdrawal model of catamenial epilepsy has been used to investigate therapies for catamenial epilepsy. Neurosteroids had enhanced activity in the perimenstrual catamenial epilepsy model. Hence, we suggest that “neurosteroid replacement” may be an effective therapy for catamenial epilepsy [69, 75, 76]. A neurosteroid could be administered in a “pulse” prior to menstruation and then withdrawn, or continuously administered throughout the month at low doses, so to avoid possible sedative side effects. Recently, a novel plasticity of extrasynaptic δGABA-A receptors is reported in a perimenstrual model [39, 40, 77, 78]. In essence, the proposed mechanism poses that the perimenstrual decline in neurosteroids triggers the selective overexpression of δGABA-A receptors in the dentate gyrus granule cells. This compensatory increase in δGABA-A receptors may act as a “Trojan horse” for the exogenous neurosteroid to inhibit seizures. Indeed, this special sensitivity to neurosteroids which women with perimenstrual epilepsy exhibit is in agreement with the findings from the NIH progesterone trial, in which the responder group was women with the perimenstrual type catamenial epilepsy [71]. Therefore, the extrasynaptic mechanism represents a molecular rationale for neurosteroid therapy of catamenial epilepsy.
Status Epilepticus
Status epilepticus (SE) is a grave neurologic emergency with significant morbidity and mortality, especially among patients who do not respond to first- and second-line therapeutic options. Traditional benzodiazepine agents such as midazolam target synaptic GABA-A receptors, but have little effect on extrasynaptic isoforms which are responsible for tonal inhibition [36]. It has been shown that functional inactivation of synaptic GABA-A receptors via active internalization is the physiologic mechanism by which benzodiazepine resistance rapidly emerges in refractory SE [79, 80]. Therefore, neurosteroid compounds like AP and its synthetic derivatives, which potentiate both phasic and tonic current, have been proposed as potential alternatives [12, 58, 81]. A randomized, double-blind, placebo-controlled, add-on, Phase II clinical trial is underway to evaluate SGE-102, an intravenous formulation of AP [82]. It includes adult patients in refractory status epilepticus for < 24 hours, defined as having failed first- and second-line treatments. At this point, the patients are randomized to receive midazolam IV + SGE-102 or midazolam IV + placebo. Midazolam will be tapered first, with SGE-102 or placebo continued through hour 120. This trial considers as its primary endpoint a comparison of the proportion of subjects still free of seizures at hour 120, and will also collect additional data at later time points, as well as on safety and pharmacokinetics. Though novel, this methodology has been devised such that the trial will have acceptable significance with regard to its outcomes. Specifically, the sample size parameters have been set to provide 90% predictive power to distinguish between the experimental and control outcomes at an alpha of 0.05.
Infantile Spasms
A devastating disorder occurring during the neurodevelopmental period of infancy, infantile spasms (IS), also known as West syndrome, is an epileptiform condition brought on by an idiopathic encephalopathy with a variety of triggers. Although IS is self-limiting, its dramatic presentation and association with significant developmental delays have led to considerable interest in expanding the currently limited repertoire of therapeutic approaches. Typical anti-seizure drugs are ineffective, but adrenocorticotropic hormone and vigabatrin are known to alleviate symptoms in select patients, leading to the speculation that that neurosteroid imbalances and GABAergic derangements may play a role in the pathophysiology of IS [83, 84]. To facilitate this analysis, a rat model of IS was developed using betamethasone and NMDA to prime and trigger the IS-like state, along with scoring criteria to assess seizure severity [85]. In this setting, a preclinical study explored the potential for GX to address IS-like disease manifestations [86]. GX doses of 25 and 50 mg/kg significantly delayed and decreased the severity of NMDA-induced spasms, whether primed with betamethasone or not, and minimized observable behavioral changes associated with repeated spasm bouts, such as alterations in exploratory behavior.
Synthetic neurosteroids have been proposed as a new, non-hormonal therapeutic approach for IS [87]. Indeed, recent clinical trials of GX support a role for neurosteroids in the treatment of IS [60, 88, 89]. Two open-label trials of GX in infantile spasms have been reported with indications of efficacy in both cases. Overall, about 30% of 79 patients with highly refractory IS (ages 6 months to 15 years) showed substantial (>50%) reductions in spasm frequency. Fifteen children with active refractory IS were treated with GX according to an escalating dosage schedule. During a 2-month GX maintenance period, ten of these children experienced a 25–50% decrease in spasm frequency.
Fragile X Syndrome
Fragile X syndrome (FXS) is a common single-gene loss-of-function disorder characterized by cognitive deficits, dysmorphic facies, and macro-orchidism, which has been closely linked to autism-spectrum disorders [90]. It is caused by CGG trinucleotide repeat expansion, with a repeat number >200 in the FMR1 gene considered diagnostic for disease. Individuals with 55–200 repeats are considered to have an FXS premutation, which is frequently clinically silent during childhood but can develop in late-adulthood into fragile X-associated tremor/ataxia syndrome. FXS affects about 100,000 people in the US. Patients with FXS show autism-like symptoms including cognitive impairment, anxiety and mood swings, behavioral and learning challenges. Many patients with FXS have seizures. Reliable mouse models of premutation have been developed in which behavioral, developmental, and histopathological findings comparable to human FXS have been observed [91]. One recent pre-clinical analysis uses a 170 CGG repeat premutation mouse model to demonstrate functional impairments in pre-CGG neurons such as clustered burst electrical firing behavior, indicating an imbalance of excitatory and inhibitory neurotransmission in affected tissues [92]. Because this imbalance was theorized to stem at least in part from deficits in GABAergic function, positive allosteric modulation of GABAA receptors using AP was explored. AP treatment was found to reversibly mitigate the functional impairments identified in this model, restoring WT firing behavior. Conversely, GABA-A receptor blockade of WT neurons using picrotoxin phenocopied the clustered burst firing pattern observed in the premutation model. These findings provide further evidence for a GABA-mediated mechanism for the electrophysiological derangements observed in models of FXS, and lend support to AP as a possible ameliorative agent worthy of further investigation as a new therapeutic intervention in this setting.
GX will be tested in a Phase II proof-of-concept trial for FXS through a research grant from the U.S. Department of Defense (ClinicalTrials.gov Identifier NCT01725152). It is thought that by normalizing GABA function, neurosteroids can reduce anxiety and related behaviors associated with FXS. About 60 subjects will be enrolled and titrated up to a maximum dose of 1,800 mg/day of GX or placebo over a two-week period followed by four weeks of treatment. At the end of the first treatment period and following a washout period, subjects will be crossed over to the other treatment for a similar two-week titration period followed by four weeks of treatment. The primary outcome measure of the study is Clinical Global Impression Rating Scale for Improvement. Secondary outcome measures include the Aberrant Behaviors Checklist and ratings scales for specific behaviors associated with childhood FXS. Results are expected to be released in 2016.
Premenstrual Mood Disorder
Premenstrual dysphoric disorder is a severe form of premenstrual syndrome affecting 3–8% of menstruating women [93]. It is characterized by marked, often disabling changes in affect and behavior as well as intense emotional lability in the luteal phase of the menstrual cycle. This onset of symptoms generally occurs after ovulation, lasting until just after menses. Although no consistent ovarian hormone level dysfunction has been conclusively linked to PMDD symptoms, it has been shown that while GnRH-agonist ovarian suppression can mitigate symptoms, progesterone administration can precipitate them [94]. Fluctuating levels of AP, a progesterone-derived neurosteroid, have been shown to produce anxiety-like behaviors [95], and are believed to trigger affective dysregulation in women with mood disorders [96], providing a possible mechanism for progesterone-mediated symptom potentiation in this population. A recent randomized, double-blind, placebo-controlled, Phase I clinical trial sought to stabilize AP levels by administering the 5α-reductase inhibitor dutasteride [97] (NCT00082043). 16 women with and 16 without PMDD (ages 30–50) were randomized to either treatment or placebo, and received 2.5 mg of dutasteride (or placebo oral capsule) daily for one month. In the 3 months prior to treatment, all participants completed a previously described mood-related symptom assessment evaluation nightly. This the daily rating form scores symptom intensity on a scale ranging from 1–6 [98]. The ratings take into account the cardinal symptoms of PMDD, including irritability, sadness, and anxiety, and changes to these baseline measurements in response to dutasteride or placebo were used as the primary outcome measure. Dutasteride administration produced significant reductions in symptom severity, especially in week 4 (late luteal phase), indicating that neurosteroid level stabilization may be a worthwhile avenue for PMDD pharmacotherapy.
Chronic Pain
Chronic pain is a potentially debilitating condition that is especially significant in veterans of active duty in the USA military, of whom 40–50% may experience these symptoms [99]. The vulnerable nature of this population to chronic pain has encouraged the development of new pharmacologic alternatives for analgesia with proven efficacy in these individuals. Copious preclinical evidence suggests that AP plays a role in analgesia [100] as well as neuropathic pain [101]. To further evaluate the relevance of AP to chronic pain in the US veteran population, a clinical trial was completed to assess the association of pain symptoms with serum AP levels [102]. This study scored self-reported pain symptoms according to the Symptom Checklist-90-R (SCL-90-R) which includes headache, chest pain, and muscle soreness in a cohort of 485 male veterans of Iraq or Afghanistan, and compared these values to serum AP levels. A significant inverse correlation was found between serum AP levels and self-reported aggregate total pain; specifically, patients with higher levels of self-reported muscle soreness were found to have lower serum AP levels. These data bolster the theory that AP may act as an endogenous analgesic, and raise further interest in determining the clinical efficacy of AP as an exogenous intervention in this population.
To that end, a Phase II clinical trial is currently recruiting 90 subjects for a randomized, double-blind, placebo-controlled intervention study to evaluate the utility of pregnenolone, an AP precursor, in alleviating chronic low back pain in veterans (NCT01898013). This parallel-assignment study will randomly divide participants into equal intervention and placebo groups; pregnenolone (or placebo) dose will be gradually scaled up to 500 mg/day according to a pre-determined schedule. Responses will be measured using an 11-point Likert ordinal pain scale, with the primary outcome measure designated as the weekly mean of 24 h average pain severity in each group. This represents the most ambitious clinical investigation yet undertaken towards demonstrating proven clinical efficacy for neurosteroid in achieving analgesia, with immediate implications in the treatment of US veterans suffering from chronic pain.
Alcohol Dependence
Alcohol abuse and dependence are pervasive mental disorders that pose a significant sociological burden, with a combined lifetime prevalence of 18.6% among American adults [103]. As the neuromodulatory role of AP and related neurosteroids in GABAergic neuron function has become clearer, mounting preclinical evidence suggests that these compounds may play an important role in mediating at least some of the acute effects of ethanol intoxication. Alcohol consumption at moderate-to-high levels (1–2 g/kg) results in increased AP synthesis and release in the rat brain, but this can be inhibited by pretreatment with 5-α-reductase inhibitor finasteride [104]. Acute induction of AP in the cerebral cortex in response to alcohol consumption can reach 700% of baseline [105]. Further preclinical evaluation has shown that pharmacological manipulation of neurosteroid levels can have an impact on measurable behavioral responses to alcohol and related sedative drugs. For example, an early study showed that pregnenolone and pregnenolone sulfate in “exceedingly low doses” (0.01–1 μg/kg) attenuated or blocked the anxiolytic effect of ethanol, assessed using mouse behavior in a plus-maze [106]. More recently, it was shown that inhibition of neurosteroid synthesis by administration of finasteride successfully delayed the establishment of stable ethanol preference in a mouse model [107]. Extending these findings to human subjects, a pilot study demonstrated that chronic pregnenolone administration antagonizes the acute sedative, amnestic, and anxiolytic effects of diazepam, and found the neurosteroid to be generally well-tolerated [108].
A recent clinical trial sought to further explore the connection between neurosteroid and alcohol intoxication and dependency by evaluating the effects of dutasteride administration on acute behavioral responses to alcohol ingestion as well as longer-term effects on drinking behavior [109]. This double-blind, placebo-controlled, within-subject, factorial-design study enrolled 70 men, divided into light drinkers (LD; n=37) and heavy drinkers (HD; n=33), based on number of standard drinks consumed weekly. Alcohol (placebo or 0.8mg/kg) and drug (placebo or 4mg dutasteride) were administered according to a uniform procedure, with the drug being administered 2–4 days prior to the alcohol laboratory session. Physiologic response to dutasteride was indexed using serum androstanediol glucuronide levels before dutasteride administration and again at the beginning of each laboratory session. Subjective effects were measured at baseline and regular intervals post-treatment using self-report questionnaires as established in the literature: the Biphasic Alcohol Effects Scale (BAES) and the Alcohol Sensation Scale (SS). A secondary Timeline Follow-Back analysis was also performed to evaluate the effects of dutasteride exposure on naturalistic drinking behavior. Dutasteride treatment showed marked reductions on the BAES sedation and SS anesthesia subscale, with significantly less self-reported sedation/relaxation in response to pretreatment. While dutasteride had no persistent effects on drinking behavior in the LD group, heavy drinkers reported fewer episodes of “heavy drinking days” (>4 standard drinks in one day) in the 2 weeks following dutasteride exposure, as well as fewer drinks overall in the first week. Dutasteride was well-tolerated in this study and was in fact found to be associated with fewer drink-related adverse responses. Overall, these findings lend further support to a clinically important role for 5α-reduced neurosteroids in mediating acute alcohol effects and maintaining long-term dependency, and provide a mechanism by which clinical intervention may be achieved.
Another active Phase I clinical trial explores the correlation between degree of acute alcohol intoxication and magnitude of alcohol-induced increases in serum levels of AP, THDOC, and GABA (NCT00608686). This randomized, double-blind, placebo-controlled study also evaluates the effect of oral administration of 30mg pregnenolone on behavioral measures of acute alcohol intoxication, which it is predicted to antagonize. This analysis seeks to translate key preclinical findings demonstrating a neurosteroid-mediated mechanism for acute alcohol effects to the human setting, and develop another potential avenue for pharmacologic intervention in this setting. Although the specific mechanisms remain unclear, there is growing evidence that pharmacologic manipulation of neurosteroid levels, whether by 5α-reductase inhibitors or by administration of precursors, may emerge as a new therapeutic option for patients struggling with alcohol dependency and abuse.
Alzheimer’s Disease
Alzheimer’s disease (AD) is a common progressive neurodegenerative disorder characterized by the accumulation of β-amyloid plaques and neurofibrillary tangles. Although the complex etiology of this disease has thus far thwarted many therapeutic approaches, recent reports have demonstrated the therapeutic potential of AP in this setting via a neurodegenerative mechanism [110, 111]. In the murine AD model, 3xTgAD, AP achieved successful restoration of normal cognitive function concurrent with a reduction in molecular markers of disease. AP meets several criteria for suitability as a therapeutic agent, including low molecular weight, minimal hydrogen bond formation, favorable pharmacokinetics, and therapeutic efficacy at a sub-sedative dose. Furthermore, extensive analysis regarding route of administration, pharmacokinetics, neurogenic efficacy, tolerability, and dosing have already been performed in preparation for clinical trials, with broader translational implications for AP [110].
A Phase I interventional, randomized, parallel-assignment, double-blind, placebo-controlled clinical trial is currently underway to determine the safety and tolerability of AP in mild cognitive impairment and AD (NCT02221622), with a particular emphasis on determining the maximally-tolerated dose. Approximately 24 enrollees will be assigned to 3 groups of 8, of which 6 will receive IV infusions of AP and 2 will receive IV placebo, once per week for 12 weeks. Experiments involving each group are to be performed sequentially, with the AP dose administered increasing from 2–6mg in each successive cohort. Higher doses will only be administered once the lower dose is shown to be safe and well-tolerated. Tolerability metrics will include clinical laboratory measurements, adverse events, brain MRI, and clinical assessment, with cognitive tests and AD assessment scales providing secondary outcome measures. This study has the potential to provide key clinical data essential to the implementation of AP as accepted pharmacotherapy for AD.
Traumatic Brain Injury
Traumatic brain injury (TBI) occurs when an external force such as a jolt, blow, or projectile causes injury to the head and disrupts normal brain function. Although it causes over 1.5 million hospitalizations and emergency department visits each year [112], there are no currently available pharmaceutical products that have FDA approval for the treatment of TBI. Many preclinical studies have probed the role of neurosteroid in TBI and shown promising results, but the majority of this work has focused on progesterone therapy. AP has emerged as perhaps the most promising compound in this area, mediating neuroprotective effects such as reduction of ischemic infarcts and inhibition of the mitochondrial permeability transition pore, as well as acute reduction in proinflammatory cytokines following TBI [113]. Reduction in serum levels of pregnenolone and its neurosteroid metabolites (including AP) were previously found in US military veterans with TBI, so a pilot, proof-of-concept, randomized, placebo-controlled clinical trial was conducted to evaluate the clinical benefit of adjunctive pregnenolone therapy in this population [113] (NCT00623506). A total of 22 patients diagnosed with mild TBI completed the study, with 11 participants per group (placebo or pregnenolone). Dosing was gradually increased to 500 mg pregnenolone or placebo by mouth daily. Primary outcome measures were indices of cognitive function including the Brief Assessment of Cognition composite z-score (BACS) and the Clinician-Administered PTSD scale (CAPS). Mean changes in composite BACS and total CAPS scores disclosed no statistically significant differences between the experimental and control arms, although cluster D symptoms of the CAPS index (e.g. irritability, anger outbursts, sleep difficulties, hypervigilance) did show a non-significant reduction in pregnenolone-treated patients. Additionally, changes in serum pregnenolone and AP showed an inverse correlation with reduction in cluster D CAPS symptomology on an individual basis; patients with the highest serum levels of neurosteroid following treatment had the largest reductions in cluster D symptoms. Although the true efficacy of adjunctive pregnenolone in TBI remains unclear, these pilot data were sufficiently promising to prompt a larger randomized placebo-controlled trial using a similar adjunctive pregnenolone dosing scheme in the same population (NCT01336413). This Phase II clinical trial is currently enrolling and seeks to include 140 participants. Executive functioning as assessed with the Tower of London test will serve as the primary outcome measure, with additional documentation of CAPS cluster D symptoms and serum levels of AP and other GABAergic neurosteroids.
An additional Phase II, double-blind, placebo-controlled, randomized clinical trial is currently enrolling that seeks to evaluate the safety and effectiveness of intravenous AP injection in the treatment of TBI (NCT01673828). Unlike the aforementioned trials, this study aims to evaluate the utility of neurosteroid administration in the acute setting (treatment beginning within 8 hours of injury), and will include subjects diagnosed with moderate to severe TBI. AP (or placebo) will be administered via continuous intravenous infusion for 5 days (4 days treatment + 1 day de-escalation), with patients randomized to the experimental arm receiving either low or high dose AP solutions. The primary outcome measure for this study is Glasgow Outcome Score Extended score, with secondary outcome measures including other indices of neurobehavioral outcome along with mortality. The anticonvulsant, neuroprotective properties of AP may successfully limit acute brain excitotoxicity in this continuous infusion regimen, providing a new therapeutic option for this debilitating disease.
Bipolar Disorder
Bipolar disorder (BPD) is a common neurobiological disorder in which patients can experience major depressive episodes (MDEs) as defined by the diagnostic criteria in DSM-IV. 15.7 million American adults age 18 or older had at least 1 MDE in 2014, of which over 10 million experienced severe impairment. The pathophysiology of BPD may show key differences from major depressive disorder, rendering traditional antidepressant pharmacotherapy less effective for BPD depressive episodes [114]. These differences have spurred interest in the development of new approaches tailored to the BPD setting. A recent randomized, double-blind, placebo-controlled clinical trial investigated the therapeutic value of oral pregnenolone therapy (500 mg/day) in alleviating symptoms of depressive episodes in 73 enrolled BPD patients, as scored on the Hamilton Rating Scale for Depression (HRSD) and Inventory of Depressive Symptomatology Self-Report (IDS-SR) along with other indices [115]. Additionally, baseline and post-treatment serum neurosteroid levels were obtained to monitor pregnenolone, AP, and pregnanolone. Patients randomized to the experimental arm reported statistically significant reduction over time in HRSD scores as compared to the placebo arm, with a noticeably larger reduction occurring in women. Depression remission rates as determined by the IDS-SR were significantly higher after pregnenolone treatment (61% vs 37%). Observed reductions in depression scores were correlated with large increases in serum neurosteroid levels from baseline. Additionally, the study disclosed that pregnenolone was well-tolerated with a minimal side effect burden that was not statistically distinguishable from the placebo control group within the 12-week observation window. These findings offer credible evidence that pregnenolone or other neurosteroid compounds may provide much needed therapeutic relief for patients suffering from BPD.
Smoking Cessation
Cigarette smoking is associated with a multitude of health risks and is the most common preventable cause of disease in the USA. Despite these well-known risks, roughly 20% of Americans continue to smoke. New therapeutic approaches to smoking cessation, with an emphasis on new pharmacotherapy, are active areas of current research. A growing body of preclinical and clinical evidence reveals the complex interplay between drug abuse behaviors and neurosteroids such as AP. In particular, smoking cessation outcomes in adult women may be substantially influenced by serum AP levels, which have been shown to vary across the menstrual cycle: for example, decreases in AP during smoking cessation attempts in the follicular phase are associated with increased risk of relapse [116]. A randomized, open-label clinical trial is currently enrolling participants to evaluate several aspects of the relationship between smoking symptomatology and AP and its precursors (NCT01811225). The nonintervention arm of this study will measure serum concentrations of progesterone and AP (among other biomarkers), as well as nicotine withdrawal/craving as indexed by the Minnesota Nicotine Withdrawal Scale, in the setting of pregnant women smoking ad libitum. The experimental arm will assess responses in these same metrics to overnight nicotine abstinence and either low- or high-dose progesterone in the setting of women who have used oral contraceptives for at least 3 months prior to enrollment. This study may provide much-needed clinical data to enhance the current understanding of the role of neurosteroid in smoking dependence, with specific applications to populations in which the risks of foregoing cessation are higher.
A proof-of-concept clinical trial has been undertaken to determine the clinical potential of GX in smoking cessation (NCT01857531). This open-label, interventional study evaluated the efficacy and tolerability of oral GX in conjunction with nicotine patches. GX was ramped up to 1200mg in the pre-quit period (first 4 weeks) and tapered after post-quit week 2. Nicotine patches were initiated at 21mg/day in weeks 3 and 4 of the pre-quit period and tapered in the post-quit period. The primary outcome measure was percentage change in expired air carbon monoxide (CO) from baseline at the end of the second week, to assess the impact of GX on ad libitum smoking. Other outcome measures included percentage change in expired air CO at later time points, as well as number of participants successfully maintaining abstinence at various time points in the post-quit period. Preliminary results disclose a nearly 40% decline in expired air CO from baseline at the 4 week time point. No serious adverse events were recorded; other side effects were relatively benign, with fatigue being the most common reported event. These findings demonstrate the tolerability of GX in the smoking cessation setting, and should be used to guide estimation of effect sizes for larger, placebo-controlled trials in the future.
Migraine
GX has been tested in over 400 patients with migraine headaches by CoCensys, Inc. However, the sponsor has abandoned further development of GX or related lead neurosteroid compounds for migraine indications. The scientific rationale for clinical testing of a neurosteroid therapy for migraine is poorly understood. There are two types of migraines: migraine with aura and migraine without aura. The menstrual migraine occurs generally during the perimenstrual period. However, the molecular pathophysiology of migraine is unclear. Although cortical spreading depression has been proposed as a neural basis of some migraines, there is little compelling evidence for GABAergic deficits during acute migraine attacks [117].
Side Effects and Other Therapeutic Aspects
Treatment with exogenously administered natural neurosteroids or synthetic analogs such as ganaxolone is reported to be generally safe and well tolerated [6,71,89]. The most common side effect of neurosteroids is reversible sedation, which is an extension of their therapeutic effects at GABA-A receptors [60]. Adverse events reported by some patients include dizziness, fatigue, and somnolence. There are some concerns with metabolites of natural neurosteroids such as AP. Unlike steroid hormones, neurosteroids are not themselves active at intracellular steroid receptors that regulate gene transcription. However, it is likely that AP and other steroids that contain a free C3-hydroxyl group can undergo metabolic conversion by 3α-HSOR isoenzymes to the hormonally-active intermediate dihydroprogesterone [130]. It is likely that chronic administration of these compounds may result in accumulation of C3-keto steroid metabolites, which can bind to steroid receptors and thereby cause serious hormonal side effects. Such neurosteroid treatments may influence hormone cycles in women. These limitations are successfully surmounted by synthetic agents like ganaxolone that do not undergo this back conversion, potentially avoiding hormonal side effects. Synthetic analogs have several advantages over natural neurosteroids for chronic administration. Synthetic neurosteroids may have greater bioavailability and longer half-life than natural neurosteroids that are rapidly inactivated and eliminated by glucuronide or sulfate conjugation at the C3-hydroxyl group.
Sex differences are evident in the actions of neurosteroids. AP and related neurosteroids exhibit striking sex differences in their anticonvulsant potency [70, 119]. Therefore, men and women stand to benefit differently from treatment of epilepsy and other condition with neurosteroids. Differences in neurosteroids levels and their receptor targets in males and females may influence the neurosteroid sensitivity. There is evidence of greater sensitivity of females to neurosteroids than male; therefore, dosage adjustments are necessary to limit the side effects while achieving the desired therapeutic outcomes.
Concluding Remarks
Neurosteroids continue to attract the attention of neuropsychiatric investigators because of their potent effects on GABA-A receptor function and the mounting evidence of their clinical potential. AP and related neurosteroids are strong sedatives, anxiolytics and anticonvulsants. Several synthetic agents have been developed to target neuronal GABA-A receptors. There is resurgence in neurosteroid therapeutics as at least four lead molecules have entered advanced clinical trials for a number of CNS disorders. An improved mechanistic understanding of neurosteroid interactions at synaptic and extrasynaptic GABA-A receptors is opening new horizons in the clinical potentials of neurosteroids. Despite decades of research with a variety of synthetic agents, there is no single FDA-approved neurosteroid drug for therapeutic use; however, there are currently many ongoing trials that will produce a better understanding of the clinical uses of these compounds. The outcome of current trials will open a trajectory for neurosteroid therapeutics. In addition to epilepsy, it is thought that neurosteroid’s actions offer numerous indications such as Alzheimer’s disease, Fragile X syndrome, and other neuropsychiatric conditions.
There are many outstanding questions on neurosteroid therapeutics (see Outstanding Questions). A retrospective analysis failed to provide clear clues as to why big pharma companies have long abandoned the clinical development of neurosteroids. It is baffling that we have not seen a return of promising neurosteroid anesthetic agents, but some plausible explanations may include poorly characterized molecular targets, unacceptable side effects, mixed efficacy outcomes, and market forces. Of these, lack of a compelling scientific rationale appears the main reason for mixed outcomes, aside from uncertain market potential due to rapid introduction of better agents. As discovery of neurosteroid analogs continues, new agents that are more specific to extrasynaptic GABA-A receptors could help to further optimize the therapeutic efficacy for niche indications such as status epilepticus and chemical neurotoxicity. Unlike natural neurosteroids, synthetic analogs have many advantages such as improved bioavailability, acceptable pharmacokinetics, and lack of active metabolites that can cause reproductive side effects as evident from ganaxolone therapy.
Outstanding Questions.
How do neurosteroids regulate the membrane expression of the α4- and δ extrasynaptic GABA-A receptor subtypes that mediate tonic inhibition? How does such plasticity affect menstrual disorders such as catamenial epilepsy, menstrual migraine, and postpartum depression?
What is the rationale for the use of neurosteroids for refractory chronic epilepsy? Why has ganaxolone showed less-than-robust control of complex partial seizures? Is there a clear PK-PD relationship at potential neuronal targets?
Which of the various neurological symptoms of epilepsy syndromes can be effectively targeted by GABAergic neurosteroids?
Why did progesterone fail to take off in epileptic trials? Is there any mechanistic reason behind this unexpected outcome? Is this related to progesterone interaction with progesterone receptors (PRs), which have been shown recently to promote seizure susceptibility?
At what time points are neurosteroid-based interventions most beneficial to recovery of TBI and prevention of long-term effects of neuronal injury?
Are there clinical interventions that can augment the neurosteroids in the brain after brain injury or stroke that prevent the negative clinical outcomes?
Despite the intense efforts of researchers to identify specific ligands for the extrasynaptic GABA-A receptor, few have been found. What is the functional significance of a receptor with a large percentage of non-specific ligands, and what are the clinical/pharmacological implications of identifying specific ligands?
Recent advances in the field of neurosteroid mechanisms are opening new horizons for neurotherapeutics. For example, neurosteroids are found to be more effective anticonvulsants than benzodiazepines because their target receptor remains unaffected by the complex dynamics of seizures [118]. Presently there is an unmet medical need for medical countermeasures against nerve agent intoxication seizures in the delayed setting where benzodiazepines have proven to be ineffective. Neurosteroids represent a novel treatment strategy for such benzodiazepine-resistant seizures. Mechanistically, neurosteroids are poised to be much more effective countermeasures for acute refractory seizures than benzodiazepines because they may act as “circuit breakers” rather than “dimming switches” like benzodiazepines (due to subunit-limiting allosteric ceiling effect and lack of tonic current activation). Seizures appear to originate in local microcircuits and then propagate from that initial zone to engage microcircuits in distal nodes, a process that can be disrupted or even shunted by neurosteroids, which may act as strong circuit breakers by augmenting tonic inhibition within the network path including neuronal body, axons and dendrites. This is clearly evident from neurosteroid’s ability to directly open the extrasynaptic GABA-A receptors, resulting in powerful augmentation of tonic currents and neuronal protection [35].
Trends.
Dysfunction of extrasynaptic GABA-A receptors may cause profound impact in many CNS conditions.
Extrasynaptic GABA-A receptors are emerging as novel targets for epilepsy, pain, insomnia and mood disorders. Neurosteroids activates GABA-A receptor function and thus are attractive therapeutic agents.
Clinical trials are currently assessing neurosteroids for treatment of diverse CNS disorders, such as epilepsy, status epilepticus (SE), Fragile X syndrome, TBI and Alzheimer’s disease.
Neurosteroids can effectively control refractory SE as they affect both synaptic and extrasynaptic GABA-A receptors. New analogs are being identified based on neurosteroid pharmacophore map.
Acknowledgments
This research was supported by NIH grants NS052158, NS051398 and U01 NS083460 (to DSR), and the TAMHSC Summer Research Fellowship (to WAE). The author thanks Victoria Golub for editing the manuscript.
Glossary
- Neurosteroid
Refers to steroids that are synthesized locally within the brain and that rapidly alter neuronal excitability.
- Neuroactive steroid
Encompasses both naturally occurring neurosteroids and their synthetic analogs that rapidly modulate neuronal excitability in the CNS.
- Allosteric agonist
An agonist that potentiates the response of an agonist by binding to a different site on the receptor by changing the affinity of the receptor for the agonist. The effect is saturable (ceiling) because potentiation reaches a limiting value when the allosteric site is fully occupied.
- Catamenial epilepsy
It is a form of pharmacoresistant epilepsy in women in which seizures are clustered around specific points in the menstrual cycle, most often around perimenstrual (C1) or periovulatory (C2) period.
- Phasic inhibition
The post-synaptic inhibitory current conductance that occurs with synaptic release of GABA followed by transient activation of synaptic GABA-A receptors.
- Tonic inhibition
The extra-synaptic inhibitory current conductance that occurs with ambient or extracellular GABA, which persistently activates extra- and peri-synaptic GABA-A receptors.
- Tolerance
Decreased response to repeated or chronic administration of the same drug.
- Status epilepticus
A neurological emergency characterized by continuous seizure activity or multiple seizures without regaining consciousness for more than 30 min. It may be manifested in two types, generalized convulsive and nonconvulsive type.
- ORG-20599
A water-soluble synthetic neurosteroid with allosteric GABA-A receptor activity. 2β,3α,5α-21-Chloro-3-hydroxy-2-(4-morpholinyl)pregnan-20-one methanesulfonate.
- THIP
4,5,6,7-tetrahydroisoxazolo-[4,5,c]-pyridin-3-ol (THIP), also called gaboxadol, is a δGABA-A receptor-preferring agonist. It has sedative and hypnotic activity, but its clinical development for insomnia was halted due to serious side effects.
- DS2
((4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridin-3-yl]benzamide) is a highly-selective experimental ligand of extrasybnaptic δGABA-A receptors.
- TSPO
A 19-kDa cholesterol transporter protein, which was formerly called a peripheral-type benzodiazepine receptor. It is a key protein in the biosynthesis of neurosteroids. Activation of TSPO by endogenous signals and ligands facilitates increased production of neurosteroids by transferring cholesterol from the outer mitochondrial membrane to the inner membrane where P450scc is located.
Footnotes
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Progr Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy DS. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front Endocrinol. 2011;2:38. doi: 10.3389/fendo.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selye H. On the hormonal activity of a steroid compound. Science. 1941;94:94. doi: 10.1126/science.94.2430.94. [DOI] [PubMed] [Google Scholar]
- 4.Selye H, Masson G. Additional steroids with luteoid activity. Science. 1942;96:358. doi: 10.1126/science.96.2494.358. [DOI] [PubMed] [Google Scholar]
- 5.Clarke RS. Anaesthesia and carbohydrate metabolism. Br J Anesthesiol. 1973;45:237–243. doi: 10.1093/bja/45.3.237. [DOI] [PubMed] [Google Scholar]
- 6.Gyermek L, Soyka LF. Steroid anesthetics. Anesthesiology. 1975;42:331–344. doi: 10.1097/00000542-197503000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Harrison NL, Simmonds MA. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- 8.Majewska MD, et al. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 9.Reddy DS. Pharmacology of endogenous neuroactive steroids. Crit Rev Neuobiol. 2003;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- 10.Hogenkamp DJ, Tran MB, Yoshimura RF, Johnstone TB, Kanner R, Gee KW. Pharmacological profile of a 17β-heteroaryl-substituted neuroactive steroid. Psychopharmacology. 2014;231:3517–3524. doi: 10.1007/s00213-014-3494-5. [DOI] [PubMed] [Google Scholar]
- 11.Reddy DS, Woodward R. Ganaxolone: a prospective overview. Drugs of the Future. 2004;29:227–242. [Google Scholar]
- 12.Reddy DS, Kulkarni SK. Development of neurosteroid-based novel psychotropic drugs. Progr Med Chem. 2000;37:135–175. doi: 10.1016/s0079-6468(08)70059-6. [DOI] [PubMed] [Google Scholar]
- 13.Reddy DS. Physiological role of adrenal deoxycorticosterone-derived neuroactive steroids in stress-sensitive conditions. Neuroscience. 2006;138:911–920. doi: 10.1016/j.neuroscience.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Whissell PD, et al. Altered expression of δGABA-A receptors in health and disease. Neuropharmacology. 2015;88:24–35. doi: 10.1016/j.neuropharm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Coulter DA, Carlson GC. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Progr Brain Res. 2007;163:235–243. doi: 10.1016/S0079-6123(07)63014-3. [DOI] [PubMed] [Google Scholar]
- 16.Glykys J, et al. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA-A receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230:151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chisari M, et al. The sticky issue of neurosteroids and GABA-A receptors. Trends Neurosci. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosie AM, et al. Neurosteroid binding sites on GABA-A receptors. Pharmacol Therap. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Akk G, et al. The influence of the membrane on neurosteroid actions at GABA-A receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S59–66. doi: 10.1016/j.psyneuen.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell EA, et al. Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem Int. 2008;52:588–595. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Reddy DS. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol Sci. 2003;24:103–106. doi: 10.1016/S0165-6147(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 23.Hosie AM, et al. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 24.Hosie AM, et al. Conserved site for neurosteroid modulation of GABA-A receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 25.Kokate TG, et al. Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Therap. 1994;270:1223–1229. [PubMed] [Google Scholar]
- 26.Upasani RB, et al. 3 alpha-Hydroxy-3 beta-(phenylethynyl)-5 beta-pregnan-20-ones: synthesis and pharmacological activity of neuroactive steroids with high affinity for GABAA receptors. J Med Chem. 1997;40:73–84. doi: 10.1021/jm9605344. [DOI] [PubMed] [Google Scholar]
- 27.Reddy DS, Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J Pharmacol Exp Therap. 2010;334:1031–1041. doi: 10.1124/jpet.110.169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Covey DF, et al. Enantioselectivity of pregnanolone-induced gamma-aminobutyric acid(A) receptor modulation and anesthesia. J Pharmacol Exp Therap. 2000;293:1009–1016. [PubMed] [Google Scholar]
- 29.Qian M, et al. Neurosteroid analogues. 18 Structure-activity studies of ent-steroid potentiators of gamma-aminobutyric acid type A receptors and comparison of their activities with those of alphaxalone and allopregnanolone. J Med Chem. 2014;57:171–190. doi: 10.1021/jm401577c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility. J Neurosci. 2002;22:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puia G, et al. Neurosteroids act on recombinant human GABA-A receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 32.Reddy DS, Rogawski MA. Neurosteroids as endogenous regulators of seizure susceptibility and use in the treatment of epilepsy. Epilepsia. 2010;51:84. doi: 10.1111/j.1528-1167.2010.02870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown N, et al. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA-A receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meera P, et al. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA-A receptors. J Neurophysiol. 2011;106:2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carver C, Reddy DS. Neurosteroid structure-activity relationships for functional activation of extrasynaptic δGABA-A receptors. J Pharmacol Exp Therap. 2016;357:188–204. doi: 10.1124/jpet.115.229302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy SD, et al. Antiseizure Activity of Midazolam in Mice Lacking delta-Subunit Extrasynaptic GABA(A) Receptors. J Pharmacol Exp Therap. 2015;353:517–528. doi: 10.1124/jpet.114.222075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA-A receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanna E, et al. Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci. 2009;29:1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X, et al. Estrous cycle regulation of extrasynaptic delta-containing GABA-A receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Therap. 2013;346:146–160. doi: 10.1124/jpet.113.203653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carver CM, et al. Perimenstrual-like hormonal regulation of extrasynaptic delta-containing GABA-A receptors mediating tonic inhibition and neurosteroid sensitivity. J Neurosci. 2014;34:14181–14197. doi: 10.1523/JNEUROSCI.0596-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mihalek RM, et al. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spigelman I, et al. Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABA(A) receptor delta subunit. J Neurophysiol. 2003;90:903–910. doi: 10.1152/jn.01022.2002. [DOI] [PubMed] [Google Scholar]
- 43.Stell BM, et al. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA-A receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandit S, et al. Dual mechanisms diminishing tonic GABA-A inhibition of dentate gyrus granule cells in Noda epileptic rats. J Neurophysiol. 2013;110:95–102. doi: 10.1152/jn.00727.2012. [DOI] [PubMed] [Google Scholar]
- 45.Dibbens LM, et al. GABRD encoding a protein for extra- or peri-synaptic GABA-A receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet. 2004;13:1315–1319. doi: 10.1093/hmg/ddh146. [DOI] [PubMed] [Google Scholar]
- 46.Feng HJ, et al. Delta subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of alpha4beta2delta GABA-A receptors. J Neurosci. 2006;26:1499–1506. doi: 10.1523/JNEUROSCI.2913-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reddy DS. Mass spectrometric assay and physiological-pharmacological activity of androgenic neurosteroids. Neurochem Int. 2008;52:541–553. doi: 10.1016/j.neuint.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy DS. Role of neurosteroids in catamenial epilepsy. Epilepsy research. 2004;62:99–118. doi: 10.1016/j.eplepsyres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Reddy DS. Pharmacology of catamenial epilepsy. Meth Find Exp Clin Pharmacol. 2004;26:547–561. doi: 10.1358/mf.2004.26.7.863737. [DOI] [PubMed] [Google Scholar]
- 50.Kaminski RM, et al. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–827. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaminski RM, et al. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia. 2004;45:864–867. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- 52.Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. Th J Pharmacol Exp Therap. 2000;295:1241–1248. [PubMed] [Google Scholar]
- 53.Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:337–344. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- 54.Tsuda M, et al. Modulation of the decrease in the seizure threshold of pentylenetetrazole in diazepam withdrawn mice by the neurosteroid 5alphapregnan-3alpha,21-diol-20-one (alloTHDOC) Addict Biol. 1997;2:455–460. doi: 10.1080/13556219772516. [DOI] [PubMed] [Google Scholar]
- 55.Devaud LL, et al. Sensitization of gamma-aminobutyric acid A receptors to neuroactive steroids in rats during ethanol withdrawal. Th J Pharmacol Exp Therap. 1996;278:510–517. [PubMed] [Google Scholar]
- 56.Kokate TG, et al. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. T J Pharmacol Exp Therap. 1998;287:553–558. [PubMed] [Google Scholar]
- 57.Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Therap. 2000;294:909–915. [PubMed] [Google Scholar]
- 58.Reddy DS, et al. Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacology. 2010;59:573–581. doi: 10.1016/j.neuropharm.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reddy DS, Mohan A. Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J Neurosci. 2011;31:650–658. doi: 10.1523/JNEUROSCI.4488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monaghan EP, et al. Ganaxolone: a novel positive allosteric modulator of the GABA-A receptor complex for the treatment of epilepsy. Expert Opin Investig Drugs. 1999;8:1663–1671. doi: 10.1517/13543784.8.10.1663. [DOI] [PubMed] [Google Scholar]
- 61.Tsai J, et al. Outpatient flexible dose, open-label add-on ganaxolone in treatment-refractory pediatric epilepsy. American Epilepsy Society Annual Meeting Abstract, #2.196.2006. [Google Scholar]
- 62.Pieribone VA, et al. Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy. Epilepsia. 2007;48:1870–1874. doi: 10.1111/j.1528-1167.2007.01182.x. [DOI] [PubMed] [Google Scholar]
- 63.Giller E, et al. Inpatient flexible dose, open-label add-on ganaxolone in treatment-refractory pediatric epilepsy. American Epilepsy Society Annual Meeting Abstract, #2.177.2006. [Google Scholar]
- 64.Tsai J, et al. An open-label extension study to evaluate the safety, tolerability, and efficacy of ganaxolone as add-on therapy in adults with uncontrolled partial onset seizures. American Epilepsy Society Annual Meeting Abstract, #1.288.2010. [Google Scholar]
- 65.Tsai J, et al. Ganaxolone is safe and well-tolerated as add-on therapy in adults in uncontrolled partial seizures. American Epilepsy Society Annual Meeting Abstract, #2.258.2011. [Google Scholar]
- 66.Herzog AG, et al. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 67.Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reddy DS. Neuroendocrine aspects of catamenial epilepsy. Hormones & Behav. 2013;63:254–266. doi: 10.1016/j.yhbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reddy DS. Neurosteroids and their role in sex-specific epilepsies. Neurobiol Disease. 2014;72(Pt B):198–209. doi: 10.1016/j.nbd.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reddy DS. Gender differences in antiseizure sensitivity of neurosteroids in the pilocarpine model of status epilepticus. Epilepsia. 2009;50(Suppl 11):126. # 1.255. [Google Scholar]
- 71.Herzog AG, et al. Progesterone vs placebo therapy for women with epilepsy: A randomized clinical trial. Neurology. 2012;78:1959–1966. doi: 10.1212/WNL.0b013e318259e1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herzog AG. Catamenial epilepsy: Update on prevalence, pathophysiology and treatment from the findings of the NIH Progesterone Treatment Trial. Seizure. 2015;28:18–25. doi: 10.1016/j.seizure.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 73.Reddy DS, et al. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- 74.McAuley JW, et al. A Prospective Evaluation of the Effects of a 12-Week Outpatient Exercise Program on Clinical and Behavioral Outcomes in Patients with Epilepsy. Epilepsy & Behavior : E&B. 2001;2:592–600. doi: 10.1006/ebeh.2001.0271. [DOI] [PubMed] [Google Scholar]
- 75.Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gangisetty O, Reddy DS. Neurosteroid withdrawal regulates GABA-A receptor alpha4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience. 2010;170:865–880. doi: 10.1016/j.neuroscience.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reddy DS, et al. A mouse kindling model of perimenstrual catamenial epilepsy. J Pharmacol Exp Therap. 2012;341:784–793. doi: 10.1124/jpet.112.192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reddy DS, et al. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005;48:14–24. doi: 10.1016/j.neuropharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 79.Naylor DE, et al. Trafficking of GABA-A receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacob TC, et al. GABA-A receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rogawski MA, et al. Neuroactive steroids for the treatment of status epilepticus. Epilepsia. 2013;54(Suppl 6):93–98. doi: 10.1111/epi.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reddy K, et al. SGE-102: a novel therapy for refractory status epilepticus. Epilepsia. 2013;54(Suppl 6):81–83. doi: 10.1111/epi.12286. [DOI] [PubMed] [Google Scholar]
- 83.Reddy DS. The clinical potentials of endogenous neurosteroids. Drugs Today. 2002;38:465–485. doi: 10.1358/dot.2002.38.7.820115. [DOI] [PubMed] [Google Scholar]
- 84.Riikonen R, et al. Does vigabatrin treatment for infantile spasms cause visual field defects? An international multicentre study. Dev Med Child Neurol. 2015;57:60–67. doi: 10.1111/dmcn.12573. [DOI] [PubMed] [Google Scholar]
- 85.Chachua T, et al. Validation of the rat model of cryptogenic infantile spasms. Epilepsia. 2011;52:1666–1677. doi: 10.1111/j.1528-1167.2011.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yum MS, et al. A potential effect of ganaxolone in an animal model of infantile spasms. Epilepsy Res. 2014;108:1492–1500. doi: 10.1016/j.eplepsyres.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 87.Rogawski MA, Reddy DS. Neurosteroids and infantile spasms: the deoxycorticosterone hypothesis. Int Rev Neurobiol. 2002;49:199–219. doi: 10.1016/s0074-7742(02)49014-9. [DOI] [PubMed] [Google Scholar]
- 88.Kerrigan JF, et al. Ganaxolone for treating intractable infantile spasms: a multicenter, open-label, add-on trial. Epilepsy Res. 2000;42:133–139. doi: 10.1016/s0920-1211(00)00170-4. [DOI] [PubMed] [Google Scholar]
- 89.Nohria V, Giller E. Ganaxolone. Neurotherapeutics. 2007;4:102–105. doi: 10.1016/j.nurt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jacquemont S, et al. Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol. 2007;6:45–55. doi: 10.1016/S1474-4422(06)70676-7. [DOI] [PubMed] [Google Scholar]
- 91.Berman RF, et al. Mouse models of the fragile X premutation and fragile X-associated tremor/ataxia syndrome. J Neurodev Disord. 2014;6:25. doi: 10.1186/1866-1955-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cao Z, et al. Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum Mol Genet. 2012;21:2923–2935. doi: 10.1093/hmg/dds118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Women’s Health. 2013;9:537–556. doi: 10.2217/whe.13.62. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt CL, et al. Transfer of cryopreserved-thawed embryos: the natural cycle versus controlled preparation of the endometrium with gonadotropin-releasing hormone agonist and exogenous estradiol and progesterone (GEEP) Fertil Steril. 1989;52:609–616. doi: 10.1016/s0015-0282(16)60973-1. [DOI] [PubMed] [Google Scholar]
- 95.Shen H, et al. Short-term steroid treatment increases delta GABA-A receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schiller CE, et al. Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology. 2014;231:3557–3567. doi: 10.1007/s00213-014-3599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martinez PE, et al. 5alpha-Reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41:1093–1102. doi: 10.1038/npp.2015.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Endicott J, et al. Premenstrual changes: patterns and correlates of daily ratings. J Affect Disord. 1986;10:127–135. doi: 10.1016/0165-0327(86)90035-2. [DOI] [PubMed] [Google Scholar]
- 99.Cifu DX, et al. Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND Veterans. J Rehabil Res Dev. 2013;50:1169–1176. doi: 10.1682/JRRD.2013.01.0006. [DOI] [PubMed] [Google Scholar]
- 100.Winfree CJ, et al. Analgesic effects of intrathecally-administered 3alpha-hydroxy-5alpha-pregnan-20-one in a rat mechanical visceral pain model. Life Sci. 1992;50:1007–1012. doi: 10.1016/0024-3205(92)90095-7. [DOI] [PubMed] [Google Scholar]
- 101.Aouad M, et al. Etifoxine stimulates allopregnanolone synthesis in the spinal cord to produce analgesia in experimental mononeuropathy. Eur J Pain. 2014;18:258–268. doi: 10.1002/j.1532-2149.2013.00367.x. [DOI] [PubMed] [Google Scholar]
- 102.Naylor JC, et al. Allopregnanolone levels are inversely associated with self-reported pain symptoms in U.S. Iraq and Afghanistan-era veterans: Implications for biomarkers and therapeutics. Pain Med. 2015 doi: 10.1111/pme.12860. [DOI] [PubMed] [Google Scholar]
- 103.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 104.VanDoren MJ, et al. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morrow AL, et al. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23:1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- 106.Melchior CL, Ritzmann RF. Pregnenolone and pregnenolone sulfate, alone and with ethanol, in mice on the plus-maze. Pharmacol Biochem Behav. 1994;48:893–897. doi: 10.1016/0091-3057(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 107.Ford MM, et al. Inhibition of 5alpha-reduced steroid biosynthesis impedes acquisition of ethanol drinking in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:1408–1416. doi: 10.1111/j.1530-0277.2008.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meieran SE, et al. Chronic pregnenolone effects in normal humans: attenuation of benzodiazepine-induced sedation. Psychoneuroendocrinology. 2004;29:486–500. doi: 10.1016/s0306-4530(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 109.Covault J, et al. Dutasteride reduces alcohol’s sedative effects in men in a human laboratory setting and reduces drinking in the natural environment. Psychopharmacology. 2014;231:3609–3618. doi: 10.1007/s00213-014-3487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Irwin RW, et al. Allopregnanolone preclinical acute pharmacokinetic and pharmacodynamic studies to predict tolerability and efficacy for Alzheimer’s disease. PloS one. 2015;10:e0128313. doi: 10.1371/journal.pone.0128313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nature Reviews in Endocrinolology. 2013;9:241–250. doi: 10.1038/nrendo.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Faul M, et al. Traumatic Brain Injury in the United States: Emergency department visits, hospitalizations, and deaths 2002–2006. Centers for Disease Control and Prevention, National Cneter for Injury Prevention and Control; 2010. [Google Scholar]
- 113.Marx CE, et al. Neurosteroids and Traumatic Brain Injury: Translating Biomarkers to Therapeutics; Overview and Pilot Investigations in Iraq and Afghanistan Era Veterans. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. 2016. [PubMed] [Google Scholar]
- 114.Sachs GS, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356:1711–1722. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- 115.Brown ES, et al. A randomized, double-blind, placebo-controlled trial of pregnenolone for bipolar depression. Neuropsychopharmacology. 2014;39:2867–2873. doi: 10.1038/npp.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Allen AM, et al. Allopregnanolone association with psychophysiological and cognitive functions during acute smoking abstinence in premenopausal women. Exp Clin Psychopharmacol. 2015;23:22–28. doi: 10.1037/a0038747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reddy DS. The pathophysiological and pharmacological basis of current drug treatment of migraine headache. Expert Rev Clin Pharmacol. 2013;6:271–288. doi: 10.1586/ecp.13.14. [DOI] [PubMed] [Google Scholar]
- 118.Reddy SD, Reddy DS. Midazolam as an anticonvulsant antidote for organophosphate intoxication--A pharmacotherapeutic appraisal. Epilepsia. 2015;56:813–821. doi: 10.1111/epi.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reddy DS, et al. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Therap. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- 120.Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3alpha-androstanediol and 17beta-estradiol. Neuroscience. 2004;129:195–207. doi: 10.1016/j.neuroscience.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 121.Reddy DS, Rogawski MA. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res. 2010;89:254–260. doi: 10.1016/j.eplepsyres.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gasior M, et al. Acute and chronic effects of the synthetic neuroactive steroid, ganaxolone, against the convulsive and lethal effects of pentylenetetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology. 2000;39:1184–1196. doi: 10.1016/s0028-3908(99)00190-2. [DOI] [PubMed] [Google Scholar]
- 123.Kaminski RM, Gasior M, Carter RB, Witkin JM. Protective efficacy of neuroactive steroids against cocaine kindled-seizures in mice. Eur J Pharmacol. 2003;474:217–222. doi: 10.1016/s0014-2999(03)02086-7. [DOI] [PubMed] [Google Scholar]
- 124.Carter RB, et al. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Therap. 1997;280:1284–1295. [PubMed] [Google Scholar]
- 125.Reddy DS. Anticonvulsant activity of the testosterone-derived neurosteroid 3α-androstanediol. Neuroreport. 2004;15:515–518. doi: 10.1097/00001756-200403010-00026. [DOI] [PubMed] [Google Scholar]
- 126.Belelli D, et al. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Eur J Pharmacol. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- 127.Liptakova S, et al. Effect of ganaxolone on flurothyl seizures in developing rats. Epilepsia. 2000;41:788–793. doi: 10.1111/j.1528-1157.2000.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 128.Kokate TG, et al. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35:1049–1056. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 129.Briyal S, Reddy DS. Neuroactive steroid therapy of status epilepticus in epilepsy rats. Epilepsia. 2008;49(Suppl 7):3.055. [Google Scholar]
- 130.Rupprecht R, et al. Progesterone receptor-mediated effects of neuroactive steroids. Neuron. 1993;11:523–530. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- 131.Rupprecht R, et al. Steroid receptor-mediated effects of neuroactive steroids: characterization of structure activity relationship. Eur J Pharmacol. 1996;303:227–234. doi: 10.1016/0014-2999(96)00036-2. [DOI] [PubMed] [Google Scholar]