Abstract
Introduction
Concerns have been raised regarding the potential association between proton pump inhibitor (PPI) use and dementia.
Objective
This study aimed to examine this association in an Asian population.
Methods
Patients initiating PPI therapy between January 1, 2000 and December 31, 2003 without a prior history of dementia were identified from Taiwan’s National Health Insurance Research Database. The outcome of interest was all-cause dementia. Cox regression models were applied to estimate the hazard ratio (HR) of dementia. The cumulative PPI dosage stratified by quartiles of defined daily doses and adjusted for baseline disease risk score served as the primary variables compared against no PPI use.
Results
We analyzed the data of 15726 participants aged 40 years or older and free of dementia at baseline. PPI users (n = 7863; average follow-up 8.44 years) had a significantly increased risk of dementia over non—PPI users (n = 7863; average follow-up 9.55 years) (adjusted HR [aHR] 1.22; 95% confidence interval: 1.05–1.42). A significant association was observed between cumulative PPI use and risk of dementia (P for trend = .013). Subgroup analysis showed excess frequency of dementia in PPI users diagnosed with depression (aHR 2.73 [1.91–3.89]), hyperlipidemia (aHR 1.81 [1.38–2.38]), ischemic heart disease (aHR 1.55 [1.12–2.14]), and hypertension (aHR 1.54 [1.21–1.95]).
Conclusions
An increased risk for dementia was identified among the Asian PPI users. Cumulative PPI use was significantly associated with dementia. Further investigation into the possible biological mechanisms underlying the relationship between dementia and PPI use is warranted.
Introduction
Dementia is a chronic, progressive, multifactorial neurodegenerative disorder characterized by a decline in cognitive function. With the increase in the aging population, the World Health Organization estimates the proportion of dementia cases in people aged 60 years and older will reach 22% worldwide by 2050[1], with Asia estimated to account for 59% of the cases worldwide[2]. The consequent high demand for medical therapy and care needed to treat cumulative cognitive decline will have considerable socioeconomic impact. The estimated worldwide costs of treating dementia were estimated to be US$604 billion in 2010[3]. Thus, the prevention of dementia in populations at increased risk (e.g., the elderly) may help reduce the burden caused by dementia on people and healthcare systems. Therefore, it is no surprise that commonly used drugs that could potentially increase or decrease the risk of dementia in the elderly as a consequence of their long-term use have been examined in epidemiological studies.
Evidence suggests that the precipitation of β-amyloid (Aβ) peptide in the central nervous system can lead to the development of dementia[4]. Proton pump inhibitors (PPIs), which act as remarkable and long-lasting reducers of gastric acid production, are prescribed for the treatment for acid-related conditions such as gastroesophageal reflux disease and peptic ulcers[5, 6]. Their use has increased, especially among the elderly[7, 8]. PPI use might lower cognition by enhancing Aβ levels in the brains of mice by affecting the enzymes β- and γ-secretases[9] or by modulating the degradation of Aβ by lysosomes in microglia[10–13]. Lam et al[14] reported a significant association of previous and current PPI use with vitamin B12 deficiency in a population-based sample. Vitamin B12 deficiency has been associated with cognitive decline [15]. A prospective, longitudinal, multicenter cohort study of elderly primary care patients in Germany, including 3327 community-dwelling persons aged 75 years or older, found a significant association between PPI use and incident dementia (hazard ratio [HR], 1.38 [95%CI, 1.04–1.83])[13]. Another prospective cohort study, derived from data supplied by the largest German statutory health insurer, reported that avoiding the use PPI may reduce the risk of dementia[16]. These studies, mostly based on western populations, show increased interest in whether PPIs can increase the incidence and progression of dementia. In this study, we investigated potential association between proton pump inhibitor (PPI) use and dementia in an Asian population using Taiwan’s National Health Insurance Research Database (NHIRD) to follow the development of dementia in users and non-users of PPIs in a Taiwanese population over a >10-year period (1997–2010).
Methods
Data source
The present study was conducted using claims data from the National Health Insurance Research Database (NHIRD), which is managed by the National Health Research Institute (NHRI) in Taiwan. Taiwan’s National Health Insurance (NHI) provides reimbursements for healthcare costs for 99% of the population in Taiwan (approximately 23 million people). The NHIRD contains comprehensive healthcare information, including demographic data of insured individuals, dates of clinical visits, diagnostic codes, and prescription details. The data of this study was obtained from the Longitudinal Health Insurance Database (LHID) 2000, a subset of the NHIRD. The LHID 2000 dataset contains historical ambulatory and inpatient care data for one million randomly sampled beneficiaries enrolled in the NHI system in 2000. The LHID 2000 database allows researchers to follow the medical service utilization history of these patients. The claims found in the LHID 2000 and NHIRD do not differ significantly in age, sex, or healthcare costs.
Study patients
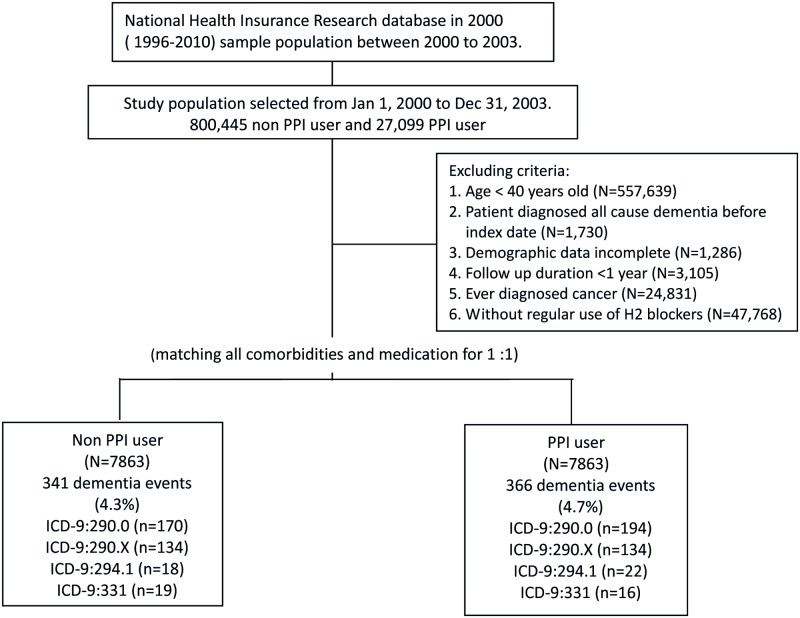
Patients who had ever received PPI between January 1, 2000 and December 31, 2003, were identified for the PPI cohort (PPI users) and were compared with a comparison cohort comprised of patients who had never been treated with PPI (hereafter, non—PPI users); these patients were randomly sampled from the remaining enrollees of the LHID 2000 data set and were 1:1 matched for age, sex, propensity score, and index year. The date of initial use of PPI for each patient was assigned as their index date. Initiation was defined as being free from any PPI therapy for 12 months prior to the first prescription (index date). Patients were excluded if they were less than 40 years of age (N = 557 639), had all-cause dementia diagnosed before the index date (N = 1730), had incomplete demographic data (N = 3105), had follow-up durations less than 1 year, were ever diagnosed with cancer (N = 24 831), or currently/previously regularly used H2 blockers (more than 3 months in 1 year) (N = 47 768) (Fig 1).
Fig 1. Study flowchart.
Cumulative exposure of PPI
Drug use information was obtained from the outpatient pharmacy prescription database. It included prescribed drug dosage, date of prescription, supply days, and total number of pills dispensed. Because patients might discontinue or restart drug therapy, we assumed that patients’ exposure to each studied drug contributed both cumulatively and continuously to their long-term risk of dementia. Defined daily dose (DDD) is the assumed average maintenance dose per day for a drug used for its main indication in adults[17]. To investigate the effect of dose, the cumulative use of PPI was calculated as total prescribed DDD (i.e., the same as total dispensed DDD under this system) analysis. When a dementia event occurred, the cumulative PPI dosage was recorded as a total of DDD from drug initiation to the day that the dementia event occurred. For those who were still at risk (event free and uncensored), the cumulative doses were recorded and ranked at each event time. Participants were then classified into mutually exclusive dosage categories on the basis of quartiles of cumulative dosage distribution of the “risk set” at that time[18]. As the PPI dosage accumulated during the follow-up period, a participant could be reassigned to either a higher or a lower quartile.
Ascertainment of dementia
The outcome of interest was defined as a diagnosis of ICD-9-CM: 290.0 (senile dementia, uncomplicated), 290.4x (arteriosclerotic dementia), 294.1 (dementia in conditions classified elsewhere), and 331.0 (Alzheimer disease). Patients diagnosed with dementia were required to have at least two outpatient visits or one inpatient hospitalization for dementia, and a diagnosis made by a neurologist or psychiatrist. Patients were followed from the index date to the earliest outcome of occurrence, death, and disenrollment from the NHI or the end of the study date (December 31, 2010), whichever occurred first. This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-EXEMPT (II)-20160028). Because the patient identifiers are scrambled to researchers to protect patient confidentiality, the requirement for written or verbal consent from patients for data linkage study was waived.
Covariate ascertainment and adjustment
Inpatient and outpatient files from the year prior to the index date were used to ascertain whether they had comorbidities, including diabetes mellitus (ICD-9-CM: 250.xx), hypertension (ICD-9-CM: 401x), hyperlipidemia (ICD-9-CM: 272x), peripheral vascular disease (ICD-9-CM: 443), ischemic heart disease (ICD-9-CM: 410.x-414.x), depression (ICD-9-CM: 296.x-, 300.4, 311), and ischemic stroke (ICD-9-CM: 433–438) as well as the Charlson’s comorbidity index(CCI)[19]. We categorized CCI into 3 levels ≦1, 2, and ≧3. CCI was determined for each subject from claims data for outpatient visits or hospitalizations at baseline. The CCI is a scoring system that includes weighting factors on important concomitant diseases; it has been validated for the use with ICD-9-CM coded administrative database [20]. Demographic data, including age, sex, and urbanization, were extracted. Comorbidities were defined in a patient if he or she was diagnosed for any of the aforementioned diseases on at least two outpatient claims or one inpatient claim during the exposure period. We included drugs as confounding agents if they could potentially accelerate or reduce inflammation or cognitive function in the model. These included anticoagulants, nonsteroidal anti-inflammatory agents (NSAIDs), antiplatelet agents, antidiabetic agents, antihypertensives, and statins. Exposure to these drugs was defined as having a prescription for one of them from at least one day after the index date to the occurrence of any event related to this study, being disenrolled from the NHI program, death, or the end of the study period (December 31, 2010), whichever occurred first.
Statistical analysis
Pearson chi-square test was used to evaluate differences in categorical data between PPI users and non—PPI users, including demographic data and comorbidities. Cox hazards regression analysis was performed to examine the risk of dementia among PPI users compared with non—PPI users during the follow-up period. The DDD recommended by WHO was used to quantify the use of PPI. Cumulative DDD was estimated as the sum of dispensed DDDs of PPI from January 1, 2000 to the data of a diagnosis of dementia or until the end of the study. PPI users were categorized into users of extremely low doses (<28 DDDs), low doses (28–48 DDDs), moderate doses (49–83 DDDs), and high doses (>84 DDDs). Several covariables such as age, gender, urbanization, Charlson’s index, and all comorbidities and comedications were adopted in the statistical analysis model. Hazard ratios (HRs) and 95% confidence intervals (CIs), using the comparison cohort of non—PPI users as the reference, were calculated to show the risk of dementia in PPI users and in the dose—response analysis of cumulative PPI use. Subgroup analysis was done restricted to aged older than 60 y/o patients, who were more likely to develop dementia. Data analysis was performed using the SAS 9.3 statistical package; all P-values were 2-sided, and P < .05 was considered significant.
Results
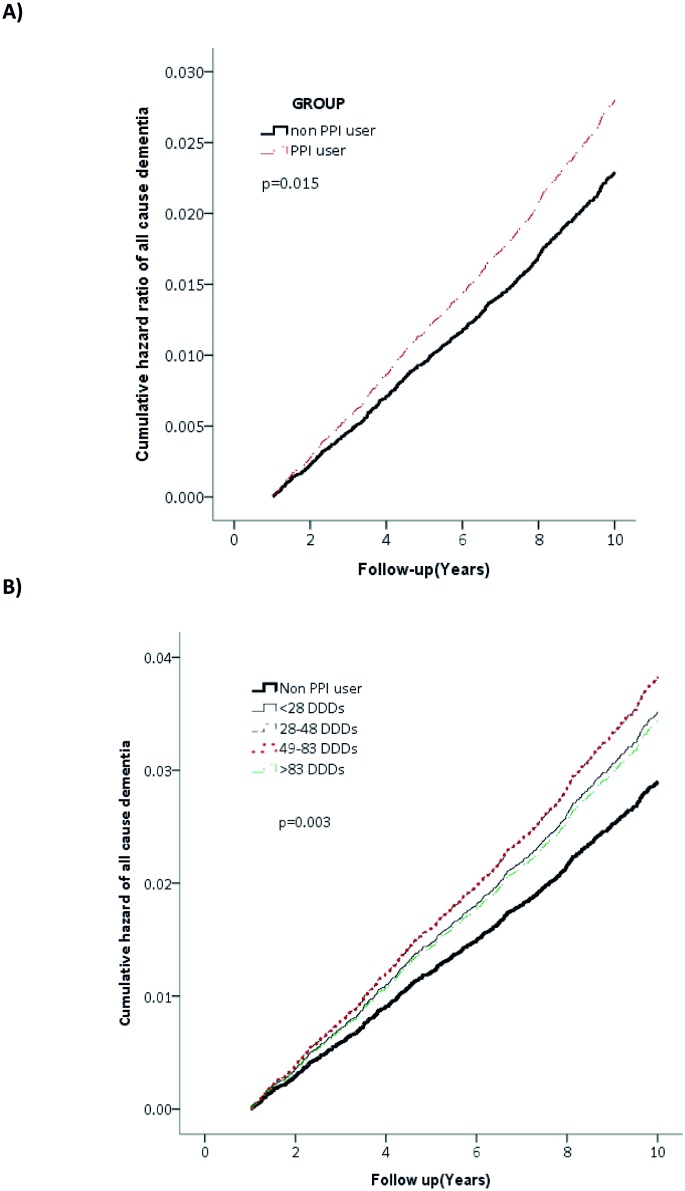
A total of 7863 PPI initiators from January 1, 2000, to December 31, 2003, were identified (Fig 1). After propensity score matching, 7863 PPI users were matched to the 7863 non—PPI users for the final analysis. The characteristics of both groups are listed in Table 1. All covariates were comparable after matching (Table 1). Among the study participants, 707 all-cause dementia cases occurred with an average follow-up of 9.0 years. The crude incidence rate was 5.51 per 1000 person-years among PPI users and 4.54 among non—PPI users. PPI users had a slightly higher risk of developing dementia than non-PPI users after adjusting for the covariates listed in Table 1. (HR, 1.22 [95%CI, 1.05–1.42]; Table 2) Furthermore, we found a significant association between cumulative PPI use and all-cause dementia (P for trend = .013; Table 2). The findings were similar among patients of inclusion age older than 60 y/o. (Table A in S1 File) The survival curve of the PPI use and incident dementia is depicted in Fig 2. Fig 2 depicts all-cause dementia by (A) use and (B) defined daily dose (DDD) of proton pump inhibitors (PPIs).
Table 1. Basic characteristics of PPI users and non—PPI users (N = 15,726).
| Variables | Non PPI user (N = 7863) | PPI user (N = 7863) | P value | ||
|---|---|---|---|---|---|
| N | (%) | N | (%) | ||
| Age | |||||
| 40–49 | 3316 | (42.2) | 3168 | (40.3) | 0.186 |
| 50–59 | 1912 | (24.3) | 2046 | (26.0) | |
| 60–69 | 1364 | (17.3) | 1356 | (17.2) | |
| ≧70 | 1271 | (16.2) | 1293 | (16.4) | |
| Mean (±SD) | 55.33 | (±12.23) | 55.65 | (±12.37) | 0.102 |
| Gender | |||||
| Female | 3199 | (40.7) | 3238 | (41.2) | 0.527 |
| Male | 4664 | (59.3) | 4625 | (58.8) | |
| Urbanization | |||||
| Urban & Suburban | 5880 | (74.8) | 5811 | (73.9) | 0.208 |
| Rural | 1983 | (25.2) | 2052 | (26.1) | |
| Comorbidities | |||||
| Diabetes | 862 | (11.0) | 814 | (10.4) | 0.215 |
| Hyperlipidemia | 1035 | (13.2) | 1020 | (13.0) | 0.723 |
| hypertension | 1925 | (24.5) | 1924 | (24.5) | 0.985 |
| Depression | 391 | (5.0) | 370 | (4.7) | 0.435 |
| Ischemic heart disease | 314 | (4.0) | 306 | (3.9) | 0.743 |
| Cerebral vascular disease | 172 | (2.2) | 184 | (2.3) | 0.520 |
| CCI score | |||||
| ≦1 | 6796 | (86.4) | 6795 | (86.4) | 0.112 |
| 2 | 840 | (10.7) | 800 | (10.2) | |
| ≧3 | 227 | (2.9) | 268 | (3.4) | |
| Medication | |||||
| Anticoagulant agents | 35 | (0.4) | 33 | (0.4) | 0.808 |
| Antiplatete agents | 233 | (3.0) | 234 | (3.0) | 0.963 |
| Antidiabetic agents | 549 | (7.0) | 539 | (6.9) | 0.753 |
| Antihypertension agents | 1465 | (18.6) | 1434 | (18.2) | 0.524 |
| statin | 184 | (2.3) | 171 | (2.2) | 0.485 |
| NSAIDs | 586 | (7.5) | 601 | (7.6) | 0.651 |
PPI: proton pump inhibitor
SD: standard deviation
NSAIDs: nonsteroidal anti-inflammatory agents
CCI score: Charlson’s comorbidity index score
Table 2. Risk of all-cause dementia among non—PPI users and PPI users (N = 15,726).
| No. cases | Per 1,000 Person year | cHR | (95%CI) | p value | aHR | (95%CI) | p value | |
|---|---|---|---|---|---|---|---|---|
| Non PPI user | 341 | 4.54 | Ref. | Ref. | ||||
| PPI user | 366 | 5.51 | 1.25 | (1.08–1.46) | 0.003 | 1.22 | (1.05–1.42) | 0.009 |
| Cumulative DDDs | ||||||||
| <28 | 72 | 4.38 | 0.99 | (0.77–1.28) | 0.962 | 1.21 | (0.94–1.56) | 0.153 |
| 28–48 | 83 | 5.15 | 1.17 | (0.92–1.49) | 0.209 | 1.32 | (1.04–1.68) | 0.023 |
| 49–83 | 108 | 5.85 | 1.33 | (1.07–1.65) | 0.011 | 1.33 | (1.07–1.65) | 0.011 |
| ≧84 | 103 | 6.73 | 1.54 | (1.23–1.92) | <0.001 | 1.19 | (0.95–1.48) | 0.133 |
| p for trend | <0.001 | 0.013 |
Adjusted for age, gender, urbanization, all comorbidities, CCI score, and medication.
PPI: proton pump inhibitor
cHR: crude hazard ratio
aHR: adjusted hazard ratio
DDD: defined daily dose
CCI score: Charlson’s comorbidity index score
Fig 2. All-cause dementia by A) use and B) defined daily dose (DDD) of proton pump inhibitors (PPIs).
A subgroup analysis was performed for the most used PPI subtypes (omeprazole, pantoprazole, and lansoprazole) in Taiwan during 2000–2003. After adjusting for covariates, we found a similar elevated risk of dementia for omeprazole (HR, 1.30 [95%CI, 1.09–1.54]) and similar but not significantly elevated risk for pantoprazole (HR, 1.36 [95%CI, 0.98–1.89]) and lansoprazole (HR, 1.20 [95%CI, 0.98–1.46]). (Table B in S1 File) The survival curve of subtype PPI use and incident dementia is presented in Fig A of S1 File.
Male PPI users had significantly higher risk of developing dementia (HR, 1.24 [95%CI, 1.01–1.52]), whereas female users did not (HR, 1.21 [95%CI, 0.97–1.50]), compared with their non—PPI user counterparts. (Table C in S1 File)
Compared with non—PPI users, PPI users without diabetes mellitus (HR, 1.29 [95%CI, 1.09–1.52]), depression (HR, 1.20 [95%CI, 1.02–1.40]), or cerebral vascular disease (HR, 1.25 [95%CI, 1.07–1.46]) and those with hyperlipidemia (HR, 1.49 [95%CI, 1.06–2.10]) and ischemic heart disease (HR, 2.18 [95%CI, 1.31–3.63]) had significantly higher risk of developing dementia. PPI users not taking anticoagulant agents (HR, 1.23 [95%CI, 1.06–1.43]), antiplatelet agents (HR, 1.20 [95%CI, 1.02–1.40]), antidiabetic agents (HR, 1.28 [95%CI, 1.09–1.51]), statins (HR, 1.18 [95%CI, 1.03–1.40]), or NSAIDs (HR, 1.20 [95%CI, 1.02–1.41]) had significantly higher risk of developing dementia than non—PPI users. In contrast, PPI users taking antihypertension agents (HR, 1.46 [95%CI, 1.14–1.85]) had significantly higher risk of developing dementia than non—PPI users. (Table C in S1 File)
Table 3 shows the additive effect of PPI use and covariates. Depression (aHR, 2.73 [95%CI, 1.91–3.89]) and hyperlipidemia (aHR, 1.81 [95%CI, 1.38–2.38]) were associated with the highest increased risk for incident dementia. In addition, being more than 70 years old was also associated with increased risk of dementia (aHR, 1.38 [95%CI, 1.02–1.87]). Other comorbidities were also significantly associated with elevated the risk of dementia. They were hypertension (aHR, 1.54 [95%CI, 1.21–1.95] and ischemic heart disease (aHR, 1.55 [95%CI, 1.12–2.14]). In addition, PPI use along with the following comedications had a considerable effect on risk of dementia: antiplatelet agents (aHR, 1.91 [95%CI, 1.37–2.66]), antihypertensives (aHR, 1.36 [95%CI, 1.07–1.38]), statins (aHR, 1.59 [95%CI, 1.00–2.53]), and NSAIDs (aHR, 1.19 [95%CI, 1.21–2.09])
Table 3. The additive effects of covariates on all cause dementia between non PPI user and PPI user (N = 15,726).
| Non PPI user | PPI user | |||
|---|---|---|---|---|
| aHR (95% CI) | p value | aHR (95% CI) | p value | |
| Age | ||||
| <70 | Ref. | 1.11(0.88–1.40) | 0.375 | |
| ≧70 | 1.06(0.79–1.43) | 0.707 | 1.38(1.02–1.87) | 0.035 |
| Comorbidites | ||||
| Hyperlipidemia | ||||
| No | Ref. | 1.18(0.99–1.39) | 0.053 | |
| Yes | 1.29(0.97–1.71) | 0.082 | 1.81(1.38–2.38) | <0.001 |
| Hypertension | ||||
| No | Ref. | 1.19(0.96–1.46) | 0.109 | |
| Yes | 1.22(0.96–1.55) | 0.106 | 1.54(1.21–1.95) | <0.001 |
| Depression | ||||
| No | Ref. | 1.19(1.02–1.40) | 0.028 | |
| Yes | 1.78(1.24–2.56) | 0.002 | 2.73(1.91–3.89) | <0.001 |
| Ischemic heart disease | ||||
| No | Ref. | 1.51(0.98–1.34) | 0.080 | |
| Yes | 0.71(0.47–1.08) | 0.113 | 1.55(1.12–2.14) | 0.008 |
| Medication | ||||
| Antiplatete agents | ||||
| No | Ref. | 1.19(1.01–1.39) | 0.035 | |
| Yes | 1.19(0.80–1.75) | 0.389 | 1.91(1.37–2.66) | <0.001 |
| Antihypertension agents | ||||
| No | Ref. | 1.10(0.91–1.33) | 0.346 | |
| Yes | 0.94(0.73–1.22) | 0.648 | 1.36(1.07–1.38) | 0.014 |
| Statin | ||||
| No | Ref. | 1.20(1.03–1.40) | 0.021 | |
| Yes | 0.91(0.52–1.59) | 0.741 | 1.59(1.00–2.53) | 0.049 |
| NSAIDs | ||||
| No | Ref. | 1.19(1.01–1.41) | 0.034 | |
| Yes | 1.15(0.86–1.55) | 0.349 | 1.19(1.21–2.09) | 0.001 |
Adjusted for age, gender, urbanization, all comorbidities, CCI score, and medication.
PPI: proton pump inhibitor
aHR: adjusted hazard ratio
NSAIDs: non-steroidal anti-inflammatory agents
CCI score: Charlson’s comorbidity index score
Discussion
This study based on claims data made available by Taiwan’s National Insurance Research Database (NHIRD) revealed a significant increased risk of dementia associated with the use of PPIs in an Asian population, confirming the findings of two German studies using AgeCoDe and AOK datasets[16, 21].
PPIs have been used since the early nineties for the treatment of gastrointestinal disorders associated with excessive synthesis of gastric acid[22]. This class of drugs exerts a stronger acid-suppressing effect than other traditional therapies, e.g., histamine-2(H2) receptor antagonists[22]. PPI use has increased significantly over the last 15 years, particularly in elderly populations [7, 8]. Despite initial reports of the safety of PPI use, some recent epidemiological studies have reported adverse effects with their use, including short-term effects (e.g., Clostridium difficile infection and pneumonia[23]) and/or long-term effects (e.g., osteoporosis, hip fractures, nutritional deficiencies [24], the risk of cardiovascular morbidity and mortality [25, 26], and renal failure[27]). Although these epidemiological studies are limited in their design, there is a need to be concerned about these complications among older patients, who are at particularly high risk.
The pharmacoepidemiological results of the current Asian study and the recent two German studies indicate an association between PPI and dementia. The exact mechanism though which PPIs might influence the development of dementia is still unknown. However, evidence indicates that PPIs first cross the blood—brain barrier[28] and interact with certain brain enzymes that could increase Aβ production[9] in the brain and decreasing its degradation[13]. PPIs may also bind to tau protein[28]. Thus, although Aβ is considered the most relevant factor related to the development of dementia, tau protein probably plays a role as well [28]. Another possible mechanism of PPI-induced dementia could be vitamin B-12 deficiency resulting from chronic PPI use[14]. Deficiency of this essential compound has been associated with cognitive decline [29, 30]. Furthermore, some evidence indicates that chronic exposure of human endothelial cells to PPIs accelerates endothelial aging, leading to dementia[31].
Our results are comparable to those based on AOK data, which was a claims data analysis adjusted for almost all of the potential confounding factors[16]. but not to the AgeCoDe study [21], which included the ApoE4 allele status and educational level. We found PPI users to be clearly at significant elevated risk for developing dementia compared with non—PPI users regardless of age, sex, and comorbidity or comedication. Furthermore, increased risk of dementia was noted in the presence of comorbidities of hyperlipidemia, hypertension, depression, and ischemic heart disease. Numerous studies have suggested a link between depression and dementia [32–36]. In our study, PPI users with depression were also found to have a higher risk for developing dementia. Furthermore, PPI users also had higher risk of developing dementia with concomitant use of antiplatelet agents or statins compared with other comorbidities and comedications. This might be explained by poor vascular status.
Our subgroup analyses for the three most used PPIs (omeprazole, pantoprazole, and lansoprazole) during 2000–2003 in Taiwan showed similar effect sizes, though there was only significantly pronounced risk of dementia associated with the use of omeprazole. Omeprazole is the first PPI to be approved (in 1988); at that time, it was found to have faster, stronger, and longer acid reduction than the histamine H2 receptor antagonists. Subsequently, lansoprazole (1991), pantoprazole (1994), rabeprazole (1999), and esomeprazole (2001) were approved [37]. PPIs have become a mainstream treatment of gastric acid-related diseases as well as a first-line medication[38]. In Taiwan, the time that pharmaceuticals are approved and listed is usually 2–3 years later than the global listing time, so the most popular PPIs during the recruiting period (2000–2003) were omeprazole, pantoprazole, and lansoprazole, which are different from the Germany claims data (omeprazole, pantoprazole, and esomeprazole)[16]. PPIs have dramatically improved the management options available for patients with acid-related disorders. Most patients with gastroesophageal reflux disease have excellent outcomes when initially prescribed PPIs, though some patients may need to switch to a different PPI during treatment. In our subgroup analysis, we found PPI rotations to be popular in clinical practice in Taiwan. We tried to reduce this confounding factor by adjusting for the other subtype PPI use.
The proportion of elderly people in Taiwan has risen over the past three decades from 4.1% in 1980 to 11.5% in 2013, the highest rate of aging worldwide [39]. Dementia will, therefore, be a major public health problem Taiwan. Another problem in the elderly population is polypharmacy. Some drugs may lead to injury of gastric mucosa (e.g., aspirin and NSAID), which, in turn, lead to tremendously increased use of PPIs among the elderly [7, 8]. This is a crucial issue given the very high prevalence of polypharmacy and long-term use among elderly populations who are already at greater risk of dementia.
The strength of this study is that it is a national cohort study based on Taiwan’s NHIRD, which contains data From Taiwan’s compulsory and universal healthcare system which has high coverage rate in Taiwan. This allowed us to perform our analysis in a real-life setting in an unselected patient population. In addition, patient dropout was avoided and selection bias or recall bias minimized because of the use of routine database records.
The study also has several limitations. One is that it was a retrospective review of the medical records and did not include results from formal cognitive function testing. Another limitation is that Alzheimer’s disease (AD) and vascular dementia often coexist as a mixed dementia. We could not differentiate between different dementia etiologies using the NHIRD because there is no distinguishing information available from the diagnostic codes of ICD-9-cm. This fact may not be a major limitation since the AgeCoDe study [21] found that there was only subtle differences between all dementia patients and AD patients and mixed dementia in most dementia cases [40, 41]. The diagnoses of dementia in NHIRD were based on ICD-9-CM codes and were determined by relevant specialists and physicians, according to standard clinical criteria. The data on the diagnoses of dementia can thus be considered reliable. However, the degree or stage of dementia was not available in this claims data. Still another limitation is that the NHIRD lacks detailed information on smoking habits, dietary preference, occupational exposure, educational level and socioeconomic status, all of which are potential risk factors for dementia. Moreover, all data in the NHIRD are anonymous; therefore, relevant clinical variables such as serum laboratory data, genetic factors (e.g., ApoE4 allele carrier), imaging and pathology results were also unavailable. Finally, although the findings of this study can be generalized to the Taiwanese population, applicability to other ethnicities should be revalidated.
Conclusions
This population-based cohort study found Asian PPI users to be at increased risk for dementia and that cumulative PPI use was significantly associated with dementia. This finding should be further examined through randomized, prospective clinical trials.
Supporting information
(DOC)
Acknowledgments
This study was supported by grants from the Ministry of Science and Technology(MOST103-2314-B-037-004-MY3), and Research Center for Environmental Medicine, Kaohsiung Medical University (KMU-TP104A34), and Kaohsiung Medical University Hospital (KMUH105-5R64). The authors are thankful for the assistance provided by the Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Municipal Ta-Tung Hospital. None of these organizations had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants from the Ministry of Science and Technology (MOST103-2314-B-037-004-MY3), Research Center for Environmental Medicine, Kaohsiung Medical University (KMU-TP104A34), and Kaohsiung Medical University Hospital (KMUH105-5R64). The authors are thankful for the assistance provided by the Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Municipal Ta-Tung Hospital. None of these organizations had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Ageing and life course, Facts about ageing, World Health Organization. 30 Sep 2014. http://www.who.int/ageing/about/facts/en/.
- 2.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3(3):186–91. Epub 2007/07/01. 10.1016/j.jalz.2007.04.381 [DOI] [PubMed] [Google Scholar]
- 3.Wimo A, Jonsson L, Bond J, Prince M, Winblad B. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1–11.e3. Epub 2013/01/12. 10.1016/j.jalz.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 4.De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. Journal of Cell Science. 2000;113(11):1857–70. [DOI] [PubMed] [Google Scholar]
- 5.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. The American journal of gastroenterology. 2013;108(3):308–28; quiz 29. Epub 2013/02/20. 10.1038/ajg.2012.444 [DOI] [PubMed] [Google Scholar]
- 6.Londong W, Barth H, Dammann HG, Hengels KJ, Kleinert R, Muller P, et al. Dose-related healing of duodenal ulcer with the proton pump inhibitor lansoprazole. Alimentary pharmacology & therapeutics. 1991;5(3):245–54. Epub 1991/06/01. [DOI] [PubMed] [Google Scholar]
- 7.Hollingworth S, Duncan EL, Martin JH. Marked increase in proton pump inhibitors use in Australia. Pharmacoepidemiology and drug safety. 2010;19(10):1019–24. Epub 2010/07/14. 10.1002/pds.1969 [DOI] [PubMed] [Google Scholar]
- 8.Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ (Clinical research ed). 2008;336(7634):2–3. Epub 2008/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badiola N, Alcalde V, Pujol A, Munter LM, Multhaup G, Lleo A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837 Epub 2013/03/23. 10.1371/journal.pone.0058837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majumdar A, Cruz D, Asamoah N, Buxbaum A, Sohar I, Lobel P, et al. Activation of Microglia Acidifies Lysosomes and Leads to Degradation of Alzheimer Amyloid Fibrils. Molecular Biology of the Cell. 2007;18(4):1490–6. 10.1091/mbc.E06-10-0975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pillay CS, Elliott E, Dennison C. Endolysosomal proteolysis and its regulation. The Biochemical journal. 2002;363(Pt 3):417–29. Epub 2002/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattsson JP, Vaananen K, Wallmark B, Lorentzon P. Omeprazole and bafilomycin, two proton pump inhibitors: differentiation of their effects on gastric, kidney and bone H(+)-translocating ATPases. Biochimica et biophysica acta. 1991;1065(2):261–8. Epub 1991/06/18. [DOI] [PubMed] [Google Scholar]
- 13.Fallahzadeh MK, Borhani Haghighi A, Namazi MR. Proton pump inhibitors: predisposers to Alzheimer disease? Journal of clinical pharmacy and therapeutics. 2010;35(2):125–6. Epub 2010/05/12. 10.1111/j.1365-2710.2009.01100.x [DOI] [PubMed] [Google Scholar]
- 14.Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. Jama. 2013;310(22):2435–42. Epub 2013/12/12. 10.1001/jama.2013.280490 [DOI] [PubMed] [Google Scholar]
- 15.Vogiatzoglou A, Smith AD, Nurk E, Drevon CA, Ueland PM, Vollset SE, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E epsilon4: the Hordaland Homocysteine Study. Psychosomatic medicine. 2013;75(1):20–9. Epub 2012/12/06. 10.1097/PSY.0b013e3182761b6c [DOI] [PubMed] [Google Scholar]
- 16.Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: A pharmacoepidemiological claims data analysis. JAMA Neurology. 2016;73(4):410–6. 10.1001/jamaneurol.2015.4791 [DOI] [PubMed] [Google Scholar]
- 17.WHO Collaborating Centre for Drug Statistics Methodology. Defined daily dose: definition and general considerations [Internet]. 29 July 2014.
- 18.Stricker BH, Stijnen T. Analysis of individual drug use as a time-varying determinant of exposure in prospective population-based cohort studies. Eur J Epidemiol. 2010;25(4):245–51. Epub 2010/04/02. 10.1007/s10654-010-9451-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. Epub 1987/01/01. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–9. Epub 1992/06/01. [DOI] [PubMed] [Google Scholar]
- 21.Haenisch B, von Holt K, Wiese B, Prokein J, Lange C, Ernst A, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. European archives of psychiatry and clinical neuroscience. 2015;265(5):419–28. Epub 2014/10/25. 10.1007/s00406-014-0554-0 [DOI] [PubMed] [Google Scholar]
- 22.Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. European journal of clinical pharmacology. 2008;64(10):935–51. Epub 2008/08/06. 10.1007/s00228-008-0538-y [DOI] [PubMed] [Google Scholar]
- 23.de Jager CP, Wever PC, Gemen EF, van Oijen MG, van Gageldonk-Lafeber AB, Siersema PD, et al. Proton pump inhibitor therapy predisposes to community-acquired Streptococcus pneumoniae pneumonia. Alimentary pharmacology & therapeutics. 2012;36(10):941–9. Epub 2012/10/05. [DOI] [PubMed] [Google Scholar]
- 24.Khalili H, Huang ES, Jacobson BC, Camargo CA Jr., Feskanich D, Chan AT. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ (Clinical research ed). 2012;344:e372. Epub 2012/02/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah NH, LePendu P, Bauer-Mehren A, Ghebremariam YT, Iyer SV, Marcus J, et al. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS One. 2015;10(6):e0124653 Epub 2015/06/11. 10.1371/journal.pone.0124653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghebremariam YT, LePendu P, Lee JC, Erlanson DA, Slaviero A, Shah NH, et al. Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation. 2013;128(8):845–53. Epub 2013/07/05. 10.1161/CIRCULATIONAHA.113.003602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, et al. Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease. JAMA internal medicine. 2016;176(2):238–46. Epub 2016/01/12. 10.1001/jamainternmed.2015.7193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rojo LE, Alzate-Morales J, Saavedra IN, Davies P, Maccioni RB. Selective interaction of lansoprazole and astemizole with tau polymers: potential new clinical use in diagnosis of Alzheimer's disease. J Alzheimers Dis. 2010;19(2):573–89. Epub 2010/01/30. 10.3233/JAD-2010-1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Leary F, Allman-Farinelli M, Samman S. Vitamin B(1)(2) status, cognitive decline and dementia: a systematic review of prospective cohort studies. The British journal of nutrition. 2012;108(11):1948–61. Epub 2012/10/23. 10.1017/S0007114512004175 [DOI] [PubMed] [Google Scholar]
- 30.Reynolds E. Vitamin B12, folic acid, and the nervous system. The Lancet Neurology. 2006;5(11):949–60. Epub 2006/10/21. 10.1016/S1474-4422(06)70598-1 [DOI] [PubMed] [Google Scholar]
- 31.Yepuri G, Sukhovershin R, Nazari-Shafti TZ, Petrascheck M, Ghebre YT, Cooke JP. Proton Pump Inhibitors Accelerate Endothelial Senescence. Circulation research. 2016. Epub 2016/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen YH, Wu SS, Lin CH, Tsai JH, Yang P, Chang YP, et al. A Bayesian Approach to Identifying New Risk Factors for Dementia: A Nationwide Population-Based Study. Medicine. 2016;95(21):e3658 Epub 2016/05/27. 10.1097/MD.0000000000003658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modrego PJ, Ferrández J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of alzheimer type: A prospective cohort study. Archives of Neurology. 2004;61(8):1290–3. 10.1001/archneur.61.8.1290 [DOI] [PubMed] [Google Scholar]
- 34.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323–31. 10.1038/nrneurol.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jorm AF. History of depression as a risk factor for dementia: an updated review. Australian and New Zealand Journal of Psychiatry. 2001;35(6):776–81. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Wang LY, Shofer JB, et al. Temporal relationship between depression and dementia: Findings from a large community-based 15-year follow-up study. Archives of General Psychiatry. 2011;68(9):970–7. 10.1001/archgenpsychiatry.2011.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sachs G, Shin JM, Howden CW. Review article: the clinical pharmacology of proton pump inhibitors. Alimentary pharmacology & therapeutics. 2006;23:2–8. [DOI] [PubMed] [Google Scholar]
- 38.Katz PO, Scheiman JM, Barkun AN. Review article: acid-related disease—what are the unmet clinical needs? Alimentary pharmacology & therapeutics. 2006;23 Suppl 2:9–22. Epub 2006/05/17. [DOI] [PubMed] [Google Scholar]
- 39.Yang YH, Wang H, Lam L, Chan WC, Yu X, Li T, et al. Characteristics of Alzheimer's disease among patients in Taiwan, Hong Kong, and Beijing. J Alzheimers Dis. 2014;42(1):193–200. Epub 2014/05/21. 10.3233/JAD-140174 [DOI] [PubMed] [Google Scholar]
- 40.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204. Epub 2007/06/15. 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- 41.Heneka MT, Fink A, Doblhammer G. Effect of pioglitazone medication on the incidence of dementia. Annals of neurology. 2015;78(2):284–94. Epub 2015/05/15. 10.1002/ana.24439 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.