Abstract
Aim:
Since, contradictory data have been reported about the effect of diverse variants of H. pylori virulence factors on IL-8 induction, we aimed to analyze the effect of this diversity on levels of IL-8 secretion in AGS cell line.
Background:
Helicobacter pylori colonizes the human stomach and induces the activation of inflammatory cytokines, including interleukin (IL)-8, in the gastric mucosa. This induction promotes neutrophil and monocyte recruitment that causes gastric tissue damage.
Methods:
To determine whether different strains of H. pylori and their CagA variants have possible roles on IL-8 induction, polarized
AGS cell line was infected with CagA+ H. pylori strains carrying different EPIYA motifs (ABCCC and ABC) and CagA- strain for 24 hours. Difference in stimulation of IL-8 was measured by ELISA.
Results:
IL-8 secretion was elevated in the treated cells with CagA encoding strains compared with the negative one. Furthermore, a noticeably increased level of IL-8 induction was measured by the CagA-EPIYA type ABCCC encoding strain in compare to that carried EPIYA type ABC
Conclusion:
Results of this study provide new evidence about different effects of H. pylori strains and possible roles of their CagA variants on IL-8 induction. It seems that not only carriage of cagA and its expression, but also diversity in EPIYA motif be involved in IL-8 induction in the gastric epithelial cells.
Key Words: Helicobacter pylori, Cytotoxin-associated gene A, Interleukin-8, CagA variants
Introduction
Helicobacter pylori is colonized in the stomach of 35%- 70% people worldwide. It is a Gram-negative bacterium that lead to disorders ranging from gastric inflammation to gastric adenocarcinoma (1-4). Several virulence factors of H. pylori are involved in the pathogenesis of the stomach.
H. pylori chronic infection could increase expression of specific immune mediators, such as interleukins, tumor necrosis factor (alpha), and interferons in the host cells (5-8). Interleukin 8 (IL-8), a potent neutrophil chemotactic factor, plays a crucial role in the proliferation and migration of cancer cells in the infected patients. It also is able to stimulate the host specific inflammatory response through chemo attraction of neutrophils to the site of infected gastric mucosa and their activation (9, 10).
In vivo and in vitro studies showed higher IL-8 secretion in the H. pylori infected cells and mainly correlated this elevated IL-8 level with expression of functional CagA, a major virulence factor of H. pylori which is located at the end of the cag pathogenicity island (cag-PAI) (11- 16). According to the presence or absence of cagA, H. pylori strains are classified into two major sub-types, cagA-positive or cagA- negative (16, 17). Interaction between specific receptors on the gastric epithelial cells and the “needle”-like structure of type IV secretion system, T4SS, CagA is transferred into the cytosol, where it is phosphorylated by host cellular kinases on the repeat sequences known as EPIYA motifs, located in the carboxyl terminus of the protein (18-20). These process lead to an activation of signaling cascades inducing pro- inflammatory cytokines like IL-8(13, 21-23). EPIYA motifs have been classified into four types, based on the adjacent amino acid sequences. The A and B motifs are found in all strains, while the C and D motifsare present within ‘western’ strains and East Asia, respectively(24, 25). It has been suggested that East Asian CagA with one D motif is highly active and it has no requirement to increase its virulence, whereas the less active western type needs to increase its number of tyrosine phosphorylation motifs for being more virulent (26). Besides, the level of CagA phosphorylation depends on the number of EPIYA-C motifs (25, 27-30). It has been shown that co-culture of AGS cell line with Asian strains increases IL-8 secretion on AGS cell line and it may induce more inflammation within the stomach which lead to an increase in atrophic gastritis and gastric cancer (26). Since, there are contradictory reports about the effect of H. pylori infection and the number of EPIYA motifs on interleukin (IL)-8 secretion, we aimed to determine the effect of CagA negative and CagA encoding H. pylori strains and its variants on IL-8 induction in an in vitro cell culture model.
Patients and methods
Strains Used
H. pylori strains HC-113 (complete cag-PAI CagA+-EPIYA type ABC, ) and OC-149 (complete cag-PAI, cCagA+- EPIYA type ABCCC), as cagA positive H. pylori strains, and OC-236 (cag-PAI- , CagA-), as a cagA negative strain, from the microbial collection of Foodborne and Waterborne Diseases Research center, Shahid Beheshti University of Medical Sciences were used for all the analyses(accession numbers JX428768, JX428784).(31)
The strains were recovered from the stocks on Brucella agar medium supplemented with fetal calf serum (10%) (v/v), horse blood (7%), selective supplement (vancomycin 2.0 mg, polymyxin B 0.05 mg and trimethoprim 1.0 mg, Merck, Germany), and amphotericin B (3 mg/l). The plates were incubated at 37 oC for 3 to 5 days in a microaerobic atmosphere (5% O , 10% CO , and 85% N ). The grown organisms were identified as H. pylori by Gram staining, colony morphology as well as positive oxidase, catalase and urease reactions.
AGS gastric epithelial cell co-culture and IL-8 ELISA:.
The human gastric cancer AGS (ATCC CRL-1739TM) cell line (IBRC, Tehran, Iran) were seeded into six-well plates at a density of 3×105 cells per well and incubated in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, USA), 1% non- essential amino acid (Gibco, Grand Island, NY, USA), 100 U ml−1 of penicillin and 100 μg ml−1 of streptomycin (Gibco, Grand Island, NY, USA) at 37◦C in a humidified incubator (Memmert, Dusseldorf, Germany) containing 5% CO for 2 days before the addition of H. pylori strains. For comparison of the effects of CagA positives and negative H. pylori strains, gastric epithelial cell lines AGS were infected with a CagA negative H. pylori strain and CagA positives strains at MOI 100. Briefly, two hours prior to infection, cells were washed with phosphate buffered saline (PBS, 1x) and the medium was replaced with fresh, antibiotic free DMEM media. Bacterial suspensions (100 μl) were used to infect gastric epithelial cells in 2 ml total volume. After 24 h incubation, the medium was removed, centrifuged at 15000 g for 10 min and the amount of secreted IL-8 into the medium was determined using Human IL-8 Elisa Ready-set- Go! ® Kit (2nd Generation, eBioscience, Korea), following the manufacturer’s protocols. All samples were measured in duplicate in at least two independent experiments.
Results
IL-8 levels in AGS cell line
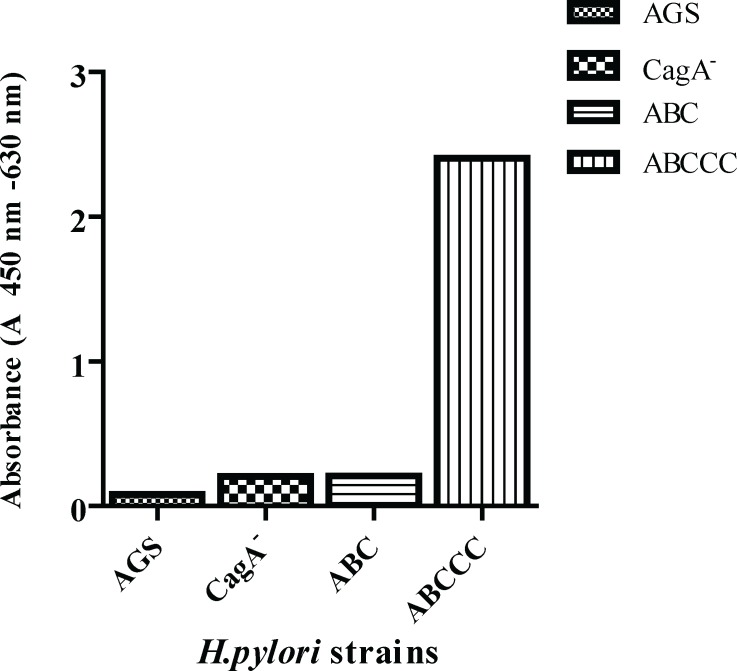
In order to quantify the effect of H. pylori CagA variants and also and the number of EPIYAC motifs site on IL-8 induction, we infected AGS cells with different strains of H. pylori. The tested strains were divided into two groups, according to the presence or absence of cagA, positive strains (complete cag PAI, CagA EPIYA type ABC and complete cag PAI, CagA EPIYA type ABCCC), and negative strain (cag PAI-, cagA−). After 24 h co-cultured, IL-8 levels were measured as described above.As a control, IL-8 concentration in the supernatants of uninfected AGS gastric epithelial cell lines was determined in the same experimental layout. As presented in Figure 1, the IL-8 secretion was elevated in H. pylori infected cells irrespective of the negative or positive strain for cagA gene. Furthermore, IL-8 amount was five folds higher in the infected cell line with cagA EPIYA type ABCCC in compare to the infected cells with the cagA- EPIYA type ABC encoding strain
Figure 1.
Effect of H.pylori strains containing CagA with variable numbers of functional EPIYA motifs on the secretion of IL-8 levels in the supernatants of AGS cells determined by ELISA
Discussion
Our results showed that IL-8 secretion was higher in infected cells in compare to uninfected cells, irrespective of the presence or absence of cagA gene. Infection with H. pylori is associated with an increased risk of gastric disease. H.pylori strains may induce more inflammation within the stomach and this may lead to an increase in atrophic gastritis and gastric cancer. After H. pylori infection, a number of genes especially inflammation genes are over expressed in host cells. IL-8, a chemo attractant for neutrophils, is a part of cancer progression. This cytokine release angiogenic growth factors, activates macrophage and immune responses at the tumor site, causes migration and survival of endothelial cells, and potentiates the epithelial-mesenchymal transition (9, 32). Ling, et al. showed that H. pylori infection correlates with up-regulated IL-8 expression and its level was found to be associated with invasion, lymph node spreading and clinical stages. These observations indicate that high levels of IL-8 may be associated with a poor prognosis and may be indicative of more aggressive gastric cancer (12). Therefore, it is tempting to consider IL-8 as a prognostic and predictive cancer biomarker. The result of this study also shows that although the amount of IL-8 secreted in this study was not so high, the IL-8 secretion was higher in infected cells with cagA positive strains than uninfected or infected cells with a negative one. Since our results demonstrated that CagA- EPIYA motifs ABCCC increased IL-8 level in compare to EPIYA motifs ABC, it seems that phosphorylation of CagA at terminal EPIYA-C motifs contributes to IL-8 secretion in AGS cells. However, there are studies that reported contrary results (33-35). Several in vitro and in vivo studies reported that H. pylori induces IL-8 through cagA-dependent and -independent manners (30, 36-38). Although different efficiency of IL-8 secretion was observed between different cagA-type strains, cagA was not always the responsible factor for IL-8 secretion (36,37).
In addition to cagA, H. pylori peptidoglycans also leading to IL-8 induction through activating NF-kB pathway (13, 18, 39). Papadakos et al showed that following infection of gastric epithelial cells with H. pylori strains, a higher activation of NF-kB was observed in the presence on phosphorylated EPIYA-C motifs and in lower levels, in the absence of cagA expression and CagA phosphorylation or in the cases where EPIYA-C motifs are totally absent (33) Others proposed that CagA multimerization (CM) motif, the highly conserved amino acid sequence FPLKRHDKVDDLSK, is contributed in IL-8 activation (40,41). Recently it has been suggested that CagL through activation of MAPKs and NF- kB induces secretion of interleukin-8 (IL-8) independently of CagA translocation and peptidoglycan (42). Zhang et al showed that the levels of IL-8 induced by H. pylori strains is noticeably differed at an early phase, irrespective of cagA- specific sequences. They suggested that other H. pylori factors, in addition to cagA, affect the enhancement of IL-8 secretion in early H. pylori infection phase (24). However, diversity of the level of secreted IL-8 by H. pylori remains a controversial issue, as a result of contradictory reports (43- 45). More studies is needed in order to prove this argument. In conclusion, we have demonstrated that the presence of functional CagA protein plays a central role in IL-8 secretion in epithelial cells and the level of IL-8 secreted is amplified with increasing numbers of CagA-EPIYAC motifs.
Acknowledgements
We would like to appreciate all staff of Foodborne and Waterborne Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran. This study was part of a PhD thesis, which supported by Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran and Faculty of Paramedical Sciences, Department of Basic Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321–33. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306–23. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Ashtari S, Pourhoseingholi MA, Molaei M, Taslimi H, Zali MR. The prevalence of Helicobacter pylori is decreasing in Iranian patients. Gastroenterol Hepatol Bed Bench. 2015;8:S23. [PMC free article] [PubMed] [Google Scholar]
- 4.Pourhoseingholi M, Moghimi-Dehkordi B, Safaee A, Hajizadeh E, Solhpour A, Zali M. Prognostic factors in gastric cancer using log-normal censored regression model. Indian J Med Res. 2009;129:262. [PubMed] [Google Scholar]
- 5.Sobala G, Crabtree J, Dixon M, Schorah C, Taylor J, Rathbone B, et al. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut. 1991;32:1415–8. doi: 10.1136/gut.32.11.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gionchetti P, Vaira D, Campieri M, Holton J, Menegatti M, Belluzzi A, et al. Enhanced mucosal interleukin-6 and-8 in Helicobacterpylori-positive dyspeptic patients. Am J Gastroenterol. 1994;89:883–7. [PubMed] [Google Scholar]
- 7.Misiewicz J. Current insights in the pathogenesis of Helicobacterpylori infection. Eur J Gastroenterol Hepatol. 1995;7:701. [PubMed] [Google Scholar]
- 8.Neto AC, Rasmussen LT, de Labio RW, de Queiroz VF, de AC Smith M, Viani GA, et al. Gene polymorphism of interleukin 1 and 8 in chronic gastritis patients infected with Helicobacterpylori. J Venom Anim Toxins Incl Trop Dis. 2014;20:1. doi: 10.1186/1678-9199-20-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KE, Khoi PN, Xia Y, Park JS, Joo YE, Kim KK, et al. Helicobacter pylori and interleukin-8 in gastric cancer. World J Gastroenterol. 2013;19:8192–202. doi: 10.3748/wjg.v19.i45.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aihara M, Tsuchimoto D, Takizawa H, Azuma A, Wakebe H, Ohmoto Y, et al. Mechanisms involved in Helicobacter pylori- induced interleukin-8 production by a gastric cancer cell line, MKN45. Infect Immun. 1997;65:3218–24. doi: 10.1128/iai.65.8.3218-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peek Jr RM, Miller GG, Tham KT, Perez-Perez GI, Zhao X, Atherton JC, et al. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995;73:760–70. [PubMed] [Google Scholar]
- 12.Ling X, Zhang H, Shen C, Yan W, Wang P, Feng J, et al. H pylori infection is related to mitochondrial microsatellite instability in gastric carcinogenesis. Infect Agent Cancer. 2016;11:1. doi: 10.1186/s13027-016-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt S, Kwok T, Hartig R, König W, Backert S. NF-κB activation and potentiation of proinflammatory responses by theHelicobacter pylori CagA protein. Proc Natl Acad Sci U SA. 2005;102:9300–5. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ando T, Kusugami K, Ohsuga M, Shinoda M, Sakakibara M, Saito H, et al. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am J Gastroenterol. 1996;91(6):1150–6. [PubMed] [Google Scholar]
- 15.Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 16.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou P, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–5. [PubMed] [Google Scholar]
- 17.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993;90:5791–5. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–74. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 19.Kwok T, Zabler D, Urman S, Rohde M, Hartig R, Wessler S, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862–6. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- 20.Schätzle S, Specht M, Waidner B. Coiled Coil Rich Proteins (Ccrp) influence molecular pathogenicity of Helicobacter pylori. PloS One. 2015;10:e0121463. doi: 10.1371/journal.pone.0121463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaoka Y, Kikuchi S, El-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123:414–24. doi: 10.1053/gast.2002.34781. [DOI] [PubMed] [Google Scholar]
- 22.Harris P, Mobley H, Perez-Perez G, Blaser M, Smith P. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology. 1996;111:419–25. doi: 10.1053/gast.1996.v111.pm8690207. [DOI] [PubMed] [Google Scholar]
- 23.Su YL, Yang JC, Lee H, Sheu F, Hsu CH, Lin SL, et al. The C-Terminal disulfide bonds of Helicobacter pylori GroES are critical for IL-8 Secretion via the TLR4-dependent pathway in gastric epithelial cells. J Immunol. 2015;194:3997–4007. doi: 10.4049/jimmunol.1401852. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Takeuchi H, Nishioka M, Morimoto N, Kamioka M, Kumon Y, et al. Relationship of IL-8 production and the CagA status in AGS cells infected with Helicobacter pylori exposed to low pH and activating transcription factor 3 (ATF3) Microbiol Res. 2009;164:180–90. doi: 10.1016/j.micres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Higashi H, Tsutsumi R, Fujita A, Yamazaki S, Asaka M, Azuma T, et al. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci U S A. 2002;99:14428–33. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Argent RH, Hale JL, El-Omar EM, Atherton JC. Differences in Helicobacter pylori CagA tyrosine phosphorylation motif patterns between western and East Asian strains, and influences on interleukin-8 secretion. J Med Microbiol. 2008;57:1062–7. doi: 10.1099/jmm.0.2008/001818-0. [DOI] [PubMed] [Google Scholar]
- 27.Argent RH, Kidd M, Owen RJ, Thomas RJ, Limb MC, Atherton JC. Determinants and consequences of different levels of CagA phosphorylation for clinical isolates of Helicobacter pylori Gastroenterology. 2004;127:514–23. doi: 10.1053/j.gastro.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Argent RH, Thomas RJ, Aviles-Jimenez F, Letley DP, Limb MC, El-Omar EM, et al. Toxigenic Helicobacter pylori infection precedes gastric hypochlorhydria in cancer relatives, and H pylori virulence evolves in these families. Clin Cancer Res. 2008;14:2227–35. doi: 10.1158/1078-0432.CCR-07-2022. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Argent RH, Letley DP, Thomas RJ, Atherton JC. Tyrosine phosphorylation of CagA from Chinese Helicobacterpylori isolates in AGS gastric epithelial cells. J Clin Microbiol. 2005;43:786–90. doi: 10.1128/JCM.43.2.786-790.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naito M, Yamazaki T, Tsutsumi R, Higashi H, Onoe K, Yamazaki S, et al. Influence of EPIYA-repeat polymorphism on the phosphorylation-dependent biological activity of Helicobacterpylori CagA. Gastroenterology. 2006;130:1181–90. doi: 10.1053/j.gastro.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 31.Vaziri F, Peerayeh SN, Alebouyeh M, Molaei M, Maghsoudi N, Zali MR. Determination of Helicobacter pylori CagA EPIYA types in Iranian isolates with differen gastroduodenal disorders. Infection, Genetics and Evolution. 2013;17:101–5. doi: 10.1016/j.meegid.2013.03.048. [DOI] [PubMed] [Google Scholar]
- 32.Yuan A, Chen J, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–65. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- 33.Papadakos KS, Sougleri IS, Mentis AF, Hatziloukas E, Sgouras DN. Presence of terminal EPIYA phosphorylation motifs in Helicobacter pylori CagA contributes to IL-8 secretion, irrespective of the number of repeats. PloS One. 2013;8:e56291. doi: 10.1371/journal.pone.0056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sgouras DN, Panayotopoulou EG, Papadakos K, Martinez- Gonzalez B, Roumbani A, Panayiotou J, et al. CagA and VacA polymorphisms do not correlate with severity of histopathological lesions in Helicobacter pylori-infected Greek children. J Clin Microbiol. 2009;47:2426–34. doi: 10.1128/JCM.00159-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panayotopoulou EG, Sgouras DN, Papadakos KS, Petraki K, Breurec S, Michopoulos S, et al. CagA and VacA polymorphisms are associated with distinct pathological features in Helicobacterpylori-infected adults with peptic ulcer and non-peptic ulcer disease. J Clin Microbiol. 2010;48:2237–9. doi: 10.1128/JCM.00662-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer W, Püls J, Buhrdorf R, Gebert B, Odenbreit S, Haas R. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin‐8. Mol Microbiol. 2001;42:1337–48. doi: 10.1046/j.1365-2958.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- 37.Selbach M, Moese S, Meyer TF, Backert S. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect Immun. 2002;70:665–71. doi: 10.1128/iai.70.2.665-671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nozawa Y, Nishihara K, Akizawa Y, Orimoto N, Nakano M, Uji T, et al. Protein kinase C activation by Helicobacter pylori in human gastric epithelial cells limits interleukin-8 production through suppression of extracellular signal-regulated kinase. J Pharmacol Sci. 2004;94:233–9. doi: 10.1254/jphs.94.233. [DOI] [PubMed] [Google Scholar]
- 39.Kim SY, Lee YC, Kim HK, Blaser MJ. Helicobacter pylori CagA transfection of gastric epithelial cells induces interleukin‐8. Cell Microbiol. 2006;8:97–106. doi: 10.1111/j.1462-5822.2005.00603.x. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, et al. Helicobacter pylori CagA phosphorylation- independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Ren S, Higashi H, Lu H, Azuma T, Hatakeyama M. Structural basis and functional consequence of Helicobacter pylori CagA multimerization in cells. J Biol Chem. 2006;281:32344–52. doi: 10.1074/jbc.M606172200. [DOI] [PubMed] [Google Scholar]
- 42.Gorrell RJ, Guan J, Xin Y, Tafreshi MA, Hutton ML, McGuckin MA, et al. A novel NOD1‐and CagA‐independent pathway of interleukin‐8 induction mediated by the Helicobacter pylori type IV secretion system. Cell Microbiol. 2013;15:554–70. doi: 10.1111/cmi.12055. [DOI] [PubMed] [Google Scholar]
- 43.Backert S, Naumann M. What a disorder: proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010;18:479–86. doi: 10.1016/j.tim.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Ando T, Peek RM, Lee Y-C, Krishna U, Kusugami K, Blaser MJ. Host cell responses to genotypically similar Helicobacter pylori isolates from United States and Japan. Clin Diagn Lab Immunol. 2002;9:167–75. doi: 10.1128/CDLI.9.1.167-175.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng YC, Ho SP, Shyu CL, Chang CS, Huang LR. Clarithromycin modulates Helicobacter pylori-induced activation of nuclear factor-κB through classical and alternative pathways in gastric epithelial cells. Clin Exp Med. 2014;14:53–9. doi: 10.1007/s10238-012-0217-2. [DOI] [PubMed] [Google Scholar]