Abstract
Hemodialysis (HD) patients face increased fracture risk, which is further associated with elevated risk of hospitalization and mortality. High-resolution peripheral computed tomography (HR-pQCT) has advanced our understanding of bone disease in chronic kidney disease by characterizing distinct changes in both the cortical and trabecular compartments. Increased cortical porosity (Ct.Po) has been shown to be associated with fracture in patients with osteopenia or in postmenopausal diabetic women. We tested whether the degree of Ct.Po identifies hemodialysis patients with prevalent fragility fractures in comparison to bone mineral density (BMD) assessed by dual X-ray absorptiometry (DXA). We performed a post-hoc analysis of a cross-sectional study in 76 prevalent hemodialysis patients. Markers of mineral metabolism, coronary calcification score, DXA-, and HR-pQCT-data were analyzed, and Ct.Po determined at radius and tibia. Ct.Po was significantly higher in patients with fracture but association was lost after adjusting for age and gender (tibia p = 0.228, radius p = 0.5). Instead, femoral (F) BMD neck area (p = 0.03), F T-score neck area (p = 0.03), radius (R) BMD (p = 0.03), R T-score (p = 0.03), and cortical HR-pQCT indices such as cortical area (Ct.Ar) (tibia: p = 0.01; radius: p = 0.02) and cortical thickness (Ct.Th) (tibia: p = 0.03; radius: p = 0.02) correctly classified patients with fragility fractures. Area under receiver operating characteristic curves (AUC) for Ct.Po (tibia AUC: 0.711; p = 0.01; radius AUC: 0.666; p = 0.04), Ct.Ar (tibia AUC: 0.832; p<0.001; radius AUC: 0.796; p<0.001), and F neck BMD (AUC: 0.758; p = 0.002) did not differ significantly among each other. In conclusion, measuring Ct.Po is not superior to BMD determined by DXA for identification of HD patients with fragility fracture.
Introduction
Fracture risk in chronic kidney disease increases with declining renal function [1]. Moreover, a fracture in hemodialysis patients is associated with elevated risk of hospitalization and mortality [2]. Renal osteodystrophy affects the majority of patients with advanced chronic kidney disease (CKD) and is one feature of chronic kidney disease—mineral bone disorder (CKD-MBD) syndrome [3].
Chronic kidney disease patients suffer from decreased cortical and trabecular bone mineral density, cortical thinning, and disturbed trabecular microarchitecture [4–7]. High-resolution peripheral quantitative computed tomography (HR-pQCT) revealed predominant decreases in bone mineral density in the cortical compartment, decreased radial cortical area and cortical thickness, and elevations in cortical porosity of both tibia and radius [8, 9]. Changes in cortical porosity have been shown to have a significant impact on the resistance to fracture [10–13].
There are numerous studies, both retrospective and prospective, on the value of DXA on the assessment of fracture risk in CKD [14–21]. Jamal et al. [22] performed pQCT measurements of the radius in dialysis patients and reported decreased cortical density, cortical area, and cortical thickness of the radius to be associated with fractures. In a previous study by our group, HR-pQCT parameters at the tibia discriminated dialysis patients with fractures from those without fractures [4].
Although higher cortical porosity as a measure of deteriorated cortical bone architecture is well recognized in chronic kidney disease throughout all stages, the presumed clinical value of this parameter is less well defined in this patient population. Based on our hypothesis that fracture patients exhibit higher cortical porosity, we investigated whether measuring cortical porosity identifies patients with prevalent low-trauma fractures. We further compared this parameter to common cortical HR-pQCT and DXA-derived parameters for their ability to identify fragility fractures in a representative Mid-European dialysis patient cohort.
Materials and methods
The study protocol was approved by the ethics committee of the Medical University of Vienna and adhered to the Declaration of Helsinki. All hemodialysis patients from our department were invited to participate. Exclusion criteria were age under 18 and pregnancy. None of the participants were on antiresorptive bone medication. Written informed consent was obtained from all participating patients. We performed a post-hoc analysis of a cross-sectional study with 76 patients on maintenance hemodialysis who were recruited between 2008 and 2010. Results of subsets of this cohort have been published previously [4, 23]. Patient charts were reviewed for demographic and laboratory data and for history of low-trauma fractures of any age at the time of HR-pQCT measurement. Low-trauma resulting in fragility fractures was defined as a fall from standing-height or lower. In addition, lateral spine x-rays and chest x-rays were screened for previously unrecognized fractures at the time of HR-pQCT measurement [24]. Survival data until 2014 were retrieved from the Austrian Dialysis and Transplant Registry (ÖDTR) [25].
HR-pQCT
HR-pQCT was performed on all patients at both tibia and radius on an XtremeCT scanner from Scanco Medical, Switzerland, and analyzed as described previously [4, 26]. The tibia of the non-dominant leg and the radius of the arm that was either devoid of or carried a dysfunctional arteriovenous fistula were analyzed. Only apparently intact areas without history of fracture were used for the examination. 110 CT slices were obtained at each site, from which a 9-mm long 3D-volume was reconstructed. Image analysis was performed as outlined by Burghardt et al. (reviewed in [27]). Periosteal boundary and cortical and trabecular compartments were semi-automatically contoured as described [28]. A threshold-based algorithm and edge enhancement distinguished trabecular structures from cortical bone [29]. Calculation of parameters was performed as described [30]. Dcomp represents apparent cortical density in mg/cm3 hydroxyapatite (HA), Ct.Ar cortical area in mm2, Tb.Ar trabecular area in mm2, D100 average bone density in mg/cm3 HA of both cortical and trabecular compartment, Ct.Th cortical thickness (the mean cortical volume divided by the outer surface) in mm, Dtrab trabecular density in mg HA/cm3, BV/TV (%) bone volume fraction in % [100 x (Dtrab in mg HA/cm3)/1200 mg HA/cm3], TbN trabecular number per mm, TbTh trabecular thickness [(BV/TV)/TbN], and Tb1/NSD (mm) trabecular separation. Contouring of the cortical compartment for determination of cortical porosity was manually corrected when overt misalignment with the trabecular compartment occurred [31, 32]. Cortical porosity was calculated as ratio of cortical pore volume (empty space) to total cortical volume (sum of empty space and mineralized matrix).
Coronary calcium scoring
Assessment of calcium burden was performed on an ECG-gated 16-slice scanner (Siemens Somatom16, Siemens, Forchheim, Germany) [33]. All coronary artery calcification (CAC) data sets were analyzed by a single technician with more than 5 years of experience in cardiac CT imaging using a commercially available software package (“Syngo CaScore”; Siemens Healthcare, Forchheim, Germany). Patients with coronary artery stents were excluded from analysis as the stent graft would have yielded false-high calcification scores.
Dual-energy X-ray Absorptiometry (DXA)
Bone mineral density measurements (BMD) were performed with dual-energy X-ray absorptiometry on a QDR-4500 scanner (Hologic, Waltham, MA), using the manufacturer's recommended standard procedures [33].
Laboratory parameters
Bone alkaline phosphatase (bAP) was measured using an immunosorbent enzyme-linked assay (Metra Biosystems, Behring Diagnostic, Eschborn, Germany). C-telopeptide pyridinoline cross-links of type I collagen (CTX, CrossLaps) and 25-hydroxy-cholecalciferol (25(OH)D) were measured by electro-chemiluminescence (Modular and Elecsys Systems, Roche, Switzerland). Calcitriol (1,25(OH)2D) was analyzed after chromatographic separation by radioimmunoassay (DiaSorin). Parathyroid hormone (PTH) was measured using a second generation assay (Elecsys PTH intact, Roche, Switzerland). Calcium and phosphate were quantified by routine clinical chemistry analyzers (Olympus, Japan). All measurements were performed according to Good Laboratory Practice (GLP) standards at the Institute of Laboratory Medicine of the General Hospital in Vienna.
Statistics
All statistical computations were performed using IBM SPSS Statistics for Windows (Version 23). Metric data were described using mean and SD or as otherwise indicated. Spearman’s rank correlation was used to assess bivariate correlations between cortical porosity (separately for tibia and radius) and age in addition to coronary calcium score. Point biserial correlations were used for the correlation between cortical porosity and fracture (yes/no) as well as mortality. In case of significant results associations were adjusted for age and sex using either multiple binary logistic regression (for fractures and mortality) or multiple linear regression (for coronary calcification). We performed factor analysis to avoid multiple testing and multiplicity corrections in a cohort of limited sample size and of in large parts highly correlated parameters. Receiver operating characteristic (ROC) analyses and subsequent De-Long testing were performed to compare area under the curves (AUC) of indicated parameters to discriminate patients with and without fractures. A p-value equal or below 0.05 was assumed to indicate significant results.
Radius Ct.Po could not be analyzed in one patient due to insufficient quality of acquisition data. One patient’s set of measurements of the tibia had to be removed because of preceding paraplegia for many years due to a childhood accident. Coronary calcium burden was unavailable in 15 patients, and DXA BMD measurements of the radius were available only in a subset of 51 patients. Therefore, we excluded radius BMD measurements in the ROC analysis.
Results
Demographic baseline data stratified according to fracture status are shown in Table 1. Patients with fractures were older with higher female proportion, and also had lower serum phosphate levels. 22 patients (29%) with current or history of low-trauma fracture were observed at the time of analysis. We observed 3 rib fractures, 5 femoral neck fractures, and 19 vertebral fractures; 5 patients had 2 documented fractures. We did not observe significant between group differences with respect to diabetes, PTH, history of corticoid use, use of active Vitamin D medication, or Cinacalcet.
Table 1. Patient demographic data.
| No Fracture | Fracture | p | |
|---|---|---|---|
| N | 54 | 22 | |
| Age (years median) | 55 ± 12.7 | 71 ± 12.7 | <0.001 |
| Sex F/M | 17/37 | 15/7 | 0.005 |
| BMI (kg/m2 median) (75) | 25.3 (22.8–27.9) | 22.6 (20.1–26.5) | 0.08 |
| Dialysis (months median) (72) | 33.8 (12.2–69.4) | 50.5 (14.7–65) | 0.55 |
| bAP (ng/mL median) (62) | 24.1 (15.1–30.1) | 24.1 (15–35.5) | 0.84 |
| PTH (pg/mL median) (74) | 206 (158–339) | 217 (87–346) | 0.51 |
| Calcium (mmol/L) (75) | 2.22 ± 0.16 | 2.24 ± 0.15 | 0.75 |
| Phosphate (mmol/L) (75) | 1.9 ± 0.42 | 1.68 ± 0.28 | 0.01 |
| CTX (ng/mL median) (49) | 1.89 (1.35–2.76) | 1.72 (1.3–2.1) | 0.33 |
| 25(OH)D (nmol/L median) (72) | 38.1 (32.1–50.8) | 36 (23.4–42.4) | 0.33 |
| 1,25(OH)2D (pg/mL median) (73) | 9.6 (6.7–15.8) | 13.9 (8.4–17.5) | 0.24 |
| Vitamin K antagonist (%) (72) | 1.9 | 5 | 0.481 |
| Cinacalcet (%) | 13 | 22.7 | 0.312 |
| Smoker (%) (65) | 30.4 | 10.5 | 0.119 |
| Diabetes (%) (75) | 33.3 | 28.6 | 0.787 |
| History of glucocorticoid use (%) (75) | 29.6 | 14.3 | 0.241 |
| Active Vitamin D medication (%) (75) | 49.1 | 59.1 | 0.458 |
| CT-scan coronary calcification (%) (61) | 69.6 | 80 | 0.524 |
| Hip fractures (%) (70) | 0 | 22.7 | n.a |
| Deceased by the time of data analysis (%) | 29.6 | 72.7 | 0.001 |
bAP: bone specific alkaline phosphatase; PTH: parathyroid hormone; CTX: C-terminal telopeptide; BMI: body mass index; 25(OH)D: 25-hydroxy-Vitamin D3; 1,25(OH)2D: 1, 25-hydroxy-Vitamin D3; shown are mean and standard deviation or median and interquartile range (25/75). Numbers in parentheses indicate sample number if different from total N. Reference ranges: bAP—men: 6–30 ng/mL and women: 6–26 ng/mL; PTH—15–65 pg/mL; CTX—men: 0.08–0.35 ng/mL and women: 0.09–0.44 ng/mL; 25(OH)D—28–107 nmol/L; 1,25(OH)2D—25–66 pg/mL; calcium—2.2–2.65 mmol/L; phosphate: 0.91–1.45 mmol/L.
Two thirds of the patients had documented coronary artery calcification of varying severity. Although there was no association of the degree of coronary calcification with fractures, we noted some univariate correlation of Ct.Po with coronary calcification score (tibia: r = 0.342, p = 0.007; radius: r = 0.366, p = 0.004) that did not persist after adjusting for age and gender in logistic regression (tibia: p = 0.98; radius: p = 0.3). By the time of data analysis approximately twice as many patients had died in the fracture group which is also reflected by positive associations of Ct.Po with mortality in unadjusted (tibia: r = 0.318, p = 0.005; radius: r = 0.31, p = 0.007), but not in adjusted analysis (tibia: p = 0.7; radius: p = 0.37).
Table 2 shows a selection of cortical and trabecular parameters derived from peripheral HR-pQCT analysis and bone density measurements from dual energy X-ray absorptiometry stratified according to fracture status and sex. There was a trend to higher Ct.Po in men (tibia: r = 0.159, p = 0.17; radius: r = 0.182, p = 0.12) and higher values were observed in patients with fracture compared to patients without fractures in both genders. Fig 1 shows representative images of tibial (A) and radial (B) bone in a dialysis patient with high cortical porosity.
Table 2. Patient HR-pQCT morphometric data stratified according to fracture status and gender.
| No fracture | Fracture | |||
|---|---|---|---|---|
| Tibia | Female | Male | Female | Male |
| D100 (mg/cm3) (75) | 248 ± 57.3 | 250 ± 60 | 180 ± 45.7 | 239 ± 83.7 |
| Tb.Ar (mm2) (75) | 554 ± 108 | 718 ± 112 | 597 ± 118 | 734 ± 224 |
| Dtrab (mg HA/cm3) (75) | 133 ± 40.9 | 146 ± 43.3 | 108 ± 40.6 | 151 ± 29.5 |
| tTbN (1/mm) (75) | 1.53 ± 0.371 | 1.82 ± 0.461 | 1.35 ± 0.428 | 1.85 ± 0.307 |
| tTb.Sp (mm) (75) | 0.62 ± 0.181 | 0.505 ± 0.142 | 0.688 ± 0.218 | 0.483 ± 0.082 |
| Tb1/NSD (mm) (75) | 0.331 ± 0.163 | 0.252 ± 0.114 | 0.427 ± 0.28 | 0.229 ± 0.057 |
| tTb.Th (mm) (75) | 0.072 ± 0.012 | 0.067 ± 0.016 | 0.069 ± 0.024 | 0.069 ± 0.012 |
| Ct.Ar (mm2) (75) | 94.8 ± 21.7 | 119 ± 32.6 | 58.6 ± 26 | 87.6 ± 47.6 |
| Dcomp (mg HA/cm3) (75) | 834 ± 71 | 804 ± 65.4 | 712 ± 103 | 726 ± 125 |
| Ct.Th (mm) (75) | 0.951 ± 0.259 | 1.04 ± 0.297 | 0.58 ± 0.269 | 0.794 ± 0.501 |
| tBV/TV (%) (75) | 11.1 ± 3.4 | 12.2 ± 3.6 | 9 ± 3.4 | 12.6 ± 2.5 |
| Ct.Po (%) (75) | 5.53 ± 3.26 | 8.46 ± 3.24 | 9.73 ± 4.04 | 10.6 ± 2.98 |
| Radius | ||||
| D100 (mg/cm3) | 279 ± 82.6 | 279 ± 93.4 | 217 ± 56.2 | 265 ± 72.6 |
| Tb.Ar (mm2) | 195 ± 47 | 282 ± 74.1 | 201 ± 42.3 | 272 ± 52.1 |
| Dtrab (mg HA/cm3) | 114 ± 45.8 | 148 ± 50.6 | 101 ± 50.3 | 152 ± 39.7 |
| tTbN (/mm) | 1.59 ± 0.357 | 1.87 ± 0.389 | 1.36 ± 0.523 | 1.71 ± 0.789 |
| tTb.Sp (mm) (75) | 0.599 ± 0.151 | 0.487 ± 0.146 | 0.692 ± 0.262 | 0.452 ± 0.099 |
| Tb1/NSD (mm) (75) | 0.283 ± 0.102 | 0.254 ± 0.164 | 0.438 ± 0.252 | 0.235 ± 0.125 |
| tTb.Th (mm) | 0.058 ± 0.012 | 0.065 ± 0.016 | 0.061 ± 0.014 | 0.063 ± 0.009 |
| Ct.Ar (mm2) | 46.8 ± 14.8 | 54.3 ± 20.1 | 30.3 ± 9.33 | 44.2 ± 17.9 |
| Dcomp (mg HA/cm3) | 845 ± 84.7 | 798 ± 96.5 | 748 ± 98.1 | 760 ± 104 |
| Ct.Th (mm) | 0.721 ± 0.25 | 0.686 ± 0.287 | 0.466 ± 0.168 | 0.554 ± 0.24 |
| tBV/TV (%) | 9.5 ± 3.8 | 12.3 ± 4.2 | 8.2 ± 4.2 | 12.7 ± 3.3 |
| Ct.Po (%) (75) | 2.02 ±1.21 | 3.61 ± 2.38 | 4.26 ± 2.75 | 4.72 ± 2.3 |
| DXA | ||||
| F BMD total (g/cm2) (70) | 0.803 ± 0.151 | 0.856 ± 0.165 | 0.664 ± 0.128 | 0.772 ± 0.109 |
| F T total (69) | -1.15 ± 1.23 | -1.17 ± 1.13 | -2.29 ± 1.05 | -1.75 ± 0.734 |
| F BMD neck (g/cm2) (71) | 0.682 ± 0.148 | 0.697 ± 0.157 | 0.557 ±0.094 | 0.572 ± 0.095 |
| F T neck (71) | -1.5 ± 1.33 | -1.76 ± 1.21 | -2.63 ± 0.854 | -2.65 ± 0.718 |
| LS BMD (g/cm2) (69) | 0.902 ± 0.148 | 0.993 ± 0.172 | 0.85 ± 0.164 | 0.968 ± 0.191 |
| LS T (69) | -1.37 ± 1.46 | -0.897 ± 1.56 | -1.79 ± 1.48 | -1.12 ± 1.76 |
| R BMD (g/cm2) (51) | 0.47 ± 0.062 | 0.545 ± 0.094 | 0.365 ± 0.064 | 0.475 ± 0.086 |
| R T (51) | -2.02 ± 1.16 | -2.73 ± 1.8 | -3.94 ± 1.22 | -4.1 ± 1.64 |
Ct.Ar: cortical area; Tb.Ar: trabecular area; Dtrab: trabecular density; TbN: trabecular number; tTb.Sp: trabecular separation; Tb1/NSD: standard deviation of 1/Tb.N; tTb.Th: trabecular thickness; D100: average bone density; Dcomp: apparent cortical bone density; Ct.Th: cortical thickness; tBV/TV: trabecular bone volume/tissue volume; Ct.Po: cortical porosity; BMD: bone mineral density; FN: femoral neck; LS: lumbar spine; R: radius; T: T-score. Numbers in parentheses indicate sample number if different from total N.
Fig 1. HR-pQCT sections of tibia and radius.
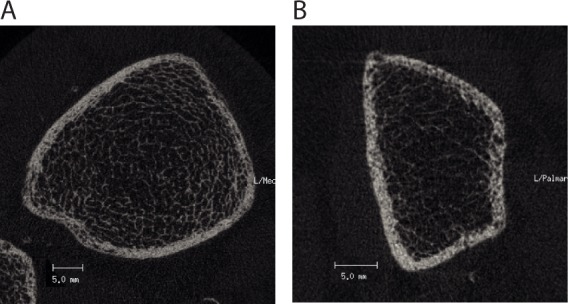
Representative images of tibia (A) and radius (B) from hemodialysis patients with high cortical porosity. (A): tBV/TV (%): 14.6, Ct.Po (%): 11.54; (B): tBV/TV (%): 7.7, Ct.Po (%): 11.49.
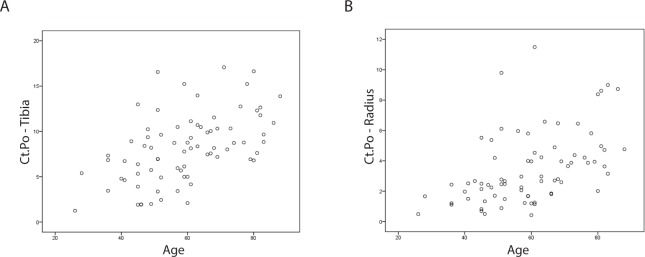
There was a strong correlation of Ct.Po with age (tibia: r = 0.524, p<0.001; radius: r = 0.541, p<0.001) (Fig 2A and 2B). We observed associations of Ct.Po with the presence of documented fractures at both sites in unadjusted analysis. However, this association did not persist after adjusting for age and gender in logistic regression analysis (Table 3).
Fig 2.
Association of Ct.Po and age in hemodialysis patients in the tibia (A) and radius (B).
Table 3. Presence of fracture discriminated by DXA and cortical HR-pQCT parameters.
| Beta | p-value | Beta* | p-value* | |
|---|---|---|---|---|
| Tibia | ||||
| Ct.Po | 0.193 | 0.011 | 0.126 | 0.228 |
| Cr.Ar | -0.042 | <0.001 | -0.028 | 0.013 |
| D100 | -0.014 | 0.020 | -0.014 | 0.020 |
| Dcomp | -0.013 | <0.001 | -0.009 | 0.030 |
| Ct.Th | -3.714 | <0.001 | -2.380 | 0.027 |
| Radius | ||||
| Ct.Po | 0.221 | 0.041 | 0.112 | 0.500 |
| Cr.Ar | -0.067 | 0.001 | -0.053 | 0.023 |
| D100 | -0.008 | 0.034 | -0.009 | 0.061 |
| Dcomp | -0.007 | 0.019 | -0.005 | 0.161 |
| Ct.Th | -3.653 | 0.005 | -3.695 | 0.017 |
| DXA | ||||
| F total BMD | -5.417 | 0.007 | -4.781 | 0.063 |
| F total T | -0.657 | 0.012 | -0.621 | 0.060 |
| F neck BMD | -7.298 | 0.008 | -6.643 | 0.032 |
| F neck T | -0.746 | 0.032 | -0.740 | 0.032 |
| LS BMD | -3.031 | 0.096 | -3.332 | 0.162 |
| LS T | -0.254 | 0.192 | -0.328 | 0.187 |
| R BMD | -16.112 | 0.001 | -14.037 | 0.029 |
| R T | -0.622 | 0.005 | -0.708 | 0.033 |
Significant parameters in the unadjusted model were used in a multiple regression model adjusted for age and gender; Ct.Po: cortical porosity; Ct.Ar: cortical area; D100: average bone density; Dcomp: apparent cortical bone density; Ct.Th: cortical thickness; BMD: bone mineral density; F: femur; LS: lumbar spine; R: radius; T: T-score.
*…adjusted model for age and gender.
To relate these findings to the performance of other DXA- and HR-pQCT-based parameters, we analyzed DXA-derived BMD data and cortical HR-pQCT parameters. Dcomp, Ct.Ar, D100, and Ct.Th of tibia and Ct.Ar and Ct.Th of radius and F BMD neck, F T neck, R BMD, and R T remained significantly associated with low-trauma fractures after adjustment for age and gender (Table 3).
As Ct.Po lost its association in adjusted analysis, we performed a post-hoc power analysis. Based on the distribution of patients with and without fracture in our cohort, a total sample size of 1532 (no fracture: 1021; fracture: 511) would be required to reach statistically significant differences for Ct.Po at the tibia (power: 80%, p<0.05, two-sided) between these two groups. For Ct.Po at the radius approximately 55000 patients would be necessary.
To assess sensitivity-specificity profiles of the above tested parameters, we calculated receiver-operating-characteristic (ROC) curves (Table 4). All tested parameters except lumbar spine (LS) BMD and LS T showed areas under the curve (AUC) which significantly differed from the line of equality (AUC of 0.5). None of the analyzed indices including Ct.Po differed significantly from the AUC of BMD in the femoral neck region (Fig 3 and Table 4).
Table 4. ROC areas under the curve (AUC) for HR-pQCT parameters including Ct.Po.
| AUC | p-value* | |
|---|---|---|
| Tibia | ||
| Ct.Po | 0.711 | 0.010 |
| Cr.Ar | 0.832 | <0.001 |
| Ct.Th | 0.797 | <0.000 |
| D100 | 0.735 | 0.004 |
| Dcomp | 0.791 | <0.001 |
| Radius | ||
| Ct.Po | 0.666 | 0.043 |
| Cr.Ar | 0.796 | <0.001 |
| Ct.Th | 0.749 | 0.002 |
| D100 | 0.683 | 0.026 |
| Dcomp | 0.697 | 0.017 |
| DXA | ||
| F total BMD | 0.749 | 0.002 |
| F total T | 0.734 | 0.004 |
| F neck BMD | 0.758 | 0.002 |
| F neck T | 0.732 | 0.005 |
| LS BMD | 0.649 | 0.070 |
| LS T | 0.606 | 0.196 |
*…vs. line of equality (AUC = 0.5); ROC: receiver-operating-characteristic; Ct.Po: cortical porosity; Ct.Ar: cortical area; D100: average bone density; Dcomp: apparent cortical bone density; Ct.Th: cortical thickness; BMD: bone mineral density; F: femur; LS: lumbar spine; T: T-score.
Fig 3. ROC curves: F neck BMD, Ct.Ar, and Ct.Po of tibia and radius discriminating fracture.
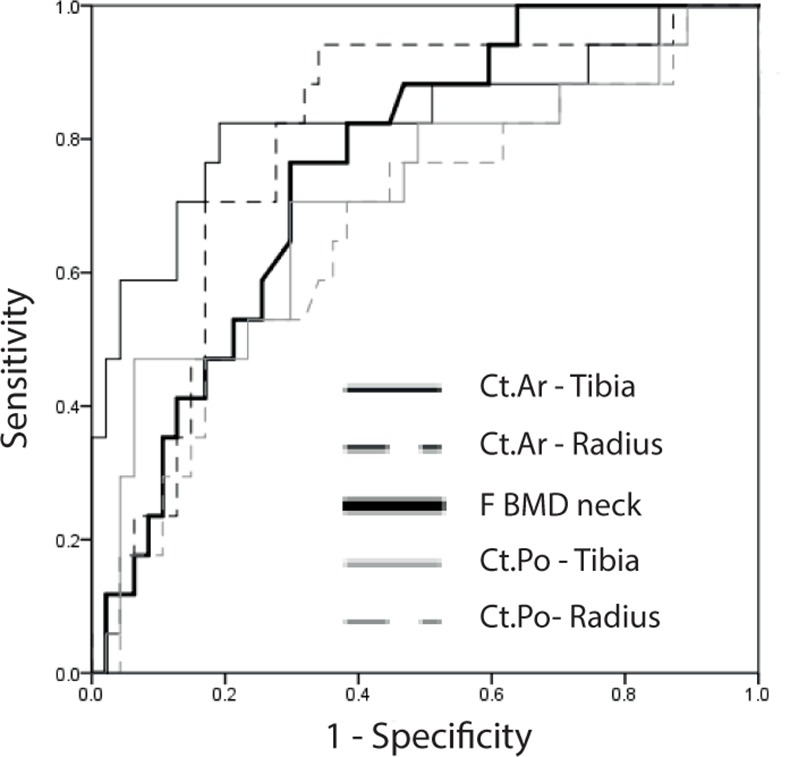
Higher area under the curve (AUC) indicates a more favorable sensitivity-specificity profile. The ROC curves of Ct.Po (radius and tibia) are compared to the ROC curves with the highest AUC among HR-pQCT parameters (Ct.Ar of radius and tibia) and densitometric parameters (F neck BMD). For exact AUC values see Table 4; De-Long comparisons of ROC curves: Tibia: Ct.Po vs. Ct.Ar: p = 0.19; Ct.Po vs. F BMD neck: p = 0.6; Ct.Ar vs. F BMD neck: p = 0.27; Radius: Ct.Po vs. Ct.Ar: p = 0.2; Ct.Po vs. F BMD neck: p = 0.34; Ct.Ar vs. F BMD neck: p = 0.59; ROC: receiver-operating-characteristic; Ct.Po: cortical porosity; Ct.Ar: cortical area; BMD: bone mineral density; F: femur.
Discussion
To the best of our knowledge, this is the first study to investigate the association between cortical porosity and fractures in patients with dialysis-dependent end-stage renal disease and to subsequently relate this parameter to other HR-pQCT indices and DXA parameters. We found that Ct.Po is higher in dialysis patients with prevalent fragility fractures as indicated by univariate correlation. However, significance was lost after adjusting for significant confounders. Rather, cortical area and other cortical HR-pQCT- and DXA-derived parameters discriminated HD patients with low-trauma fractures in the adjusted analysis.
The lack of a direct association between Ct.Po and fracture status is in contrast to findings reported in other patient populations: Ct.Po has been shown to identify postmenopausal diabetic women with fragility fractures [12]. In another study Ct.Po discriminated women with fractures but only in the subgroup with osteopenia and not in the group with overt osteoporosis [10]. This might indicate that increased Ct.Po exerts a detrimental effect on cortical bone strength before substantial decreases in bone mass occur [13]. However, in patients with severely reduced bone mass with cortical thinning such as the dialysis population studied here, the relative contribution of Ct.Po to mechanical stability of bone might become smaller. In addition, it must be recognized that the pathophysiology of renal osteodystrophy as part of CKD-MBD syndrome, which also significantly influences bone quality, differs substantially from osteopenia and osteoporosis [34].
We confirm previous reports from non-CKD cohorts by finding a high association between cortical porosity and age in dialysis patients as well [32, 35, 36]. We also observed some significant associations between Ct.Po with CAC and mortality in univariate analyses, which were most likely mediated by age as they did not prevail in multivariate analysis. However, these analyses were exploratory in nature; larger studies would be required to ascertain as to whether Ct.Po associates with CAC or mortality independently of age.
HD patients with fracture are at substantially increased risk for adverse outcomes as described in a recent large observational study [2]. Not only is the overall fracture rate higher in HD patients compared to the general population, but also rates of death and re-hospitalization increase after a fracture event [2]. Clinically, DXA is currently the most commonly used technique to determine BMD and assess fracture risk in osteoporosis patients. There is some debate as to the role of DXA in CKD, HD, and renal transplant patients in assessing fracture risk, which has been addressed by a substantial number of studies [14–16, 18, 20].
BMD was prospectively shown to predict fractures in females with low parathyroid hormone (PTH); it also discriminated prevalent spine fractures in HD patients [17]. According to a prospective study of CKD patients not on dialysis both BMD and HR-pQCT indices indicated risk for future fracture [19]. This is also consistent with findings from a large cohort of elderly participants with and without CKD [21].
There is considerable interest in a potential diagnostic role of HR-pQCT in renal osteodystrophy [7–9, 22, 37]. We have previously shown that HR-pQCT parameters of the tibia in this prevalent hemodialysis cohort discriminate HD patients with fragility fractures [4]. Interestingly, Ct.Th at the radius reached only borderline significance (p = 0.06) in the preceding report. In this cohort, which contains additional patients and fracture data, we observed significant correlations of Ct.Ar and Ct.Th with fracture status at both sites (Table 3).
Our study has limitations. A significant number of patients suffered from long-standing renal-insufficiency and secondary hyperparathyroidism. We did not perform bone-biopsies that could further delineate the turnover-state of the bones, which likely has major impact on cortical bone architecture over time. However, as this information is rarely available in clinical routine, our findings are well applicable in an unselected dialysis population of the kind under study.
Cortical thinning as well as changes in the trabecular compartment might result in unusual anatomic circumstances at the endocortical border that are not found to this extent in other populations studied. We performed an automated cortex-contouring process and minimized manual intervention, except when overt misalignments occurred (inner contour lines falsely extending far into trabecular bone). However, endocortical bone resorption and increased porosity close to the endocortical area might lead to underestimation of Ct.Po when the original endocortical border is no longer clearly identified.
We do not have information regarding when the fracture occurred in relation to the time of HR-pQCT measurement when fracture data were collected. As we screened all available radiographic material of the study population, a significant proportion of patients were likely unaware of the fracture or when it was acquired. Owing to this and the retrospective nature of this study, our study precludes any conclusion as to whether increased cortical porosity was causative for the observed fractures. However, although measurement of Ct.Po did not outperform conventional densitometry, our data are well in agreement of an important physiological role of Ct.Po in fracture resistance.
Although our post-hoc power analysis showed that a far greater patient number would be necessary to demonstrate possible associations of Ct.Po with low-trauma fracture, sample size was large enough to readily confirm such associations with F BMD neck, Ct.Ar, and Ct.Th. This argues against a superior clinical role for Ct.Po in fracture discrimination in hemodialysis patients. This is further corroborated by the ROC analysis, where we assessed how Ct.Po, cortical HR-pQCT, and DXA-derived parameters compare in their ability to correctly classify fragility fractures in HD patients. We observed only small differences between AUCs of Ct.Po and F BMD neck that did not reach statistical significance and are also unlikely to reflect clinical relevance. It merits further study whether restricting HR-pQCT/Ct.Po analysis to patients of a certain age range or according to clinical pre-assessment of fracture risk would alter these results.
In conclusion, cortical porosity of the tibia or radius assessed by HR-pQCT is of no added value in comparison to BMD measurement by DXA for identification of prevalent HD patients with fragility fracture.
Supporting Information
(XLSX)
Acknowledgments
We thank Prof. R. Kramar for providing clinical outcome data through the Austrian Dialysis and Transplant Registry.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Naylor KL, McArthur E, Leslie WD, Fraser LA, Jamal SA, Cadarette SM, et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. 2014;86(4):810–8. 10.1038/ki.2013.547 [DOI] [PubMed] [Google Scholar]
- 2.Tentori F, McCullough K, Kilpatrick RD, Bradbury BD, Robinson BM, Kerr PG, et al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2014;85(1):166–73. PubMed Central PMCID: PMCPMC3910091. 10.1038/ki.2013.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes CKDMBDWG. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;(113):S1–130. 10.1038/ki.2009.188 [DOI] [PubMed] [Google Scholar]
- 4.Cejka D, Patsch JM, Weber M, Diarra D, Riegersperger M, Kikic Z, et al. Bone microarchitecture in hemodialysis patients assessed by HR-pQCT. Clin J Am Soc Nephrol. 2011;6(9):2264–71. PubMed Central PMCID: PMCPMC3358993. 10.2215/CJN.09711010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamal S, Cheung AM, West S, Lok C. Bone mineral density by DXA and HR pQCT can discriminate fracture status in men and women with stages 3 to 5 chronic kidney disease. Osteoporos Int. 2012;23(12):2805–13. 10.1007/s00198-012-1908-y [DOI] [PubMed] [Google Scholar]
- 6.Nickolas TL, Cremers S, Zhang A, Thomas V, Stein E, Cohen A, et al. Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol. 2011;22(8):1560–72. PubMed Central PMCID: PMCPMC3148711. 10.1681/ASN.2010121275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nickolas TL, Stein E, Cohen A, Thomas V, Staron RB, McMahon DJ, et al. Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol. 2010;21(8):1371–80. PubMed Central PMCID: PMCPMC2938588. 10.1681/ASN.2009121208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickolas TL, Stein EM, Dworakowski E, Nishiyama KK, Komandah-Kosseh M, Zhang CA, et al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res. 2013;28(8):1811–20. PubMed Central PMCID: PMCPMC3720694. 10.1002/jbmr.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trombetti A, Stoermann C, Chevalley T, Van Rietbergen B, Herrmann FR, Martin PY, et al. Alterations of bone microstructure and strength in end-stage renal failure. Osteoporos Int. 2013;24(5):1721–32. 10.1007/s00198-012-2133-4 [DOI] [PubMed] [Google Scholar]
- 10.Bala Y, Zebaze R, Ghasem-Zadeh A, Atkinson EJ, Iuliano S, Peterson JM, et al. Cortical porosity identifies women with osteopenia at increased risk for forearm fractures. J Bone Miner Res. 2014;29(6):1356–62. PubMed Central PMCID: PMCPMC4156822. 10.1002/jbmr.2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer L, Valentinitsch A, DiFranco MD, Schueller-Weidekamm C, Kienzl D, Resch H, et al. High-resolution peripheral quantitative CT imaging: Cortical porosity, poor trabecular bone microarchitecture, and low bone strength in lung transplant recipients. Radiology. 2015;274(2):473–81. 10.1148/radiol.14140201 [DOI] [PubMed] [Google Scholar]
- 12.Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013;28(2):313–24. PubMed Central PMCID: PMCPMC3534818. 10.1002/jbmr.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeni YN, Brown CU, Wang Z, Norman TL. The influence of bone morphology on fracture toughness of the human femur and tibia. Bone. 1997;21(5):453–9. [DOI] [PubMed] [Google Scholar]
- 14.Akaberi S, Simonsen O, Lindergard B, Nyberg G. Can DXA predict fractures in renal transplant patients? Am J Transplant. 2008;8(12):2647–51. 10.1111/j.1600-6143.2008.02423.x [DOI] [PubMed] [Google Scholar]
- 15.Atsumi K, Kushida K, Yamazaki K, Shimizu S, Ohmura A, Inoue T. Risk factors for vertebral fractures in renal osteodystrophy. Am J Kidney Dis. 1999;33(2):287–93. [DOI] [PubMed] [Google Scholar]
- 16.Grotz WH, Mundinger FA, Gugel B, Exner V, Kirste G, Schollmeyer PJ. Bone fracture and osteodensitometry with dual energy X-ray absorptiometry in kidney transplant recipients. Transplantation. 1994;58(8):912–5. [DOI] [PubMed] [Google Scholar]
- 17.Iimori S, Mori Y, Akita W, Kuyama T, Takada S, Asai T, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—a single-center cohort study. Nephrol Dial Transplant. 2012;27(1):345–51. 10.1093/ndt/gfr317 [DOI] [PubMed] [Google Scholar]
- 18.Jamal SA, Chase C, Goh YI, Richardson R, Hawker GA. Bone density and heel ultrasound testing do not identify patients with dialysis-dependent renal failure who have had fractures. Am J Kidney Dis. 2002;39(4):843–9. 10.1053/ajkd.2002.32006 [DOI] [PubMed] [Google Scholar]
- 19.West SL, Lok CE, Langsetmo L, Cheung AM, Szabo E, Pearce D, et al. Bone mineral density predicts fractures in chronic kidney disease. J Bone Miner Res. 2015;30(5):913–9. 10.1002/jbmr.2406 [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi T, Kanno E, Tsubota J, Shiomi T, Nakai M, Hattori S. Retrospective study on the usefulness of radius and lumbar bone density in the separation of hemodialysis patients with fractures from those without fractures. Bone. 1996;19(5):549–55. [DOI] [PubMed] [Google Scholar]
- 21.Yenchek RH, Ix JH, Shlipak MG, Bauer DC, Rianon NJ, Kritchevsky SB, et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7(7):1130–6. PubMed Central PMCID: PMCPMC3386677. 10.2215/CJN.12871211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamal SA, Gilbert J, Gordon C, Bauer DC. Cortical pQCT measures are associated with fractures in dialysis patients. J Bone Miner Res. 2006;21(4):543–8. 10.1359/jbmr.060105 [DOI] [PubMed] [Google Scholar]
- 23.Cejka D, Jager-Lansky A, Kieweg H, Weber M, Bieglmayer C, Haider DG, et al. Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrol Dial Transplant. 2012;27(1):226–30. 10.1093/ndt/gfr270 [DOI] [PubMed] [Google Scholar]
- 24.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–48. 10.1002/jbmr.5650080915 [DOI] [PubMed] [Google Scholar]
- 25.Prischl FC, Auinger M, Saemann M, Mayer G, Rosenkranz AR, Wallner M, et al. Diabetes-related end-stage renal disease in Austria 1965–2013. Nephrol Dial Transplant. 2015;30(11):1920–7. 10.1093/ndt/gfv113 [DOI] [PubMed] [Google Scholar]
- 26.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90(12):6508–15. 10.1210/jc.2005-1258 [DOI] [PubMed] [Google Scholar]
- 27.Burghardt AJ, Link TM, Majumdar S. High-resolution computed tomography for clinical imaging of bone microarchitecture. Clin Orthop Relat Res. 2011;469(8):2179–93. PubMed Central PMCID: PMCPMC3126972. 10.1007/s11999-010-1766-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis KA, Burghardt AJ, Link TM, Majumdar S. The effects of geometric and threshold definitions on cortical bone metrics assessed by in vivo high-resolution peripheral quantitative computed tomography. Calcif Tissue Int. 2007;81(5):364–71. 10.1007/s00223-007-9076-3 [DOI] [PubMed] [Google Scholar]
- 29.Laib A, Ruegsegger P. Comparison of structure extraction methods for in vivo trabecular bone measurements. Comput Med Imaging Graph. 1999;23(2):69–74. [DOI] [PubMed] [Google Scholar]
- 30.Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6(5–6):329–37. [PubMed] [Google Scholar]
- 31.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–28. PubMed Central PMCID: PMCPMC2926164. 10.1016/j.bone.2010.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res. 2010;25(5):983–93. PubMed Central PMCID: PMCPMC3153365. 10.1359/jbmr.091104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cejka D, Weber M, Diarra D, Reiter T, Kainberger F, Haas M. Inverse association between bone microarchitecture assessed by HR-pQCT and coronary artery calcification in patients with end-stage renal disease. Bone. 2014;64:33–8. 10.1016/j.bone.2014.03.048 [DOI] [PubMed] [Google Scholar]
- 34.Malluche HH, Porter DS, Monier-Faugere MC, Mawad H, Pienkowski D. Differences in bone quality in low- and high-turnover renal osteodystrophy. J Am Soc Nephrol. 2012;23(3):525–32. PubMed Central PMCID: PMCPMC3294305. 10.1681/ASN.2010121253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shanbhogue VV, Brixen K, Hansen S. Age- and Sex-Related Changes in Bone Microarchitecture and Estimated Strength. A Three-Year Prospective Study Using HR-pQCT. J Bone Miner Res. 2016. [DOI] [PubMed] [Google Scholar]
- 36.Vilayphiou N, Boutroy S, Sornay-Rendu E, Van Rietbergen B, Chapurlat R. Age-related changes in bone strength from HR-pQCT derived microarchitectural parameters with an emphasis on the role of cortical porosity. Bone. 2016;83:233–40. 10.1016/j.bone.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 37.Nishiyama KK, Pauchard Y, Nikkel LE, Iyer S, Zhang C, McMahon DJ, et al. Longitudinal HR-pQCT and image registration detects endocortical bone loss in kidney transplantation patients. J Bone Miner Res. 2015;30(3):554–61. 10.1002/jbmr.2358 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.