Abstract
Objective
Pathologies in the heart-brain axis might, independently or in combination, accelerate the process of brain parenchymal loss. We aimed to investigate the association of serum N-terminal brain natriuretic peptide (NT-proBNP), as a marker of cardiac dysfunction, and carotid intima media thickness (CIMT), as a marker of carotid atherosclerosis burden, with structural brain changes.
Approach and Results
In the longitudinal population-based AGES–Reykjavik Study, we included 2430 subjects (mean age 74.6, 41.4% male) with baseline data on NT-proBNP and CITM (assessed by Ultrasound imaging). Participants underwent a high-resolution brain MRI at baseline and five years later to assess total brain (TBV), grey matter (GMV) and white matter (WMV) volumes. Each unit higher log-transformed NT-proBNP was associated with 3.6 ml (95% CI: −6.0, −1.1) decline in TBV and 3.5 ml (95% CI: −5.7, −1.3) decline in GMV. Likewise, each millimeter higher CIMT was associated with 10.8 ml (95% CI: −17.3, −4.2) decline in TBV and 8.6 ml (95% CI: −14.4, −2.8) decline in GMV. There was no association between NT-proBNP and CIMT and changes in WMV. Compared to participants with low NT-proBNP and CIMT, participants with both high NT-proBNP and CIMT had 3.8 ml (95% CI: −6.0, −1.6) greater decline in their TBV and 4ml (95% CI: −6.0, −2.0) greater decline in GMW. These associations were independent of socio-demographic and cardiovascular factors.
Conclusions
Older subjects with both cardiac dysfunction and carotid atherosclerosis are at an increased risk for brain parenchymal loss. Accumulated pathologies in the heart-brain axis might accelerate brain atrophy.
Keywords: Natriuretic peptide, carotid, brain, white matter, grey matter
Introduction
Current evidence indicates that individuals with high burden of cardiovascular comorbidities, even independent of cerebrovascular diseases, run a greater risk of brain atrophy 30. The brain is a highly vascular organ and requires a constant and well regulated levels of blood flow to maintain its structural integrity 31. It is well-established that the intact function of the heart and extra-cranial vessel is crucial for regulation of the cerebral circulation 32. Hence, accumulation of pathologies in the heart-brain axis can potentially increase the risk of brain parenchymal loss.
Previous patient-based studies have consistently reported that advanced cardiac and carotid pathologies are associated with accelerated brain structural changes 33. Patients with congestive heart failure 34 and carotid stenosis6 more frequently develop brain parenchymal loss and it has been shown that patients who develop cardiac arrest, after resuscitation, suffer from an extensive reduction of their brain parenchymal volume in particular gray matter 7. Although it is widely accepted that cardiovascular pathologies in the heart-brain axis are relevant for brain health, up to now limited robust longitudinal data from general populations exists to substantiate this common preconception. Despite the evidence from patients’ populations, it remains to be known whether community-dwelling older subjects with less severe or subclinical cardiac impairment or carotid atherosclerosis are also at a greater risk for accelerated brain structural changes. Furthermore, it is unclear whether cumulative pathologies in the heart and carotid arteries result in higher degrees of brain parenchymal loss.
In this population-based study of older subjects, we aim to investigate whether higher levels of serum N-terminal brain natriuretic peptide (NT-proBNP), as a marker of left ventricular dysfunction 8, 9, and common carotid intima media thickness (CIMT), reflecting atherosclerosis burden in the carotid artery 10, independently or in combination are linked with accelerated structural brain changes.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Baseline mean age of the participants was 74.6 years and 41.4% were male. Median NT-proBNP level was 124.9 ng/L and mean value of CIMT was 1.0 mm. Average total brain tissue, grey matter and white matter volumes were 1077.8 ml, 686.7 ml and 391.1 ml respectively (Table 1). Cross-sectional analyses showed that higher NT-proBNP was associated with lower total brain volume as well as grey matter volume (Suppl. Table-II). There was no cross-sectional association between CIMT and brain volumes at baseline (Suppl. Table-III).
Table 1.
Baseline characteristics of study participants (n=2430)
| Characteristics | Values |
|---|---|
| Age, mean (SD) | 74.6 (4.8) |
| Male, n (%) | 1005 (41.4) |
| Low education*, n (%) | 487 (20.0) |
| Current smoker, n (%) | 269 (11.1) |
| Hypertension**, n (%) | 1886 (77.6) |
| Diabetes mellitus, n (%) | 225 (9.3) |
| Body mass index, kg/m2, mean (SD) | 27.2 (4.1) |
| Systolic blood pressure, mmHg, mean (SD) | 141.3 (19.8) |
| Diastolic blood pressure, mmHg, mean (SD) | 74.2 (9.4) |
| Total cholesterol, mmol/L, mean (SD) | 5.6 (1.1) |
| eGFR, ml/min, mean (SD) | 66.8 (18.5) |
| Coronary heart disease, n (%) | 449 (18.5) |
| Atrial fibrillation, n (%) | 256 (10.6) |
| Stroke, n (%) | 99 (4.6) |
| Dementia | 31 (1.3) |
| Serum NT-proBNP (ng/L), median (IQR) | 124.9 (70.5–229.3) |
| Carotid intima media thickness (mm), mean (SD) | 1.0 (0.1) |
| Total brain tissue volume, (ml), mean (SD) | 1077.8 (102.6) |
| Grey matter volume, (ml), mean (SD) | 686.7 (62.5) |
| White matter volume, (ml), mean (SD) | 391.1 (47.9) |
Abbreviations: n: number, SD: standard deviation, IQR: interquartile range, eGFR: estimated glomerular filtration rate
Primary school education or less
History of hypertension or use of antihypertensive medications
In the longitudinal analyses, each unit higher log-transfomed serum NT-proBNP was associated with 3.9 ml (95% confidence interval (CI): −6.0, −1.8) decrease in total brain parenchymal volume (Table 2, Model 1). Similarly, each unit higher log-transformed serum NT-proBNP was associated with 3.7 ml (95% CI: −5.6, −1,8) decrease in grey matter volume. Although a trend was observed in the association between higher serum NT-proBNP and higher white matter volume loss, the associations did not reach statistical significance (p=0.09). Further adjustment of the analyses for socio-demographic and cardiovascular risk factors did not essentially change these associations (Table 2, Model 2).
Table 2.
Changes in brain parenchymal volumes in relation to serum NT-proBNP
| Serum NT-proBNP (ng/L) | |||||
|---|---|---|---|---|---|
|
| |||||
| Low N=812 5–87.7 |
Middle N=796 87.8–179.4 |
High N=822 179.5–4795 |
Beta* (95% CI) | P-value | |
| Δ Total brain volume, ml, mean (SE) | |||||
| Model-1 | −38.1 (0.7) | −40.3 (0.7) | −41.0 (0.7) | −3.9 (−6.0, −1.8) | <0.001 |
| Model-2 | −41.3 (1.7) | −43.8 (1.7) | −44.0 (1.7) | −3.6 (−6.0, −1.1) | 0.004 |
| Δ Grey matter volume, ml, mean (SE) | |||||
| Model-1 | −14.7 (0.6) | −16.4 (0.6) | −16.7 (0.7) | −3.7 (−5.6, −1,8) | <0.001 |
| Model-2 | −17.5 (1.6) | −19.6 (1.6) | −19.6 (1.6) | −3.5 (−5.7, −1.3) | 0.001 |
| Δ White matter volume, ml, mean (SE) | |||||
| Model-1 | −23.1 (0.4) | −23.9 (0.4) | −24.7 (0.4) | −1.1 (−2.3, 0.18) | 0.093 |
| Model-2 | −27.2 (1.1) | −27.3 (1.1) | −27.8 (1.0) | −0.3 (−1.7, 1.1) | 0.659 |
Abbreviations: SE: standard error
Beta (95% CI) and p-values are calculated using the continuous value of log-transformed NT-proBNP levels
Δ: Second measure - First measure
Model-1: adjusted for age, sex and baseline brain volumes
Model-2: adjusted for age, sex, baseline brain volumes, education, current smoker, hypertension (medical records or antihypertensive medication), diabetes mellitus, systolic blood pressure, coronary heart disease, stroke, total cholesterol, body mass index, atrial fibrillation and glomerular filtration rate
All the analyses are adjusted for intracranial volumes and coil type.
In the longitudinal analyses, each millimeter higher CIMT was associated with 13.3 ml (95% CI: −19.3, −7.2) decrease in total brain parenchymal volume. Similarly, each millimeter higher CIMT was associated with 12.6 ml (95% CI: −18.1, −7.0) decrease in grey matter volume (Table 3, Model 1). There was no association between higher CIMT and decrease in white matter volume (p=0.84). Further adjustments for socio-demographic and cardiovascular risk factors only slightly changed the magnitude of the associations (Table 3, Model 2).
Table 3.
Changes in brain parenchymal volumes in relation to carotid intima media thickness
| Carotid intima media thickness | |||||
|---|---|---|---|---|---|
|
| |||||
| Low N=810 0.62–0.9 mm |
Middle N=811 0.91–1.0 mm |
High N=809 1.02–2.03 mm |
Beta* (95% CI) | P-value | |
| Δ Total brain volume, ml, mean (SE) | |||||
| Model-1 | −37.4 (0.7) | −39.6 (0.7) | −42.3 (0.7) | −13.3 (−19.3, −7.2) | <0.001 |
| Model-2 | −41.4 (1.7) | −42.8 (1.7) | −45.7 (1.7) | −10.8 (−17.3, −4.2) | 0.001 |
| Δ Grey matter volume, ml, mean (SE) | |||||
| Model-1 | −13.8 (0.7) | −15.2 (0.6) | −18.7 (0.6) | −12.6 (−18.1, −7.0) | <0.001 |
| Model-2 | −17.4 (1.6) | −17.9 (1.6) | −21.4 (1.6) | −8.6 (−14.4, −2.8) | 0.004 |
| Δ White matter volume, ml, mean (SE) | |||||
| Model-1 | −23.8 (0.4) | −24.5 (0.4) | −23.5 (0.4) | −0.4 (−4.1, 3.3) | 0.842 |
| Model-2 | −27.6 (1.0) | −28.1 (1.0) | −27.0 (1.0) | −1.3 (−5.1, 2.5) | 0.501 |
Abbreviations: SE: standard error, CIMT: carotid intima media thickness
Beta (95% CI) and p-values are calculated using the continuous value of carotid intima media thickness
Δ: Second measure - First measure
Model-1: adjusted for age, sex and baseline brain volumes
Model-2: adjusted for age, sex, baseline brain volumes, education, current smoker, hypertension, diabetes mellitus, systolic blood pressure, coronary heart disease, stroke, total cholesterol, body mass index, atrial fibrillation and glomerular filtration rate.
All the analyses are adjusted for intracranial volumes and coil type.
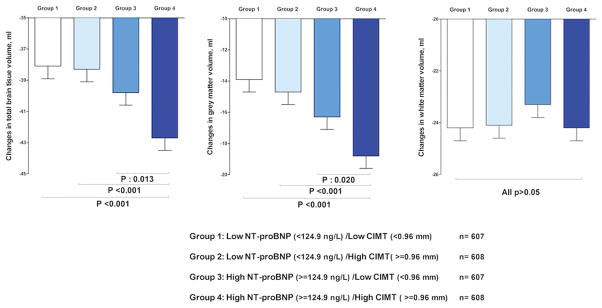
Subjects with high NT-proBNP and high CIMT had the greatest total brain parenchymal and grey matter volume loss compared to the other groups (all p<0.05). For both total and gray matter volumes, the largest difference was observed between subjects with low NT-proBNP and CIMT and subjects with high NT-proBNP and CIMT (both p<0.001). In contrast, there was not such an association in relation to decline in white matter volume (Figure 1). Further adjustments of the analyses for socio-demographic and cardiovascular risk factors revealed similar associations (Suppl. Table-IV). Compared to participants with low NT-proBNP and CIMT, participants with both high NT-proBNP and CIMT had 3.8 ml (95% CI: −6.0, −1.6) higher decline in their TBV and 4ml (95% CI: −6.0, −2.0) higher decline in GMW. The interaction between NT-proBNP and CIMT in relation to brain volume loss was significant for total brain parenchymal volume (p=0.04) and suggestive for grey matter volume (p=0.15) indicating that subjects with both high NT-proBNP and CIMT had greatest decline in brain parenchymal volumes. In a sensitivity analysis we excluded participants who had stroke and/or dementia (n=128). We observed similar findings after this exclusion (data not shown). In a series of extra analyses, we tested the associations using inverse probability weighting method. Repeating the analyses with this method revealed similar findings with larger effect estimates (Suppl. Tables V, VI, VII).
Figure 1.
Brain parenchymal loss in relation to cardiac and carotid measures. Changes in brain volumes in relation to levels of N-terminal pro brain natriuretic peptide (NT-proBNP) and carotid intima media thickness (CIMT). Bars represent mean changes (standard error) in four years of follow up (second MRI measures minus first) adjusted for age, sex, baseline brain volumes, intracranial volume and coil type. Probability values are calculated for the difference between high NT-proBNP/high CIMT and other groups.
Discussion
In this prospective cohort study of community dwelling older subjects, we showed that elevated NT-proBNP, as a marker of left ventricular dysfunction 8, and CIMT, as a measure of atherosclerosis burden at the carotid artery 10, are associated with steeper decline in the structural brain volumes. We found that combination of high NT-proBNP and CIMT is linked with greatest risk for accelerated structural brain changes.
Different lines of evidence from epidemiological and neuroimaging studies indicate that long-lasting exposure to cardiovascular risk factors, independent of cerebrovascular accidents, are associated with higher risk of brain parenchymal loss and atrophy11–13. Although the exact mechanism behind this association is not fully known, an increasing body of evidence suggests that cerebral hypoperfusion is a key factor linking cardiovascular morbidities with accelerated brain structural changes14. The brain is a demanding organ consuming about 20% of total body oxygen and 25% of total body glucose. Therefore, integrity of the brain depends on adequate supply of oxygen and energy through blood flow15. It is known that multiple systemic and cerebrovascular mechanisms act in concert to maintain cerebral blood flow in a stable range despite fluctuations in the systemic perfusion16. However, in the presence of prolonged low systemic perfusion, due to pathologies in the heart and large vessels, these regulatory mechanisms might fail to safeguard the brain and eventually cerebral hypoperfusion occurs.
The heart is the generator of the cerebral perfusion pressure and intra and extra-cranial vessels modify the cerebral perfusion as they deliver the blood to the brain17. Given the increasing prevalence of pathologies affecting the heart and cerebropetal arteries in the elderly, these pathologies can be expected to contribute to changes in the brain as people age. Although previous studies showed that individual pathologies at the levels of heart or carotid arteries are independently associated with adverse brain outcomes18, the current study shows that accumulated pathologies in the heart and carotid arteries might reinforce each other and put older subjects at an extra risk for accelerated brain parenchymal loss. We observed that impaired cardiac function and carotid atherosclerosis are mainly linked to grey matter volume decline rather than white matter volume decline. The brain receives about 15% of cardiac output and it is well-known that more than two thirds of total cerebral blood flow is directed toward grey matter because of its high metabolic demand 19. Therefore, it is expected that grey matter, more than white matter, becomes vulnerable to pathologies in the heart-brain axis that jeopardize blood flow to the brain.
Adequate and constant cerebral perfusion is safeguarded by the so-called cerebral autoregulation. Cerebral autoregulation encompasses a series of complex physiologic functions to match the fluctuating systemic supply of blood flow with the high energy demands of the brain20. Although such a regulatory mechanism acts quickly in the face of immediate changes in the systemic perfusion pressure, it is yet to be determined whether cerebral autoregulation can also handle the long-lasting hypoperfusion state due to established pathologies in the heart-brain axis21. Previous studies have shown that cardiovascular risk factors are associated with lower cerebral blood flow22. Hence, chronic cerebral hypoperfusion might be a key mechanism behind the link between cardiac and carotids pathologies and accelerated brain parenchymal loss. In addition to the plausible roles of hypo-perfusion in this link, it needs to be acknowledged that impaired cardiac function and advanced atherosclerosis might also reflect a global systemic vascular condition that affects not only the large vessels but also small vessels 23. In addition, other pathways such pro-inflammatory state and activation of the renin angiotensin system might play contribute in this link. Both high systemic inflammation and activation of renin angiotensin system have been explained in association with higher cardiovascular burden and atherosclerosis, which can independently affect the brain and speed up the pace of brain parenchymal loss 24, 25.
The growing numbers of older people and increasing prevalence of age-related disorders of the brain such as dementia warrant further studies to identify novel strategies to decelerate pace of brain ageing 26, 27. Although the pivotal roles of the heart and extracranial arteries in providing adequate oxygen, glucose and nutrient for the brain are well-established, limited evidence exists regarding the contribution of the heart-brain axis in the development and progression of abnormal brain ageing in older adults. Findings of this study might highlight that older adults with higher loads of multiple pathologies in the heart-brain axis should be considered as high risk groups for accelerated brain ageing. While previous reports indicate that in normal ageing subjects experience 0.32% annual decline in their total brain volumes28, in our study, subjects with both high NT-proBNP and CIMT had about one percent annual brain parenchymal loss. The magnitude of this change becomes even clearer when it is compared with annual brain parenchymal loss of patients with Alzheimer’s disease which is two percent 29. Collectively, our results warrant closer collaboration between cardiologists and neurologists in identification of older patients at highest risk for accelerated brain ageing. Once clinicians detect pathologies at the cardiac level should search for concomitant abnormalities in the other parts of the heart-brain axis and vice versa.
This study has a number of strengths and limitations. Availability of repeated neuroimaging data and information regarding the cardiovascular status of more than 2000 community dwelling older men and women are among the major strengths of this study. As a limitation, we could only include subjects who survived up to the follow up session, which might limit generalizability of our results. Nonetheless, as the inverse probability weighting analyses suggested, it is expected that our findings might even underestimate magnitude of the associations since survivors are generally healthier. It needs to be pointed out that despite all the adjustments for the conventional cardiovascular risk factors, we cannot exclude possible roles of unmeasured confounders on the associations. In this study we used the mean of far and near wall CIMT to assess the degree of carotid atherosclerosis. While this method has been validated before, it is generally recognized that the far wall CIMT might better reflect the true thickness of the carotid wall. We assessed the left ventricular function using serum NT-proBNP. Although it has been shown that NT-proBNP is a reliable marker for left ventricular dysfunction 9, future studies are needed to confirm the link between impaired cardiac function and brain parenchymal loss using imaging techniques such as echocardiography and cardiac MRI.
This study shows that high serum NT-proBNP, as a marker for impaired cardiac function, and intima media thickness reflecting atherosclerosis burden in carotid arteries are linked with accelerated structural brain changes. Combination of high NT-proBNP and high CIMT was associated with greatest brain parenchymal loss. This study suggests that pathologies at the levels of the heart and carotid arteries act in concert and, instead of discipline-specific evaluations by cardiologists and neurologists, older subjects might further benefit from a comprehensive evaluation of the heart-brain axis in relation to adverse brain outcomes. Findings of this study call for further research on the cumulative effects of cardiac and carotid abnormalities on the brain parenchymal loss. Our data in a general population of older subjects need to be replicated in middle aged and younger adults when interventions might have a bigger impact for brain health in the subsequent years. Furthermore, the exact mechanisms behind the observed associations need to be further explored by experiments on animal models and interventional approaches targeting multiple components of the heart-brain axis to better understand the interplays of various cardiovascular mechanisms that affect brain structural integrity.
Methods
Study population
This study was performed in the framework of the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study. The AGES–Reykjavik study consists of a population-based cohort of community dwelling older adults born between 1907 and 1935 and living in Reykjavik in 1967 when the Icelandic Heart Association initiated the Reykjavik Study. In the first (baseline) phase of AGES–Reykjavik Study (2002–2006), 5,764 (42% male) participants were examined. For each subject the baseline examination was completed in three clinic visits, with a participant’s full examination finished within a 4- to 6-week time window. Participants were followed for five years and data acquisition in the follow-up exam was done between 2007 and 2012. Details of the AGES–Reykjavik study have been reported previously1. The AGES–Reykjavik Study was approved by the National Bioethics Committee in Iceland that acts as the institutional review board for the Icelandic Heart Association and by the National Institute on Aging intramural institutional review board. At the baseline examination 4397 subjects had data on NT-proBNP, CIMT and brain MRI. In this longitudinal study, we included 2430 participants for whom data on serum NT-proBNP, CIMT and brain MRI (both baseline and follow-up sessions) were available. Compared to the baseline population, included participants were relatively younger and had less cardiovascular co-morbidities (Suppl. Table-I).
Serum NT-proBNP
Blood samples were drawn in the first clinical visit. The laboratory of the Icelandic Heart Association measured serum NT-proBNP using the Elecsys proBNPII sandwich immunoassay using two monoclonal antibodies on a Cobas e411 instruments (Roche Diagnsotics, Basel, Switzerland). The analyses were performed using a single lot of reagents. The manufacturer’s controls were used to monitor quality control with limits of acceptability defined by the manufacturer. The low control coefficient of variation (CV) was 2,7% and high control CV was 3,2 %. The limit of sensitivity was 5 ng/L.
Carotid intima-media thickness (CIMT)
CIMT was assessed using Ultrasound images which were acquired with a Sequoia C256 (Siemen Medical Systems, Erlangen, Germany). Standard B-mode images of the CIMT were obtained for the predefined segment of each common carotid artery (CCA; right and left) at defined interrogation angles using Meijers arc. Standard images were recorded from four angles at each site. The mean CIMT of the near and far walls were determined from a single image at each interrogation angle for both the right and left CCA. The average of all these CIMT values comprised the CIMT outcome parameter. The details of intima-media thickness analysis protocol were described previously 2.
Brain Imaging
At baseline and follow up sessions, all the participants underwent a high-resolution brain MRI scanning acquired on a study-dedicated 1.5-T Signa Twinspeed system (GE Healthcare). The imaging protocol has been described previously 1, 3 and included 3D spoiled-gradient recalled T1-weighted, fast spin echo proton density/T2-weighted, fluid-attenuated inversion recovery (FLAIR) and echo-planar imaging gradient echo T2*-weighted sequences. All images were acquired to give full brain coverage with slices angled parallel to the anterior commissure–posterior commissure line in order to give reproducible image views in the oblique-axial plane. Total brain, white and grey matter volumes were computed automatically with an algorithm based on the Montreal Neurological Institute pipeline. The AGES-Reykjavik/Montreal Neurological Institute pipeline has been modified to accommodate full brain coverage including cerebellum and brainstem, multispectral images (T1-weighted 3D spoiled-gradient recalled sequence, FLAIR and proton density/T2-weighted fast spin echo sequences), high throughput and minimal editing. The segmentation pipeline, its components and accuracy have been described in detail elsewhere 4. There was a coil upgrade soon after the study began. In a quality control study we scanned 33 subjects with the old and new coil. Although there were no detectable differences in the parameters we calculated, we enter coil type into models to reflect the change of protocol. Changes in brain structural volumes were calculated as the difference between follow up measures and baseline measures.
Other covariates
Level of education and smoking status were assessed by questionnaires. Diabetes was defined as a history of diabetes, use of glucose-modifying medication, or fasting blood glucose of ≥ 7 mmol/L. Hypertension was defined as measured systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg, or self-reported doctor’s diagnosis of hypertension, or using antihypertensive medications. History of stroke was recorded using questionnaires and medical reports. Prevalent coronary heart disease was defined as self-reported history of coronary artery disease or coronary artery bypass surgery or angioplasty or angina pectoris on the Rose Angina Questionnaire, hospital records or evidence on ECG of possible or probable myocardial infarction. The diagnosis of atrial fibrillation was made by a twelve lead electrocardiogram (ECG) performed during the AGES-Reykjavik study comprehensive examination based on the Minnesota codes. Additionally, hospital discharge diagnosis codes from all hospitals in Reykjavik from January 1987 until the day of the study examination were reviewed for the diagnosis of atrial fibrillation (ICD-9 code 427.9 or ICD-10 code I48).
Statistical analyses
Characteristics of the study participants are reported as mean (standard deviation) or median (interquartile range) for continuous variables and number (percentage) for categorical variables. Analyses on the association of NT-proBNP and CIMT with brain structural volumes were done using linear regression models. Given the skewed distribution of serum NT-proBNP, the log-transformed NT-proBNP was used in the analyses. In addition, analysis of covariance was applied to calculate the adjusted means and standard errors for the brain volumes in tertiles of NT-proBNP and CIMT. We performed our analyses in two steps. In the first step, all the analyses were adjusted for age and sex. In the next step, the analyses were additionally adjusted for socio-demographic and cardiovascular factors including education, current smoker, hypertension and/or use of antihypertensive medications, diabetes mellitus, systolic blood pressure, history of coronary heart disease, history of stroke, total cholesterol, body mass index, atrial fibrillation and estimated glomerular filtration rate. All models were corrected for total intracranial volume and MRI coil type. Furthermore, the longitudinal associations were adjusted for baseline brain volumes. The continuous variables were added to the statistical models in their original format without categorization. To test whether subjects with both high NT-proBNP and CMIT are at the highest risk for decline in brain volumes, we divided the cohort into four groups split at the median values of NT-proBNP and CIMT. We tested for group differences and specifically whether the high NT-proBNP/high CIMT group had significantly higher change in brain volumes as compared to the other groups. Further, in fully adjusted model; we tested for the interaction between categories of NT-proBNP and CIMT. To boost our statistical signals for detection of interactions, we a priori considered a p value <0.1 to suggest a significant interaction to follow-up on with a stratified analysis. For the rest of analyses p<0.05 was set as the level to reject the null hypothesis. To assess the sensitivity of our results to missing data to the follow up we repeated the analyses with inverse probability weighting method5. Using this method, observations are weighted by the inverse of the probability of an individual that is in the baseline participating in the follow up session. All analyses were carried out using SPSS software (version 20.0.0, SPSS Inc., Chicago, IL).
Supplementary Material
Highlights.
Markers of cardiac impairment and carotid atherosclerosis are associated with accelerated brain parenchymal loss.
The combination of pathologies at the levels of the heart and carotid arteries poses an accentuated risk for brain atrophy.
Older subjects can further benefit from a comprehensive evaluation of the heart-brain axis, instead of a discipline-specific approach, in relation to adverse brain outcomes.
Acknowledgments
Sources of Funding
This study was funded by the National Heart, Lung, and Blood Institute Intramural Research Program (Z01 HL004607-08 CE), the National Institute on Aging Intramural Research Program (N01-AG-12100), Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). The study was approved by the Icelandic National Bioethics Committee (VSN: 00-063) and the Medstar Research Institute (project 2003-145). Behnam Sabayan is partly supported by a grant from Internationale Stichting Alzheimer Onderzoek (ISAO).
Disclosure
None
Abbreviations
- NT-proBNP
N-terminal brain natriuretic peptide
- CIMT
carotid intima media thickness
- CI
Confidence interval
References
- 1.Knopman DS, Mosley TH, Catellier DJ, Sharrett AR Atherosclerosis Risk in Communities S. Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology. 2005;65:876–81. doi: 10.1212/01.wnl.0000176074.09733.a8. [DOI] [PubMed] [Google Scholar]
- 2.Raichle ME, Gusnard DA. Appraising the brain’s energy budget. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10237–9. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panerai RB. Cerebral autoregulation: from models to clinical applications. Cardiovascular engineering. 2008;8:42–59. doi: 10.1007/s10558-007-9044-6. [DOI] [PubMed] [Google Scholar]
- 4.Marshall RS, Lazar RM. Pumps, aqueducts, and drought management: vascular physiology in vascular cognitive impairment. Stroke; a journal of cerebral circulation. 2011;42:221–6. doi: 10.1161/STROKEAHA.110.595645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogels RL, van der Flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Brain magnetic resonance imaging abnormalities in patients with heart failure. European journal of heart failure. 2007;9:1003–9. doi: 10.1016/j.ejheart.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Muller M, van der Graaf Y, Algra A, Hendrikse J, Mali WP, Geerlings MI, Group SS. Carotid atherosclerosis and progression of brain atrophy: the SMART-MR study. Annals of neurology. 2011;70:237–44. doi: 10.1002/ana.22392. [DOI] [PubMed] [Google Scholar]
- 7.Horstmann A, Frisch S, Jentzsch RT, Muller K, Villringer A, Schroeter ML. Resuscitating the heart but losing the brain: brain atrophy in the aftermath of cardiac arrest. Neurology. 2010;74:306–12. doi: 10.1212/WNL.0b013e3181cbcd6f. [DOI] [PubMed] [Google Scholar]
- 8.Bay M, Kirk V, Parner J, Hassager C, Nielsen H, Krogsgaard K, Trawinski J, Boesgaard S, Aldershvile J. NT-proBNP: a new diagnostic screening tool to differentiate between patients with normal and reduced left ventricular systolic function. Heart. 2003;89:150–4. doi: 10.1136/heart.89.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macheret F, Boerrigter G, McKie P, Costello-Boerrigter L, Lahr B, Heublein D, Sandberg S, Ikeda Y, Cataliotti A, Bailey K, Rodeheffer R, Burnett JC., Jr Pro-B-type natriuretic peptide(1-108) circulates in the general community: plasma determinants and detection of left ventricular dysfunction. Journal of the American College of Cardiology. 2011;57:1386–95. doi: 10.1016/j.jacc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Groot E, van Leuven SI, Duivenvoorden R, Meuwese MC, Akdim F, Bots ML, Kastelein JJ. Measurement of carotid intima-media thickness to assess progression and regression of atherosclerosis. Nature clinical practice Cardiovascular medicine. 2008;5:280–8. doi: 10.1038/ncpcardio1163. [DOI] [PubMed] [Google Scholar]
- 11.Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, Smith S, Schmidt R. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64:1704–11. doi: 10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed] [Google Scholar]
- 12.Carmelli D, Swan GE, Reed T, Wolf PA, Miller BL, DeCarli C. Midlife cardiovascular risk factors and brain morphology in identical older male twins. Neurology. 1999;52:1119–24. doi: 10.1212/wnl.52.6.1119. [DOI] [PubMed] [Google Scholar]
- 13.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–8. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature reviews Neuroscience. 2011;12:723–38. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erecinska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respiration physiology. 2001;128:263–76. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 16.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–43. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritz K, van Buchem MA, Daemen MJ. The heart-brain connection: mechanistic insights and models. Neth Heart J. 2013;21:55–7. doi: 10.1007/s12471-012-0348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Buchem MA, Biessels GJ, Brunner la Rocca HP, de Craen AJ, van der Flier WM, Ikram MA, Kappelle LJ, Koudstaal PJ, Mooijaart SP, Niessen W, van Oostenbrugge R, de Roos A, van Rossum AC, Daemen MJ. The heart-brain connection: a multidisciplinary approach targeting a missing link in the pathophysiology of vascular cognitive impairment. J Alzheimers Dis. 2014;42(Suppl 4):S443–51. doi: 10.3233/JAD-141542. [DOI] [PubMed] [Google Scholar]
- 19.Willie CK, Smith KJ. Fuelling the exercising brain: a regulatory quagmire for lactate metabolism. J Physiol. 2011;589:779–80. doi: 10.1113/jphysiol.2010.204776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–85. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- 21.Ruland S, Aiyagari V. Cerebral autoregulation and blood pressure lowering. Hypertension. 2007;49:977–8. doi: 10.1161/HYPERTENSIONAHA.107.087502. [DOI] [PubMed] [Google Scholar]
- 22.Pase MP, Grima NA, Stough CK, Scholey A, Pipingas A. Cardiovascular disease risk and cerebral blood flow velocity. Stroke. 2012;43:2803–5. doi: 10.1161/STROKEAHA.112.666727. [DOI] [PubMed] [Google Scholar]
- 23.Nitkunan A, Lanfranconi S, Charlton RA, Barrick TR, Markus HS. Brain atrophy and cerebral small vessel disease: a prospective follow-up study. Stroke; a journal of cerebral circulation. 2011;42:133–8. doi: 10.1161/STROKEAHA.110.594267. [DOI] [PubMed] [Google Scholar]
- 24.Rosano C, Marsland AL, Gianaros PJ. Maintaining brain health by monitoring inflammatory processes: a mechanism to promote successful aging. Aging and disease. 2012;3:16–33. [PMC free article] [PubMed] [Google Scholar]
- 25.Kehoe PG, Passmore PA. The renin-angiotensin system and antihypertensive drugs in Alzheimer’s disease: current standing of the angiotensin hypothesis? J Alzheimers Dis. 2012;30(Suppl 2):S251–68. doi: 10.3233/JAD-2012-111376. [DOI] [PubMed] [Google Scholar]
- 26.Trollor JN, Valenzuela MJ. Brain ageing in the new millennium. Aust N Z J Psychiatry. 2001;35:788–805. doi: 10.1046/j.1440-1614.2001.00969.x. [DOI] [PubMed] [Google Scholar]
- 27.Abbott A. Cognition: The brain’s decline. Nature. 2012;492:S4–5. doi: 10.1038/492S4a. [DOI] [PubMed] [Google Scholar]
- 28.Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Archives of neurology. 2003;60:989–94. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 29.Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, Sexton G, Kaye JA. Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology. 2003;61:487–92. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
References
- 1.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. American journal of epidemiology. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonsson H, Helgadottir GP, Aspelund T, Eiriksdottir G, Sigurdsson S, Ingvarsson T, Harris TB, Launer L, Gudnason V. Hand osteoarthritis in older women is associated with carotid and coronary atherosclerosis: the AGES Reykjavik study. Ann Rheum Dis. 2009;68:1696–700. doi: 10.1136/ard.2008.096289. [DOI] [PubMed] [Google Scholar]
- 3.Scher AI, Gudmundsson LS, Sigurdsson S, Ghambaryan A, Aspelund T, Eiriksdottir G, van Buchem MA, Gudnason V, Launer LJ. Migraine headache in middle age and late-life brain infarcts. JAMA. 2009;301:2563–70. doi: 10.1001/jama.2009.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigurdsson S, Aspelund T, Forsberg L, Fredriksson J, Kjartansson O, Oskarsdottir B, Jonsson PV, Eiriksdottir G, Harris TB, Zijdenbos A, van Buchem MA, Launer LJ, Gudnason V. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik study. Neuroimage. 2012;59:3862–70. doi: 10.1016/j.neuroimage.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Statistical methods in medical research. 2013;22:278–95. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.