Abstract
Background
Si-Mo-Tang oral liquid (SMT) has been widely used to treat functional dyspepsia (FD), but the effectiveness is still controversial. A systematic review and meta-analysis of randomized controlled trials (RCTs) were performed to assess the efficacy and adverse effects of SMT for FD.
Methods
Investigators searched for articles with publication dates to June 21, 2016, from 9 English and Chinese electronic databases. Comparisons were SMT alone or SMT in combination with western medicine as experimental intervention, and western medicine or placebo as the control. We used the Cochrane collaboration tool for assessing risk of bias to evaluate methodologies. Data were synthesized with RevMan 5.3 software. (PROSPERO Registration #CRD42016042003)
Results
Twenty-seven RCTs were included in the review, involving 2,713 participants: 1,383 subjects were in the experimental group and 1,330 in the control group. SMT showed a significant improvement in clinical efficacy (RR 1.14; 95% CI 1.09, 1.20; P<0.00001), but the heterogeneity was also significant (P = 0.0002, I2 = 56%). Because of the different interventions in the 2 groups, we performed subgroup and sensitivity analyses to investigate potential sources of heterogeneity. The heterogeneity was smaller after subgroup analysis and the exclusion of a study by Zhu from 2009. The corresponding pooled RR has no obvious change (RR 1.17; 95% CI 1.13, 1.21; P<0.00001). Subgroup analysis by age and drugs administered in control interventions between SMT and western medicine also showed improvement in the efficacy rate. But a data synthesis that excluded high risk of bias in the blinding of participants and personnel showed no significant difference (RR 1.14; 95% CI 0.97, 1.35; P = 0.12). Three studies measured gastric emptying. Two of these studies reported no significant difference between the experimental and control groups, while 1 study showed that SMT reduced the time of gastric emptying. The relapse rate and adverse effects had no difference between 2 groups.
Conclusions
This meta-analysis suggests that SMT is an effective and safe therapy option for patients with FD. However, because of the high clinical heterogeneity, poor quality, high risk of bias and small sample size of some included studies, further standardized large-scale and strictly designed studies are needed.
Introduction
Functional dyspepsia (FD), a relapsing and remitting disorder, is defined as the presence of 1 or more of the following: pain or burning in the epigastrium, postprandial fullness, early satiation and no evidence of structural disease to explain these symptoms [1]. The global prevalence of FD in the community is between 5% and 11%[1]. FD influences patients’ quality of life, work and other daily activities [2]. It also has substantial financial implications for patients, health care organizations, and society [1,3].
Although great progress has been achieved in the understanding and treatment of FD [4], conventional treatment remains suboptimal. A survey aimed at investigating the current treatments used in functional gastrointestinal disorders (FGIDs) found that treatment of FGIDs was based on symptoms relieved by conventional drugs. However, increasingly, complementary and alternative medicine is used [5].
Traditional Chinese medicine (TCM) utilizes a typically symptoms-based approach, with history-proven therapeutic efficacy [6]. Si-Mo-Tang oral liquid (SMT), a Chinese patent medicine product, is produced by Hansen Pharmaceutical Company and Hunan Wuma Pharmaceutical Company. SMT originated from an ancient and classic formula designed approximately 800 years ago, and is primarily comprised of 4 different kinds of herbs [7]. SMT has been prescribed by TCM practitioners for a longer period of time, and has a good clinical effect. But most studies about the efficacy of SMT has been reported in Chinese, and not readable by any non-Chinese. In addition, the current state of evidence of SMT in treating FD is insufficient. Therefore, we conducted a systematic review and meta-analysis of RCTs to determine whether or not SMT is beneficial to patients with FD.
Because of the widespread application of SMT, participants were included in the review regardless of personal characteristics, like age or sex. Si-Mo-Tang oral liquid alone or in combination with western medicine was used in experimental groups, and a placebo or positive medicine was administered in control groups. Outcomes contained clinical efficacy rates, effects on gastric emptying, relapse rates, safety profiles and adverse events.
Methods
Reports were in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (S1 Checklist).
Registration number
We have registered a protocol for this systematic review and meta-analysis in PROSPERO(available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016042003, S1 Protocol).
Search strategy
For the systematic review and meta-analysis, the following databases were searched: PUBMED(1990 to June 21,2016), EMBASE(1980 to June 21,2016), Cochrane Library, BMJ Clinical Evidence and International Clinical Trials Registry Platform (to June 21,2016), China National Knowledge Infrastructure database (1979 to June 21,2016), Chinese Biomedical Literature database (1978 to June 21,2016), Wanfang database (1990 to June 21,2016), and VIP database (1989 to June 21,2016). The free text search elements were “functional dyspepsia,” “epigastric pain syndrome,” “postprandial discomfort syndrome,” “Pi-Man in Chinese,” “Ji-Zhi in Chinese” and “Wei-Tong in Chinese.” Additionally, the subject heading “dyspepsia” was searched. These terms were combined with the operator AND with the free text term “Si-Mo-Tang.” For example, in the Wanfang database, the combined search terms were “functional dyspepsia or epigastric pain syndrome or postprandial discomfort syndrome or Pi-Man or Ji-Zhi or Wei-Tong” and “Si-Mo-Tang.” Papers published in English and Chinese were evaluated. Only studies with full text were reviewed. Investigators contacted the authors of any articles without full text. A recursive manual search of cited references was performed to identify other relevant studies.
Data selection
Studies were considered to be eligible for inclusion if they met the following criteria: (i) patients were diagnosed with FD by the ROME I, II, III or IV criteria and on clinical grounds regardless of age, sex, inpatient or outpatient; (ii)the study was performed as a RCT; (iii) Si-Mo-Tang oral liquid alone or in combination with western medicine was compared with placebo or positive controlled studies; (iv) criteria for successful treatment were clearly clarified; (v) treatment lasted for 7 days or more. Studies meeting the following criteria were excluded: duplication (the same data of patients with the same authors published in different journals); lack of information of diagnostic criteria, interventions or outcomes were not defined or suitable; lack of important information of participants’ characteristics; full text could not be obtained; academic fraud or errors; and studies not meeting the inclusion criteria for other reasons.
Data abstraction
Detailed information abstracted from the studies included the name of first author, year of publication, age of subjects, number of participants, sex, details of intervention, outcome measures, effectiveness and ineffectiveness number (based on the alleviation of symptoms, effectiveness means that the symptoms were relieved more than 30%, ineffectiveness means less than 30%) and duration of treatment. Data were extracted as intention-to-treat analyses (ITT analyses) [8], in which drop-outs were assumed as treatment failures in experimental groups and as efficiency in control groups. Assessment of methodological quality was conducted with the Cochrane Collaboration tool [9].
Eligibility assessment of the literature search, study selection, data abstraction and analysis of study quality were performed independently by YH and YB to avoid bias. Articles were screened repeatedly to confirm, and data were checked for internal consistency. Disagreements between evaluators were resolved by discussing with JY and ZZ.
Data synthesis and analysis
Meta-analysis was carried out using Review Manager software (version 5.3), provided by the Cochrane Collaboration. Dichotomous data were presented as risk ratio (RR) and continuous outcomes as mean difference (MD), both with a 95% confidence interval (CI). The chi-squared test for heterogeneity was performed before the results were pooled, and heterogeneity was presented as significant when I2was over 50% or P<0.1 [10]. A random effect model was used for the meta-analysis if there was significant heterogeneity, and a fixed effect model was used when the heterogeneity was not significant [10]. A subgroup analysis was conducted when the heterogeneity was high, and a sensitivity analysis was done to investigate potential sources of heterogeneity by omitting each trial in turn [11]. Forest plots were used for comparisons, and funnel plots were used to evaluate publication bias.
Results
Description of included studies
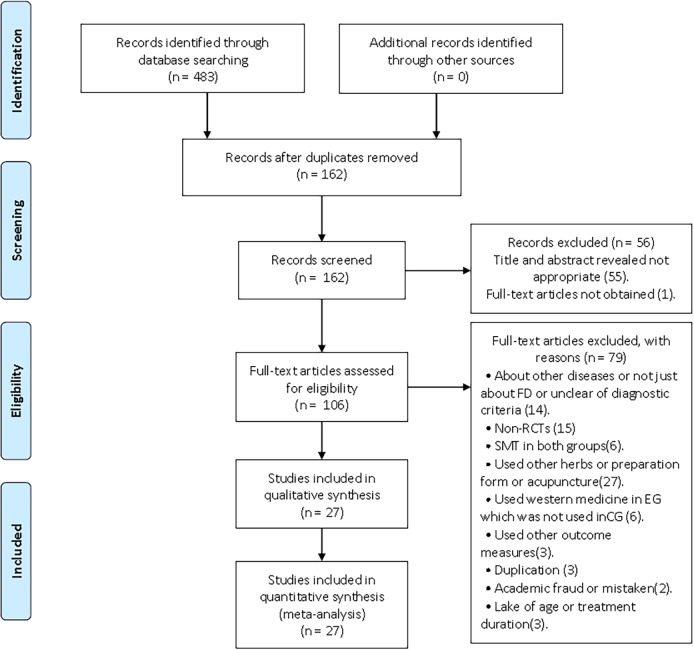
A total of 483 studies were identified by both electronic and manual searches of cited references. Of these, 162 articles were duplicated, 55 articles were excluded for inappropriate titles and abstracts, and 1 full-text article could not be obtained. After further screening, a total of 27 studies satisfied the criteria. A flow chart of the study selection process was showed in Fig 1.
Fig 1. Flow chart of study selection process.
PRISMA flow chart of the selection process of SMT-treated FD. SMT = Si-Mo-Tang oral liquid, FD = functional dyspepsia.
In the 27 trials, 1 study was a postgraduate candidate thesis, and the remaining were journal articles. All studies were conducted in China. One was a multi-center study, and the rest were single-center. Total numbers of subjects of the individual studies varied from 30 to 201, with a total of 2,713 participants included. A description of the characteristics of the included studies can be found in Table 1. The interventions were given orally. Si-Mo-Tang oral liquid was given in doses from 3–20 ml three times a day according to age. Other medicines depended on age and weight according to the drug use instructions.
Table 1. Summary of the characteristics of the included studies.
| Number | Sex | Age | Intervention | Effectiveness | Ineffectiveness | Duration | |||
|---|---|---|---|---|---|---|---|---|---|
| First Author | EG/CG | M(EG/CG) | F(EG/CG) | (year) | EG | CG | EG/CG | EG/CG | (day) |
| Cai 2010[12] | 101/100 | 38/39 | 63/61 | 18–65 | SMT | Domperidone | 81/74 | 20/26 | 14 |
| Ji2010[13] | 100/100 | 57/55 | 43/45 | 3–12 | SMT | Domperidone | 96/81 | 4/19 | 7 |
| Shen 2012[14] | 46/46 | / | / | 20–63 | SMT | Trimebutine+Famotidine | 45/37 | 1/9 | 28 |
| Tu 2009[15] | 55/55 | 25/28 | 30/27 | 4–12 | SMT | Domperidone | 50/42 | 5/13 | 7 |
| Wang 2003[16] | 62/62 | 37/35 | 25/27 | 18–72 | SMT | Domperidone | 58/56 | 4/6 | 14 |
| Wang 2000[17] | 37/36 | 17/14 | 20/22 | 18–66 | SMT | Cisapride | 27/28 | 10/8 | 28 |
| Wei 1999[18] | 69/67 | / | / | >18 | SMT | Cisapride | 57/58 | 12/9 | 14 |
| Xiao 2012[19] | 30/30 | / | / | 18–65 | SMT | Domperidone | 28/26 | 2/4 | 14 |
| Zheng 2005[20] | 50/48 | 28/30 | 22/18 | 16–68 | SMT | Domperidone+Oryzanol | 46/35 | 3/14 | 14 |
| Zhu 2009[21] | 50/50 | 13/12 | 37/38 | 20–68 | SMT | Domperidone+Amitriptyline | 35/40 | 15/10 | 28 |
| Ye 2005[22] | 50/50 | 16/18 | 34/32 | 20–58 | SMT | Placebo | 44/18 | 6/32 | 7 |
| Zhou 2014[23] | 30/30 | / | / | 18–65 | SMT | Domperidone | 27/26 | 1/3 | 14 |
| Zhang 2011[24] | 33/39 | 17/18 | 16/21 | 17–72 | SMT | Cisapride | 32/37 | 1/2 | 14 |
| Li 2012[25] | 42/42 | 16/19 | 23/22 | 18–65 | SMT | Domperidone | 36/35 | 3/6 | 14 |
| Meng 2014[26] | 60/60 | 26/28 | 34/32 | 4–13 | SMT | Domperidone | 55/47 | 5/13 | 7 |
| Xie 2016[27] | 45/45 | 24/26 | 21/19 | 1–5 | SMT+Lactobacillin | Lactobacillin | 42/34 | 3/11 | 14 |
| Zhang 2016[28] | 46/46 | 21/24 | 25/22 | 39–51 | SMT+LCBLEC | LCBLEC | 40/31 | 2/9 | 56 |
| Tian 2014[29] | 15/15 | 9/7 | 6/8 | 5–14 | SMT+Domperidone suspension | Domperidone | 15/13 | 0/2 | 14 |
| Wen 2011[30] | 108/54 | 56/28 | 52/26 | 5–14 | SMT+Domperidone | Domperidone | 104/46 | 4/8 | 14 |
| Li 2011[31] | 96/96 | 110 | 82 | 23–71 | SMT+Domperidone | Domperidone | 90/72 | 6/24 | 28 |
| Zhang 2015[32] | 58/55 | 30/29 | 28/26 | 39–48 | SMT+Trimebutine | Trimebutine | 49/34 | 9/21 | 28 |
| Jiang 2010[33] | 43/43 | 15/28 | 14/29 | 34–68 | SMT+CBSEFGM | CBSEFGM | 38/26 | 5/17 | 28 |
| Yu 2011[34] | 34/34 | 14/16 | 20/19 | 29–41 | SMT+Domperidone | Domperidone | 31/25 | 3/9 | 28 |
| Wu 2014[35] | 29/29 | 16/15 | 13/14 | 3–14 | SMT+Domperidone | Domperidone | 28/24 | 1/5 | 14 |
| Hu 2015[36] | 30/30 | 18/14 | 12/16 | 23–67 | SMT+Mosapride | Mosapride | 28/22 | 2/8 | 28 |
| Li Lin 2012[37] | 35/35 | 20/21 | 15/14 | 46–54 | SMT+Domperidone+Amitriptyline | Domperidone+Amitriptyline | 33/25 | 2/10 | 28 |
| Tang 2010[38] | 27/33 | 14/15 | 13/18 | 2-28day | SMT | Lactasin Tablets | 24/23 | 3/19 | 7 |
”/” = not mentioned, CG = control group, EG = experimental group, SMT = Si-Mo-Tang oral liquid, LCBLEC: Live Combined Bifidobacterium, Lactobacillus and Enterococcus Capsules, CBSEFGM: Combined Bacillus Subtilis and Enterococcus Faecium Granules with Multivitamins.
Methodological quality of included studies
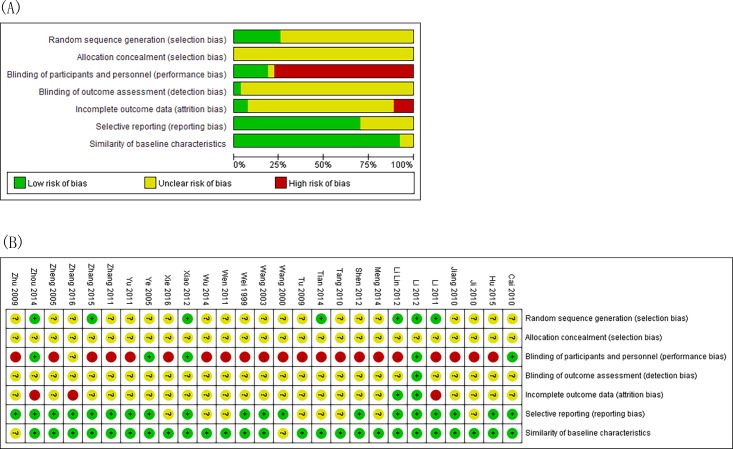
The risk of bias assessment in the studies is shown in Fig 2. Risk of bias was found across studies for random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessment, incomplete outcome data, selective outcome reporting, and similarity of baseline characteristics [9, 10]. All studies mentioned randomization, but just 6 articles had a detailed description of random sequence generation. None of them discussed allocation concealment. Five studies described blinding of patients, but the other studies did not describe the details. Less study mentioned the details about blinding of outcome assessment. Six studies mentioned follow-up; and 3 of these reported the drop-out or withdrawal information, but did not use ITT analysis. The author reported that characteristics of subjects in different groups have similar baseline (age, sex, race, and disease course) among the studies.
Fig 2. The risk of bias assessment with the Cochrane Collaboration tool.
(A)Risk of bias graph. (B)Risk of bias summary.
Primary outcome: Clinical efficacy rate
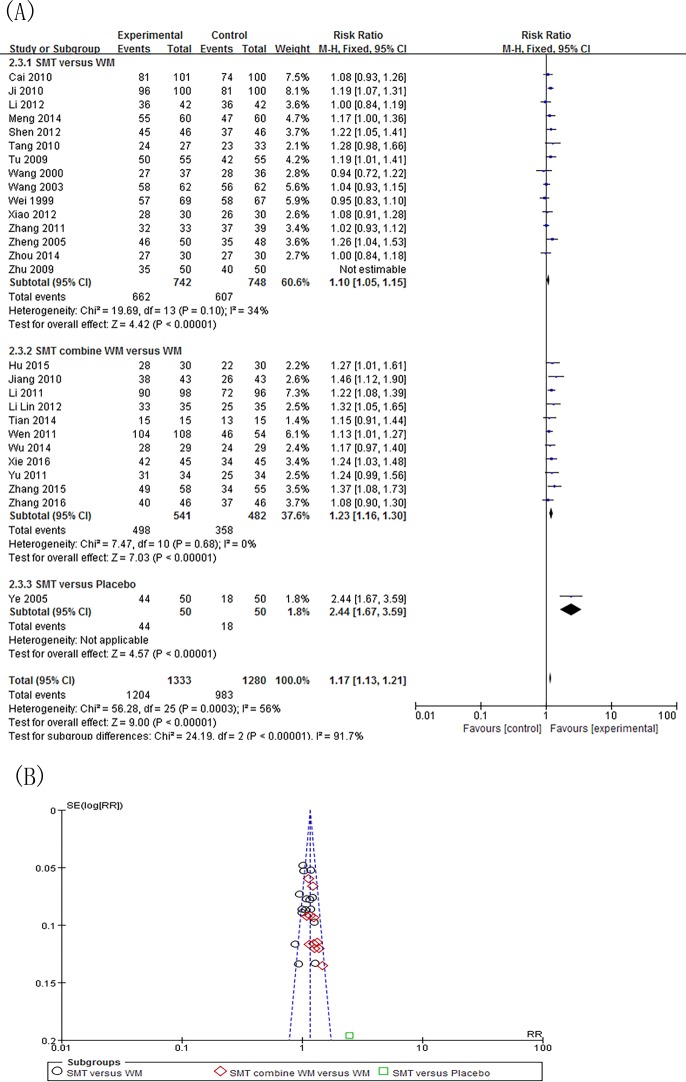
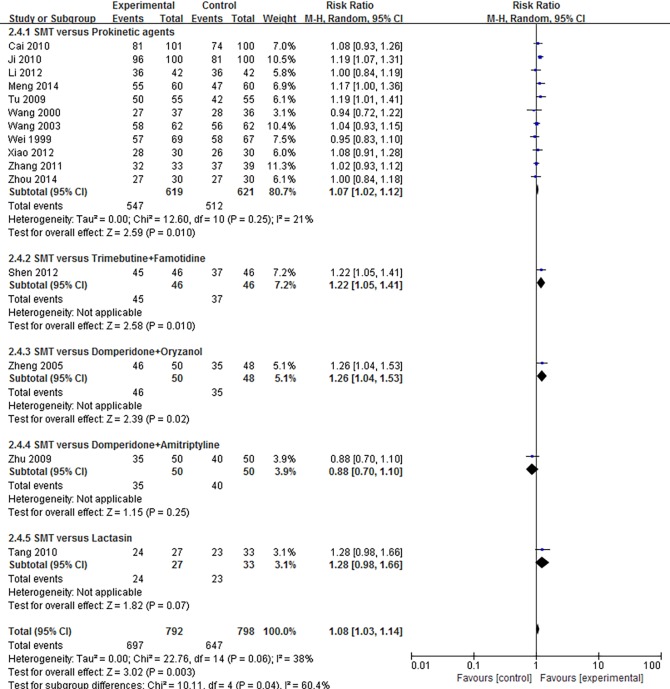
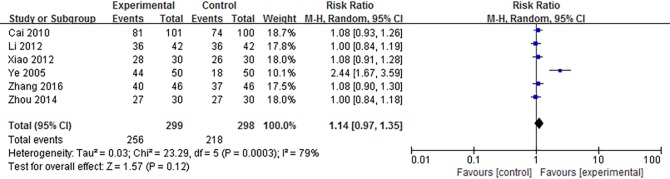
Twenty-7 independent studies reported a clinical efficacy rate defined by symptom relief for SMT-treated FD. Participants in the experimental group totaled 1,383, and 1,330 in the control group. SMT showed significant improvement in efficacy (RR 1.14; 95% CI 1.09, 1.20; P<0.00001), but the heterogeneity was significant (P = 0.0002, I2 = 56%) (S1 Fig).We performed a subgroup analysis to investigate potential sources of heterogeneity. The included studies fell into 3 subgroups: SMT versus western medicine, SMT versus placebo, and SMT combined with western medicine versus western medicine alone. We then performed a sensitivity analysis, and determined that the Zhu (2009)[21] study was the main origin of heterogeneity in the subgroup analysis. The heterogeneity was smaller after subgroup analysis and exclusion of the study in question. However, the corresponding pooled RR had no obvious change (RR 1.17; 95% CI 1.13, 1.21; P<0.00001) (Fig 3A).The funnel plot demonstrates no apparent asymmetry, suggesting that publication bias is unlikely (Fig 3B). Further examination of the control interventions in the “SMT versus western medicine” subgroups showed that interventions could be categorized by type, such as prokinetic agents, antidepressants, and microbial preparation. Subgroup analysis by different categories of control interventions showed that SMT improved efficacy rate (RR 1.08; 95% CI 1.03, 1.14; P = 0.003) (Fig 4).Subgroup analysis by age also demonstrated the clinical efficacy of SMT (RR 1.16; 95% CI 1.12, 1.20; P<0.00001) (S2 Fig). Due to the importance of blinding of participants and personnel during SMT treatment, we also conducted a data synthesis excluding high risk of bias in the blinding of participants and personnel. Results show that there is no significant difference in the effective rate after SMT treatment (RR 1.14; 95% CI 0.97, 1.35; P = 0.12) (Fig 5).
Fig 3. Efficacy rate and publication bias of SMT-treated FD after subgroup of SMT alone or combine with WM versus placebo or WM and sensitivity analysis.
(A)Forest plot of comparison: the efficacy rate of symptoms. (B) Funnel plot of publication bias of the included studies. SMT = Si-Mo-Tang oral liquid, WM = western medicine, FD = functional dyspepsia.
Fig 4. Forest plot of the efficacy rate of SMT-treated FD after subgroup analysis of the different kinds of control interventions among SMT versus WM.
SMT = Si-Mo-Tang oral liquid, WM = western medicine, FD = functional dyspepsia.
Fig 5. Forest plot of the efficacy rate of SMT-treated FD after excluding poor quality studies.
SMT = Si-Mo-Tang oral liquid, WM = western medicine, FD = functional dyspepsia.
Second outcomes
Gastric emptying
Three included studies reported gastric emptying, but the data could not be incorporated because Cai (2010)[12] reported efficacy rate, while Ye (2005)[22] referred to the time of gastric emptying, and Zhu (2009)[21] used the emptying rate. Cai and Zhu reported no significant difference between experimental and control group, while Ye reported that SMT significantly reduced the time of gastric emptying.
Relapse rate
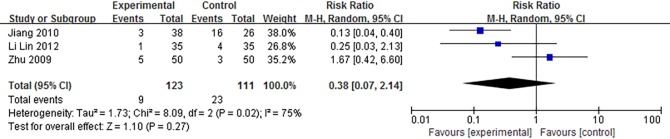
Three articles discussed a follow-up time. The observation period of Zhu[21] was 8 weeks, Li Lin (2012)[37] was 2 months, and Jiang (2010) [33] was 1 year. The relapse rate was 7.3% for the experimental group and 20.7% for the control group. But as shown in Fig 6, meta-analysis of the 3 studies showed no significant difference between 2 groups.
Fig 6. Forest plot of relapse rate of SMT-treated FD between experimental and control group.
SMT = Si-Mo-Tang oral liquid, FD = functional dyspepsia.
Safety profile and adverse events
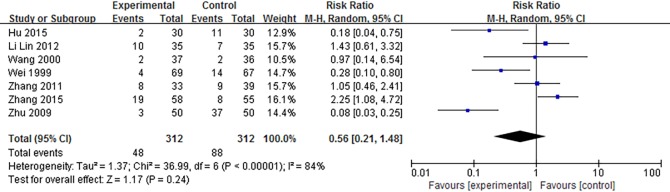
The safety profile was evaluated in all 27 studies. Eight trials did not mention adverse events. Eleven studies reported no adverse effects in the experimental group during SMT treatment. In the 11 studies of the control group, 7 showed no adverse effects, 3 trials had no mention of adverse events, and 1 research study by Cai[12] revealed that 3 of 41 patients had experienced nausea and dizziness while being treated with Domperidone. A trial by Jiang (2010)[33] reported that 1 of 43 patients appeared to have diluted stool in the experimental group. Another 7 studies showed adverse events in both groups. These adverse events in experimental groups included nausea, dizziness, somnolence, weakness, dry mouth, diarrhea and diluted stool. In the control groups, symptoms also included spastic abdominal pain. The authors, however, said no serious incidents occurred, most of the events did not require special treatment, and were alleviated with time or dose reduction. A meta-analysis of the 7 studies in Fig 7 shows that there was no significant difference between 2 groups (P = 0.24). In addition, 23 of the included studies reported that participants in both groups had no organic or systemic disease, such as liver and kidney function damage, while 4 of these studies did not mention the information. Among the 19 studies that reported adverse effects, no case had impact on renal and hepatic function.
Fig 7. Forest plot of safety profile and adverse events of SMT-treated FD.
SMT = Si-Mo-Tang oral liquid, FD = functional dyspepsia.
Discussion
This is the first attempt to synthesize clinical data of Si-Mo-Tang oral liquid for functional dyspepsia. In this systematic review, 27 studies were included involving 2713 participants: 1383 versus 1330 between experimental and control group. SMT showed a significant improvement of clinical efficacy rate, and no significant different of relapse rate and adverse effects. Three studies referred gastric emptying, 2 studies reported no significant different between experimental and control group, while 1 showed SMT reduced the time of gastric emptying. The heterogeneity was significant for the primary outcome. We performed a subgroup and sensitivity analysis and found that the Zhu (2009)[21] study may be the main origin of heterogeneity. After subgroup analysis and exclusion of the study, the heterogeneity was effectively decreased while the corresponding pooled RR was not substantially altered. We checked all the included studies carefully and found that there were differences between Zhu and the other groups. In Zhu’s study, besides the experimental and control group in Table 1, there were groups of SMT, Domperidone and Amitriptyline treatment. The third group showed the best efficacy rate. Subgroup analysis by age and different kinds of drugs also showed an improvement in the efficacy rate. But a data synthesis excluding high risk of bias in the blinding of participants and personnel showed no significant difference.
The pathogenesis of FD has not been fully clarified. Numerous mechanisms are involved in the development of FD, including gastric motility and compliance, visceral hypersensitivity, Helicobacter pylori infection, altered gut microbiome and psychosocial dysfunction [39]. It is well known that gastric motility and compliance are a common pathogenesis of FD. Several motility disorders have been reported in patients with dyspepsia. These include delayed gastric emptying, rapid gastric emptying, antral hypomotility, gastric dysrhythmias, and impaired gastric accommodation in response to a meal [39]. Prokinetic agents (such as Domperidone, Cisapride, Mosapride in Table 1) were used widely. The mechanism of SMT is related to its pathogenesis. Ghrelin is a peptide hormone that is involved in gastrointestinal motility and secretion, and therefore, may play a role in functional dyspepsia. Nitric Oxide may play a role as a mediator for ghrelin secretion [40,41]. Kazemi M, Eshraghian A, Hamidpour L, etc. (2015) compared the change of serum ghrelin levels in relation to meal-time between patients with FD and a control group, and found that ghrelin may have an important role in inducing symptoms in FD patients [40]. Several studies explored the possible mechanism of SMT, and found that SMT can regulate the level of Ghrelin and NO in patients with FD [23,42,43].
This Chinese patent medicine is composed of Radix Aucklandiae (Muxiang in Chinese), Fructus Aurantii (Zhiqiao in Chinese), Areca catechu Linn (Binglang in Chinese) and Lindera aggregata (Sims) Kosterm (Wuyao in Chinese) [44].Evidence for the effectiveness of SMT for FD can also be identified in modern pharmacological studies. Guo H, Zhang J, Gao W, etc.(2014) focused on the effects of the methanol extract Radix Aucklandiae (RA ext) on the gastrointestinal tract. They concluded that the mechanism of RA ext might be the root of inhibitory activity. In vitro, RA ext mediated possibly through the combination of Ca2+ antagonist and anticholinergic mechanisms, which provides scientific basis for the clinical use of Radix Aucklandiae [45]. Jiang Y, Bai X, Zhu X, etc.(2014) revealed that Fructus Aurantii can enhance gastrointestinal motility by altering 5-HT and vasoactive intestinal peptide expression levels in the rat GI tract [46].Wang Y and Huang T used 95% ethanol for herbal extraction, and found that Areca catechu Linn possessed lower anti-Helicobacter pylori effects [47]. Guo J, Nie Z, Zhang M, etc. (2012) found that the extraction of Lindera aggregata (Sims) Kosterm has the effect of relaxing the isolated ileum of guinea pigs [48]. Although several mechanisms have been proposed, the pathogenesis of functional dyspepsia and the mechanism of SMT-treated FD remains unclear [39].Further studies both in vitro and in vivo need to be conducted to better understand the drug mechanism.
The methodological quality of included studies was showed in Fig 2. Most trails had similar baseline characteristics to ensure the reliability of the research. But there were some flaws in the quality of the included studies. None of the included studies mentioned allocation concealment and fewer provided random sequence generation so the selection bias was high. Few studies mentioned details about the blinding of outcome assessment. Most studies did not describe the details of blinding that may be caused by the different dosage forms between each group, but there were still 5 studies pills and oral liquid. Another flaw was the lack of ITT analysis, which may lead to incomplete outcome data. However, we extracted data as ITT analyses, assuming all drop-outs to be treatment failures, which could reduce the risk of attrition bias [11].
Several possible limitations of this review are worthy of comment. First, while we used a wide range of search strategies to minimize publication bias, some linguistic biases may exist due to language limitations. Second, because of the relatively small sample sizes and the short duration in most studies, the detection of a statistically significant difference between SMT and control group may be limited. It still needs to be demonstrated whether or not the effect size of SMT remains the same when applied in future large-scale studies. Third, the majority of the RCTs had limitations that included the paucity of detailed methodology, non-standardized evaluation of efficacy, and the suboptimal quality of the study design. Therefore, there is a need for further well-designed, long-term, and strictly designed RCTs to explore the effects of SMT for FD.
Conclusions
This review suggests that SMT is an effective and safe therapy option for patients with FD. However, due to the high clinical heterogeneity, poor quality, high risk of bias and small sample size of some included studies, further standardized preparation, large-scale and strictly designed studies are needed.
Supporting information
(DOC)
(PDF)
Efficacy rate of symptoms of SMT-treated FD. SMT = Si-Mo-Tang oral liquid, FD = functional dyspepsia.
(TIF)
SMT = Si-Mo-Tang oral liquid, FD = functional dyspepsia.
(TIF)
Acknowledgments
The authors thank Dr. Hao Chen (The Second College of Clinical Medicine, Nanjing University of Chinese Medicine, Nanjing, China) for methodology guidance of meta-analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant number: 81373608). The website is http://www.nsfc.gov.cn/. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Talley NJ, Ford AC. Functional Dyspepsia. The New England journal of medicine. 2015;373(19):1853–1863. 10.1056/NEJMra1501505 [DOI] [PubMed] [Google Scholar]
- 2.Sander G, Mazzoleni L, Francesconi C, Balbinotto G, Mazzoleni F, Wortmann A, et al. Influence of organic and functional dyspepsia on work productivity: the HEROES-DIP study. Value Health. 2011;14(5):S126–S129. [DOI] [PubMed] [Google Scholar]
- 3.Lacy B, Weiser K, Kennedy A, Crowell M, Talley N. Functional dyspepsia: the economic impact to patients. Alimentary Pharmacology & Therapeutics. 2013;38(2):170–177. [DOI] [PubMed] [Google Scholar]
- 4.Talley NJ. Functional dyspepsia: new insights into pathogenesis and therapy. The Korean Journal of Internal Medicine. 2016;31(3):444–456. 10.3904/kjim.2016.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahner E, Bellentani S, de Bastiani R, Tosetti C, Cicala M, Esposito G, et al. A survey of pharmacological and nonpharmacological treatment of functional gastrointestinal disorders. United European Gastroenterology Journal. 2013;1(5):385–393. 10.1177/2050640613499567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling W, Li Y, Jiang W, Sui Y, Zhao HL. Common Mechanism of Pathogenesis in Gastrointestinal Diseases Implied by Consistent Efficacy of Single Chinese Medicine Formula. Medicine. 2015;94(27):e1111 10.1097/MD.0000000000001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheng YZ, Wang WT, Wei C. Si-Mo-Tang oral liquid: China, 94110842.2. 1995-08-09.
- 8.Jie Luo, Leng WD. Theory & practice of systematic review/meat-analysis. 5th ed. Beijing: Military Medical Science Press; 2013. [Google Scholar]
- 9.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Library; 2016. Available: http://handbook.cochrane.org/ [Google Scholar]
- 10.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao Y, Liu Y, Huang S, Sun X, Tang Y, Cheng J, et al. The efficacy of Shugan Jianpi Zhixie therapy for diarrhea-predominant irritable bowel syndrome: a meta-analysis of randomized, double-blind, placebo-controlled trials. PloS one 2015;10(4):e0122397 10.1371/journal.pone.0122397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai GX, Bu XC. Clinical efficacy of Si-Mo-Tang on functional dyspepsia with syndrome of incoordination between the liver and spleen and its effect on motilin and substance P in plasma. China Journal of Traditional Chinese Medicine and Pharmacy.2010;25(6):856–859. [Google Scholar]
- 13.Ji XH. Evaluation of the effect of Si-Mo-Tang on functional dyspepsia in children. Chinese Modern Doctor. 2010;48(29):157–158. [Google Scholar]
- 14.Shen ZD. Clinical efficacy of Si-Mo-Tang in 92 cases of functional dyspepsia. Frontiers of Medicine. 2012(21):316–317. [Google Scholar]
- 15.Tu Y. Si-Mo-Tang oral liquid in the treatment of 55 cases of functional dyspepsia. Jiangxi Journal of traditional Chinese Medicine. 2009;40(6):50. [Google Scholar]
- 16.Wang JH. Si-Mo-Tang oral liquid in the treatment of 62 cases of functional dyspepsia. Clinical Journal of Practical Integrated Traditional Chinese and Western Medicine. 2003;3(1):33. [Google Scholar]
- 17.Wang QZ, Tian Y, Xu XY. Clinical effect of Si-Mo-Tang and Cisapride on functional dyspepsia. Chinese Journal of Spleen and Stomach. 2000;8(1):29. [Google Scholar]
- 18.Wei SH, Yang ZE. The effect of Si-Mo-Tang and Cisapride in the treatment of functional dyspepsia. Acta Medicinae Sinica. 1999;12(6):758. [Google Scholar]
- 19.Xiao ZH, Liu BY, Cai GX, Yi J, Lin XY. Clinical observation of Si-Mo-Tang in the treatment of 60 cases of functional dyspepsia. Journal of Guiyang College of Traditional Chinese Medicine. 2012;34(2):14–16. [Google Scholar]
- 20.Zheng Y, Wei Q, Li JX. The effect of Si-Mo-Tang on functional dyspepsia in 98 cases. Hebei Journal of Traditional Chinese Medicine. 2005;27(11):815–815. [Google Scholar]
- 21.Zhu YL, Qin YM, Liu ZE. Combined use of Si-Mo-Tang, Domperidone and Amitriptyline to treat functional dyspepsia: a report of 60 cases. World Chinese Journal of Digestology. 2009;17(29):3028–3033. [Google Scholar]
- 22.Ye S, Huang H, Zhao YP, Xu ZJ.Treatment of 50 patients with dyskinetic functional dyspepsia with Si-Mo-Tang oral liquid. Herald of Medicine 2005;24(8):679–681. [Google Scholar]
- 23.Zhou SN, Cai GX, Wan S. Clinical efficacy of Si-Mo-Tang on functional dyspepsia with Qi stagnancy of both liver and spleen and its effect on Nitrie Oxide, Acetylcholinesterase, Cholecystokinin and substance P in serum. Journal of Emergency in Traditional Chinese Medicine. 2014;23(10):1791–1792,1833. [Google Scholar]
- 24.Zhang Y.Clinical observation of Cisapride combined with Si-Mo-Tang on functional dyspepsia. China Healthcare Innovation. 2011;06(13):22,44. [Google Scholar]
- 25.Li YJ. The Meta-analysis of Si-Mo-Tang on patients with functionaldyspepsia and the study on clinical efficacy of Si-Mo-Tang onpatients with functional dyspepsia with qi stagnancy of both liverand spleen.M.Sc. Thesis, Hunan University of Traditional Chinese Medicine. 2012. Available:http://www.cnki.net/KCMS/detail/detail.aspx?QueryID=17&CurRec=1&recid=&filename=1012401545.nh&dbname=CMFD2012&dbcode=CMFD&pr=&urlid=&yx=&v=MjA1MzdJUjhlWDFMdXhZUzdEaDFUM3FUcldNMUZyQ1VSTHlmWU9SbkZDbmxWTDNCVkYyNkhMZTRIOVRJcXBFYlA=
- 26.Meng FC. Si-Mo-Tang oral liquid in the treatment of dyspepsia in 60 cases of children. Hunan Journal of Traditional Chinese Medicine. 2014;30(10):65–66. [Google Scholar]
- 27.Xie YB. Si-Mo-Tang combined with Lactobacillinin the treatment of dyspepsia in children. Strait Pharmaceutical Journal. 2016(04):211–212. [Google Scholar]
- 28.Zhang MM. Clinical observation of functions dyspepsia combined Si-Mo-Tang oral liquid with Live Combined Bifidobacterium, Lactobacillus and Enterococcus Capsules. New traditional Chinese Medicine. 2016(05):83–85. [Google Scholar]
- 29.Tian LX, Yang HR, Niu LN. Combination of Traditional Chinese and Western Medicine in the treatment of functional dyspepsia in 30 cases of children. Medicine & People. 2014;27(9):222–223. [Google Scholar]
- 30.Wen H, Liu XL. Clinical observation on 108 cases of children with functional dyspepsia treated by integrated Traditional Chinese and Western Medicine. The Medical Journal of Industrial Enterprise. 2011;24(5):60–61. [Google Scholar]
- 31.Li SX. Clinical research of treating functional dyspepsia with Domperidone and Si-Mo-Tang oral liquid. Clinical Journal of Chinese Medicine. 2011;03(23):59–60. [Google Scholar]
- 32.Zhang K. Effect of Si-Mo-Tang and Trimebutine in treating postprandial distress syndrome of functional dyspepsia. Chinese Medical Innovation. 2015(19):126–128. [Google Scholar]
- 33.Jiang HS. The effect and nursing strategy of Si-Mo-Tangassociated with Combined Bacillus Subtilis and Enterococcus Faecium Granules with Multivitamines in treating functional dyspepsia. Chinese Journal of Modern Drug Application. 2010;04(17):187–188. [Google Scholar]
- 34.Yu DH. Effect of Domperidone and Si-Mo-Tang oral liquid in 68 cases with dyskinetic functional dyspepsia. Chinese Community Physicians (Medical). 2011;13(14):148–149. [Google Scholar]
- 35.Wu L. Clinical research of Traditional Chinese and Western Medicine in the treatment of children with functional dyspepsia. Journal of North Pharmacy. 2014(12):92–92. [Google Scholar]
- 36.Hu P. Clinical observation of Si-Mo-Tang combined with Mosapride in the treatment of 60 cases of functional dyspepsia. Chinese Journal of Modern Drug Application 2015;9(24):143–144. [Google Scholar]
- 37.Li L. Si-Mo-Tang, Dmperidone and Amitriptyline in the treatment of functional dyspepsia in 35 cases. Chinese Journal of Gerontology. 2012;32(13):2853–2854. [Google Scholar]
- 38.Tang W, Chen ZL, Wen CY. Clinical observation of Si-Mo-Tang oral liquid in the treatment of 27 cases of neonatal functional dyspepsia. Guiding Journal of Traditional Chinese Medicine and Pharmacy. 2010;16(10):33–35. [Google Scholar]
- 39.Longstreth GF, Lacy BE. Functional dyspepsia in adults. Up To Date. 2016. Available: http://www.uptodate.com/contents/functional-dyspepsia-in-adults?source=search_result&search=Functional+dyspepsia+in+adults&selectedTitle=2~150
- 40.Kazemi M, Eshraghian A, Hamidpour L, Taghavi S. Changes in serum ghrelin level in relation to meal-time in patients with functional dyspepsia. United European gastroenterology journal 2015;3(1):11–6. 10.1177/2050640614563373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohno T, Mochiki E, Kuwano H. The roles of motilin and ghrelin in gastrointestinal motility. International Journal of Peptides 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Li B, Zhong Z. Effect of Si-Mo-Tang on the expression of Ghrelin both in the plasma and gastric mucosal of functional dyspepsia patients. Chinese Journal of Traditional Medical Science and Technology. 2013;20(3):222–223. [Google Scholar]
- 43.Huang JD, Jian XX, Bai QZ. Effect of Si-Mo-Tang on the expression of Ghrelin and Nitric Oxide in plasma of the patients with functional dyspepsia. Journal of Clinical Medicine. 2015(21):4341–4341,4344. [Google Scholar]
- 44.Yi Y, Cheng X, Liu L, Hu G, Wang Z, Deng Y, et al. Simultaneous determination of synephrine, arecoline, and norisoboldine in Chinese patent medicine Si-Mo-Tang oral liquid preparation by strong cation exchange high performance liquid chromatography. Pharmaceutical biology 2012;50(7):832–8. 10.3109/13880209.2011.637505 [DOI] [PubMed] [Google Scholar]
- 45.Guo H, Zhang J, Gao W, Qu Z, Liu C. Gastrointestinal effect of methanol extract of Radix Aucklandiae and selected active substances on the transit activity of rat isolated intestinal strips. Pharmaceutical biology 2014;52(9):1141–9. 10.3109/13880209.2013.879601 [DOI] [PubMed] [Google Scholar]
- 46.Jiang Y, Bai X, Zhu X, Li J. The effects of Fructus Aurantii extract on the 5-hydroxytryptamine and vasoactive intestinal peptide contents of the rat gastrointestinal tract. Pharmaceutical biology 2014;52(5):581–5. 10.3109/13880209.2013.854396 [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Huang T. Screening of anti-Helicobacter pylori herbs deriving from Taiwanese folk medicinal plants. FEMS immunology and medical microbiology 2005;43(2):295–300. 10.1016/j.femsim.2004.09.008 [DOI] [PubMed] [Google Scholar]
- 48.Guo JS, Nie ZW, Zhang M, Chen J, Liu HY. The influence of the extraction of Lindera aggregata (Sims) Kosterm on the isolated ileum of guinea pigs. Lishizhen Medicine and Materia Medica Research. 2012(01):56–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
Efficacy rate of symptoms of SMT-treated FD. SMT = Si-Mo-Tang oral liquid, FD = functional dyspepsia.
(TIF)
SMT = Si-Mo-Tang oral liquid, FD = functional dyspepsia.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.