Abstract
Objectives
Limited data are available on sepsis in low-resource settings, particularly outside of urban referral centers. We conducted a prospective observational single-center cohort study in May 2013 to assess the presentation, management and outcomes of adult and pediatric patients admitted with sepsis to a community hospital in rural Uganda.
Methods
We consecutively screened all patients admitted to medical wards who met sepsis criteria. We evaluated eligible patients within 24 hours of presentation and 24–48 hours after admission, and followed them until hospital discharge. In addition to chart review, mental status evaluation, peripheral capillary oxygen saturation, and point-of-care venous whole blood lactate and glucose testing were performed.
Results
Of 56 eligible patients, we analyzed data on 51 (20 adults and 31 children). Median age was 8 years (IQR 2–23 years). Sepsis accounted for a quarter of all adult and pediatric medical ward admissions during the study period. HIV prevalence among adults was 30%. On enrollment, over half of patients had elevated point-of-care whole blood lactate, few were hypoglycemic or had altered mental status, and one third were hypoxic. Over 80% of patients received at least one antibiotic, all severely hypoxic patients received supplemental oxygen, and half of patients with elevated lactate received fluid resuscitation. The most common causes of sepsis were malaria and pneumonia. In-hospital mortality was 3.9%.
Conclusions
This study highlights the importance of sepsis among adult and pediatric patients admitted to a rural Ugandan hospital and underscores the need for continued research on sepsis in low resource settings.
Introduction
Infectious diseases are among the leading causes of morbidity and mortality worldwide, with the greatest burden in low-income countries (LICs) [1][2]. In Uganda, a sub-Saharan African LIC of 37.8 million people, HIV/AIDS, pneumonia, diarrheal diseases, and malaria account for 4 of the top 5 causes of death [1][3]. Sepsis, the dysregulated host response to infection, is a common syndrome encountered by clinicians treating patients with infection. Despite what is known about the epidemiology of these infectious diseases at the population level, few data are available to inform the clinical management of sepsis in low-resource settings such as Uganda [4].
The Surviving Sepsis Campaign (SSC) has published guidelines for management of patients with severe sepsis and septic shock [5]. While these guidelines have become the standard of care in high-resource settings, much of the world remains unable to routinely implement them either in part or in full [6–10]. While efforts have been made to provide modified recommendations and approaches for limited-resource settings [2,11–18], a deeper understanding of the current state of care in more varied low-resource settings is paramount to allow clinicians and public health practitioners to improve care and better target research efforts.
While studies describing the management of sepsis in low-resource settings have been published previously, many of these have excluded pediatric patients, evaluated patients with acute febrile illness instead of sepsis specifically, focused on patients with just one infectious disease such as malaria or pneumonia, or limited their patient population to those in urban referral centers [19–27]. Beyond observational studies, others have published results of interventional trials for sepsis or shock conducted in sub-Saharan Africa [17,28,29]. Important work has been done in Uganda evaluating the presentation, management and outcomes of adult patients with severe sepsis presenting to urban referral hospitals [30–40]. Few studies have evaluated non-neonatal pediatric sepsis in Uganda or sepsis in rural or non-tertiary care hospitals in LICs. To address these knowledge gaps, we conducted a prospective observational single-center cohort study with the primary objective of describing the epidemiology, management, and outcomes of sepsis in adults and children presenting to a rural Ugandan hospital.
Methods
Study design
We conducted a prospective observational cohort study at Bwindi Community Hospital, a private not-for-profit 112-bed hospital serving a catchment area of approximately 100,000 patients in rural southwest Uganda. This is the only hospital in the area and serves a very poor and rural community. The hospital has both inpatient and outpatient facilities including well-developed HIV treatment and prevention services. Hospital capabilities include full obstetric services, surgical facilities with a general surgeon available 24 hrs per day, and dedicated adult and pediatric wards with medical officers on-site during the day and available by call overnight. There is a reliable supply of IV fluids and sterile supplies; basic antibiotics including penicillins, gentamycin, and ceftriaxone; IV dextrose; antimalarials and tuberculosis treatments; and supplemental oxygen in the form of 6L concentrators (2 per ward, sometimes requiring splitting of the flow between patients). The hospital has basic laboratory services, basic radiography services, and one of the only blood banks in the region. Malaria is diagnosed with rapid diagnostic testing and blood smear. While limited microbiology testing such as gram stain is available, microbiological culture and sensitivity testing is not available on-site, nor are there more advanced imaging technologies such as computed tomography (CT). The nearest referral hospital is approximately 180km away.
We consecutively enrolled all adult (age ≥ 18yrs) and pediatric (ages 28d – 17yrs) patients admitted to the inpatient medical wards who met sepsis criteria May 4–26, 2013. Sepsis was defined as the presence of the systemic inflammatory response syndrome (SIRS) and suspected infection. For adults, SIRS was defined as two of the four following criteria: temperature greater than 38°C (100.4°F) or less than 36°C (96.8°F), heart rate of more than 90 beats per minute, respiratory rate of more than 20 breaths per minute or arterial carbon dioxide tension (PaCO2) of less than 32mm Hg, and abnormal white blood cell count (>12,000/μL or < 4,000/μL or >10% immature [band] forms) [41]. For children, SIRS was defined as two of the four following criteria: temperature greater than or equal to 38.5°C or less than 36°C, heart rate > 2 standard deviations (SD) above normal for age in the absence of external stimulus, respiratory rate > 2 SD above normal for age, and white blood cell count elevated or depressed for age, or > 10% bands [42]. Although standard definitions for pediatric SIRS use core temperature, only axillary temperatures are obtained at the study hospital, and thus axillary temperature were substituted. Of note, to meet pediatric SIRS criteria, children must have a temperature or WBC abnormality in addition to at least one other SIRS criterion. Surgical patients, women admitted to the maternity ward, and neonates (less than 28 days of age) were excluded, as were patients who did not meet sepsis criteria, did not provide consent, or were not enrolled within 24 hours of admission.
Potential subjects were identified through chart review for all new admissions to the adult and pediatric wards at least twice daily. Qualifying patients and/or their legal representatives were then approached for consent and study enrollment. Data were collected through chart review as well as through direct patient assessment by study staff as soon as possible within 24 hours of presentation and again 24–48 hours after admission. Additional chart review was performed upon hospital discharge. Information obtained from chart review included demographic information; admission and discharge diagnoses; vital signs; laboratory findings (CBC with differential, basic chemistry, HIV status, CD4 count, malaria rapid test and smear, and gram stain); results of radiology studies; type and quantity of administered IV fluid or antimicrobials; administration of supplemental oxygen; complications; disposition; and length of stay. Additional data obtained through direct patient assessment included age-appropriate AVPU and Glasgow Coma scores (GCS), peripheral capillary oxygen saturation (SpO2), point-of-care lactate testing of whole venous blood (Lactate Plus lactate meter, Nova Biomedical), and point-of-care glucose testing of whole venous blood (ACCU-CHECK Aviva glucometer, Roche). All abnormal glucose, lactate, and SpO2 results identified were reported to the patient’s treating clinician within one hour of the test.
Statistical analysis
Data was recorded manually in case report forms. We performed statistical analysis using Stata/IC 13. Continuous variables with normal distributions are expressed as means with standard deviations (SD), continuous variables with non-normal distributions are expressed as medians with interquartile range (IQR), and categorical variables are expressed as counts with percentages.
Human subjects and ethics
Ethical approval was obtained from the Human Subjects Division of the University of Washington (Seattle, WA, USA) and the Mbarara University of Science and Technology Institutional Review Committee (Mbarara, Uganda). Additional study approval was obtained from the Ugandan National Council of Science and Technology (UNCST). Informed consent was obtained from all adult patients and a parent of all pediatric patients (less than 18 years of age) in Rukiga, the local language. In the event that an adult patient was unable to provide consent due to altered mental status, their legal representative provided informed consent. In the event that patients or their legal representatives were able to read, consent was obtained in writing. In the event that patients or their legal representatives were unable to read, the consent form was read aloud by an interpreter. Patients or representatives who were unable to sign their name drew an “X” on the consent form signature line or marked the space with a thumbprint and a witness signed the form.
Results
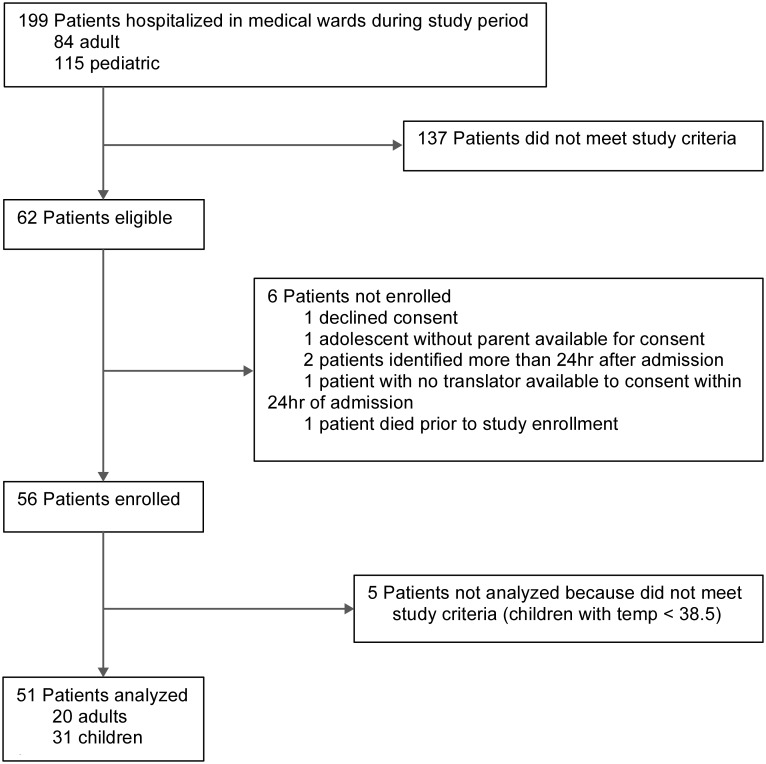
During the study period, 84 adult and 115 pediatric patients were admitted to the adult and pediatric medical wards (Fig 1). Of these, 62 met enrollment criteria and 56 patients were enrolled in the study. One patient who met study criteria died prior to enrollment. Five patients were subsequently excluded from the analysis as re-review indicated that they did not meet inclusion criteria. Therefore, of 56 eligible patients we analyzed data for 51 patients (20 adults and 31 children), representing 23.8% (20/84) of all adult and 27% (31/115) of all pediatric patients admitted to the hospital during the study period.
Fig 1. Patient identification.
The characteristics of subjects at enrollment are given in Table 1. The median age was 8 years (IQR 2–23 years). Among adults, the median age was 32 years (IQR 22–42 years). Among children, the median age was 3 years (IQR 2–7 years). There was a roughly equal distribution of males and females in both the adult and pediatric cohorts. All of the study subjects were of the Bantu ethnic majority. Only eight patients (16%) had a CBC drawn upon admission, and thus most adult and all pediatric patients met sepsis criteria for study enrollment exclusively based on vital sign abnormalities. No children had significant co-morbidities. Among adults, the most common co-morbidity was HIV (n = 6, 30.0%). The most common chief complaints among adults were fever (75.0%) and neurologic symptoms (65.0%). The most common chief complaints among children were fever (93.5%) and cardiopulmonary symptoms (71.0%).
Table 1. Characteristics of patients.
| Variable | Adults | Pediatrics |
|---|---|---|
| Number of patients, n (%) | 20 (39.2%) | 31 (60.8%) |
| Male gender, n (%) | 11 (55.0%) | 14 (45.2%) |
| Ethnicity, n (%) | ||
| Bantu | 20 (100.0%) | 31 (100.0%) |
| Batwa | 0 (0.0%) | 0 (0.0%) |
| Median age, yr [IQR] | 32 [22–42] | 3 [2–7] |
| SIRS criteria, n (%) | ||
| Heart rate | 18 (90.0%) | 19 (61.3%) |
| Respiratory rate | 13 (65.0%) | 29 (93.5%) |
| Temperature | 15 (75.0%) | 31 (100.0%) |
| White blood cell count | 2 (10.0%) | 0 (0.0%) |
| Co-morbidities, n (%) | ||
| Diabetes | 1 (5.0%) | 0 (0.0%) |
| HIV | 6 (30.0%) | 0 (0.0%) |
| Tuberculosis | 1 (5.0%) | 0 (0.0%) |
| Chief complaint, n (%) | ||
| Anorexia | 0 (0.0%) | 10 (32.3%) |
| Cardiopulmonarya | 9 (45.0%) | 22 (71.0%) |
| Fever | 15 (75.0%) | 29 (93.5%) |
| Gastrointestinalb | 9 (45.0%) | 9 (29.0%) |
| Musculoskeletalc | 3 (15.0%) | 2 (6.5%) |
| Neurologicd | 13 (65.0%) | 10 (32.3%) |
| Upper respiratory symptomse | 0 (0.0%) | 4 (12.9%) |
aChest pain, cough, dyspnea, hemoptysis.
bAbdominal pain, diarrhea, vomiting.
cArthralgias, back pain, extremity pain, myalgias.
dAltered mental status, dizziness, headache, seizure.
eCoryza, rhinorrhea.
The characteristics of subjects at the time of enrollment (within 24 hours of admission) are given in Table 2. One adult (5.0%) and two children (6.5%) were hypoglycemic. The majority of subjects had evidence of hypoperfusion, especially adults, eight (40%) of whom had a lactate > 4 mmol/L. Of those adults with initial blood pressure recorded, six (30%) were hypotensive (SBP < 90 mmHg), two of whom had a lactate > 4 mmol/L and three of whom had a lactate > 2 and ≤ 4 mmol/L. Blood pressure was not recorded in children. Nine adults (45%) and 11 children (35.5%) were hypoxemic (SpO2 < 94%). The majority of patients had normal mental status upon assessment but altered mental status was more common in children.
Table 2. Severity of illness at 24 and 48 hours.
| Variable | Adults | Pediatrics | ||
|---|---|---|---|---|
| 24 hrs N = 20 |
48 hrs N = 16 |
24 hrs N = 31 |
48 hrs N = 30 |
|
| Hypoglycemia, BG < 70 mg/dL, n (%)a | 1 (5.0%) | 0 (0.0%) | 2 (6.5%) | 3 (10%) |
| Hypoperfusion, n (%)b | ||||
| Mild, lactate > 2 and ≤ 4 mmol/L | 6 (30.0%) | 8 (50.0%) | 14 (45.2%) | 13 (43.3%) |
| Severe, lactate > 4 mmol/L | 8 (40.0%) | 3 (18.8%) | 5 (16.1%) | 2 (6.7%) |
| Hypotension, SBP < 90mmHg, n (%)c | 6 (30.0%) | 4 (25.0%) | - | - |
| Hypoxia, SpO2 < 94%, n (%)d | 9 (45.0%) | 1 (6.3%) | 11 (35.5%) | 5 (16.7%) |
| Mental status | ||||
| AVPU score, n (%) | ||||
| A | 16 (80.0%) | 15 (93.8%) | 20 (64.5%) | 28 (93.3%) |
| V | 2 (10.0%) | 0 (0.0%) | 5 (16.1%) | 0 (0.0%) |
| P | 1 (5.0%) | 0 (0.0%) | 5 (16.1%) | 1 (3.3%) |
| U | 1 (5.0%) | 1 (6.3%) | 1 (3.2%) | 1 (3.3%) |
| Glasgow Coma Score, median [IQR] | 15 [15–15] | 15 [15–15] | 15 [11–15] | 15 [15–15] |
aMissing value for 1 adult at 24 hrs.
bMissing values for 1 adult at 24 hrs and 1 adult at 48 hrs.
cPediatric blood pressure not available. Missing values for 6 adults at 24 hrs and 2 adults at 48 hrs.
dDue to lack of access to infant-specific oximetry probes, missing values for 6 children at 24 hrs and 7 children at 48 hrs.
On follow-up assessment (24–48 hours after admission), four adults (20%) and one (3.2%) pediatric patient had been discharged home in improved condition; none had died or been transferred. Of those patients still hospitalized, no adults and three children (10%) were hypoglycemic. Compared to enrollment, fewer patients had severe hypoperfusion but eleven adults (68.8%) and 15 children (50.0%) had persistently elevated lactates. A quarter of adults remained hypotensive. Prevalence of hypoxia decreased in both adults and children. Nearly all subjects had a normal mental status.
The majority of adult (80%) and pediatric (83.9%) patients received at least one antibiotic during their admission. Antibiotics most commonly prescribed were ceftriaxone in adults (45%) and chloramphenicol (38.7%), ceftriaxone (29%), gentamicin (29%) and ampicillin (25.8%) in children (Table 3). Eight adults (40%) and eight children (25%) received an antimalarial agent. All adult and pediatric patients who were severely hypoxic (SpO2 < 90%) received supplemental oxygen support. Fluid resuscitation was defined as ≥2 L of isotonic intravenous fluid in adults or ≥ 20 mL/kg in children within the first 24hr of admission. At least half of adults and children with evidence of severe hypoperfusion received fluid resuscitation.
Table 3. Patient management.
| Variable | Adults N = 20 |
Pediatrics N = 31 |
|
|---|---|---|---|
| Antimicrobials received during admission | |||
| Antibiotic, n (%) | 16 (80.0%) | 26 (83.9%) | |
| Amoxicillin | 1 (5.0%) | 1 (3.2%) | |
| Ampicillin | 0 (0.0%) | 8 (25.8%) | |
| Ceftriaxone | 9 (45.0%) | 9 (29.0%) | |
| Chloramphenicol | 3 (15.0%) | 12 (38.7%) | |
| Ciprofloxacin | 1 (5.0%) | 0 (0.0%) | |
| Dicloxacillin | 1 (5.0%) | 0 (0.0%) | |
| Doxycycline | 1 (5.0%) | 0 (0.0%) | |
| Erythromycin | 0 (0.0%) | 1 (3.2%) | |
| Gentamicin | 2 (10.0%) | 9 (29.0%) | |
| Penicillin G | 0 (0.0%) | 1 (3.2%) | |
| Trimethoprim-Sulfamethoxazole | 3 (15.0%) | 0 (0.0%) | |
| Antihelminth, n (%) | 1 (5.0%) | 2 (6.5%) | |
| Albendazole | 1 (5.0%) | 1 (3.2%) | |
| Mebendazole | 0 (0.0%) | 1 (3.2%) | |
| Antimalarial, n (%) | 8 (40.0%) | 8 (25.0%) | |
| Artemether | 5 (25.0%) | 8 (25.8%) | |
| Quinine | 5 (25.0%) | 0 (0.0%) | |
| Antiviral, n (%) | 0 (0.0%) | 0 (0.0%) | |
| Oxygen support if hypoxic, n (%)a | |||
| SpO2 < 94% | 4/9 (44.4%) | 9/11 (81.2%) | |
| SpO2 < 90% | 3/3 (100.0%) | 5/5 (100%) | |
| IV fluid bolus if hypoperfused, n (%)b | |||
| Mild, venous lactate > 2 and ≤4 mmol/L | 3/7 (42.9%) | 8/14 (57.1%) | |
| Severe, venous lactate > 4 mmol/L | 4/8 (50.0%) | 3/5 (60.0%) | |
aIn first 24hr of admission.
bMild hypoperfusion defined as systolic blood pressure < 90mmHg (in adults) or venous lactate > 2 and ≤ 4 mmol/L in the first 24hr of admission. Severe hypoperfusion defined as systolic blood pressure < 70 mmHg (in adults) or venous lactate > 4 mmol/L in the first 24hr of admission. Fluid bolus defined as ≥ 2L isotonic crystalloid in first 24hr in adults and ≥ 20 mL/kg isotonic crystalloid in the first 24hr in pediatrics.
In adults the most common causes of sepsis, as documented in the medical record, were malaria (35%) and pneumonia (20%) (Table 4). The most common causes of sepsis in children were pneumonia (48.4%) and malaria (29%). Seven children (22.6%) were diagnosed with viral upper respiratory tract infections (URTI); two of these had co-infection with malaria or bacterial infections. Four of five (80%) of the children with viral URTI alone had evidence of hypoperfusion (lactate > 2 mmol/L) at enrollment. Two adult (10%) and no pediatric patients died in hospital or were discharged home in moribund condition. No adults and one child (3.2%) were transferred to other medical facilities. The patient who was transferred was in serious condition and was transferred to the nearest referral hospital with CT capabilities, approximately 180km away. The median hospital length of stay was 2.5 days for adults (IQR 2.0–3.5) and 2 days for children (IQR 2–4).
Table 4. Patient outcomes.
| Variable | Adults N = 20 |
Pediatrics N = 31 |
|---|---|---|
| Discharge diagnosis, n (%) | ||
| Acute otitis media | 0 (0.0%) | 2 (6.5%) |
| Brucellosis | 0 (0.0%) | 1 (3.2%) |
| Encephalitis | 0 (0.0%) | 2 (6.5%) |
| Gastroenteritis | 1 (5.0%) | 1 (3.2%) |
| Idiopathic abdominal paina | 1 (5.0%) | 0 (0.0%) |
| Malaria | 7 (35.0%) | 9 (29.0%) |
| Pharyngotonsillitis | 0 (0.0%) | 1 (3.2%) |
| Pneumonia | 4 (20.0%) | 15 (48.4%) |
| Prostatitis | 1 (5.0%) | 0 (0.0%) |
| Puerperal sepsis | 1 (5.0%) | 0 (0.0%) |
| Sepsis with unspecified source | 3 (15.0%) | 0 (0.0%) |
| Toxoplasmosis | 1 (5.0%) | 0 (0.0%) |
| Tuberculosis | 1 (5.0%) | 0 (0.0%) |
| Typhoid fever | 1 (5.0%) | 1 (3.2%) |
| Viral upper respiratory tract infection | 1 (5.0%) | 7 (22.6%) |
| Length of stay, median days [IQR] | 2.5 [2.0–3.5] | 2.0 [2.0–4.0] |
| Disposition, n (%) | ||
| Home in improved condition | 17 (85.0%) | 30 (96.8%) |
| Home to die | 1 (5.0%) | 0 (0.0%) |
| Died in hospital | 1 (5.0%) | 0 (0.0%) |
| Transferred to other facility | 0 (0.0%) | 1 (3.2%) |
| Left against advice | 1 (5.0%) | 0 (0.0%) |
aThis patient left against medical advice prior to complete evaluation.
Discussion
This study demonstrates that sepsis is a common reason for hospital admission in rural Uganda, accounting for about a quarter of all medical admissions. Adult patients are young and HIV is a common co-morbidity. In both adults and children, sepsis is frequently attributed to malaria and pneumonia. Despite high rates of hypoxemia and hypoperfusion on presentation, hospital mortality is low.
This study adds important knowledge to the fields of adult and pediatric tropical medicine and global health. Many previous studies of sepsis in sub-Saharan Africa have been conducted with urban patient populations, usually at major regional or national hospitals, and have frequently excluded children. Our results differ from prior studies in a number of ways. First, subjects in our study were relatively well, with a lower prevalence of HIV and other chronic diseases relative to that reported elsewhere in the literature [21–23,26,30,31,34,43]. The inpatient HIV testing rate is > 85% in this hospital, making it unlikely that a significant number of study patients had undiagnosed HIV. The lower HIV prevalence in this cohort may be in part due to the hospital’s aggressive HIV testing and treatment strategy and the rural nature of the population. The overall HIV prevalence in the Southwestern Uganda region is 8.0% (as of 2011) [44], whereas the prevalence of HIV among 349 pediatric (under 16yr) and 558 adult inpatients tested through this hospital in 2013 was only 0.9% in children and 5.0% in adults [45]. Additionally, chronic comorbidities among study subjects are likely underestimates because most patients in this community do not get preventative care and conditions such as diabetes, tuberculosis and hypertension are frequently not diagnosed until complications develop. Our inclusion of patients with non-severe sepsis is an important contrast to other studies of sepsis in sub-Saharan Africa which restricted inclusion criteria to subjects with severe sepsis or shock [17,28].
Second, we found a higher prevalence of malaria among our cohort than others have reported elsewhere in Uganda [31,32,35,37]. There may be several reasons for this. All patients diagnosed with malaria underwent rapid testing and blood smear by treating providers to confirm parasitemia but this was not independently tested. Additionally, the more rural nature of the area compared to the sites of other studies may account for our higher malaria prevalence than previously reported [37].
Third, the low mortality rate that we identified is a significant contrast to the high case-fatality rates reported elsewhere [30–33,35], and is likely in part due to the relatively well patient population enrolled. However, it is unlikely that this entirely explains our findings, as a large number of patients had evidence of end-organ dysfunction upon initial evaluation. While Bwindi Community Hospital is the only hospital in this area, it is private and well-resourced relative to public hospitals of similar size in Uganda.
Most patients in this study received antimicrobials and, where indicated, supplemental oxygen. Fluid resuscitation was less frequent even in more severely ill patients: only 50% of adults and 60% of children with severely elevated venous lactate received IV fluid boluses. One unique feature of the study site is that nurses frequently initiate IV fluids and antibiotics for ill patients who present directly to the wards overnight.
Strengths of our study are that we consecutively screened for all patients with sepsis requiring medical admission during the study period. Enrollment of eligible patients was high. Full medical record review was completed on all patients and no patients were lost to follow up. There was minimal missing data, and is noted where applicable. Therefore, although our study is small, our results are likely to be robust. In addition, our study is one of only a few to specifically describe pediatric sepsis in sub-Saharan Africa.
This study has several limitations. The study was designed to be strictly observational, but the presence of medically-trained study staff and the collection of primary data likely affected patient management and outcomes. We collected—and reported to treating clinicians—clinical data which were not routinely measured in non-study patients (e.g. pulse oximetry, point of care glucose, and point of care venous lactate). In several cases, enrolled patients then received interventions such as supplemental oxygen, IV dextrose or IV fluid boluses based on study findings.
Another limitation of this study was our strict application of the definition of pediatric sepsis [42] to this limited-resource setting. At least five pediatric patients were initially enrolled but were not included in the analysis because their temperature was elevated but less than 38.5°C, the cutoff for SIRS criteria in children [42]. We maintained this standard temperature cutoff despite the site’s use of axillary rather than core temperature measurement for pediatric patients, and thus likely missed several patients with true sepsis physiology who may have had a core temperature that met inclusion requirements. Additionally, because the study site does not commonly perform CBCs on children (only 2 of 31 pediatric patients included in analysis had CBCs performed at all, neither of which met criteria for sepsis) and the pediatric sepsis definition requires either elevated temperature or WBC derangement, it’s likely that several other patients with sepsis physiology but no fever were excluded. Additionally, we excluded pregnant women, and did not screen postpartum women admitted to the Maternity Ward or septic patients requiring surgical intervention. We excluded neonates because neonatal sepsis is a separate syndrome with unique etiology, presentation, management guidelines, and outcomes that significantly differ from sepsis in older children. These limitations may have resulted in underestimates of the prevalence of sepsis and mortality rates from sepsis, and potentially widens an existing gap in research on these populations.
The small size of our study population and the low mortality rate precluded determination of correlates of death or of hospital length of stay. Additionally, likely due to the small size of this study, we failed to enroll any Batwa patients. This is a marginalized minority population, and the presentation and outcomes of sepsis has not previously been described in this community. Although the study hospital is a major source of medical care for the Batwa community in southwest Uganda, failure to enroll any of these individuals was probably due to the small study size.
Since this study was performed, an updated consensus definition for adult sepsis has been released [46]. Notably, the definition for pediatric sepsis used in this study is consistent with current definitions. Although our study used the adult sepsis definition that was current at the time the study was performed, we acknowledge that our use of a now-outdated definition limits the future comparability and generalizability of our study.
Lastly, while the external validity of this study is high in terms of presentation of sepsis among adult and pediatric patients in similar rural areas in Sub-Saharan African LICs, given that this was a private hospital which is relatively well resourced, the generalizability of sepsis management and outcomes may be limited.
This study is one of the first to evaluate the presentation, management and outcomes of sepsis among both adults and children in a rural community in Uganda. Our results highlight the burden of sepsis in this population. Furthermore, our findings emphasize the necessity of continued sepsis research in sub-Saharan Africa and other low resource settings around the world.
Supporting information
(XLS)
Acknowledgments
We would like to thank the Bwindi Community Hospital administration, medical staff, and patients for facilitating and participating in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Dr. Rudd was supported by a grant from the INTERSECT-Ellison Fellowship (http://depts.washington.edu/intrsect/fellowship/) and by the National Heart, Lung, and Blood Institute (NIH-NHLBI T32 HL007287; http://www.nhlbi.nih.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Dr. Tutaryebwa received no specific funding for this work. Dr. West received no specific funding for this work.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380: 2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng AC, West TE, Limmathurotsakul D, Peacock SJ. Strategies to reduce mortality from bacterial sepsis in adults in developing countries. PLoS Med. 2008;5: 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators et al. Global, regional, and national age—sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385: 117–171. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis—Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193: 253–272. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit Care Med. 2013;41: 580–637. 10.1097/CCM.0b013e31827e83af [DOI] [PubMed] [Google Scholar]
- 6.Jacob ST, West TE, Banura P. Fitting a square peg into a round hole: are the current Surviving Sepsis Campaign guidelines feasible for Africa? Crit Care. 2011;15: 117 10.1186/cc9981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baelani I, Jochberger S, Laimer T, Otieno D, Kabutu J, Wilson I, et al. Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: a self-reported, continent-wide survey of anaesthesia providers. Crit Care. BioMed Central Ltd; 2011;15: R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baelani I, Jochberger S, Laimer T, Rex C, Baker T, Wilson IH, et al. Identifying resource needs for sepsis care and guideline implementation in the democratic republic of the Congo: A cluster survey of 66 hospitals in four eastern provinces. Middle East J Anesthesiol. 2012;21: 559–576. [PubMed] [Google Scholar]
- 9.Bataar O, Lundeg G, Tsenddorj G, Jochberger S, Grander W, Baelani I, et al. Nationwide survey on resource availability for implementing current sepsis guidelines in Mongolia. Bull World Health Organ. 2010;88: 839–846. 10.2471/BLT.10.077073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahavanakul W, Nickerson EK, Srisomang P, Teparrukkul P, Lorvinitnun P, Wongyingsinn M, et al. Feasibility of modified surviving sepsis campaign guidelines in a resource-restricted setting based on a cohort study of severe S. aureus sepsis. PLoS One. 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker JU, Theodosis C, Jacob ST, Wira CR, Groce NE. Surviving sepsis in low-income and middle-income countries: new directions for care and research. Lancet Infect Dis. Elsevier Ltd; 2009;9: 577–582. [DOI] [PubMed] [Google Scholar]
- 12.Jacob ST, Lim M, Banura P, Bhagwanjee S, Bion J, Cheng AC, et al. Integrating sepsis management recommendations into clinical care guidelines for district hospitals in resource-limited settings: the necessity to augment new guidelines with future research. BMC Med. 2013;11: 107 10.1186/1741-7015-11-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dünser MW, Festic E, Dondorp A, Kissoon N, Ganbat T, Kwizera A, et al. Recommendations for sepsis management in resource-limited settings. Intensive Care Med. 2012;38: 557–574. 10.1007/s00134-012-2468-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollach G, Namboya F. Preventing Intensive Care Admissions for Sepsis in Tropical Africa (PICASTA): An Extension of the International Pediatric Global Sepsis Initiative: An African Perspective. Pediatr Crit Care Med. 2013;14: 561–70. 10.1097/PCC.0b013e318291774b [DOI] [PubMed] [Google Scholar]
- 15.Papali A, McCurdy MT, Calvello EJB. A “Three Delays” Model for Severe Sepsis in Resource-Limited Countries. J Crit Care. Elsevier Inc.; 2015;30: 861.e9–861.e14. [DOI] [PubMed] [Google Scholar]
- 16.Bass CM, Sajed DR, Adedipe AA, West TE. Pulmonary ultrasound and pulse oximetry versus chest radiography and arterial blood gas analysis for the diagnosis of acute respiratory distress syndrome: a pilot study. Crit Care. 2015;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews B, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, Bernard GR. Simplified Severe Sepsis Protocol: A Randomized Controlled Trial of Modified Early Goal-Directed Therapy in Zambia. Crit Care Med. 2014;42: 2315–2324. 10.1097/CCM.0000000000000541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortiz JR, Jacob ST, Eoin West T. Clinical care for severe influenza and other severe illness in resource-limited settings: The need for evidence and guidelines. Influenza Other Respi Viruses. 2013;7: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagher GA, Saadeldine M, Bachir R, Zebian D, Chebl RB. Descriptive analysis of sepsis in a developing country. Int J Emerg Med. 2015;8: 19 10.1186/s12245-015-0068-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banajeh SM. Outcome for Children Under 5 Years Hospitalized with Severe Acute Lower Respiratory Tract Infections in Yemen : A 5 Year Experience. J Trop Pediatr. 1998;44: 343–346. [DOI] [PubMed] [Google Scholar]
- 21.Archibald LK, McDonald LC, Nwanyanwu O, Kazembe P, Dobbie H, Tokars J, et al. A hospital-based prevalence survey of bloodstream infections in febrile patients in Malawi: implications for diagnosis and therapy. J Infect Dis. 2000;181: 1414–1420. 10.1086/315367 [DOI] [PubMed] [Google Scholar]
- 22.Bell M, Archibald LK, Nwanyanwu O, Dobbie H, Tokars J, Kazembe PN, et al. Seasonal variation in the etiology of bloodstream infections in a febrile inpatient population in a developing country. Int J Infect Dis. 2001;5: 63–69. [DOI] [PubMed] [Google Scholar]
- 23.Nadjm B, Mtove G, Amos B, Walker NF, Diefendal H, Reyburn H, et al. Severe febrile illness in adult hospital admissions in Tanzania: A prospective study in an area of high malaria transmission. Trans R Soc Trop Med Hyg. Royal Society of Tropical Medicine and Hygiene; 2012;106: 688–695. [DOI] [PubMed] [Google Scholar]
- 24.Chimese SM, Andrews B, Lakhi S. The Etiology And Outcome Of Adult Patients Presenting With Sepsis To The University Teaching Hospital, Lusaka, Zambia. Med J Zambia. 2012;39: 19–22. [PMC free article] [PubMed] [Google Scholar]
- 25.Chisti MJ, Salam MA, Bardhan PK, Faruque ASG, Shahid ASMSB, Shahunja KM, et al. Severe Sepsis in Severely Malnourished Young Bangladeshi Children with Pneumonia: A Retrospective Case Control Study. PLoS One. 2015;10: e0139966 10.1371/journal.pone.0139966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norrgren H, Cardoso AN, da Silva ZJ, Andersson S, Dias F, Biberfeld G, et al. Increased prevalence of HIV-2 infection in hospitalized patients with severe bacterial diseases in Guinea-Bissau. Scand J Infect Dis. 1997;29: 453–459. [DOI] [PubMed] [Google Scholar]
- 27.Waitt PI, Mukaka M, Goodson P, SimuKonda FD, Waitt CJ, Feasey N, et al. Sepsis carries a high mortality among hospitalised adults in Malawi in the era of antiretroviral therapy scale-up: A longitudinal cohort study. J Infect. Elsevier Ltd; 2015;70: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011; [DOI] [PubMed] [Google Scholar]
- 29.Jacob ST, Banura P, Baeten JM, Moore CC, Meya D, Nakiyingi L, et al. The impact of early monitored management on survival in hospitalized adult Ugandan patients with severe sepsis: a prospective intervention study. Crit Care Med. 2012;40: 2050–2058. 10.1097/CCM.0b013e31824e65d7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asiimwe SB, Okello S, Moore CC. Frequency of Vital Signs Monitoring and its Association with Mortality among Adults with Severe Sepsis Admitted to a General Medical Ward in Uganda. PLoS One. 2014;9: e89879 10.1371/journal.pone.0089879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacob ST, Moore CC, Banura P, Pinkerton R, Meya D, Opendi P, et al. Severe sepsis in two Ugandan hospitals: A prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PLoS One. 2009;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ssekitoleko R, Pinkerton R, Muhindo R, Bhagani S, Moore CC. Aggregate evaluable organ dysfunction predicts in-hospital mortality from sepsis in Uganda. Am J Trop Med Hyg. 2011;85: 697–702. 10.4269/ajtmh.2011.10-0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore CC, Jacob ST, Pinkerton R, Meya DB, Mayanja-Kizza H, Reynolds SJ, et al. Point-of-care lactate testing predicts mortality of severe sepsis in a predominantly HIV type 1-infected patient population in Uganda. Clin Infect Dis. 2008;46: 215–222. 10.1086/524665 [DOI] [PubMed] [Google Scholar]
- 34.Mayanja BN, Todd J, Hughes P, Van Der Paal L, Mugisha JO, Atuhumuza E, et al. Septicaemia in a population-based HIV clinical cohort in rural Uganda, 1996–2007: Incidence, aetiology, antimicrobial drug resistance and impact of antiretroviral therapy. Trop Med Int Heal. 2010;15: 697–705. [DOI] [PubMed] [Google Scholar]
- 35.Ssekitoleko R, Jacob ST, Banura P, Pinkerton R, Meya DB, Reynolds SJ, et al. Hypoglycemia at admission is associated with inhospital mortality in Ugandan patients with severe sepsis. Crit Care Med. 2011;39: 2271–2276. 10.1097/CCM.0b013e3182227bd2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachou H, Tumwine JK, Mwadime RKN, Tylleskär T. Risk factors in hospital deaths in severely malnourished children in Kampala, Uganda. BMC Pediatr. 2006;6: 7 10.1186/1471-2431-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auma MA, Siedner MJ, Nyehangane D, Nalusaji A, Nakaye M, Mwanga-Amumpaire J, et al. Malaria is an uncommon cause of adult sepsis in south-western Uganda. Malar J. 2013;12: 146 10.1186/1475-2875-12-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacob ST, Pavlinac PB, Nakiyingi L, Banura P, Baeten JM, Morgan K, et al. Mycobacterium tuberculosis Bacteremia in a Cohort of HIV-Infected Patients Hospitalized with Severe Sepsis in Uganda-High Frequency, Low Clinical Sand Derivation of a Clinical Prediction Score. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doka NI, Jacob ST, Banura P, Moore CC, Meya D, Mayanja-Kizza H, et al. Enrichment of HIV-1 Subtype AD Recombinants in a Ugandan Cohort of Severely Septic Patients. PLoS One. 2012;7: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore CC, Jacob ST, Pinkerton R, Banura P, Meya DB, Reynolds SJ, et al. Treatment of severe sepsis with artemether-lumefantrine is associated with decreased mortality in ugandan patients without malaria. Am J Trop Med Hyg. 2009;80: 723–728. [PubMed] [Google Scholar]
- 41.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6: 2–8. 10.1097/01.PCC.0000149131.72248.E6 [DOI] [PubMed] [Google Scholar]
- 43.Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, Yang LY, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis. 2011;52: 341–348. 10.1093/cid/ciq103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uganda AIDS Commission. The HIV and AIDS Uganda Country Progress Report 2014. 2015;
- 45.Bwindi Community Hospital. Bwindi Community Hospital Annual Report 2013/2014. 2014;
- 46.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315: 801–810. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.