Abstract
Nanoparticulate delivery of biocides has the potential to decrease levels of exposure to non-target organisms, and miminize long-term exposure that can promote the development of resistance. Silica nanoparticles are an ideal vehicle since they are inert, biocompatible, biodegradable, and thermally and chemically stable. Encapsulation of biocides within nanoparticulates can improve their stability and longevity and maximize the biocidal potential of hydrophobic volatile compounds. Herein, we have shown that the plant secondary metabolites allyl isothiocyanate and cinnamaldehyde demonstrated increased antimicrobial activity against Escherichia coli in planktonic form, when packaged into mesoporous silica nanoparticles. Furthermore, the biocide-loaded nanoparticles showed activity against Pseudomonas aeruginosa biofilms that have inherent resistance to antimicrobial agents. The delivery platform can also be expanded to traditional biocides and other non-conventional antimicrobial agents.
Keywords: mesoporous silica nanoparticles, biocides, allyl isothiocyanate, cinnamaldehyde, planktonic bacteria, biofilms
1. Introduction
Biocides, including antimicrobials and particularly antibacterials, are essential in healthcare, and ubiquitous in many commercial applications to kill or inhibit the growth of microorganisms. However, specific biocides can be unsafe to humans or the environment at high dosages, and long-term exposure promotes development of resistance, leading to increasing concerns with regards to antibiotic failure. Thus, there is a need for improved delivery of biocidal agents that are effective against harmful organisms, while minimizing unnecessary long-term exposure to high doses of antimicrobials and the concomitant risk of developing resistance. One approach to achieve this is to use nanoparticles to encapsulate and deliver biocides, maximizing biocidal efficiency while permitting lower doses. Silica nanoparticles are attractive candidates as inorganic, nanoscale (less than or equal to 100 nm in diameter) materials that have received considerable attention in recent years, particularly in medicine, due to their inert, biocompatible, biodegradable, thermally and chemically stable properties [1–4] as well as their inexpensive synthesis [5]. In particular, mesoporous silica nanoparticles (MSNPs) have received interest as carriers for many applications, including pharmaceutical drug delivery, gene therapy, enzyme immobilization, catalytic chemistry, ion exchange, biosensing and bioimaging [6–12]. Furthermore, the ordered pore network, high pore volume and surface area, and silanol-containing surface [13] as well as the biodegradation of silica in the natural environment into relatively harmless silicic acid by-products make this an attractive proposition. This study expands upon the advantages conferred by MSNPs to deliver novel antimicrobial agents and evaluates their activity against bacteria in planktonic form and in biofilms.
MSNPs have pores ranging from 2 to 50 nm in diameter into which compounds of interest can be loaded [14]. Particle size, porosity and surface properties can also be predictably controlled and tailored to match the physico-chemical properties of guest compounds as well as the overall needs of specific applications [8]. Functional groups, targeting ligands and stimuli-sensitive molecules can be conjugated to both the internal surfaces of the pores and the outer surface in order to optimize loading of guest compounds, control the release profile, improve dispersity or direct the carriers to targeted sites [9,15,16].
It has been reported that plant secondary metabolites are promising candidates as novel biocides. Chemical conflict between microbial invaders and plants has gone on for millions of years, resulting in the evolution of an enormous array of plant-derived compounds that are toxic towards potential pathogens. As such, the plant kingdom offers substantial discovery potential in terms of developing alternative killing agents. These secondary metabolites are (i) highly effective in killing microbes while being non-toxic to humans at antimicrobially active concentrations [17] and (ii) safer and non-damaging to the natural environment because they are originally sourced from plant material. However, hindered by their volatile nature, immiscibility and tendency to degrade in aqueous solutions, plant secondary metabolites typically do not reach their maximum antimicrobial activity in real-world applications.
Several of the limitations of these compounds could potentially be mitigated by novel delivery strategies. In particular, the channels that form the nanosized pores within MSNPs act as physical, protective barriers that surround guest molecules; hence, MSNPs are capable of stabilizing volatile antimicrobial agents, minimizing their loss through vaporization and enhancing the effectiveness of each antimicrobial treatment by retaining more of the loaded agent in the system [3]. In addition, as MSNPs are intrinsically hydrophilic, they can increase the bioavailability of plant compounds by dispersing freely and evenly in aqueous solutions, obviating the need for additional emulsifiers or solvents, improving the delivery of hydrophobic agents to target cells [1]. Furthermore, the high surface area-to-volume ratio of MSNPs helps to maximize the bioavailability of the loaded compound during exposure to microbes [8].
In this study, we used allyl isothiocyanate (AIT) and cinnamaldehyde (CNAD) as model plant antimicrobial agents to assess the efficacy of MSNPs as carriers to improve their biocidal activity. AIT and CNAD are volatile oils at room temperature, with low solubility, but have both been reported to be highly antimicrobial in comparison with other classes of plant secondary metabolites [18].
The objectives of this study were therefore (i) to synthesize MSNPs with a high loading capacity; (ii) to characterize the size, size distribution, morphology and porosity of the synthesized MSNPs; (iii) to load MSNPs with AIT and CNAD and determine release profiles of the two plant compounds; (iv) to assess the antimicrobial efficiency of AIT- and CNAD-loaded MSNPs as compared to that of AIT and CNAD as free agents; and (v) to assess the efficacy of the loaded particles against biofilms.
2. Material and methods
2.1. Synthesis of mesoporous silica nanoparticles with hexagonally ordered mesopores
MSNPs were synthesized via hot hydrolysis in an aqueous, base-catalysed sol–gel system as described by Hom et al. [8], with some modifications. In a typical synthesis, 200 mg cetyltrimethylammonium bromide (CTAB) (99%; Sigma-Aldrich, UK) was dissolved in a 96 ml deionized water and 700 µl 2 M NaOH in a 250 ml round-bottom flask at 80°C (pH 12.4), stirring at 500 r.p.m. After temperature stabilization, 1 ml tetraethylorthosilicate (Sigma-Aldrich) was added. After stirring for 15 min, 254 µl 3-(trihydroxysilyl) propylmethylphosphonate was added to the suspension to modify the silica surface with phosphonate groups to reduce aggregation. After stirring for another 2 h at 80°C, the suspension was centrifuged at 9000 r.p.m. for 5 min. Resulting nanoparticles were washed twice with methanol (Sigma-Aldrich). To ensure that the resulting nanoparticles could be loaded with hydrophobic molecules, the surfactant CTAB was subsequently removed by distillation by refluxing in 40 ml methanol plus 2 ml 37% hydrochloric acid (Sigma-Aldrich) overnight at 80°C using a water-cooled coil condenser.
2.2. Mesoporous silica nanoparticle characterization
2.2.1. Transmission electron microscopy analysis
Synthesized MSNPs were imaged via transmission electron microscopy (TEM) with JEOL JEM 2010 operating at 200 kV for bright field imaging, with small angle tilts (−20° to +20°) to inspect pore orientation. Specimens were prepared by drop-casting MSNPs onto holey carbon-coated TEM copper grids (carbon film on 3 mm 300 mesh, Agar Scientific, UK), allowing them to air-dry overnight before imaging.
Particle and pore sizes were measured using Digital Micrograph™ software. Nanoparticles were measured in two directions, perpendicular to each other, by analysing the region of interest on a histogram of the TEM image, in line plot display. The size of each individual nanoparticle was the mean value of the two measurements, and the mean size of all collective MSNPs was the average of all particle sizes measured. Pore/channel diameters were measured similarly. Owing to the rigidity of the material, pore size remains constant before and after drug loading [19]; therefore, pore size measurements were only made directly after MSNP synthesis.
2.3. Loading and release of allyl isothiocyanate and cinnamaldehyde
AIT (250 µl, ρ = 1.01 g ml−1; Sigma-Aldrich) was dissolved in 1.75 ml dimethyl sulfoxide (DMSO) (Sigma-Aldrich), and then loaded into 20 mg MSNPs suspended in 7 ml phosphate-buffered saline (PBS) via diffusion in a 15 ml glass vial. Contents were sonicated with a probe (Vibra-Cell VCX 130, Sonics & Materials Inc., USA) for 3 min at 5 s intervals, then left to stir at 250 r.p.m. for 24 h. After loading, MSNPs were transferred into six microfuge tubes and centrifuged at 12 000 r.p.m. for 3 min. The concentration of AIT and CNAD in the supernatant was analysed by liquid chromatography (LC, see below for details) and compared to a calibration curve to calculate the loading capacity of the nanoparticles. A control in the absence of MSNPs was used to account for the oil that is lost from the system via evaporation.
After this, MSNPs were washed twice with 1.3 ml 50 : 50 (v/v) methanol: distilled water and once with 1.3 ml PBS to remove any excess AIT on the surface of the MSNPs and to ensure the removal of DMSO, which can be potentially toxic to bacterial cells. In the case of CNAD (Sigma-Aldrich), 500 µl CNAD (ρ = 1.05 g ml−1) was dissolved in 1.5 ml DMSO and loaded into 20 mg of MSNPs in 7 ml PBS using the same protocol.
Washed MSNPs were resuspended in 10 ml PBS. Resulting AIT- and CNAD-loaded MSNP suspensions were incubated at 30°C at 150 r.p.m. to allow the molecules to be released into the PBS via diffusion. Release was monitored every 30 min for 4 h and then again at 24 h, via liquid–liquid extraction and subsequent gas chromatography with flame ionization detection (GC-FID) analysis. Five separate release experiments were performed for each plant compound, and three samples per time point were prepared in each experiment.
To evaluate the volatility of each compound during release, 10 µl of AIT and CNAD were dissolved in 10 ml PBS with and without 20 mg of MSNPs and incubated at 30°C at 150 r.p.m. The volatility test was performed in triplicate and compound concentration was monitored over 24 h via LC analysis.
2.3.1. GC-FID and liquid chromatography
Volatility and loading tests were evaluated via LC analysis, using an Agilent Technologies 1120 Compact LC, equipped with a Zorbax Eclipse Plus C18 column (Agilent Technologies, UK). Samples were analysed using isocratic ratio 80 : 20 (acetonitrile: H2O), UV detector (λ = 270 nm), flow of 1 ml min−1 and an injection volume of 10 µl (3 µl for loading tests) over 4 min. Calibration curves for both compounds were constructed using these LC parameters to determine the loading capacity of the MSNPs.
To estimate the loading capacity of the MSNPs, the oil that remained in the supernatant after the loading process was analysed via LC. Owing to the volatility of the compounds, a control sample was also set up in parallel which contained essential oils in the same solvent system, but in the absence of nanoparticles. The difference in the amount of essential oils in the supernatant between the control sample and the experimental sample was considered to be the amount loaded into the MSNPs.
The method to evaluate the release of the compounds from the MSNPs was adapted from Uchiyama et al. [20]: ethyl acetate (EtOAc) was used as an organic solvent to extract AIT and CNAD from PBS solution and at each sampling time point, 550 µl MSNP suspension was transferred into a microfuge tube and centrifuged at 13 000 r.p.m. for 2 min. The PBS supernatant (500 µl) was then transferred to a new microfuge tube, to which 500 µl EtOAc was added. This 1 : 1 (v/v) EtOAc: PBS solution was shaken at 220 r.p.m. for 20 min to extract the plant compounds out from aqueous solution into the organic phase. Samples were then centrifuged at 13 000 r.p.m. for 2 min to separate the two immiscible liquids, and 220 µl of the top EtOAc layer was transferred into a GC vial. The sample was then injected into the GC-FID instrument to be analysed.
A GC-2010 (Shimadzu) GC-FID instrument with Perkin Elmer Clarus GC ovens was used to quantify the release of AIT and CNAD from 20 mg of loaded MSNPs. The analytical methods for both compounds were developed experimentally (electronic supplementary material, table S1) using a Shimadzu GC-2010, Perkin Elmer Clarus GC and Shim-5MS 5% phenyl (equiv.) polysilphenylene-siloxane column. Retention time for AIT was 6.668 min and 7.078 min for CNAD.
Standard curves of AIT and CNAD were established by preparing solutions of known concentrations ranging from 0.5 to 3000 mg l−1 in EtOAc for both compounds. Each standard solution of known concentration was analysed with GC-FID under the respective analytical methods described above.
2.4. Bacterial viability assays
Escherichia coli (NCIMB 8879) cultures grown in nutrient broth were harvested at mid-log growth phase, washed twice and resuspended in PBS. The turbidity of the E. coli suspension was adjusted to match 0.5 McFarland standard (OD600 = 0.132), which corresponded to bacterial density of 108 CFU ml−1.
The AIT- and CNAD-loaded MSNP release studies performed revealed that the maximum concentrations detected in solution as a result of being released from 2 mg ml−1 of loaded MSNPs were 362.7 mg l−1 for AIT and 34.8 mg l−1 for CNAD. To directly compare the killing efficiencies of AIT and CNAD in free form versus encapsulated in pores of MSNPs, when used at the same concentrations, E. coli suspensions grown and adjusted as described above were each incubated with the following treatments for 18 h at 32°C with 120 r.p.m. agitation: (i) no treatment (negative control), (ii) 362.7 mg l−1 AIT, (iii) 2 mg ml−1 AIT-loaded MSNPs, (iv) 1 mg ml−1 AIT-loaded MSNPs, (v) 34.8 mg l−1 CNAD, and (vi) 2 mg ml−1 CNAD-loaded MSNPs. Bacterial viability was assessed employing both the Miles and Misra [21] plate count method and TEM.
2.5. Transmission electron microscopy imaging of Escherichia coli to asses bacterial viability
After treatment with free oils and loaded MSNPs, E. coli suspensions were centrifuged, washed, resuspended in 5 ml distilled water and further diluted 150-fold. Specimens were prepared by drop-casting 10 µl of each diluted sample onto holey carbon-coated TEM copper grids (carbon film on 3 mm 300 mesh, Agar Scientific, UK), allowing them to dry overnight. Triplicates were prepared for each treatment. Resulting specimens were imaged via TEM with JEOL JEM 2010 at 200 kV for bright field imaging.
2.6. Confocal imaging of bacterial biofilms
Confocal laser scanning microscopy (CLSM) was used to visualize Pseudomonas aeruginosa biofilms grown in sterile FluoroDish™ tissue and cell culture dishes (35 mm diameter) with cover glass bottom (23 mm diameter; World Precision Instruments, UK). Control biofilms were grown for 48 h at 30°C in 2 ml sterile 10% tryptic soy broth (TSB) (Sigma-Aldrich) in PBS with 1/100 (vol/vol) P. aeruginosa inocula (OD600 = 0.132). After 24 h of growth, medium in the culture dish was removed and carefully replaced with fresh sterile 10% TSB in PBS so as not to disturb the biofilm forming on the surface of the cover glass. This was done to provide fresh nutrients to support growth of nascent biofilm, and to remove any planktonic cells in the spent media to avoid competition with the biofilm cells for nutrients. Confluent and adherent biofilms were visible after 48 h of growth. At this point, the pre-formed biofilms were subjected to various treatments listed in table 1 (2 ml for each) for 24 h, with PBS as the solvent. Control samples were treated with 2 ml sterile PBS.
Table 1.
The treatments that P. aeruginosa biofilms were subjected to prior to CLSM imaging.
| treatment | |||
|---|---|---|---|
| AIT (mg l−1) | AIT-loaded MSNPs (mg ml−1) | CNAD (mg l−1) | CNAD-loaded MSNPs (mg ml−1) |
| 600 | 2 | 600 | 2 |
| 350 | 1 | 350 | 1 |
| 0.5 | 35 | 0.5 | |
CLSM images were collected directly from the cover glass at the bottom of the cell culture dish using a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany) with a 1.2-numerical-aperture C-Apochromat 40× water immersion lens (Carl Zeiss MicroImaging GmbH, Jena, Germany). Before imaging, biofilms were stained with 1 : 1 (v : v) mixture of Syto® 9 and PI dyes from the LIVE/DEAD® BacLight™ bacterial viability kit (Molecular Probes®, Invitrogen™, USA). Stock stain solution was prepared by dissolving 30 µl 3.34 mM Syto® 9 and 30 µl 20 mM PI in 9.94 ml distilled water. Biofilm samples were stained with 1 ml stock dye solution for 15 min in the dark to fluorescently stain the cells, then washed once with PBS to remove any excess dye.
Biofilm samples were excited with a 488 nm line from a 30 mW argon ion laser operating with a tube current of 6.1 A attenuated to 0.3–0.7% of full power in multitrack mode. Fluorescence emission was detected with 500–530 nm (green, alive) and 585–615 nm (red, dead) band-pass filters. Three-dimensional images were collected over square areas with image size of 1024 × 1024 pixels (76.8 × 76.8 µm) in x and y with variable number of z-sections since the thickness of each biofilm differed from one another due to the intrinsic variance of biological samples and the different types of treatment each biofilm was subjected to. Pixel spacing was 70 nm and pixel dwell time was 1.6 µs.
Three biological replicates were prepared for each treatment, each was imaged at three different locations of the biofilm (at the least), and the entire experiment was repeated four separate times. CLSM images were rendered using Zeiss LSM Image Browser v. 4.2.0.121 software.
3. Results
3.1. Mesoporous silica nanoparticle characterization
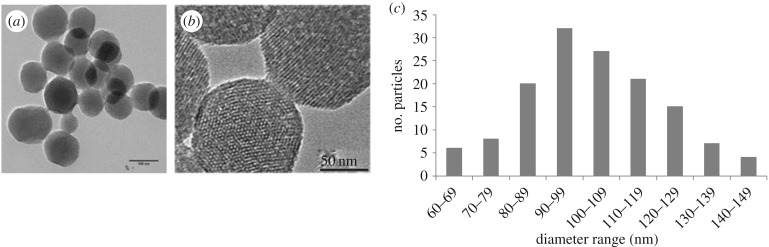
MSNPs were imaged with bright field TEM in different orientations to determine the shape and dimension of the particles as well as the pore characteristics (figure 1a). Mesopores showed high rotational symmetry about the centre axis in a hexagonal arrangement when imaged through the axis of the central pore. When the electron beam was perpendicular to the centre axis (figure 1b), the MSNPs showed the pores to penetrate directly through the entire volume of the nanoparticle. From such images, a synthesized MSNP may be described as a polyhedron with constituent pore channels arranged in a highly ordered manner.
Figure 1.
Typical TEM image of MSNPs (a) showing external diameter and overall spherical shape. (b) Close-up image of MSNPs showing two adjacent MSNPs, one in spot contrast view with pores arranged hexagonally, and the second showing the line array of pores running through the entire volume of the nanoparticle. (c) Particle size distribution of MSNPs obtained from measurements of TEM images.
Particle diameters were determined from TEM images. The distribution of nanoparticle size is displayed in figure 1c. Mean particle diameter was 103.64 ± 18.78 nm, with an average aspect ratio of 1.12 (n = 140). Size distribution of the MSNPs followed a unimodal Gaussian curve (s.d. = 18.78), which shows a robust and reproducible synthesis method.
The sizes of the pores were also measured from TEM images: mean pore diameter was 2.03 ± 0.19 nm (n = 170 from 15 individual nanoparticles). Mean channel diameter was 2.02 ± 0.17 nm (n = 70 from 15 different nanoparticles). Pore and channel width measurements were highly consistent, and a low s.d. indicates uniformity and regularity.
3.2. Loading and release profiles of allyl isothiocyanate and cinnamaldehyde
The loading of MSNPs was carried out with excess oil to maximize the loading efficiency. AIT and CNAD are adsorbed onto the silica surface and in the nanoparticles' pores by hydrogen bonds and van der Waals forces that can be broken by water to enable the release of the biocide [22]. The loading capacity of the MSNPs assessed via LC was approximately 7.4 mg AIT and approximately 0.95 mg CNAD for every 10 mg MSNPs.
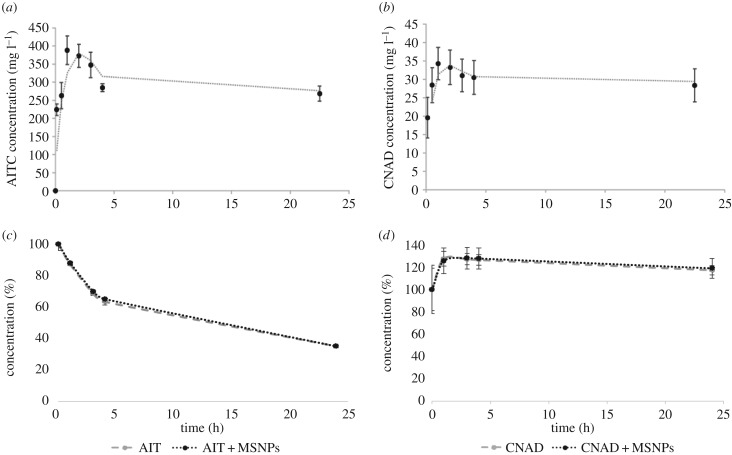
The release of both AIT and CNAD from MSNPs, assessed using GC-FID analysis, reached peak concentrations after 1 h (AIT: mean of 362.7 mg l−1; CNAD: mean of 34.3 mg l−1) (figure 2a,b). The high initial rates of release over the first hour may have been due to the steep concentration gradient established from within and outside the pores in the external solution or because of release of compounds bound to the external surface of the nanoparticle (see [19] for a discussion of the ratio of external to internal surface area). Subsequently, there was a slight and gradual decrease in concentration from 1 to 24 h for both compounds. This decrease may have been due to the highly volatile nature of both plant compounds that are easily lost from the aqueous phase via spontaneous vaporization. Henry's law volatility constant for AIT is 2.4 × 10−4 atm-m3 mol−1 and for CNAD it is 1.6 × 10−6 atm-m3 mol−1, meaning AIT has a greater tendency than CNAD to escape from the water phase into the atmosphere. This could explain why the decrease in concentration from 1 to 24 h was much greater for AIT than CNAD.
Figure 2.
Release and volatility profiles of the plant-derived compounds from MSNPs. Compounds were released from MSNPs resuspended in 10 ml PBS: (a) 20 mg AIT-loaded MSNPs; (b) 20 mg CNAD-loaded MSNPs. Volatility of 10 µl of (c) free AIT and (d) free CNAD incubated in 10 ml PBS as estimated from the decrease in concentration in the absence and presence of unloaded MSNPs. Error bars represent ±s.d.
To evaluate the volatility of the oils, AIT and CNAD were incubated with and without nanoparticles. This confirmed that AIT was highly volatile, and up to 65% of the oil in solution evaporated within 24 h (figure 2c). CNAD was less soluble than AIT and therefore the concentration of oil in solution increased during the first hour of incubation. However, after the first hour, a slight decrease in concentration demonstrated the volatility of this compound, and after 24 h around 10% of the oil in solution was lost from the system (figure 2d). The concentration of each compound and rate of evaporation were not affected by the presence of unloaded nanoparticles in the solution, suggesting that no oil became immobilized by irreversible adherence to the nanoparticles. Taken together, we infer that the decline in concentrations of oil released from the MSNPs represents a quasi-steady-state equilibrium between continued release from the MSNPs and evaporation.
While the release kinetics were similar for both compounds, much greater concentrations of AIT were detected in solution than CNAD (approx. 10-fold) as a result of diffusive release from the same amount of MSNPs (20 mg). This may have been due to a combination of several factors: firstly, AIT is a smaller molecule than CNAD and may be able to load at higher density. Considering the longest inter-atomic distance that occurs within each molecule (5.77 Å for AIT and 8.5 Å for CNAD) and adding the van der Waals radii (for AIT 1.2 Å (H) and 1.8 Å (S); for CNAD 1.2 Å (H) and 1.55 Å (O)), the smallest cube that could contain one CNAD molecule would have dimensions of approximately 6.50 × 6.50 × 6.50 Å and a volume of approximately 274.02 Å3. By contrast, the smallest cube containing an AIT molecule would have dimensions of approximately 5.06 × 5.06 × 5.06 Å and a volume of approximately 129.81 Å3. As the AIT molecules are smaller than the CNAD molecules, approximately twice as many AIT molecules could be housed in the mesopores of MSNPs as compared to CNAD molecules. Secondly, as AIT is a smaller molecule than CNAD, it may have been released more readily from pore openings due to less physical self-obstruction. Thirdly, AIT is more soluble in water (2000 mg l−1) [23] than CNAD (1420 mg l−1) [24] so it could be more easily unloaded from the MSNPs into the aqueous PBS.
3.3. Viability of allyl isothiocyanate- and cinnamaldehyde-treated bacteria
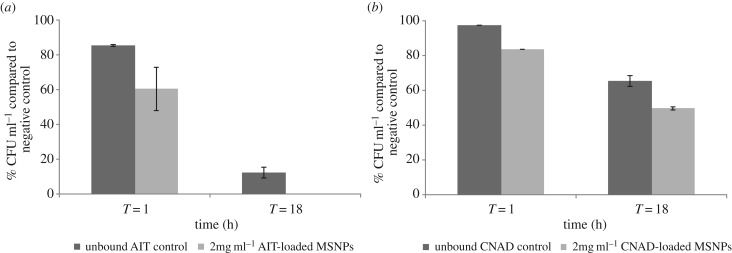
To directly compare the killing efficiency of AIT and CNAD in free form versus encapsulated in MSNPs, planktonic E. coli cells were treated with MSNPs and free oils at the equivalent concentration determined from the release profiles. At 1 h, free AIT and CNAD resulted in approximately 15% and approximately 3% lower colony counts compared with untreated controls. By 18 h, AIT reduced the colony counts to 12%. CNAD was less effective at killing cells but still caused a reduction to 65% of untreated controls.
At 1 h, 1 mg ml−1 (data not shown) and 2 mg ml−1 AIT-loaded MSNPs (figure 3a) as well as 2 mg ml−1 CNAD-loaded MSNPs (figure 3b) yielded significantly lower colony counts compared with the untreated controls (by approx. 20%, 40% and more than 15%, respectively; p ≤ 0.05). AIT- and CNAD-loaded MSNPs at a concentration of 2 mg ml−1 also resulted in a reduction in CFU ml−1 compared to the free oils (by approx. 20% and 15%, respectively; p ≤ 0.05; figure 3a,b).
Figure 3.
Miles and Misra plate count assay after exposing planktonic E. coli to biocide for 1 h and 18 h: (a) AIT treatments comparing unbound AIT (362.7 mg l−1) in free form with 2 mg ml−1 loaded MSNPs; (b) CNAD treatments comparing unbound CNAD (34.8 mg l−1) in free form with 2 mg ml−1 loaded MSNPs. CFU ml−1 values are expressed as percentages of the negative control. Error bars represent ±s.d. Data represent the mean and s.d. of triplicate biological and technical samples.
Exposure to 1 mg ml−1 AIT-loaded MSNPs (data not shown) for 1 h gave comparable results to free AIT at 362.7 mg l−1. Since 2 mg ml−1 AIT-loaded MSNPs released a maximum of 362.7 mg l−1 AIT in solution (figure 2a), it would be reasonable to assume that 1 mg ml−1 AIT-loaded MSNPs would release around 181.35 mg l−1. Based on present data, we infer that using MSNPs as a delivery system doubled the antibacterial efficacy of free form AIT after 1 h of exposure.
At the 18 h time point, both 2 and 1 mg ml−1 (data not shown) AIT-loaded MSNPs killed all planktonic bacteria while AIT in free form at 362.7 mg l−1 was significantly less effective and resulted in a more than 85% reduction in bacterial colonies counts when compared with the negative control (p ≤ 0.05; figure 3a). Based on present experimental data, the degree of antibacterial efficacy was not solely dependent on the amount of AIT-loaded MSNPs employed; but rather, the killing efficiency of AIT-loaded MSNPs was also a function of treatment time.
A similar result was observed for 2 mg ml−1 CNAD-loaded MSNPs at 18 h where colony counts were significantly reduced compared with the negative and reference controls (by approx. 50% and 15%, respectively; p ≤ 0.05; figure 3b). Untreated control samples yielded (2.365 ± 0.037) × 109 CFU ml−1
E. coli. Additionally, when the CFU/ml values generated by 2 mg ml−1 CNAD-loaded MSNPs were normalized to the 34.8 mg l−1 CNAD reference control, there was no significant difference detected between the colony count percentage at 1 h and 18 h (79.56% of reference control at 1 h, 76.75% at 18 h), which suggests that the degree to which the employment of MSNPs improved CNAD's antibacterial activity compared to the free form remained the same at 1 h and 18 h and both reduced colony counts at similar rates. We infer that there was a strong interaction between concentration and exposure time, but in all cases, the MSNP-encapsulated oils were significantly more effective than the free oils. The IC50 for CNAD-loaded MSNPs was around 2 mg ml−1, while IC50 for AIT-loaded MSNPs was  1 mg ml−1.
1 mg ml−1.
3.4. Monitoring the interaction between bacteria and allyl isothiocyanate- and cinnamaldehyde-loaded mesoporous silica nanoparticles using transmission electron microscopy
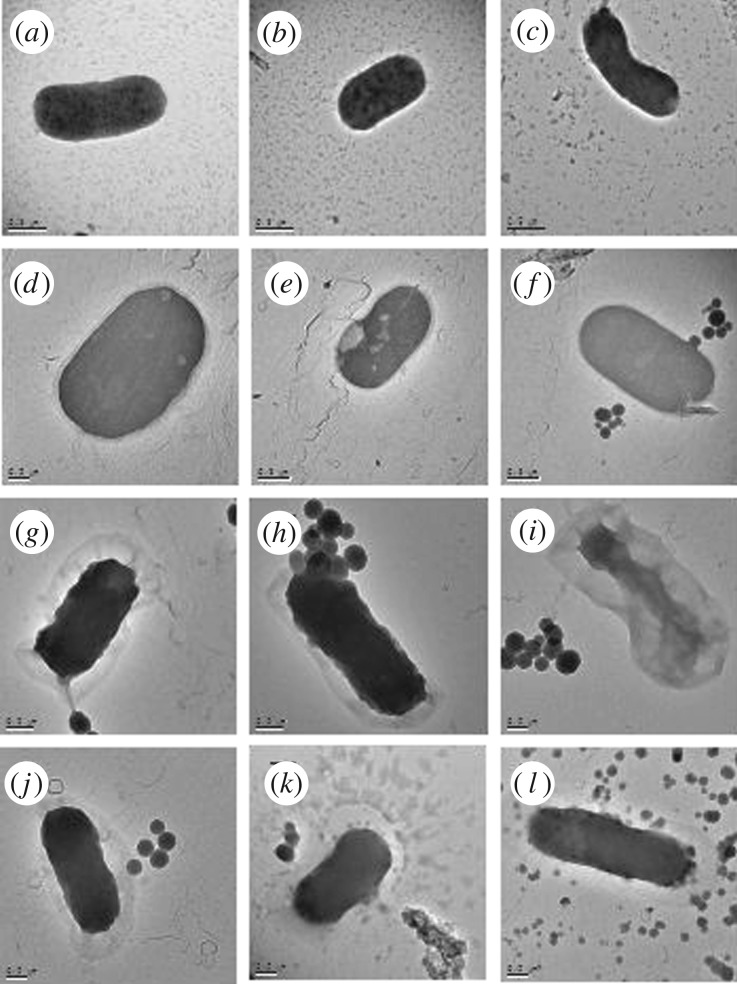
The action of MSNPs may only involve release of the volatile oils in solution or augment the delivery by physical interaction with the bacteria. To visualize the interaction, planktonic E. coli treated with AIT- and CNAD-loaded MSNPs, along with empty MSNPs (vehicle control) and with no treatment (negative control), were imaged with bright field TEM (figure 4). Untreated controls (figure 4a–c) revealed rod-shaped bacteria, with a proportion of cells dividing. Treatment with empty MSNPs also yielded cells with bacillus morphology and no obvious damage (figure 4d–f) although the bacteria appeared slightly larger and relatively more spherical compared with those in the negative control. This could be a bacterial reaction induced by the presence of foreign, but non-toxic, objects in the media. From brief visual analysis, the cells did not appear to be dividing after the treatment, unlike those in the negative controls.
Figure 4.
TEM images of planktonic E. coli. One representative image was chosen from each triplicate sample. (a–c) Controls showing rod-shaped bacteria with some cells undergoing division. (d–f) Escherichia coli exposed to 2 mg ml−1 empty MSNPs (vehicle control). MSNPS can be seen clearly in (f) surrounding the bacterium. (g–i) Planktonic E. coli exposed to 2 mg ml−1 AIT-loaded MSNPs. (j–l) Planktonic E. coli exposed to 2 mg ml−1 CNAD-loaded MSNPs. Scale bars represent 0.2 µm, except for (e,f) where the scale bar is 0.5 µm.
By contrast, E. coli treated with 2 mg ml−1 AIT-loaded (figure 4g–i) or CNAD-loaded (figure 4j–l) MSNPs appeared stressed and flaccid compared to bacteria in the untreated controls, with corrugated wall outlines suggesting cells were dead or dying.
3.5. Biofilm viability assay
To determine whether MSNPs would also enhance the effectiveness of AIT and CNAD toxicity in the more challenging but relevant case of bacterial biofilms, P. aeruginosa biofilms were subjected to the treatments listed in table 1, for 24 h. Biofilms were treated with the experimentally established maximum concentrations (approx. 350 mg l−1 AIT and approx. 35 mg l−1 CNAD) and 350 mg l−1 CNAD was also included to directly compare the anti-biofilm efficacies of the two compounds when used at the same concentration.
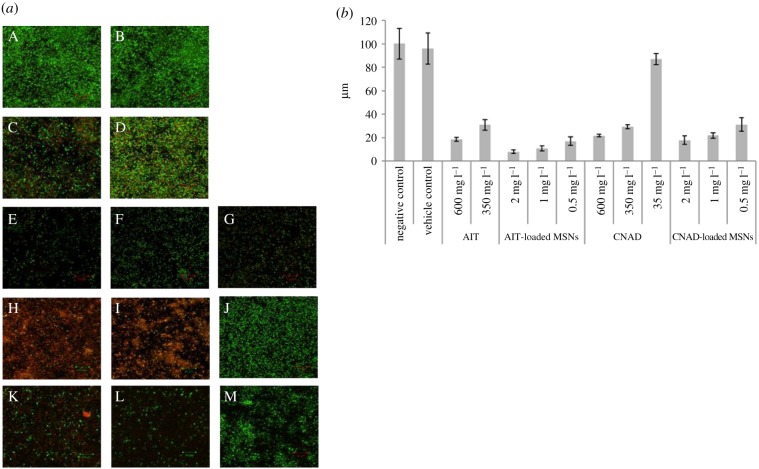
CLSM was used to evaluate the effects of AIT- and CNAD-loaded MSNPs treatments on pre-existing, 48 h-old biofilms after treated samples were stained with LIVE/DEAD® BacLight™ Bacterial Viability Stain. Maximum intensity projections of CLSM stack images are presented in figure 5a. Images are 76.8 × 76.8 µm in x- and y-axes with variable numbers of z-sections since the thickness of each biofilm differed due to the different types of treatment to each biofilm (figure 5b).
Figure 5.
(a) Maximum intensity projections of CLSM image stacks stained directly prior to CLSM with LIVE/DEAD® BacLight™ Bacterial Viability Stain (SYTO® 9, green = live cells, PI, red = dead cells). Biofilms grown for 72 h at 30°C in 2 ml 10% TSB in PBS. (A) Negative control; (B) vehicle control P. aeruginosa; 48 h-old P. aeruginosa biofilms treated for 24 h with: 2 ml AIT (C) 600 mg l−1, (D) 350 mg l−1; 2 ml AIT-loaded MSNPs (E) 2 mg ml−1, (F) 1 mg ml−1, (G) 0.5 mg; 2 ml CNAD (H) 600 mg l−1, (I) 350 mg l−1, (J) 35 mg l−1 CNAD; 2 ml CNAD-loaded MSNPs (K) 2 mg ml−1, (L) 1 mg ml−1, (M) 0.5 mg ml−1. One representative image was chosen from four independent imaging sessions, each session consisting of three biological replicates, with each replicate imaged at three different locations of the biofilm (at the least). Scale bar is 10 µm in all CLSM images presented. (b) Thickness of 48 h-old P. aeruginosa biofilms (z-axis of three-dimensional CLSM image stacks) resulting from various treatments for 24 h. Error bars represent ±s.d. The data represent the mean and s.d. of images from four independent imaging sessions, each session consisting of three biological replicates, with each replicate imaged at three different locations of the biofilm (at the least).
There was no detectable difference between the untreated control and unloaded MSNPs after 24 h treatment with 2 mg ml−1 empty MSNPs. In both cases, the bacteria were highly viable, as indicated by the dense green fluorescence signals (figure 5aA,B), and the biofilm maintained a thickness of 100.25 ± 13.13 µm and 96 ± 13.12 µm, respectively (figure 5b). Thus, empty, unloaded MSNPs had negligible effect on pre-formed P. aeruginosa biofilms.
By contrast, treatment with free AIT or CNAD at 350 mg l−1 and 600 mg l−1 significantly reduced viability and thickness of the biofilms compared with the untreated controls (p ≤ 0.05), resulting in a reduction of average biofilm thickness to 31 ± 4.42 µm and 18.5 ± 1.63 µm for AIT and of 29.375 ± 1.75 µm and 21.8 ± 1.19 µm for CNAD, respectively (figure 5b). Treatments not only reduced the number of bacteria present, but also killed a large portion of the remaining bacteria and led to voids opening up in the biofilm. Nevertheless, some bacteria in the remnant biofilm remained viable, even in the highest concentration of AIT (figure 5aC). CNAD at 350 mg l−1 and 600 mg l−1 was comparable to AIT, with both concentrations reducing biofilm thickness and effectively killing almost all remaining bacteria present in the biofilms (figure 5aH,I). However, there was no detectable difference between untreated controls (figure 5aA,B) and samples treated with 10-fold lower concentrations of CNAD (35 mg l−1, figure 5aJ). Biofilms in both cases remained viable with mean biofilm thickness of 100.25 ± 13.13 µm and 87.125 ± 4.75 µm, respectively. While equally effective in detaching adherent biofilm cells, CNAD was more effective than AIT in killing the remaining adherent bacteria when both were used in free form at the same concentration (compare panels (H,I) with (C,D) in figure 5a).
Treatment with AIT-loaded MSNPs (figure 5aE–G) significantly reduced the average thickness and disrupted the spatial integrity of the remaining biofilm when compared with the untreated control (figure 5aA), and the vehicle control (figure 5aB). The effects were more pronounced than samples exposed to free form AIT (figure 5aC,D; p ≤ 0.05), resulting in mean z-axis values of 8.125 ± 1.44 µm (2 mg ml−1 MSNPs), 11 ± 2.16 µm (1 mg ml−1 MSNPs) and 17.125 ± 3.65 µm (0.5 mg ml−1 MSNPs; figure 5b). The thinnest, least dense and most patchy biofilms of all AIT-treated samples were caused by 2 mg ml−1 AIT-loaded MSNPs. We infer from the low cell density that dead cells became detached from the biofilm during washing. Nevertheless, small patches of a few viable cells remained.
Exposure to 2 mg ml−1 AIT-loaded MSNPs (equivalent to 350 mg l−1 AIT) was significantly more effective in detaching and killing biofilm bacteria than AIT in free form (compare panel E with C and D in figure 5a) and in reducing the average thickness of biofilms (figure 5b). Even exposure to 1 mg ml−1 and 0.5 mg ml−1 AIT-loaded MSNPs was more effective than the free form in dissociating adherent cells from the biofilm and/or killing constituent cells first and then causing detachment (or a combination of both). Furthermore, biofilm thickness, cell density and abundance of green and red fluorescence signals between 0.5 mg ml−1 AIT-loaded MSNPs and 600 mg l−1 AIT in free form were similar, which showed these treatments had a similar efficacy in detaching and killing bacterial biofilms. Therefore, the efficacy of AIT was estimated to have been improved more than six times by the MSNPs.
For CNAD, there was no observable difference in viability and cell density between 2 and 1 mg ml−1 CNAD-loaded MSNPs (figure 5aK,L) and no detectable difference in biofilm thickness (18 ± 3.46 µm and 22 ± 2.45 µm, respectively; figure 5b). However, these treatments yielded significantly thinner biofilms than 350 mg l−1 free CNAD did (29.375 ± 1.75 µm; p ≤ 0.05), which is theoretically a concentration equivalent to the employment of 10 × 2 and 20 × 1 mg ml−1 CNAD-loaded MSNPs. Additionally, these two treatments yielded similar biofilm thickness to samples treated with 600 mg l−1 CNAD (21.8 ± 1.19 µm), even though they resulted in relatively more viable cells.
Interestingly, treatments with CNAD-loaded MSNPs were significantly more effective in detaching and killing biofilm bacteria than 35 mg l−1 CNAD in free form (compare panels K–M with J in figure 5a, and the average thickness of biofilms in figure 5b), even though this would be expected to result in equivalent concentrations (figure 2b). The biofilm cells remaining after treatments with 2 and 1 mg ml−1 MSNPs were sparse and mostly dead (figure 5aK,L). While 0.5 mg ml−1 CNAD-loaded MSNPs resulted in relatively denser, thicker, and more viable biofilms (figure 5aM), this treatment (equivalent to (¼) × 35 mg l−1 CNAD in free form) displayed similar efficacies to 350 mg l−1 CNAD in reducing biofilm thickness (31.25 ± 5.87 µm and 29.375 ± 1.75 µm, respectively), although it was not as effective in killing the remaining adherent cells (compare panels I and M in figure 5a). Still, these results suggest that the efficacy of CNAD in reducing biofilm thickness was improved up to 40 times by the MSNPs.
4. Discussion
This study is the first to report the utilization of MSNPs for the delivery of AIT and CNAD for antimicrobial use against planktonic E. coli and P. aeruginosa biofilms. Herein we focused on the compounds CNAD and AIT, and sought to investigate further the potential of their antimicrobial activity and specifically whether the compound could be packaged into MSNPs to improve the stability of the compound.
AIT (figure 6a) is a natural compound derived from plants in the Cruciferae family and is a potent inhibitor of a large number of pathogenic microorganisms in liquid media as well as in vapour form [25–28]. The compound has been demonstrated to be effective against E. coli O157:H7 and Salmonella Montevideo, and was an effective bactericidal agent at all growth stages in comparison to penicillin G and streptomycin which do not exhibit any bactericidal effect against stationary cells. The mode of action of AIT was determined by comparison to be most similar with that of polymixin B; showing cell membrane damage and resulting leakage of cellular metabolites [29]. We showed similar efficacy by the application of free AIT at 362.7 mg l−1 which was able to inhibit more than 85% bacterial colonies in planktonic form when compared with the untreated control after 18 h (figure 3a). At the 18 h time point, the negative control samples experimentally yielded (2.365 ± 0.037) × 109 CFU ml−1 E. coli.
Figure 6.
AIT and CNAD molecules (Chem3D). (a) AIT and (b) CNAD chemical structures. (Online version in colour.)
CNAD (figure 6b) was first isolated from cinnamon essential oil in 1834 by Dumas and Peligot, and was synthesized in the laboratory by Chiozza in 1854 [30]. The molecule consists of a phenyl group attached to an unsaturated aldehyde closely related to compounds that give rise to lignin [31]. In terms of its antibacterial action, CNAD has been reported to inhibit the growth of Clostridium botulinum [32], Staphylococcus aureus [33], E. coli O157:H7, and Salmonella enterica serovar typhimurium [34]. In this study, CNAD was tested against E. coli, and colony counts were performed after application of the free compound. Exposure to 34.8 mg ml−1 CNAD resulted in a 35% reduction in CFU ml−1 compared with the control.
While essential oils can clearly be seen to be efficacious as biocides, their application has been limited due to their volatility, which can potentially be overcome by encapsulation of the antimicrobial to retain its potency. As such, MSNPs were investigated as carriers. The nanoparticles have an exceptionally large surface area (see [29] for full characterization details), and the silica chemistry lends itself to adsorbing biocides. Other studies have shown that drug molecules can be adsorbed both onto the surface of the silica nanoparticles and inside the pores by van der Waals forces and hydrogen bonds, which can be broken by contact with water, hence enabling detachment and release of the drug [22]. In our previous studies, we have also shown that a potential chemotherapeutic, the fungal derivative Ophiobolin A, underwent significantly less degradation when adsorbed to MSNPs [35]. Other studies have reported the use of nanostructured mesoporous silica loaded with essential oils against the planktonic form of the bacteria P. aeruginosa and Staphylococcus aureus [36] as well as against the fungus Aspergillus niger [37]. Different methods for essential oil loading into MSNPs have been reported. For instance, Bernardos et al. have discussed a simple vapour adsorption method to load essential oils into nanoparticles [38]. The method described in this paper is based on diffusion and relies on the adsorption of the compounds onto the nanoparticles.
We demonstrated that both AIT and CNAD were more effective in killing bacteria when encapsulated in MSNPs than as free agents. Free AIT was demonstrated to be 10% less effective than the encapsulated form and CNAD-loaded MSNPs yielded significantly lower colony counts reaching 50% greater efficacy after 18 h. Additionally, cells exposed to both CNAD and AIT were seen to undergo plasmolysis, a phenomenon where the cytoplasmic membrane shrinks and pulls away from the cell wall due to loss of water from inside the cell when bacteria are in a hypertonic environment [39,40]. Eventually, plasmolysis can lead to the complete collapse of the cell wall (cytorrhysis) and subsequent cell death [40,41]. The images show that the MSNPs alone were not toxic to E. coli, and no internalization of the MSNPs was observed. Instead, MSNPs accumulate around the cells delivering high concentrations of antimicrobials to a critical point in the cell membrane. This accumulation could be caused by a process known as depletion, where an imbalance in osmotic pressure enhances the potential for aggregation [42].
The susceptibility to a biocide varies markedly between planktonic cells and biofilms. Biofilms are densely packed communities of microbial cells that surround themselves with extracellular polymeric substances. These sessile communities and their inherent resistance to antimicrobial agents are at the root of many persistent and chronic bacterial infections in medical and industrial scenarios.
Previous studies have used nanoparticles against biofilms. Mu et al. [43] used gold nanoparticles loaded with gentamicin to treat biofilms. Other studies have treated E. coli biofilms with essential oil capsules stabilized by nanoparticles [44] or by loading the nanoparticles with Eucalyptus globulus oil [45]. In this study, we demonstrated that both AIT and CNAD were effective against P. aeruginosa biofilms and that the essential oil efficacy is improved up to 40 times by MSNP encapsulation. The use of loaded MSNPs as delivery vehicles increases and localizes the antimicrobial concentration delivered to the biofilm due to a proximity effect of the nanoparticles to the biofilm surface. The sticky nature of the biofilm exopolysaccharides [46] helps the MSNPs to adhere to the biofilm surface, enhancing delivery and preventing dilution of the antimicrobial. In addition, MSNPs maintain the antimicrobials in their active form for a longer period and protect them from evaporation. Qualitative and quantitative analyses of biofilm viability demonstrated that the ability of both compounds to dissociate and kill constituent biofilm cells was significantly enhanced by the employment of MSNPs as a delivery system.
5. Conclusion
MSNPs proved to be an effective method of delivering hydrophobic, volatile and unstable compounds and significantly enhanced the compounds' bioactivity (in this case, antimicrobial activity). The delivery platform could potentially be expanded to traditional biocides and other non-conventional antimicrobial agents which would benefit from the directed and sustained release to target microbes. Because bioactivity is increased, lower doses of biocide would be effective. Treating bacteria with higher dosages than are necessary for cell kill increases the likelihood of resistance developing. Therefore, using MSNPs to deploy antimicrobial agents could be an effective way of maximizing biocidal potential of hydrophobic and/or volatile compounds while reducing the opportunity for spontaneous antibiotic resistance to occur. To ensure a controlled release of the biocide from the MSNPs, we have capped the nanoparticles with a responsive coating that will provide a controlled and targeted not release of the biocide. The manuscript of this work is currently under preparation.
Supplementary Material
Acknowledgements
The authors would like to thank Prof. Christopher Schofield, Dr Ian Clifton and Paolo Spingardi from the Department of Chemistry, University of Oxford, for providing the AIT and CNAD images presented in figure 6a,b.
Authors' contributions
A.C.C. carried out the microbiological work, data analysis, participated in the study design and first draft of the manuscript; M.B.C. carried out experimental work, data analysis and completed the final draft of the manuscript; H.E.T. was instrumental in silica nanoparticle synthesis, study concept and revision of the article; M.D.F. for confocal based biofilm analysis; I.P.T. conceived, designed and coordinated the study. All authors gave final approval for publication.
Competing interests
The content of this paper relates to a patent submitted by A.C.C., H.E.T. and I.P.T.
Funding
M.B.C. is funded by the National Council of Science and Technology (Conacyt, Mexico, grant no. 384507), the Schlumberger Foundation FFTF Award and the Public Secretariat of Education (SEP DGRI, Mexico). H.E.T. is grateful to the Williams Fund for ongoing support.
References
- 1.Barbé C, Bartlett J, Kong L, Finnie K, Linn HQ, Larkin M, Calleja S, Bush A, Calleja G. 2004. Silica particles: a novel drug-delivery system. Adv. Mater. 16, 1959–1966. ( 10.1002/adma.200400771) [DOI] [Google Scholar]
- 2.Lin Y-S, Haynes CL. 2009. Synthesis and characterization of biocompatible and size-tunable multifunctional porous silica nanoparticles. Chem. Mater. 21, 3979–3986. ( 10.1021/cm901259n) [DOI] [Google Scholar]
- 3.Lu J, Liong M, Zink JI, Tamanoi F. 2007. Mesoporous silica nanoparticles as delivery system for hydrophobic anticancer drugs. Small 3, 1341–1346. ( 10.1002/smll.200700005) [DOI] [PubMed] [Google Scholar]
- 4.Trewyn BG, Nieweg JA, Zhao Y, Lin VSY. 2008. Biocompatible mesoporous silica nanoparticles with different morphologies for animal cell membrane penetration. Chem. Eng. J. 137, 23–29. ( 10.1016/j.cej.2007.09.045) [DOI] [Google Scholar]
- 5.Nandiyanto ABD, Kim SG, Iskander F, Okuyama K. 2009. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Micropor. Mesopor. Mater. 120, 447–453. ( 10.1016/j.micromeso.2008.12.019) [DOI] [Google Scholar]
- 6.Davis ME. 2002. Ordered porous materials for emerging applications. Nature 417, 813–821. ( 10.1038/nature00785) [DOI] [PubMed] [Google Scholar]
- 7.Heikkila T, et al. 2007. Mesoporous silica material TUD-1 as a drug delivery system. Int. J. Pharm. 331, 133–138. ( 10.1016/j.ijpharm.2006.09.019) [DOI] [PubMed] [Google Scholar]
- 8.Hom C, Lu J, Liong M, Luo H, Li Z, Zink JI, Tamanoi F. 2010. Mesoporous silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells. Small 6, 1185–1190. ( 10.1002/smll.200901966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ispas C, Sokolov I, Andreescu S. 2009. Enzyme-functionalized mesoporous silica for bioanalytical applications. Anal. Bioanal. Chem. 393, 543–554. ( 10.1007/s00216-008-2250-2) [DOI] [PubMed] [Google Scholar]
- 10.Knopp D, Tang D, Niessner R. 2009. Review: bioanalytical applications of biomolecule-functionalized nanometer-sized doped silica particles. Anal. Chim. Acta 647, 14–30. ( 10.1016/j.aca.2009.05.037) [DOI] [PubMed] [Google Scholar]
- 11.Ozin GA, Arsenault AC. 2005. Nanochemistry: a chemical approach to nanomaterials, 1st edn Cambridge, UK: Royal Society of Chemistry Publishing. [Google Scholar]
- 12.Wang F, Guo C, Yang L-R, Liu CZ. 2010. Magnetic mesoporous silica nanoparticles: fabrication and their laccase immobilization performance. Bioresource Technol. 101, 8931–8935. ( 10.1016/j.biortech.2010.06.115) [DOI] [PubMed] [Google Scholar]
- 13.Vallet-Regί M, Balas F, Arcos D. 2007. Mesoporous materials for drug delivery. Angew. Chem. 46, 7548–7558. ( 10.1002/anie.200604488) [DOI] [PubMed] [Google Scholar]
- 14.McCusker LB, Liebau F, Engelhardf G, Schuth F. 2003. Nomenclature of structural and compositional characteristics of ordered microporous and mesoporous materials with inorganic hosts: IUPAC recommentations 2001. Micropor. Mesopor. Mater. 58, 3–13. ( 10.1016/S1387-1811(02)00545-0) [DOI] [Google Scholar]
- 15.Xia TA, Kovochich M, Liong M, Meng H, Kabehie S, George S, Zink JI, Nel AE. 2009. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano 3, 3273–3286. ( 10.1021/nn900918w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Fang Y, Bordchardt L, Kaskel S. 2011. PEGylated hollow mesoporous silica nanoparticles as potential drug delivery vehicles. Micropor. Mesopor. Mater. 141, 199–206. ( 10.1016/j.micromeso.2010.11.013) [DOI] [Google Scholar]
- 17.Chao S, Young G, Oberg C, Nakaoka K. 2008. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by essential oils. Flavour Frag. J. 23, 444–449. ( 10.1002/ffj.1904) [DOI] [Google Scholar]
- 18.Chan AC, Ager D, Thompson IP. 2013. Resolving the mechanism of bacterial inhibition by plant secondary metabolites employing a combination of whole-cell biosensors. J. Microbiol. Meth. 93, 209–217. ( 10.1016/j.mimet.2013.03.021) [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Young NP, Townley HE. 2013. Characterization and comparison of mesoporous silica particles for optimized drug delivery. Nanomater. Nanotechnol. 4, 2 ( 10.5772/58290) [DOI] [Google Scholar]
- 20.Uchiyama S, et al. 1992. Determination of methyl isothiocyanate in wine by GC and GC/MS. Food Hyg. Safety Sci. 33, 603–608. ( 10.3358/shokueishi.33.603) [DOI] [Google Scholar]
- 21.Hedges AJ. 2002. Estimating the precision of serial dilutions and viable bacterial counts. Int. J. Food Microbiol. 76, 207–214. ( 10.1016/S0168-1605(02)00022-3) [DOI] [PubMed] [Google Scholar]
- 22.Jia L, Shen J, Liu Z, Zhang D, Zhang Q, Liu G, Zheng D, Tian X. 2013. In vitro and in vivo evaluation of paclitaxel-loaded mesoporous silica nanoparticles with three pore sizes. Int. J. Pharm. 445, 12–19. ( 10.1016/j.ijpharm.2013.01.058) [DOI] [PubMed] [Google Scholar]
- 23.Yalkowsky SH, He Y, Jain P. 2010. Handbook of aqueous solubility data, 2nd edn Boca Raton, FL: CRC Press. [Google Scholar]
- 24.Valvani SC, Yalkowski SH, Roseman TJ. 1981. Solubility and partitioning IV: aqueous solubility and octanol-water partition coefficients of liquid nonelectrolytes. J. Pharm. Sci. 70, 502–507. ( 10.1002/jps.2600700510) [DOI] [PubMed] [Google Scholar]
- 25.Kanemaru K, Miyamoto T. 1990. Inhibitory effects on the growth of several bacteria by brown mustard and allyl isothiocyanate. J. Food Sci. Technol. 37, 823–829. ( 10.3136/nskkk1962.37.10_823) [DOI] [Google Scholar]
- 26.Delaquis P, Sholberg P. 1997. Antimicrobial activity of gaseous allyl isothiocyanate. J. Food Protect. 60, 943–947. ( 10.4315/0362-028X-60.8.943) [DOI] [PubMed] [Google Scholar]
- 27.Kyung KH, Fleming HP. 1997. Antimicrobial activity of sulfur compounds derived from cabbage. J. Food Protect. 60, 67–71. ( 10.4315/0362-028X-60.1.67) [DOI] [PubMed] [Google Scholar]
- 28.Lin CM, Kim J, Du WX, Wei CI. 2000. Bacterial activity of isothiocyanate against pathogens on fresh produce. J. Food Protect. 63, 25–30. ( 10.4315/0362-028X-63.1.25) [DOI] [PubMed] [Google Scholar]
- 29.Lin CM, Preston JF, Wei CI. 2000. Antibacterial mechanism of allyl isothiocyanate. J. Food Protect. 6, 703–838. ( 10.4315/0362-028x-63.6.727) [DOI] [PubMed] [Google Scholar]
- 30.Dumas J, Peligot E. 1834. Sur l'Huile de Cannelle, l'Acide Hippurique, et l'Acide Sébacique. Ann. Chim. Phys. 57, 305–334. [Google Scholar]
- 31.Boerjan W, Ralph J, Baucher M. 2003. Lignin biosynthesis. Annu. Rev. Plant Biol. 54, 519–546. ( 10.1146/annurev.arplant.54.031902.134938) [DOI] [PubMed] [Google Scholar]
- 32.Bowles BL, Miller AJ. 1993. Antibotulinal properties of selected aromatic and aliphatic aldehydes. J. Food Protect. 56, 788–794. ( 10.4315/0362-028X-56.9.788) [DOI] [PubMed] [Google Scholar]
- 33.Bowles BL, Sackitey SK, Williams AC. 1995. Inhibitory effects of flavor compounds on Staphylococcus aureus WRRC B124. J. Food Safety 15, 337–347. ( 10.1111/j.1745-4565.1995.tb00144.x) [DOI] [Google Scholar]
- 34.Helander IM, Alakomi HL, Latva-Kala K, Mattila-Sandholm T, Pol L, Smid EJ, Gorris LGM, von Wright A. 1998. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 46, 3590–3595. ( 10.1021/jf980154m) [DOI] [Google Scholar]
- 35.Morrison R, Gardiner C, Evidente A, Kiss R, Townley H. 2014. Incorporation of ophiobolin a into novel chemoembolization particles for cancer cell treatment. Pharmaceut. Res. 31, 2904–2917. ( 10.1007/s11095-014-1386-3) [DOI] [PubMed] [Google Scholar]
- 36.Voicu G, et al. 2015. Nanostructured mesoporous silica: new perspectives for fighting antimicrobial resistance. J. Nanopart. Res. 17, 201 ( 10.1007/s11051-015-3004-7) [DOI] [Google Scholar]
- 37.Janatova A, Bernardos A, Smid J, Frankova A, Lhotka M, Kourimská L, Pulkrabek J, Kloucek P. 2015. Long-term antifungal activity of volatile essential oil components released from mesoporous silica materials. Ind. Crop. Prod. 67, 216–220. ( 10.1016/j.indcrop.2015.01.019) [DOI] [Google Scholar]
- 38.Bernardos A, Marina T, Žáček P, Pérez-Esteve É, Martínez-Mañez R, Lhotka M, Kouřimská L, Pulkrábek J, Klouček P. 2014. Antifungal effect of essential oil components against Aspergillus niger when loaded into silica mesoporous supports. J. Sci. Food Agric. 95, 2824–2831. ( 10.1002/jsfa.7022) [DOI] [PubMed] [Google Scholar]
- 39.Abedon ST.1998. Lecture notes on microbiology (last accessed: 10th August 2016). See http://www.mansfield.ohio-state.edu/~sabedon/biol1080.htm .
- 40.Voet D, Voet JG, Pratt CW. 2001. Fundamentals of biochemistry. New York, NY: Wiley. [Google Scholar]
- 41.Campbell NA, Reece JB, Urry LA, Cain M, Wasserman SA, Minorsky PV, Jackson RB. 2008. Membrane structure and function biology, 8th edn Menlo Park, CA: Benjamin Cummings. [Google Scholar]
- 42.Eboigbodin KE, Biggs CA. 2008. Characterization of the extracellular polymeric substances produced by Escherichia coli using infrared spectroscopic proteomic, and aggregation studies. Biomacromolecules 9, 686–695. ( 10.1021/bm701043c) [DOI] [PubMed] [Google Scholar]
- 43.Mu H, Tang J, Liu Q, Sun C, Wang T, Duan J. 2016. Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Sci. Rep. 6, 18877 ( 10.1038/srep18877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duncan B, et al. 2015. Nanoparticle-stabilized capsules for the treatment of bacterial biofilms. ACS Nano 9, 7775–7785. ( 10.1021/acsnano.5b01696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dohare S, Dubey SD, Kalia M, Verma P, Pandey H, Singh NK, Agarwal V. 2014. Anti-biofilm activity of eucalyptus globulus oil encapsulated silica nanoparticles against E. coli biofilm. IJPSR 5, 5013–5018. [Google Scholar]
- 46.Sutherland IW. 2001. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147, 3–9. ( 10.1099/00221287-147-1-3) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.