Abstract
Background
Due to its potent effects on social behavior, including maternal behavior, oxytocin has been identified as a potential mediator of postpartum depression and anxiety. The objective of this study was to examine the relationship between peripartum synthetic oxytocin administration and the development of depressive and anxiety disorders within the first year postpartum. We hypothesized that women exposed to peripartum synthetic oxytocin would have a reduced risk of postpartum depressive and anxiety disorders compared with those without any exposure.
Methods
Population-based data available through the Massachusetts Integrated Clinical Academic Research Database (MiCARD) was used to retrospectively (2005–2014) examine this relationship and calculate the relative risk of peripartum synthetic oxytocin for the development of postpartum depressive and anxiety disorders in exposed (n=9,684) compared to unexposed (n=37,048) deliveries.
Results
Among deliveries to women with a history of prepregnancy depressive or anxiety disorder, exposure to peripartum oxytocin increased the risk of postpartum depressive or anxiety disorder by 36% (RR: 1.36; 95%CI: 1.20–1.55). In deliveries to women with no history of prepregnancy depressive or anxiety disorder, exposure to peripartum oxytocin increased the risk of postpartum depressive or anxiety disorder by 32% compared to those not exposed (RR: 1.32; 95% CI: 1.23–1.42).
Conclusions
Contrary to our hypothesis, results indicate that women with peripartum exposure to synthetic oxytocin had a higher relative risk of receiving a documented depressive or anxiety disorder diagnosis or antidepressant/anxiolytic prescription within the first year postpartum than women without synthetic oxytocin exposure.
INTRODUCTION
Postpartum depressive and anxiety disorders are diagnosed in up to 12.9% and 20.4% of women, respectively (Austin et al., 2010; Gaynes et al., 2005; Reck et al., 2008; Vesga-Lopez et al., 2008), and adversely affect mothers and their offspring (Earls, 2010; Goodman, 2007). Postpartum depression has an effect on maternal functioning and can be fatal; maternal suicide accounts for up to 20% of deaths in depressed postpartum women (Lindahl, Pearson, & Colpe, 2005). For offspring, maternal postpartum depression is associated with difficult infant and childhood temperament (Britton, 2011; Feldman et al., 2009), attachment insecurity (Forman et al., 2007), developmental delays (Deave, Heron, Evans, & Emond, 2008), changes in cognitive development (Azak, 2012; Murray & Cooper, 1997; Sohr-Preston & Scaramella, 2006), and difficulties with social interaction (Downey & Coyne, 1990). Postpartum anxiety is associated with infant irritability, poor infant sleep, and breastfeeding difficulty (Jover et al., 2014).
Despite negative impacts on both mother and child, the pathophysiology of postpartum depressive and anxiety disorders is poorly understood, with relatively few animal models (Perani & Slattery, 2014). Due to its role in the modulation of social behavior, especially affiliative bonding, oxytocin has been identified as a potential mediator of postpartum depression and anxiety (Sohye Kim et al., 2014)(Kim et al., 2014). Animal studies document a relationship between oxytocin and expressions of normal maternal behavior (Finkenwirth, Martins, Deschner, & Burkart, 2016; Nephew, 2012; Rilling & Young, 2014), as well as with animal models of postpartum psychiatric disorders (Babb, Carini, Spears, & Nephew, 2014; Murgatroyd & Nephew, 2013; Perani & Slattery, 2014) and the effects of labor induction on offspring neural development (Hashemi et al., 2013). This preclinical literature suggests that the use of synthetic oxytocin in the peripartum period may have long-term effects on mood in both mothers and their offspring and led to the recent introduction of oxytocin manipulations as potential therapeutic targets for the treatment of postpartum psychiatric disorders (Aleeca F. Bell, Erickson, & Carter, 2014; Kim et al., 2013; Strathearn, 2011).
In humans, maternal plasma levels of oxytocin are associated with maternal gaze, vocalizations, and affectionate contact (Feldman, Weller, Zagoory-Sharon, & Levine, 2007), as well as maternal representations of attachment (Aleeca F. Bell, et al., 2014; Levine, Zagoory-Sharon, Feldman, & Weller, 2007). Two clinical studies have found that low antepartum blood oxytocin levels are correlated with postpartum depression (Skrundz, Bolten, Nast, Hellhammer, & Meinlschmidt, 2011; A. M. Stuebe, Grewen, & Meltzer-Brody, 2013), and related work reports an association between prepartum depression and low oxytocin (L. Garfield et al., 2015).(Garfield et al., 2014). Genetic analyses indicate that postpartum depression may be mediated by epigenetic variation in oxytocin receptor expression (A. F. Bell et al., 2015), and a similar relationship is observed in non-maternal depressed women (Reiner et al., 2015). Failed lactation (A.M. Stuebe, Grewen, Pedersen, Propper, & Meltzer-Brody, 2011) and symptoms of depression are associated with differences in oxytocin activity (A. M. Stuebe, et al., 2013), and it has been postulated that endogenous oxytocin may protect against the adverse depressive effects of psychosocial stress on mothers (Zelkowitz et al.). A recent review underscores the importance of reproductive and perinatal stage in associations between oxytocin and depression (Suena H Massey, Backes, & Schuette, 2016). Despite these clinical observations and interest in oxytocin as a treatment for perinatal depression and anxiety, there has been little investigation of the potential effects of clinical peripartum manipulation through the use of synthetic oxytocin.
Synthetic oxytocin has been used since the 1950’s (Fields, Greene, & Franklin, 1959), and current indications for use include labor induction or augmentation and prevention or treatment of postpartum hemorrhage (“ACOG Practice Bulletin No. 107: Induction of labor,” ACOG 2009). Between 1990 and 2012, rates of labor induction increased from 9.5 to 23.3% (Osterman & Martin, 2014), with rates as high as 42% in some populations (Laughon et al., 2012). Rates of labor augmentation are estimated at 57% (Declercq, Sakala, Corry, & Applebaum, 2007). However, recent recommendations, including those from the World Health Organization, indicate the use synthetic oxytocin as the first line agent in the active management of the third stage of labor for hemorrhage prevention (Westhoff, Cotter, & Tolosa, 2013; WHO, 2012), and it is conceivable that most or all women giving birth will soon have some synthetic oxytocin exposure.
The objective of this study was to examine the relationship between peripartum synthetic oxytocin administration and the development of depressive and anxiety disorders within the first year postpartum. We hypothesized that women exposed to peripartum synthetic oxytocin would have a reduced risk of postpartum depressive and anxiety disorders compared with those without exposure. To retrospectively examine this relationship, we utilized population-based data available through the Massachusetts Integrated Clinical Academic Research Database (MiCARD) which included data from peripartum patients who received intravenous synthetic oxytocin between 2005 and 2014 at University of Massachusetts Memorial Health Care (UMMHC).
METHODS
Study Design and Data Source
This was an analysis of data from a clinical data repository of patients who delivered a single live born infant at UMMHC between January 2, 2005 and April 4, 2014. Protected health information (PHI) was accessed using individual medical record numbers provided by the MiCARD query tool, the UMMHC implementation of the Informatics for Integrating Biology & the Bedside (i2b2) platform for clinical research (Murphy et al., 2010). Through MiCARD, variables of interest were extracted from databases including Allscripts™, Soarian® Clinical Electronic Medical Records, Meditech, Payer, and IDX schedule data; all centralized electronic medical record software solutions used by UMMHC facilities. Additional data was obtained from Quantitative Sentinel (QS, General Electric Centricity Perinatal), a software product utilized for both outpatient and inpatient perinatal care.
Study Sample
Participants were identified at the delivery level, where each delivery was considered a separate unit in our analysis. Deliveries were selected based on the following inclusion criteria: live born singleton deliveries, coded with any of the eligible delivery codes (Table 1), to females between the ages of 15–50. Multiple gestation deliveries (i.e., twins, triplets, or higher order multiples) were excluded from the study. Deliveries were identified using Current Procedural Terminology (CPT) and International Classification of Diseases 9th Revision (ICD-9) diagnosis codes. We considered the first delivery to each woman in our dataset as her “index delivery.” Depression and anxiety data included any diagnosis information and/or documentation of clinically relevant antidepressant or anxiolytic medication prescription (Table 1). The University of Massachusetts Medical School Institutional Review Board (IRB) approved the study.
Table 1.
Billing, drug, and procedure codes for obstetric deliveries, oxytocin administration, depressive or anxiety disorders, diagnoses, and medications
| Condition | ICD-9 Code | CPT Code | Date of First Record in Database |
|---|---|---|---|
| Deliveries | 650, V27.0 | 59400, 59409, 59410, 59510, 59514, 59515, 59525, 59610, 59612, 59614, 59618, 59620, 59622 | 4/6/1995 |
| Depressive or anxiety disorder diagnosis | 309.00, 309.10, 309.24, 309.28, 309.90, 309.89, 309.81, 311.00 296.30, 296.31, 296.32, 296.33, 296.34, 296.35, 296.36, 296.20, 296.21, 296.22, 296.23, 296.24, 296.25, 296.26, 296.90, 296.99, 300.00, 300.01, 300.02, 300.09 | 3/27/1995 | |
| Oxytocin injectable solution drug codes | |||
| RXCUI Codes | 207315, 238013 | 10/21/2005 | |
| Psychotropic medications | |||
| Antidepressant: SSRI | citalopram, Celexa, escitalopram, Lexapro, Prozac, fluoxetine, Sarafem, Selfemra, fluvoxamine, paroxetine, Paxil, sertraline, Zoloft | 1/1/2002 | |
| Antidepressant: SNRI | desvenlafaxine, Pristiq, duloxetine, Cymbalta, venlafaxine, Effexor | ||
| Antidepressant: TCA | amitriptyline, Elavil, amoxapine, clomipramine, Anafranil, desipramine, Norpramin, doxepin, imipramine, Tofranil, nortriptyline, Pamelor, protriptyline, Vivactil, trimipramine, Surmontil | ||
| Antidepressant: MAOI | isocarboxazid, Marplan, phenelzine, Nardil, selegiline, Emsam, tranylcypromine, Parnate | ||
| Antidepressant: Other | bupropion, budeprion, Wellbutrin, buproban, maprotiline, mirtazapine, Remeron, trazodone, Desyrel, nefazodone, Serzone | ||
| Anxiolytic, benzodiazepine | alprazolam, Niravam, Xanax, clorazepate, Tranxene, chlordiazepoxide, diazepam, Dizac, Valium, estazolam, flurazepam, lorazepam, Ativan, midazolam, oxazepam, Serax, temazepam, Restoril, triazolam, Halcion, quazepam, Doral | ||
| Antidepressant/ benzodiazepine combination | amitriptyline/chlordiazepoxide | ||
Indication of exposure to synthetic oxytocin was for any peripartum clinical indication within two weeks of delivery date, including but not limited to contraction stress tests, labor augmentation or induction by oxytocin when natural labor had or had not begun, and postpartum hemorrhage prevention and treatment. Delivery data was present in the databases as early as 1995; however, the availability of oxytocin exposure information began in 2005, thus dictating our use of the 2005–2014 timeframe. Controls included deliveries without record of exposure to synthetic oxytocin, while cases were defined as deliveries with exposure to synthetic oxytocin treatment.
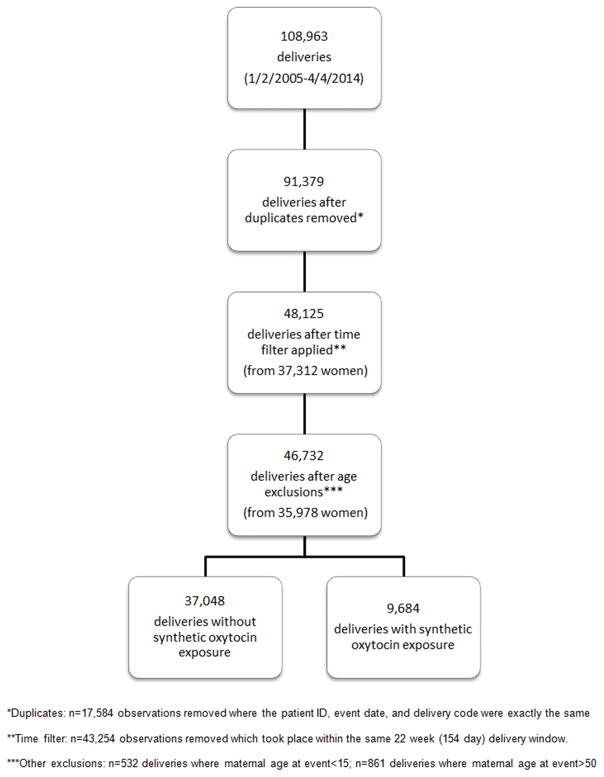
Initially, data were set up longitudinally where each delivery could exist across many records due to multiple diagnosis codes assigned to any one delivery. We removed any delivery records that had the same patient ID, event date, delivery code, and treatment (+/− synthetic oxytocin). This excluded 17,584 delivery records. CPT and ICD-9 delivery codes are conventionally used to designate deliveries that occur after 20 weeks gestational age, as deliveries occurring prior to 20 weeks gestation are considered abortions, whether spontaneous, elective, therapeutic, or otherwise. As a pregnancy due date is based on 40 weeks gestation but some can extend post-dates with the vast majority being delivered by 42 weeks gestation, we considered a ‘birth interval’ to minimally be 22 weeks (154 days) between delivery dates for each unique ID. Any delivery codes that existed within 154 days of each other for the same woman were considered part of the same delivery. After applying this birth interval filter, we excluded an additional 43,254 delivery records, likely part of the same delivery or hospital stay with delivery codes recorded on multiple/subsequent days. Finally, we excluded any records not meeting our eligibility criteria that were not flagged upon initial data query. This removed 532 deliveries where the maternal age at delivery was less than 15 and 861 deliveries where the maternal age at delivery was greater than 50.
Our final analytic sample included 46,732 unique deliveries (from 35,978 women); 37,048 deliveries without peripartum synthetic oxytocin exposure and 9,684 deliveries with peripartum synthetic oxytocin exposure (Figure 1). The delivery and psychiatric datasets were merged using an individual patient identifier available across datasets.
Figure 1.
Filtering process of data included in this study from the Massachusetts Integrated Clinical Academic Research Database (MiCARD)
Quantitative Variables
We classified depressive or anxiety disorders, defined as record of diagnosis and/or receipt of antidepressant or anxiolytic medication prescription, into three timeframes: prepregnancy: diagnoses/prescriptions greater than one year prior to the index delivery (diagnosis/prescription prior to (delivery date – 365 days)); pregnancy: diagnosis/prescription during pregnancy, or occurring between 40 weeks prior to delivery date (delivery date – 280 days) and delivery date; and postpartum: diagnosis/prescription one year following delivery (delivery date + 365 days) or 280 days between multiple deliveries (delivery date + 280 days). We retained any depressive or anxiety diagnosis or receipt of psychotropic medication prescription with a service date falling within any of these windows.
Psychiatric diagnosis ICD-9 codes were grouped into categories for analysis: 1) Adjustment disorder (various types) (309, 309.1, 309.24, 309.28, 309.89, 309.9); 2) Depressive disorder - Not Otherwise Specified (NOS) (311); 3) Major depressive disorder (MDD), various types (296.2, 296.21, 296.22, 296.23, 296.24, 296.25, 296.26, 296.3, 296.31, 296.32, 296.33, 296.34, 296.35, 296.36); 4) Episodic mood disorder (296.99); 5) Anxiety states (300.09); 6) Generalized Anxiety Disorder (GAD) (300.02); 7) Anxiety Disorder – Not Otherwise Specified (NOS) (300.00); 8) Post-traumatic Stress Disorder (PTSD) (309.81); and 9) Panic Disorder, with or without agoraphobia (300.01). Codes for adjustment disorders were included in the analysis due to variations in coding practices (Fiest et al., 2014). We examined the frequency of depressive or anxiety disorders in each of our three timeframes.
Additionally, we created a flag to distinguish caesarean and vaginal deliveries in the 24,099 delivery records coded with at least one CPT code. Caesarean deliveries were indicated with CPT codes 59510, 59514, 59515, 59618, 59620, and 59622; vaginal deliveries with CPT codes 59400, 59409, 59410 59610, 59612, and 59614.
Finally, we included the following covariates to examine descriptively, for our total sample as well as by oxytocin exposure group: maternal age, marital status (single, married, divorced/separated, and widowed), and race and ethnicity, all recorded at the time of data pull (October 2015), with the exception of maternal age which was recorded at the time of the event. We also included year of delivery grouped into five-year intervals as an additional covariate.
Statistical Analysis
As our primary interest was to examine the association between oxytocin exposure and postpartum depressive or anxiety disorder, we calculated the relative risk and 95% confidence interval of postpartum depressive or anxiety disorder by oxytocin exposure, using the postpartum timeframe. To confirm that we only identified new cases of a depressive or anxiety disorder following delivery, we split our sample by the presence or absence of a history of prepregnancy depressive or anxiety disorder and ran two separate relative risk calculations: 1) the relative risk in index deliveries (as available in the database) to women with a history of a prepregnancy depressive or anxiety disorder; and 2) the relative risk for any delivery to women without a history of a prepregnancy depressive or anxiety disorder. We then compared relative risk by mode of delivery (vaginal and cesarean).
Additionally, we conducted sensitivity analyses to examine the robustness of our initial findings. We replicated our analysis on our total sample in two subsets: first, by selecting a random delivery from each woman in our sample; and next, by repeating our analysis on all index deliveries (as available in the database), regardless of prepregnancy depressive or anxiety disorder history. We also repeated our analyses to examine the effect of depression or anxiety disorders diagnosed during pregnancy. Finally, we varied the 40-week definitions for our pregnancy and postpartum timeframes to 37 and 42 weeks (259 and 294 days, respectively). This was done to account for the vast majority of range of deliveries given our inability to determine gestational age at delivery.
All analyses were conducted in SAS Version 9.3 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Descriptive Data
The average onset of postpartum depressive and anxiety symptoms in patients who received a diagnosis or prescription was 112.52 days (+/− standard deviation 111.35). The median amount of time between delivery and diagnosis/prescription receipt was 71 days, suggesting that 50% of our patients received their diagnosis/prescription within just over 2 months following delivery. Descriptive data can be found in Figure 1 and Table 2.
Table 2.
Descriptive statistics of deliveries by peripartum exposure to oxytocin (n=46,732)
| Total Deliveries (n=46,732) | Without Exposure (n=37,048) | With Exposure (n=9,684) | |
|---|---|---|---|
|
| |||
| Age at time of delivery, years (mean ± SD, min-max) | 29.9 ± 6.2 (15–50) | 30.1 ± 6.2 (15–50) | 29.2 ± 6.2 (15–50) |
|
| |||
| Marital status, n (%) | |||
| Single | 14,210 (30.4) | 10,969 (29.6) | 3,241 (33.5) |
| Married | 24,882 (53.2) | 19,825 (53.5) | 5,057 (52.2) |
| Divorced or separated | 1,315 (2.8) | 1,068 (2.9) | 247 (2.6) |
| Widowed | 59 (0.1) | 50 (0.1) | 9 (0.1) |
| Unknown/Missing | 6,266 (13.4) | 5,136 (13.9) | 1,130 (11.7) |
|
| |||
| Delivery year, n (%) | 4,703 (48.6) | ||
| 2005–2009 | 23,869 (51.1) | 19,166 (51.7) | |
| 2010–2014 | 22,863 (48.9) | 17,882 (48.3) | |
|
| |||
| Race & ethnicity, n (%) | 4,981 (51.4) | ||
| White | 26,602 (56.9) | 21,216 (57.3) | 5,386 (55.6) |
| Black | 2,970 (6.4) | 2,247 (6.1) | 723 (7.5) |
| Other or unknown | 15,292 (32.7) | 12,131 (32.7) | 3,161 (32.6) |
| Hispanic or Latino | 1,868 (4.0) | 1,454 (3.9) | 414 (4.3) |
|
| |||
| Delivery type, n (%) | |||
| Vaginal | 17,316 (37.1) | 13,118 (35.4) | 4,198 (43.3) |
| Cesarean section | 7,683 (16.4) | 6,281 (17.0) | 1,402 (14.5) |
| Unknown/Missing | 21,733 (46.5) | 17,649 (47.6) | 4,084 (42.2) |
|
| |||
| Depressive/anxiety disorder diagnoses or receipt of psychotropic medication by timeframe*, n (%) | |||
| Prepregnancy (≥ 1 year prior to delivery**) | 3,609 (10.0) | 2,808 (9.9) | 801 (10.5) |
| During pregnancy | 1,976 (4.2) | 1,480 (4.0) | 496 (5.1) |
| ≤ 1 year postpartum | 3,211 (6.9) | 2,343 (6.3) | 868 (9.0) |
Prepregnancy: diagnosis/medication prior to (index delivery date – 365 days); pregnancy: diagnosis/ medication within (delivery date – 280 days); postpartum: diagnosis/medication within (delivery date + 280 days).
History of depressive or anxiety disorder diagnosis or dispensing of psychotropic medication calculated for index deliveries only (n=35,974).
Main Results
Results from the risk comparison models showed that relative risks (RR) and their associated 95% confidence intervals (95% CI) were higher in women with peripartum exposure to synthetic oxytocin across all subgroups. In index deliveries to women with a history of prepregnancy depressive or anxiety disorder (or documentation of antidepressant or anxiolytic prescription), exposure to peripartum oxytocin increased the risk of postpartum depressive or anxiety disorder by 36% (RR: 1.36; 95% CI: 1.20–1.55) (Table 3). In deliveries to women with no history of prepregnancy depressive or anxiety disorder, exposure to peripartum oxytocin increased the risk of postpartum depressive or anxiety disorder by 32% compared to those not exposed to peripartum oxytocin (RR: 1.32; 95% CI: 1.23–1.42) (Table 4).
Table 3.
Relative risks and 95% confidence intervals comparing risk for postpartum depressive or anxiety diagnosis or psychotropic medication and peripartum exposure to oxytocin for index deliveries to women with a history of prepregnancy depressive or anxiety diagnosis or psychotropic medication (n=3,609)
| Without exposure (n=2,808) | With exposure (n=801) | |
|---|---|---|
| No postpartum depressive or anxiety or psychotropic, n (%) | 2,166 (77.1) | 554 (69.2) |
| Postpartum depressive or anxiety disorder or psychotropic medication, n (%) | 642 (22.9) | 247 (30.8) |
| RR (95% CI) | 1.36 (1.20–1.55) | |
Table 4.
Relative risks and 95% confidence intervals comparing risk for postpartum depressive or anxiety diagnosis or psychotropic medication and peripartum exposure to oxytocin for any delivery to women with no history of prepregnancy depressive or anxiety diagnosis or psychotropic medication (n=43,123)
| Without exposure (n=34,240) | With exposure (n=8,883) | |
|---|---|---|
| No postpartum depressive or anxiety or psychotropic, n (%) | 32,539 (95.0) | 8,262 (93.0) |
| Postpartum depressive or anxiety disorder or psychotropic medication, n (%) | 1,701 (5.0) | 621 (7.0) |
| RR (95% CI) | 1.32 (1.23–1.42) | |
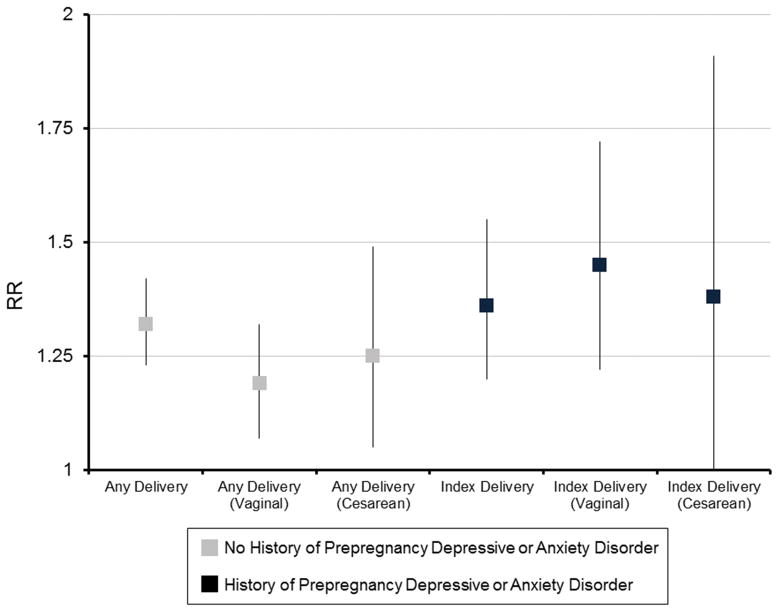
In index deliveries to women with a history of prepregnancy depressive or anxiety disorder, the relative risk of postpartum depressive or anxiety disorder was higher in women who received peripartum synthetic oxytocin compared to those who did not for both vaginal (RR: 1.45; 95% CI: 1.22–1.72) and cesarean (RR: 1.38; 95% CI: 1.00–1.91) deliveries. In deliveries to women with no history of prepregnancy depressive or anxiety disorder, exposure to synthetic oxytocin increased the relative risk of postpartum depressive or anxiety disorder by 19% in vaginal deliveries and 25% in cesarean deliveries compared to those not exposed (RR: 1.19; 95% CI: 1.07–1.32 and RR: 1.25; 95% CI: 1.05–1.49, respectively. Data not shown.)
Sensitivity Analyses
When selecting a random delivery from each woman, regardless of prepregnancy depressive or anxiety disorder history, the relative risk of postpartum depressive or anxiety disorder was 35% higher in women who received peripartum synthetic oxytocin compared to those who did not (RR: 1.35; 95% CI: 1.26–1.44).
When calculating relative risks in all index deliveries, relative risks remained similar, with a 39% increased risk of postpartum depressive or anxiety disorder in deliveries to women with exposure to peripartum synthetic oxytocin compared to deliveries to women with no exposure to peripartum synthetic oxytocin (RR: 1.39; 95% CI: 1.30–1.49).
Additionally, we examined the relative risks for only patients with an onset of depressive or anxiety symptoms during pregnancy, to determine if they would be more likely to have a postpartum episode regardless of synthetic oxytocin administration. Controlling for depressive or anxiety diagnoses during pregnancy resulted in a relative risk of 0.90 (0.77–1.05), suggesting no increased risk in the postpartum. Removing these deliveries from the main analysis resulted in a relative risk of 1.41 (95% CI: 1.32–1.51), confirming that episodes during pregnancy did not influence our findings.
Lastly, examining the full term delivery spectrum of 37–42 weeks did not change the results (data not shown).
DISCUSSION
Contrary to our hypothesis, our results indicate that women with peripartum exposure to synthetic oxytocin had a higher relative risk of receiving a documented depressive or anxiety disorder diagnosis or antidepressant/anxiolytic prescription within the first year postpartum than women without synthetic oxytocin exposure. This increased risk was still present when the analyses were restricted to index deliveries or a single random delivery in women who had multiple deliveries, and the risks were higher regardless of delivery mode.
The literature on the role of endogenous oxytocin concentrations and synthetic oxytocin administration in the peripartum period on postpartum mood is scarce, and data are conflicting. Several studies have examined the relationship between maternal plasma oxytocin and depressive symptomology (Cox et al., 2015; Garfield et al., 2015; Massey et al., 2016) found that low oxytocin during pregnancy is associated with increased depressive symptoms and thus suggest that administering exogenous oxytocin, such as the synthetic oxytocin Pitocin, may alleviate or prevent negative postpartum mood. Another study found lower oxytocin levels during breastfeeding at 8 weeks postpartum in women with depressive symptoms compared to asymptomatic women (Cox, et al., 2015). However, one study found that higher oxytocin in the third trimester predicted higher depressive symptom severity at 6 weeks postpartum, but only in women with a prior history of depression; plasma oxytocin was unrelated to symptom severity in women without a history of depression (Suena H. Massey, et al., 2016). And finally, a very recent longitudinal study of women whose plasma oxytocin was measured twice during pregnancy and at 3 different times postpartum, found that rather than absolute plasma oxytocin levels, the timing of fluctuations in oxytocin levels from pregnancy to 6 months postpartum differed significantly in women with postpartum depressive symptoms compared to those without (Jobst et al., 2016).
Even fewer studies have addressed the effects of administration of oxytocin on postpartum mood. A randomized controlled trial (RCT) which investigated the effect of timing of intravenous administration of oxytocin in primiparous laboring women with dysfunctional labor indicated that women who received oxytocin earlier in their labor had a non-significant, but higher rate of major depression, although median depression scores did not differ between groups (Hinshaw, Simpson, Cummings, Hildreth, & Thornton, 2008). Another RCT administered intranasal synthetic oxytocin in a placebo-controlled, double-blind, within-subjects design to a cohort of mothers diagnosed with postpartum depression (Mah, Van Ijzendoorn, Smith, & Bakermans-Kranenburg, 2013), and treatment resulted in lower reported mood. Our data support the idea that synthetic oxytocin administration during labor has a negative impact on postpartum mood. It is important to note that the effect of synthetic oxytocin administration on endogenous oxytocin levels is unclear, and nothing about endogenous oxytocin levels can be inferred from our dataset and the aforementioned RCTs. For example, it is possible that low endogenous oxytocin is associated with both the need for synthetic oxytocin administration during labor and higher postpartum depressive symptomatology. One recent study, however, found that the dose of synthetic oxytocin given during the labor and delivery (as determined retroactively from hospital charts) was positively correlated with both endogenous levels of oxytocin and depressive symptoms at 2 months postpartum (Gu et al., 2016), similar to the results from intravenous dosing. The current data suggest that peripartum synthetic oxytocin administration may have more prolonged negative effects on mood than demonstrated in the aforementioned studies, where depression symptoms following treatment were only measured at a single time point soon after administration.
Strengths of the present investigation include a reasonable sample size and a diverse population. Inclusion of both diagnostic and medication prescription codes acknowledges the importance of assessing both diagnoses and symptoms, in line with Research Domain Criteria (RDoC) initiative (Insel et al., 2010). The sensitivity analyses also indicate that this association is found across different parous states and within the full term delivery spectrum. As an initial investigation of associations between peripartum synthetic oxytocin and maternal mood and anxiety, it provides a theoretical and methodological framework for both expanded and more specific studies of this relationship. For example, similar medical databases searches that include Medicaid patients and/or more detailed data on Pitocin exposures would be beneficial, as well as longitudinal studies which assess oxytocin levels and mood at multiple peripartum time points. The use of the i2b2 informatics platform makes this especially relevant to the dozens of institutions that have access to this resource, increasing the potential for future multi-site investigations. This broad examination of maternal depression and anxiety during the first year postpartum extends beyond the previous investigations that only focused on the early postnatal period, including a more expansive assessment of risk that reflects growing concerns about the prevalence and adverse effects of postpartum depression throughout the first year and beyond (ACOG, 2015).
A few limitations of the study affect the interpretation of the results and potential applicability of the findings. The effects of Pitocin on oxytocin levels could not be assessed. Given that these are administrative/population health data, it is not possible to determine whether the relationship between peripartum synthetic oxytocin administration and postpartum depressive and anxiety disorders is causal. True prevalence of postpartum depressive and anxiety disorders is likely underestimated due to known under-diagnosis and thus under-documentation in the medical record (Evins, Theofrastous, & Galvin, 2000; Halbreich & Karkun, 2006). In our dataset, we did not have access to data on patients served by Medicaid due to how Medicaid data is stored in Massachusetts; outside of MiCARD. This represents a high-risk population our health system serves. In addition, given the broad range of synthetic oxytocin exposures included, it is possible that risks were higher for more substantial exposures during labor induction and augmentation. Taken together, the under-diagnosis, omission of Medicaid data, and range of synthetic oxytocin exposures make it likely that the calculated risk ratios are conservative. The data set was also missing delivery mode data for a considerable percentage of deliveries and information on potential confounding variables (such as familial history of postpartum depressive or anxiety disorders, maternal body weight and body mass index, maternal illnesses associated with depression such as gestational diabetes, Pitocin dosages and indication, labor/delivery and/or breastfeeding complications, etc.) that limited more detailed investigation of the findings and statistical adjustment.
There has been a great deal of debate recently on the significance of oxytocin in psychiatry research in animals and humans, including valuable commentary on evolutionary and theoretical social perspectives and potential implications for disorders ranging from autism to schizophrenia (Shamay-Tsoory & Young, 2016). Unfortunately, there is little discussion or even appreciation for the fact that 22% of births are induced (with 4 million births in the U.S. in 2014 (Hamilton, Martin, Osterman, C., & Matthews, 2014)) and that synthetic oxytocin is one of the most commonly used drugs in the United States (ACoPB, 2009) while little research has been done to examine the potential effects on postpartum maternal mood or anxiety. Given the paucity of data on this topic, prior literature along with the current study underscore the need for more research on the interaction between synthetic oxytocin and maternal mood in a clinical context where its use is very common and expected to increase given recommendations for all women to receive it for postpartum hemorrhage prevention (WHO, 2012). The potent behavioral effects of oxytocin in basic and clinical studies justify increased study of the potential effects of peripartum manipulation of this hormone and its receptors. There are specific needs for studies on: the longitudinal role of endogenous oxytocin in maternal mood and anxiety, detailed prospective studies of the effects of peripartum treatments on maternal mood, with an emphasis on populations at high risk for maternal mood and anxiety disorders, and clinically relevant animal studies which investigate neural mechanisms of the behavioral effects of exogenous peripartum oxytocin.
Figure 2.
Relative risks and 95% confidence intervals comparing risk for postpartum depressive or anxiety diagnosis or psychotropic medication and peripartum exposure to oxytocin by delivery type and history of prepregnancy depressive or anxiety diagnosis or psychotropic medication
Acknowledgments
ROLE OF THE FUNDING SOURCE
This study was supported by National Center for Advancing Translational Sciences, National Institutes of Health Grant (UL1TR000161) and National Institutes of Health Grant (5K23MH097794). Dr. Deligiannidis currently receives funding from the National Institutes of Health (UL1TR000161; 5K23MH097794), and SAGE Therapeutics and receives royalties from an NIH Employee Invention. Dr. Babb is supported by a National Institute of Mental Health Individual Postdoctoral Fellowship (F32MH108247). Dr. Nephew is supported by an award from the National Institutes of Health (NICHD R00 HD059943) and a Brain and Behavior Research Foundation NARSAD Young Investigator Award. Dr. Moore Simas is supported by a Centers for Disease Control and Prevention award (1U01DP006093-01).
We thank Paul Ranauro for his assistance with the MiCARD database and Bruce A. Barton, PhD for his assistance with statistical analyses.
Footnotes
CONFLICT OF INTEREST
None. The authors of this manuscript do not have conflicts of interest relevant to the subject of this manuscript. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The sponsoring agency had no further role in the study design and analysis, the writing of the report, or the decision to submit the paper for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position of the NIH.
References
- ACOG. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268–1271. doi: 10.1097/01.AOG.0000465192.34779.dc. [DOI] [PubMed] [Google Scholar]
- ACOG Practice Bulletin No. 107: Induction of labor. ACOG. Obstet Gynecol. 2009;114(2 Pt 1):386–397. doi: 10.1097/AOG.0b013e3181b48ef5. [DOI] [PubMed] [Google Scholar]
- Austin MPV, Hadzi-Pavlovic D, Priest SR, Reilly N, Wilhelm K, Saint K, Parker G. Depressive and anxiety disorders in the postpartum period: how prevalent are they and can we improve their detection? [journal article] Archives of Women’s Mental Health. 2010;13(5):395–401. doi: 10.1007/s00737-010-0153-7. [DOI] [PubMed] [Google Scholar]
- Azak S. Maternal depression and sex differences shape the infants’ trajectories of cognitive development. Infant Behav Dev. 2012;35(4):803–814. doi: 10.1016/j.infbeh.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Babb JA, Carini LM, Spears SL, Nephew BC. Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats. Horm Behav. 2014;65(4):386–393. doi: 10.1016/j.yhbeh.2014.03.005. doi: http://dx.doi.org/10.1016/j.yhbeh.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AF, Carter CS, Steer CD, Golding J, Davis JM, Steffen AD, … Connelly JJ. Interaction between oxytocin receptor DNA methylation and genotype is associated with risk of postpartum depression in women without depression in pregnancy. Front Genet. 2015;6(243) doi: 10.3389/fgene.2015.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AF, Erickson EN, Carter CS. Beyond Labor: The Role of Natural and Synthetic Oxytocin in the Transition to Motherhood. Journal of Midwifery & Women’s Health. 2014:n/a–n/a. doi: 10.1111/jmwh.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JR. Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health. 2011;51(1):55–71. doi: 10.1080/03630242.2011.540741. 934590106 [pii] [DOI] [PubMed] [Google Scholar]
- Cox EQ, Stuebe A, Pearson B, Grewen K, Rubinow D, Meltzer-Brody S. Oxytocin and HPA stress axis reactivity in postpartum women. Psychoneuroendocrinology. 2015;55:164–172. doi: 10.1016/j.psyneuen.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deave T, Heron J, Evans J, Emond A. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043–1051. doi: 10.1111/j.1471-0528.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- Declercq ER, Sakala C, Corry MP, Applebaum S. Listening to Mothers II: Report of the Second National U.S. Survey of Women’s Childbearing Experiences: Conducted January–February 2006 for Childbirth Connection by Harris Interactive(R) in partnership with Lamaze International. J Perinat Educ. 2007;16(4):9–14. doi: 10.1624/105812407X244769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey G, Coyne JC. Children of depressed parents: an integrative review. Psychological Bulletin. 1990;108(1):50–76. doi: 10.1037/0033-2909.108.1.50. [DOI] [PubMed] [Google Scholar]
- Earls MF. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126(5):1032–1039. doi: 10.1542/peds.2010-2348. [DOI] [PubMed] [Google Scholar]
- Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. [Comparative Study] American journal of obstetrics and gynecology. 2000;182(5):1080–1082. doi: 10.1067/mob.2000.105409. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry. 2009;48(9):919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. [10.1111/j.1467-9280.2007.02010.x] Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Fields H, Greene JWJ, Franklin RR. Intravenous Pitocin in Induction and Stimulation of Labor: A study of 3754 cases. Obstetrics & Gynecology. 1959;13(3):353–359. [PubMed] [Google Scholar]
- Fiest KM, Jette N, Quan H, St Germaine-Smith C, Metcalfe A, Patten SB, Beck CA. Systematic review and assessment of validated case definitions for depression in administrative data. [journal article] BMC Psychiatry. 2014;14(1):289. doi: 10.1186/s12888-014-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkenwirth C, Martins E, Deschner T, Burkart JM. Oxytocin is associated with infant-care behavior and motivation in cooperatively breeding marmoset monkeys. Horm Behav. 2016;80:10–18. doi: 10.1016/j.yhbeh.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Forman DR, O’Hara MW, Stuart S, Gorman LL, Larsen KE, Coy KC. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. [Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Development and Psychopathology. 2007;19(2):585–602. doi: 10.1017/S0954579407070289. [DOI] [PubMed] [Google Scholar]
- Garfield L, Giurgescu C, Carter CS, Holditch-Davis D, McFarlin BL, Schwertz D, … White-Traut R. Depressive symptoms in the second trimester relate to low oxytocin levels in African-American women: a pilot study. Archives of Women’s Mental Health. 2015;18(1):123–129. doi: 10.1007/s00737-014-0437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, … Miller WC. Perinatal depression: prevalence, screening accuracy, and screening outcomes. [Meta-Analysis Review] Evidence report/technology assessment. 2005;(119):1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH. Depression in Mothers. Annu Rev Clin Psychol. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Gu V, Feeley N, Gold I, Hayton B, Robins S, Mackinnon A, … Zelkowitz P. Intrapartum Synthetic Oxytocin and Its Effects on Maternal Well-Being at 2 Months Postpartum. Birth. 2016;43(1):28–35. doi: 10.1111/birt.12198. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Karkun S. Cross-cultural and social diversity of prevalence of postpartum depression and depressive symptoms. Journal of affective disorders. 2006;91(2–3):97–111. doi: 10.1016/j.jad.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Hamilton BE, Martin JA, Osterman MJ, CCS, Matthews TJ. Births: Final Data for 2014. National Vital Statistics Reports. 2014;64(12) [PubMed] [Google Scholar]
- Hashemi F, Tekes K, Laufer R, Szegi P, Tothfalusi L, Csaba G. Effect of a single neonatal oxytocin treatment (hormonal imprinting) on the biogenic amine level of the adult rat brain: could oxytocin-induced labor cause pervasive developmental diseases? Reprod Sci. 2013;20(10):1255–1263. doi: 10.1177/1933719113483010. [DOI] [PubMed] [Google Scholar]
- Hinshaw K, Simpson S, Cummings S, Hildreth A, Thornton J. A randomised controlled trial of early versus delayed oxytocin augmentation to treat primary dysfunctional labour in nulliparous women. [Comparative Study Multicenter Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t] BJOG : an international journal of obstetrics and gynaecology. 2008;115(10):1289–1295. doi: 10.1111/j.1471-0528.2008.01819.x. discussion 1295–1286. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010 doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jobst A, Krause D, Maiwald C, Härtl K, Myint A-M, Kästner R, … Müller N. Oxytocin course over pregnancy and postpartum period and the association with postpartum depressive symptoms. [journal article] Archives of Women’s Mental Health. 2016:1–9. doi: 10.1007/s00737-016-0644-2. [DOI] [PubMed] [Google Scholar]
- Jover M, Colomer J, Carot JM, Larsson C, Bobes MT, Ivorra JL, … Sanjuan J. Maternal anxiety following delivery, early infant temperament and mother’s confidence in caregiving. Span J Psychol. 2014;17:E95. doi: 10.1017/sjp.2014.87. [DOI] [PubMed] [Google Scholar]
- Kim S, Soeken TA, Cromer SJ, Martinez SR, Hardy LR, Strathearn L. Oxytocin and postpartum depression: Delivering on what’s known and what’s not. Brain research. 2014 2013;1580(0):219–232. doi: 10.1016/j.brainres.2013.11.009. doi: http://dx.doi.org/10.1016/j.brainres.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Soeken TA, Cromer SJ, Martinez SR, Hardy LR, Strathearn L. Oxytocin and postpartum depression: deliveringDelivering on what’s known and what’s not. Brain Res, 11, research. 2014;1580:219–232. doi: 10.1016/j.brainres.2013.11.009. doi: http://dx.doi.org/10.1016/j.brainres.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon SK, Zhang J, Grewal J, Sundaram R, Beaver J, Reddy UM. Induction of labor in a contemporary obstetric cohort. Am J Obstet Gynecol. 2012;206(6):23. doi: 10.1016/j.ajog.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: Individual patterns and maternal-fetal attachment. [10.1016/j.peptides.2007.04.016] Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77–87. doi: 10.1007/s00737-005-0080-1. [DOI] [PubMed] [Google Scholar]
- Mah BL, Van Ijzendoorn MH, Smith R, Bakermans-Kranenburg MJ. Oxytocin in postnatally depressed mothers: Its influence on mood and expressed emotion. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;40(0):267–272. doi: 10.1016/j.pnpbp.2012.10.005. doi: http://dx.doi.org/10.1016/j.pnpbp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Massey SH, Backes KA, Schuette SA. Plasma oxytocin concentration and depressive symptoms: a review of current evidence and directions for future research. Depression and Anxiety. 2016 doi: 10.1002/da.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SH, Schuette SA, Pournajafi-Nazarloo H, Wisner KL, Carter CS. Interaction of oxytocin level and past depression may predict postpartum depressive symptom severity. [journal article] Archives of Women’s Mental Health. 2016:1–10. doi: 10.1007/s00737-016-0616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, Nephew BC. Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology. 2013;38(2):219–228. doi: 10.1016/j.psyneuen.2012.05.020. doi: http://dx.doi.org/10.1016/j.psyneuen.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SN, Weber G, Mendis M, Gainer V, Chueh HC, Churchill S, Kohane I. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2) Journal of the American Medical Informatics Association. 2010;17(2):124–130. doi: 10.1136/jamia.2009.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Cooper P. Effects of postnatal depression on infant development. Arch Dis Child. 1997;77(2):99–101. doi: 10.1136/adc.77.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC. Behavioral roles of oxytocin and vasopressin. In: Sumiyoshi T, editor. Neuroendocrinology and Behavior. InTech; 2012. Retrieved from http://www.intechopen.com/books/neuroendocrinology-and-behavior/behavioral-roles-of-oxytocin-and-vasopressin. [DOI] [Google Scholar]
- Osterman MJ, Martin JA. Recent declines in induction of labor by gestational age. NCHS Data Brief. 2014;155:1–8. [PubMed] [Google Scholar]
- Perani CV, Slattery DA. Using Animal Models to study Postpartum Psychiatric Disorders. Br J Pharmacol. 2014;16(10):12640. doi: 10.1111/bph.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck C, Struben K, Backenstrass M, Stefenelli U, Reinig K, Fuchs T, … Mundt C. Prevalence, onset and comorbidity of postpartum anxiety and depressive disorders. Acta Psychiatrica Scandinavica. 2008;118(6):459–468. doi: 10.1111/j.1600-0447.2008.01264.x. [DOI] [PubMed] [Google Scholar]
- Reiner I, Van IMH, Bakermans-Kranenburg MJ, Bleich S, Beutel M, Frieling H. Methylation of the oxytocin receptor gene in clinically depressed patients compared to controls: The role of OXTR rs53576 genotype. J Psychiatr Res. 2015;65:9–15. doi: 10.1016/j.jpsychires.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345(6198):771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S, Young LJ. Understanding the Oxytocin System and Its Relevance to Psychiatry. Biol Psychiatry. 2016;79(3):150–152. doi: 10.1016/j.biopsych.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma Oxytocin Concentration during Pregnancy is associated with Development of Postpartum Depression. Neuropsychopharmacology. 2011;36(9):1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohr-Preston SL, Scaramella LV. Implications of timing of maternal depressive symptoms for early cognitive and language development. Clin Child Fam Psychol Rev. 2006;9(1):65–83. doi: 10.1007/s10567-006-0004-2. [DOI] [PubMed] [Google Scholar]
- Strathearn L. Maternal Neglect: Oxytocin, Dopamine and the Neurobiology of Attachment. Journal of Neuroendocrinology. 2011;23(11):1054–1065. doi: 10.1111/j.1365-2826.2011.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Grewen K, Meltzer-Brody S. Association between maternal mood and oxytocin response to breastfeeding. J Womens Health. 2013;22(4):352–361. doi: 10.1089/jwh.2012.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Grewen KM, Pedersen CA, Propper C, Meltzer-Brody S. Failed lactation and perinatal depression: Common problems with shared neuroendocrine mechanism. J Women’s Health. 2011;21(3):264–272. doi: 10.1089/jwh.2011.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Archives of general psychiatry. 2008;65(7):805–815. doi: 10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst Rev. 2013:10. doi: 10.1002/14651858.CD001808.pub2. [DOI] [PubMed] [Google Scholar]
- WHO. WHO recommendations for the prevention and treatment of postpartum haemorrhage. WHO; Geneva, Switzerland: 2012. [PubMed] [Google Scholar]
- Zelkowitz P, Gold I, Feeley N, Hayton B, Carter CS, Tulandi T, … Levin P. Psychosocial Stress Moderates the Relationships between Oxytocin, Perinatal Depression, and Maternal Behavior. Hormones and Behavior. doi: 10.1016/j.yhbeh.2014.06.014. http://dx.doi.org/10.1016/j.yhbeh.2014.06.014. [DOI] [PubMed]