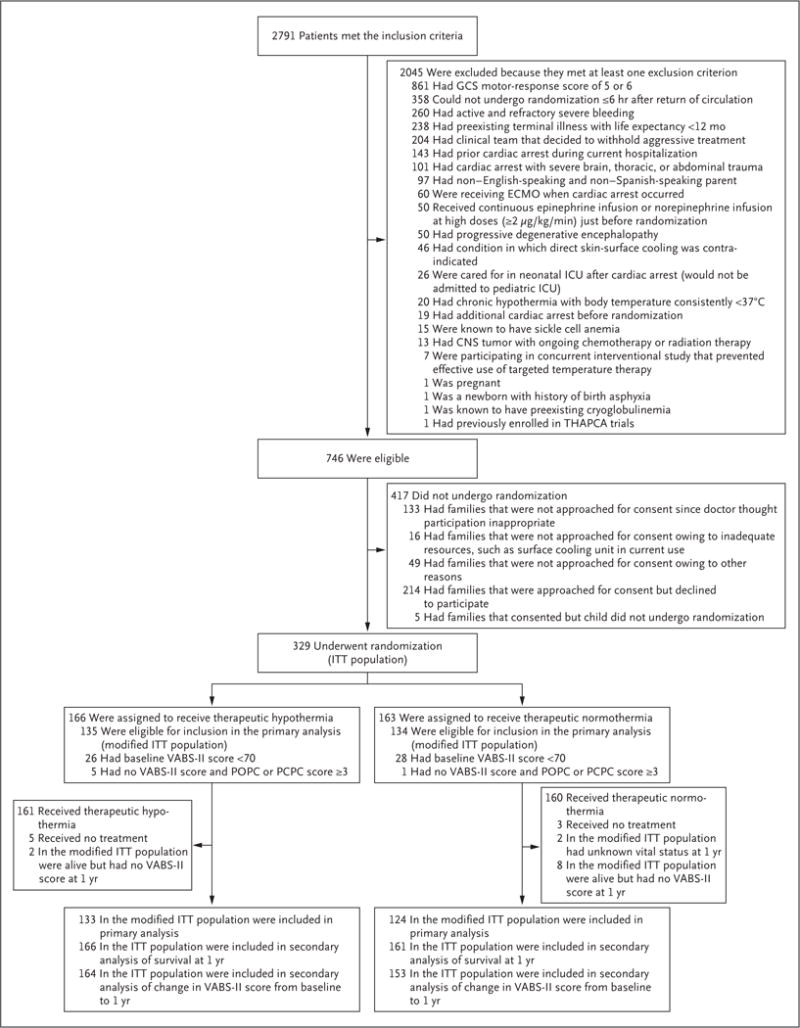
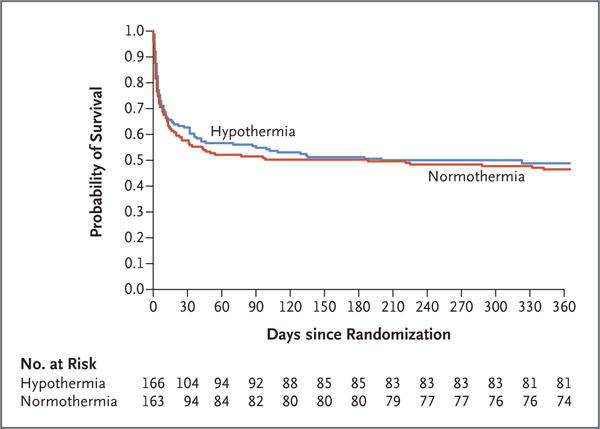
FW Moler
FW Moler
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
FS Silverstein
FS Silverstein
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
R Holubkov
R Holubkov
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
BS Slomine
BS Slomine
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
JR Christensen
JR Christensen
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
VM Nadkarni
VM Nadkarni
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
KL Meert
KL Meert
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
B Browning
B Browning
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
VL Pemberton
VL Pemberton
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
K Page
K Page
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
MR Gildea
MR Gildea
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
BR Scholefield
BR Scholefield
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
S Shankaran
S Shankaran
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
JS Hutchison
JS Hutchison
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
JT Berger
JT Berger
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
G Ofori-Amanfo
G Ofori-Amanfo
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
CJL Newth
CJL Newth
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
A Topjian
A Topjian
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
KS Bennett
KS Bennett
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
JD Koch
JD Koch
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
N Pham
N Pham
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
NK Chanani
NK Chanani
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
JA Pineda
JA Pineda
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
R Harrison
R Harrison
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
HJ Dalton
HJ Dalton
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
J Alten
J Alten
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
CL Schleien
CL Schleien
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
DM Goodman
DM Goodman
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
JJ Zimmerman
JJ Zimmerman
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
US Bhalala
US Bhalala
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
AJ Schwarz
AJ Schwarz
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
MB Porter
MB Porter
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
S Shah
S Shah
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
EL Fink
EL Fink
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
P McQuillen
P McQuillen
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
T Wu
T Wu
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
S Skellett
S Skellett
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
NJ Thomas
NJ Thomas
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
JE Nowak
JE Nowak
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
PB Baines
PB Baines
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
J Pappachan
J Pappachan
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
M Mathur
M Mathur
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
E Lloyd
E Lloyd
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
EW van der Jagt
EW van der Jagt
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
EL Dobyns
EL Dobyns
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
MT Meyer
MT Meyer
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
RC Sanders Jr
RC Sanders Jr
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
AE Clark
AE Clark
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1,
JM Dean
JM Dean
1The University of Michigan, Ann Arbor (F.W.M., F.S.S.), and Wayne State University, Detroit (K.L.M., S. Shankaran) — both in Michigan; University of Utah, Salt Lake City (R. Holubkov, B.B., K.P., M.R.G., K.S.B., A.E.C., J.M.D.); Kennedy Krieger Institute and Johns Hopkins University (B.S.S., J.R.C.) and Johns Hopkins Children’s Center (U.S.B.), Baltimore, and the National Heart, Lung, and Blood Institute, Bethesda (V.L.P.) — both in Maryland; Children’s Hospital of Philadelphia, Philadelphia (V.M.N., A.T.), University of Pittsburgh Medical Center, Pittsburgh (E.L.F.), and Penn State Children’s Hospital, Hershey (N.J.T.) — all in Pennsylvania; Birmingham Children’s Hospital, Birmingham (B.R.S.), Great Ormond Street Hospital, London (S. Skellett), Alder Hey Children’s Hospital, Liverpool (P.B.B.), and University Hospital Southampton, Southampton (J.P.) — all in the United Kingdom; Hospital for Sick Children, Toronto (J.S.H.); Children’s National Medical Center, Washington, DC (J.T.B.); Duke Children’s Hospital, Durham, NC (G.O.-A.); Children’s Hospital Los Angeles (C.J.L.N.) and Mattel Children’s Hospital UCLA (R. Harrison), Los Angeles, Children’s Hospital of Orange County, Orange (A.J.S.), University of California, San Francisco Benioff Children’s Hospital, San Francisco (P.M.), and Loma Linda University Children’s Hospital, Loma Linda (M.M.) — all in California; Children’s Medical Center Dallas, University of Texas Southwestern Medical School, Dallas (J.D.K.); University of Texas Health Sciences Center at San Antonio, San Antonio (T.W.); Children’s Healthcare of Atlanta, Atlanta (N.P., N.K.C.); Washington University, St. Louis (J.A.P.); Phoenix Children’s Hospital, Phoenix, AZ (H.J.D.); the Children’s Hospital of Alabama, Birmingham (J.A.); Children’s Hospital of New York, Columbia University Medical Center, New York (C.L.S.), and Golisano Children’s Hospital, University of Rochester Medical Center, Rochester (E.W.J.) — both in New York; Ann and Robert Lurie Children’s Hospital of Chicago, Chicago (D.M.G.); Seattle Children’s Hospital, Seattle (J.J.Z.); Kosair Children’s Hospital, University of Louisville, Louisville, KY (M.B.P.); University of Tennessee Health Science Center, Memphis (S. Shah); Children’s Hospital and Clinics of Minnesota, Minneapolis (J.E.N.); Nationwide Children’s Hospital, Columbus, OH (E.L.); Children’s Hospital Colorado, Aurora (E.L.D.); Medical College of Wisconsin, Milwaukee (M.T.M.); and Arkansas Children’s Hospital, Little Rock (R.C.S.)
1;
for the THAPCA Trial Investigators1,*