Abstract
Purpose
To correlate currently available quantitative CT measurements for airway disease with physiological indices and the body-mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) index in patients with chronic obstructive pulmonary disease (COPD).
Materials and methods
This study was approved by our institutional review board (IRB number 2778). Written informed consent was obtained from all subjects. The subjects included 188 current and former cigarette smokers from the COPDGene cohort who underwent inspiratory and expiratory CT and also had physiological measurements for the evaluation of airflow limitation, including FEF25–75%, airway resistance (Raw), and specific airway conductance (sGaw). The BODE index was used as the index of clinical symptoms. Quantitative CT measures included % low attenuation areas [% voxels ≤ 950 Hounsfield unit (HU) on inspiratory CT, %LAA−950ins], percent gas trapping (% voxels ≤ −856 HU on expiratory CT, %LAA−856exp), relative inspiratory to expiratory volume change of voxels with attenuation values from −856 to −950 HU [Relative Volume Change (RVC)−856 to −950], expiratory to inspiratory ratio of mean lung density (E/I-ratio MLD), Pi10, and airway wall thickness (WT), luminal diameter (LD) and airway wall area percent (WA%) in the segmental, subsegmental and subsubsegmental bronchi on inspiratory CT. Correlation coefficients were calculated between the QCT measurements and physiological measurements in all subjects and in the subjects with mild emphysema (%LAA−950ins <10%). Univariate and multiple variable analysis for the BODE index were also performed. Adjustments were made for age, gender, smoking pack years, FEF25–75%, Raw, and sGaw.
Results
Quantitative CT measurements had significant correlations with physiological indices. Among them, E/I-ratio MLD had the strongest correlations with FEF25–75% (r = −0.648, <0.001) and sGaw (r = −0.624, <0.001) while in the subjects with mild emphysema subsegmental WA% and segmental WA% had the strongest correlation with FEF25–75% (r = −0.669, <0.001) and sGaw (r = −0.638, <0.001), respectively. The multiple variable analyses showed that RVC−856 to −950 was an independent predictor of the BODE index showing the highest R2 (0.468) as an independent variable among the QCT measurements.
Conclusion
Quantitative CT measurements of gas trapping such as E/I-ratioMLD, correlate better with physiological indices for airway disease than those of airway such as WA% or LD. In mild emphysema, however, quantitative CT measurements of airway correlate better with the physiological indices. RVC−856 to −950 is a predictor of the BODE index.
Keywords: Chronic obstructive lung disease (COPD), CT, Airway disease, Air trapping, Quantitative CT
1. Introduction
Quantitative CT (QCT) analysis has emerged as a new approach to measure the disease severity of chronic obstructive lung disease (COPD). QCT assessment of emphysema, using the density mask technique, correlates quite well with spirometric measurements and with pathologic severity of emphysema [1,2] and spirometric evaluation [3–7] with the clinical status of COPD patients [8–13]. QCT may also estimate the degree of small airway disease of COPD indirectly by using the percent of low attenuation area less than or equal to −856 Hounsfield unit (HU) on expiratory CT (%LAA−856exp) or directly by using airway measurements, such as airway wall thickness (WT), luminal diameter (LD) and airway wall area percent (WA%). Several reports have already documented the feasibility of these airway measurements from multidetector CT acquisitions, which may reflect the severity of small airway disease [8–10,12–20]. However, it is not still fully understood which QCT measurements will better represent the severity of airway disease in COPD. Especially, our concern was whether indirect measurements (i.e. measurements of gas trapping) or direct measurements (i.e. measurements of airway) are better to reflect the severity of airway disease. Thus, this study aimed to determine which of currently available measurements is most feasible to represent the clinical severity of airway disease of COPD by evaluating the relationship between airway QCT parameters and physiological indices of airway disease, and clinical severity of COPD. We also tried to visually grade the severity of airway disease of COPD and correlated the grading system with the clinical indices and compared with airway QCT parameters.
2. Materials and methods
This study was approved by our institutional review board (IRB number 2778). Written informed consent was obtained from all subjects, and the study was compliant with the Health Insurance Portability and Affordability Act. This study was retrospectively conducted in a single institution using part of the COPDGene study data, which has prospectively been being gathered.
2.1. Study population
The study subjects consisted of 188 current and former cigarette smokers who had complete sets of QCT measurements obtained by CT according to the study protocol [21] and also had physiological airway measurements, including Raw, sGaw and FEF25–75%, measured within 90 days of CT examination between February, 2008 and December, 2010. The physiological measurements were performed according to the ATS guidelines [22,23].
2.2. Clinical parameter to estimate the severity of COPD
The body-mass index, airflow obstruction, dyspnea, and exercise capacity index (BODE) [24] was used to estimate the clinical severity of COPD. The BODE index was available in all the study subjects. The BODE index was calculated as previously reported [24].
2.3. CT examination
CT examination was performed according to the standardized COPDGene study protocol [21]. In brief, all subjects underwent volumetric CT at full inspiration and at the end of a normal expiration. All CT scans were performed with a tube potential peak of 120 kVp with a fixed mAs of 200 for inspiratory CT and 50 for expiratory CT at a gantry rotation time of 0.5 s. The reconstructed slice thicknesses were 0.625 mm and 0.60 mm for General Electric Medical Systems and Siemens scanners, respectively. Scans were acquired on LS-16 (General Electric Medical Systems) (n = 15), Definition-64(Siemens Medical Solutions) (n = 121) or Definition-AS-128 (Siemens Medical Solutions) (n = 52) scanner.
2.4. CT quantification
All CT images were analyzed using the Pulmonary Workstation 2 software (VIDA Diagnostics, Inc, Coralville, IA). Soft tissue algorithms were used for CT quantification. Both lungs as well as each lung lobe were automatically segmented with manual edits as necessary by professional technologists. Proximal vasculature and bronchi were automatically removed. Percent low attenuation areas were defined as percent lung tissue ≤ −950HU on inspiratory CT (%LAA−950ins) (Fig. 1A). Percent gas trapping areas were defined as percent lung tissue ≤ −856HU on expiratory CT (%LAA−856exp). This definition of gas trapping is based on the concept that the attenuation of normal lung parenchyma is usually around −856 HU on inspiratory CT and thus areas with CT attenuation less than −856HU on expiratory CT can be regarded as showing inadequate gas emptying (i.e. gas trapping). In addition, we measured the relative inspiratory to expiratory volume change of voxels with attenuation values from −856 to −950 HU (RVC−856 to −950) which was proposed by Matsuoka et al. as a measurement of gas trapping [25]. This measure is calculated by the following formula: RVC−856 to −950 = relative lung volume −856 HU to −950 HU on expiratory CT% −relative lung volume −856 HU to −950 HU on inspiratory CT% [relative lung volume −856 HU to −950 HU% = (lung volume between −856 HU and −950HU in voxel CT value) × 100/lung volume ≥ −950HU in voxel CT value] [25]. We also calculated the expiratory to inspiratory ratio of mean lung density (E/I-ratioMLD) [26]: the expiratory mean lung density in HU is divided by the inspiratory mean lung density, and presented as percentage. Thus, increase in gas trapping results in a higher E/I-ratioMLD.
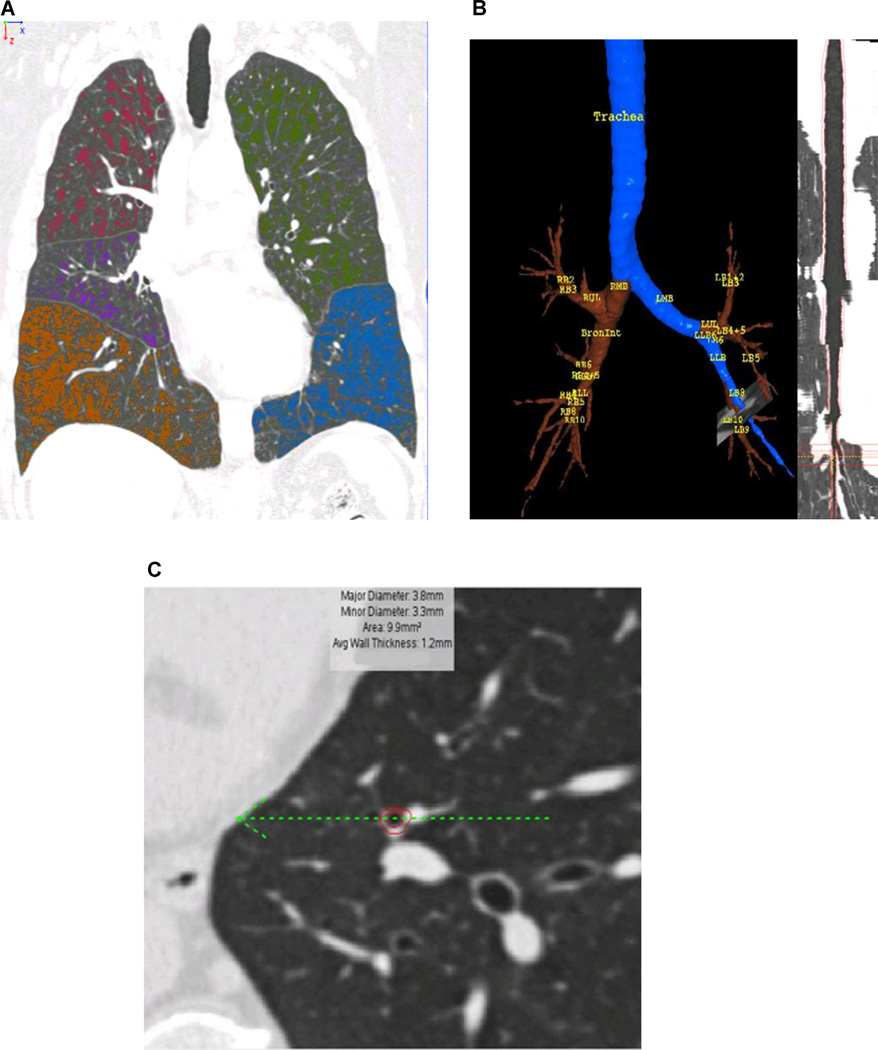
Fig. 1. Quantification of emphysema and airway dimensions.
(a) Quantitative CT of emphysema. All lung voxels ≤ −950 HU in CT value are color-coded by lobe. (b) Quantitative CT of airway dimensions. Volumetric acquisition permits segmentation of the airway tree, and curved reformatting. (c) Orthogonal cross-section of a subsegmental airway, derived from curved reformat, shows delineation of outer and inner bronchial wall, permitting calculation of airway dimensions.
The airway tree was generated using an automated region-growing technique (Fig. 1B). Detailed airway analysis was completed for the segmental bronchi in six selected airway pathways (RB1, RB4, and RB10 of the right lung and LB1, LB4 and LB10 of the left lung), as well as two generations distally. Airway wall disease was evaluated using measures of airway wall thickness, luminal diameter and airway wall area percent (% wall area/total bronchial area) (Fig. 1C). These measurements were quantified in each of 6 segmental, subsegmental and subsubsegmental bronchi. Airway measurements were obtained as averages across the middle third of each segment. We calculated mean values of airway wall thikness (WT), luminal diameter (LD) and airway wall area percent (WA%) in segmental, subsegmental and subsubsegmental bronchi of B1, B4 and B10 bronchial pathways. Square root of the wall area at an internal airway perimeter of 10 mm (Pi10) was also calculated. Pi10 could represent a standardized estimate of bronchial wall thickness. Specifically, it is the square root of bronchial wall area when bronchial internal perimeter is 10 mm, which was obtained from a regression line expressing square root of bronchial wall area as a function of bronchial internal perimeter [5]. The regression line was drawn using the data from the segmental, subsegmental and subsubsegmental levels of the 6 bronchial pathways mentioned above (i.e. 18 measurements of square root of bronchial wall area and bronchial internal perimeter in each subject).
Finally, the QCT measurements used in this study included %LAA-950ins, %LAA−856exp, RVC−856 to −950, E/I-ratioMLD, Pi10 and mean WA%, mean WT and mean LD of 4th, 5th and 6th generation bronchi (i.e. segmental, subsegmental and subsubsegmental bronchi).
2.5. Visual assessment of airway disease in COPD
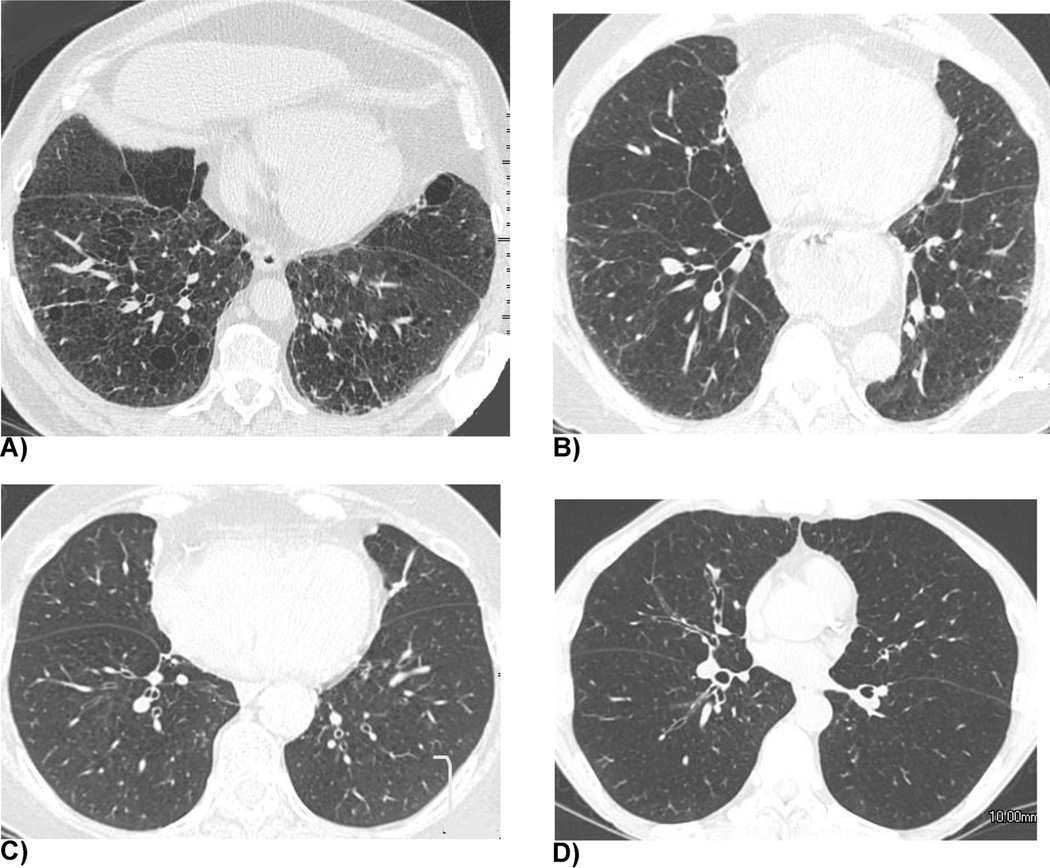
Two chest radiologists (A.N and S.K, 20 and 13 years of experience as radiologists, respectively) independently graded the severity of airway disease in COPD on thin-section inspiratory CT for quantitative analysis using the following criteria: grade 1 = absence of bronchial wall thickening; grade 2 = borderline bronchial wall thickening; grade 3 = definitive bronchial wall thickening; grade 4 = severe bronchial wall thickening (Fig. 2). We did not regard focal bronchial wall thickening as bronchial wall thickening since it more likely suggests sequelae of transbronchial infection. Final decision for each scoring was reached by consensus between the two readers.
Fig. 2. Representative cases of the 4–point grading system for the visual assessment of airway disease.
(a) Grade 1 (absence of bronchial wall thickening). A 63-year-old man with a %LAA−856exp of 44.67%. (b) Grade 2 (borderline bronchial wall thickening). A 63-year-old woman with a %LAA−856exp of 46.56%. (c) Grade 3 (definitive bronchial wall thickening). A 74-year-old woman with a %LAA−856exp of 61.71%. (d) Grade 4 (severe bronchial wall thickening). A 56-year-old man with a %LAA−856exp of 65.65%.
2.6. Data analyses
Pearson’s or Spearman’s rank correlation coefficients were calculated between QCT measurements, visual assessment of airway disease and physiological measurements as appropriate. Correlation coefficients were also calculated for the subjects with mild emphysema %LAA−950ins <10.
Univariate linear regression analyses were performed for the BODE index with an independent variable of age, gender, smoking pack-years, each physiological measurement, QCT measurement, and visual assessment of airway disease. Body mass index (BMI), %FEV1 and FEV1/FVC were not included in the independent variables since BMI and %FEV1 are constituents of the BODE index. Multiple variable analysis was also done including variables that were statistically significant on the univariate analyses. In addition, to avoid multicollinearity as much as possible, when correlation coefficients between the independent variables were greater than 0.7, each variable was separately used as an independent variable creating different regression models.
P values less than 0.05 were considered statistically significant. All statistical analyses were done by using IBM SPSS Statistics version 18 (SPSS, New York, USA).
3. Results
The subject characteristics are summarized in Table 1. QCT measurements and visual assessment of airway disease generally had significant correlations with %FEV1, FEV1/FVC, FEF25–75%, Raw and sGaw (Table 2). Amongst them, E/I-ratioMLD had the strongest correlations with these physiological indices. Indirect QCT measurements for airway disease (i.e. %LAA-856exp, RVC−856 to −950, and E/I-ratio MLD) generally correlated better with the physiological indices than direct QCT measurements (i.e. WA%, LD and WT) and visual assessment of airway disease.
Table 1.
Subject characteristics and measurements of physiological examinations and quantitative CT (n = 188).
| mean ± SD (range) | |
|---|---|
| Age | 63.7 ± 8.37(45–79) |
| Gender, male:female | 88:100 |
| Body mass index | 27.40 ± 5.93 (12.29–44.97) |
| Smoking pack years | 51.04 ± 24.06 (10.50–150.00) |
| GOLD stage 0:I:II:III:IV | 15:7:62:61:43 |
| BODE index | 3.25 (0–7) |
| %FEV1 predicted post-bronchodilator | 48.76 ± 23.57 (11–150) |
| FEV1/FVC ratio post-bronchodilator | 0.46 ± 0.15 (0.18–0.89) |
| Raw (cmH2O/L/s) | 4.04 ± 1.94 (0.81–11.59) |
| sGaw (L//s/cmH2 O/L) | 0.07 ± 0.05 (0.01–0.44) |
| FEF25–75% (L/s) | 0.65 ± 0.75 (0.11–6.16) |
| %LAA−950ins | 17.19 ± 13.13 (0.08–55.89) |
| %LAA−856exp | 42.76 ± 21.09 (2.26–76.75) |
| RVC−856 to −950 (%) | −28.94 (−75.42 to −4.83) |
| E/I-ratio MLD(%) | 91.69 (76.45–98.34) |
| Pi10 (mm) | 3.80 ± 0.11 (3.47–4.22) |
| Mean airway wall area% (subsegmental) | 65.83 ± 2.44 (57.69–71.78) |
| Mean airway wall thickness (mm) (subsegmental) |
1.41 ± 0.15 (1.05–1.79) |
| Mean luminal diameter (mm) (subsegmental) | 3.45 ± 0.51 (2.22–5.19) |
| Visual assessment of severity of airway disease* 1:2:3:4 |
9:31:64:84 |
Abbreviations: GOLD = the global initiative for obstructive lung disease, BODE = the body-mass index, airflow obstruction, dyspnea, and exercise capacity index, FEV = forced expiratory volume, FVC = forced vital capacity, Raw = airway resistance, sGaw = specific airway conductance, FEF25–75% = the average forced expiratory flow during the mid (25–75%) portion of the FVC, %LAA−950ins = percents of low attenuation areas ≤−950 Hounsfield unit on inspiratory CT, %LAA−856exp = percents of low attenuation areas ≤−856 Hounsfield unit on expiratory CT, RVC (relative volume change) −856 to −950 = change of the relative volume of voxels between −856 to −950 HU from inspiratory to expiratory CT (%) (see the text for details), E/I-ratio MLD = ratio of expiratory mean lung density to inspiratory mean lung density (%), Pi10 = square root of the wall area at an internal airway perimeter of 10 mm.
The severity of airway disease was assessed based on the degree of bronchial wall thickening. See the text for details.
Table 2.
Correlation coefficients (p value) between QCT measurements and physiological measurements.
| QCT parameter | FEV1 % predicted | FEV1/FVC ratio | FEF25–75% | Raw | sGaw |
|---|---|---|---|---|---|
| %LAA−950ins | −0.575 (<0.001) | −0.685 (<0.001) | −0.425, (<0.001) | 0.083 (0.259) | −0.397, (<0.001) |
| %LAA−856exp | −0.730, (<0.001) | −0.802 (<0.001) | −0.590 (<0.001) | 0.254 (<0.001) | −0.592 (<0.001) |
| RVC−856 to −950 | −0.765 (<0.001) | −0.733 (<0.001) | −0.606 (<0.001) | 0.266 (<0.001) | −0.515 (<0.001) |
| E/I-ratio MLD | −0.796 (<0.001) | −0.773 (<0.001) | −0.648 (<0.001) | 0.332 (<0.001) | −0.624 (<0.001) |
| Pi10 | −0.289 (<0.001) | −0.212 (0.003) | −0.298 (<0.001) | 0.304 (<0.001) | −0.328 (<0.001) |
| Mean airway wall area% (segmental) | −0.355 (<0.001) | −0.267 (<0.001) | −0.433 (<0.001) | 0.434 (<0.001) | −0.489 (<0.001) |
| Mean airway wall area% (subsegmental) | −0.438 (<0.001) | −0.338 (<0.001) | −0.541 (<0.001) | 0.531 (<0.001) | −0.496 (<0.001) |
| Mean airway wall area% (subsubsegmental) | −0.438 (<0.001) | −0.295 (<0.001) | −0.490 (<0.001) | 0.496 (<0.001) | −0.369 (<0.001) |
| Mean airway wall thickness (segmental) | 0.043 (0.555) | −0.029 (0.694) | 0.175 (0.016) | −0.253 (<0.001) | 0.052 (0.477) |
| Mean airway wall thickness (subsegmental) | 0.102 (0.102) | 0.099 (0.177) | 0.174 (0.017) | −0.240 (0.001) | 0.063 (0.390) |
| Mean airway wall thickness (subsubsegmental) | 0.0038 (0.603) | 0.057 (0.434) | 0.133 (0.069) | −0.205 (0.005) | 0.056 (0.446) |
| Mean luminal diameter (segmental) | 0.306 (<0.001) | 0.203 (0.005) | 0.445 (<0.001) | −0.496 (<0.001) | 0.395 (<0.001) |
| Mean luminal diameter (subsegmental) | 0.352 (<0.001) | 0.267 (<0.001) | 0.506 (<0.001) | −0.515 (<0.001) | 0.416 (<0.001) |
| Mean luminal diameter (subsubsegmental) | 0.324 (<0.001) | 0.247 (0.001) | 0.471 (<0.001) | −0.485 (<0.001) | 0.357 (<0.001) |
| Visual assessment of severity of airway disease | −0.264 (<0.001) | −0.247 (0.001) | −0.334 (<0.001) | 0.211 (0.004) | −0.313 (<0.001) |
Abbreviations are the same as those of Table 1.
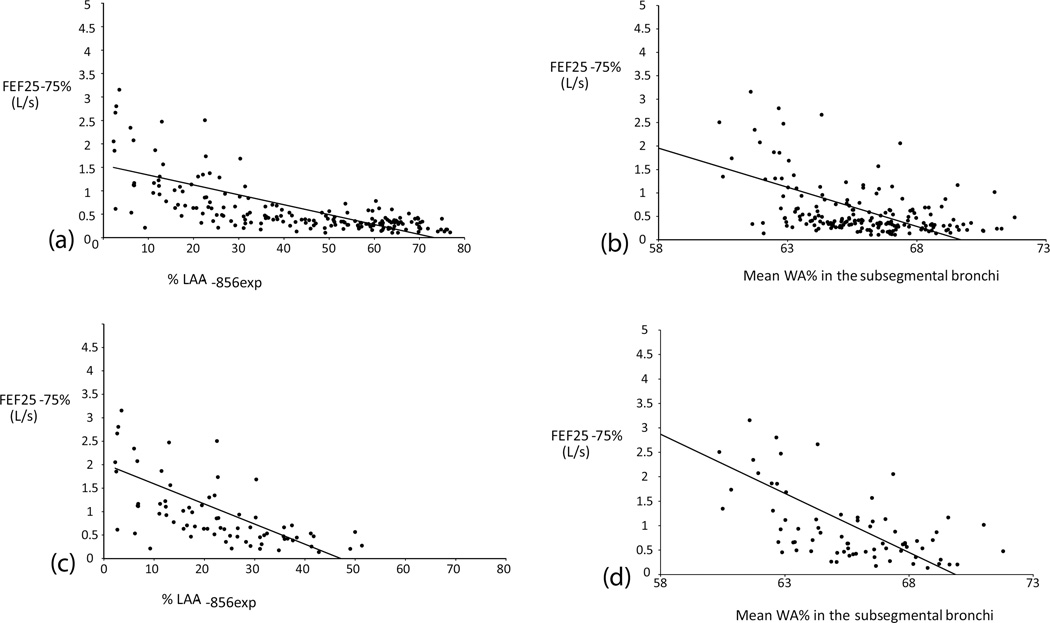
In the subjects with mild emphysema (%LAA−950ins <10%), WA% and LD more strongly correlated with FEF25–75% and Raw whereas the correlations between the indirect QCT measurements and the physiological indices were generally weaker (Table 3, Fig. 3).
Table 3.
Correlation coefficients (p value) between airway physiological measurements and QCT in the subjects with mild emphysema (%LAA−950ins < 10%, n = 74).
| QCT parameter | FEV1 % predicted | FEV1/FVC ratio | FEF25–75% | Raw | sGaw |
|---|---|---|---|---|---|
| %LAA−950ins | −0.199 (0.090) | −0.403 (<0.001) | −0.223, (0.056) | 0.104 (0.378) | −0.259 (0.026) |
| %LAA−856exp | −0.559 (<0.001) | −0.702 (<0.001) | −0.519 (<0.001) | 0.410 (<0.001) | −0.564 (<0.001) |
| RVC−856 to −950 | −0.640 (<0.001) | −0.507 (<0.001) | −0.492 (<0.001) | 0.333 (0.004) | −0.351 (0.002) |
| E/I-ratio MLD | −0.705 (<0.001) | −0.695 (<0.001) | −0.585 (<0.001) | 0.391 (0.003) | −0.542 (<0.001) |
| Pi10 | −0.463 (<0.001) | −0.392 (0.001) | −0.390 (0.001) | 0.394 (0.001) | −0.421 (<0.001) |
| Mean airway wall area% (segmental) | −0.599 (<0.001) | −0.544 (<0.001) | −0.631 (<0.001) | 0.606, (<0.001) | −0.638 (<0.001) |
| Mean airway wall area% (subsegmental) | −0.596 (<0.001) | −0.518 (<0.001) | −0.669 (<0.001) | 0.647 (<0.001) | −0.577 (<0.001) |
| Mean airway wall area% (subsubsegmental) | −0.540 (<0.001) | −0.432 (<0.001) | −0.612 (<0.001) | 0.527 (<0.001) | −0.421 (<0.001) |
| Mean airway wall thickness (segmental) | 0.149 (0.204) | −0.023 (0.845) | 0.238 (0.041) | −0.235 (0.043) | 0.107 (0.363) |
| Mean airway wall thickness (subsegmental) | 0.139 (0.239) | 0.057 (0.632) | 0.188 (0.109) | −0.132 (0.262) | −0.016 (0.892) |
| Mean airway wall thickness (subsubsegmental) | 0.067 (0.570) | 0.042 (0.721) | 0.195 (0.097) | −0.022 (0.850) | −0.006 (0.959) |
| Mean luminal diameter (segmental) | 0.473 (<0.001) | 0.365 (0.001) | 0.605 (<0.001) | −0.575 (<0.001) | 0.484 (<0.001) |
| Mean luminal diameter (subsegmental) | 0.505 (<0.001) | 0.412 (<0.001) | 0.647 (<0.001) | −0.630 (<0.001) | 0.502 (<0.001) |
| Mean luminal diameter (subsubsegmental) | 0.500 (<0.0010 | 0.397 (<0.001) | 0.625 (<0.001) | −0.495 (<0.001) | 0.419 (<0.001) |
| Visual assessment of severity of airway disease | −0.242 (0.038) | −0.278 (0.016) | −0.252 (0.030) | 0.209 (0.075) | −0.314 (0.006) |
Abbreviations are the same as those of Table 1.
Fig. 3. Representative scatter plots showing the relationships between the airway physiological indices and quantitative CT measurements.
(a) FEF25–75% – %LAA-856expin all subjects. (b) FEF25–75% – Mean airway wall area% in the subsegmental bronchi in all subjects. (c) FEF25–75% – %LAA-856exp in the subjects with mild emphysema. (d) FEF25–75% – Mean airway wall area% in the subsegmental bronchi in the subjects with mild emphysema. Note that dots are more scattered in the areas of lower %LAA-856exp on (a) and (c), which may partly be due to physiological gas trapping.
Among the levels of bronchi at which QCT data were obtained, subsegmental level measurements most frequently had the strongest correlations with FEF25–75%, Raw and sGaw. Among the airway measurements, WA% at subsegmental level generally had the strongest correlations with physiological measurements. On the univariate analyses for the BODE index, RVC−856 to −950 had the strongest correlation among the QCT measurements and physiological measurements (Table 4). The statistically significant variables were age, FEF25–75%, Raw, sGaw, %LAA-950ins, %LAA-856exp, RVC−856 to −950, E/I-ratio MLD, Pi10, WA% and LD in each level of bronchi, and visual assessment of airway disease. As %LAA-950ins, %LAA-856exp, RVC−856 to −950 and E/I-ratio MLD, and WA% and LD in each level of bronchi were highly correlated with each other with a correlation coefficient greater than 0.7 (data not shown), each measurement was included separately as an independent variable creating different regression models. A regression model including RVC−856 to −950 and WA% in the subsegmental bronchi showed the highest adjusted R2 of 0.468 and RVC−856 to −950 had the largest standardized coefficient in the equation (Table 4). When %LAA-950ins was included in the model instead of RVC−856 to −950, the R2 was 0.438.
Table 4.
Univariate and multiple variable (forced inclusion method) analyses for the BODE index.
| Univariate analysis (R2, p value) | Multiple variable analysis* (standardized coefficient, p value) Adjusted R2= 0.468 |
|
|---|---|---|
| age | 0.024, 0.048 | −0.245, <0.001 |
| Female gender | 0.000, 0.881 | |
| Smoking pack years | 0.01, 0.775 | |
| FEF25–75% | 0.228, <0.001 | −0.083, 0.374 |
| Raw | 0.115, <0.001 | 0.139, 0.083 |
| sGaw | 0.216, <0.001 | −0.008, 0.930 |
| %LAA−950ins | 0.298, <0.001 | |
| %LAA−856exp | 0.351, <0.001 | |
| RVC−856 to −950 | 0.394, <0.001 | 0.575, <0.001 |
| E/I-ratio MLD | 0.371, <0.001 | |
| Pi10 | 0.057, 0.002 | |
| **Mean airway wall area% (subsegmental) | 0.098, <0.001 | −0.030, 0.705 |
| **Mean airway wall thickness (subsegmental) | 0.010, 0.199 | |
| **Mean luminal diameter (subsubsegmental) | 0.054, 0.003 | |
| Visual assessment of severity of airway disease | 0.039, 0.012 | 0.055, 0.364 |
Abbreviations are the same as those of Table 1
The regression model showing the highest R2 is shown. See the text for details.
As for the airway QCT measurements, only the results of bronchial generations in which the highest correlations with the BODE index were observed are shown.
4. Discussion
Our study demonstrated that indirect QCT measurements for airway disease (i.e. %LAA-856exp, RVC−856 to −950, and E/I-ratio MLD) correlated better with the physiological indices for airway disease than direct airway QCT measurements (i.e. WA%, LD and WT) and visual assessment of airway disease. Among them, E/I-ratio MLD had the strongest correlations with the physiological measurements. These results suggest that the evaluation of resultant gas trapping is better than the measures of bronchi for estimating the degree of air flow limitation. Mets et al. documented that E/I-ratio MLD is most suitable for detecting gas trapping in the evaluation of various QCT measurements for gas trapping although they did not include direct airway QCT measurements in their study [18]. Schroeder et al. observed that %LAA-856exp strongly correlates with physiologic measurements of airway obstruction while direct airway QCT measurements alone are less correlated with spirometric measures of %FEV1 and FEV1/FVC [19]. Our results are consistent with their observations. However, when the subjects were confined to those with mild emphysema (%LAA−950ins <10%), WA% and LD correlated much better with the physiological indices and had stronger correlations with FEF25–75%, Raw and sGaw than the indirect QCT measurements. Conversely, the correlations between the indirect measurements and physiological indices generally became weaker in the subjects with mild emphysema. It is well known that airflow limitation in COPD is caused by not only airway disease but also pulmonary emphysema that results in loss of elastic recoil of the lung. Therefore, it is conceivable that in mild emphysema where the impact of emphysema is relatively small, the degree of airway disease estimated by direct airway measurements has a greater impact on the degree of airflow limitation. Also, physiological gas trapping that could happen to normal subjects may have compromised the estimation of gas trapping in the subjects with mild emphysema. Therefore, we think that direct QCT measurements are useful for estimating the severity of airway disease in subjects with mild emphysema. Paoletti et al. have recently found that the relationship between pulmonary function and lung attenuation is not linear and differs between milder and severer emphysema [27]. Our results may be in line with their observations.
Multiple variable analysis for the BODE index demonstrated that RVC−856 to −950 in addition to age were significant and independent predictors of the BODE index. Previous reports have documented that the extent of emphysema (%LAA) is a significant predictor of the BODE index [8,28]. In our analysis, the regression model including RVC−856 to −950 had a mildly higher R2 than that including %LAA-950ins. This is unsurprising given that RVC−856 to −950 had a stronger correlation with %FEV1 than %LAA-950ins had and %FEV1 is a constituent of the BODE index. RVC−856 to −950 might be more predictive of clinical severity of COPD as represented by the BODE index than %LAA-950ins.
We also tried to visually assess the degree of airway disease. Our visual assessment of airway disease had significant correlations with physiological indices as well as the BODE index. These results suggest the severity of airway disease in COPD could be assessed also by means of visual assessment. However, the correlations were much weaker than those with the QCT measurements. In fact, the readers confessed the difficulty in evaluating and grading the airway disease as bronchial wall thickness was not homogeneous in lungs and was too thin to precisely grade the thickness. Therefore, we think that QCT is superior to visual assessment in the assessment of the degree of airway disease in COPD.
As to the level of airway measurements, the strongest correlations with indices of airflow obstruction, including FEF25–75% and RaW, were most commonly observed in the level of subsegmental bronchi. The major site of airway obstruction in COPD has been shown to be in bronchi with an internal diameter of 2.0 mm or smaller [29], smaller than the airways evaluated in our study which typically measured 2.7 ± 0.4 mm (data not shown). However, Nakano et al. showed that dimensions of airways of this size correlated quite well with those of smaller airways, and the correlations with physiologic measures of small airways obstructions identified in the current study would support the use of airway measurements of these levels in the evaluation of COPD [30]. Hasegawa et al. found that as the airways became smaller, the correlations between QCT measurements and %FEV1 were stronger [17]. We did not find such a trend between the generation of bronchi and the correlations with physiological measurements including %FEV1. This discrepancy may be due to the differences in the methods to calculate QCT measurements or in study populations. Theoretically, as the generation of bronchi becomes higher (i.e. in more peripheral bronchi), the measurements of airway should better correlate with physiological indices of airway. However, it is also conceivable that as the generation of bronchi becomes higher measurements become more imprecise because of limited resolution of CT. Further investigation including standardization of airway QCT measurements will be required to determine which generation of bronchi is most suitable for the estimation of severity of COPD.
The correlations between WT, and FEF25–75% and Raw were not statistically significant or were very weak if significant. Although WT has been reported to correlate with the extent of small airway disease [14,17], it was not useful in estimating FEF25–75%, Raw and sGaw, and thus QCT measurements involving bronchial lumen are considered much better estimates for these physiological measurements. These results may suggest that even when airway wall is thickened airflow can be preserved if luminal area or diameter of bronchi is maintained. It is also conceivable that luminal area or diameter may be less affected by measurement errors than airway wall thickness since luminal area or diameter has a larger numerical value.
Our study had a couple of limitations. First, we used 3 different CT scanner models, which may provide slightly different CT values. Therefore, differences in CT scanners may have affected our QCT results. Second, expiratory CT was obtained at a low dose (50 mAs) to reduce radiation exposure to the patients in our study. Increased image noise may also have affected CT quantification. Third, we didn’t visually evaluate the extent of gas trapping by using expiratory CT, which may be a better estimate to evaluate the severity of airway disease given the results of CT quantification. However, we had felt before the start of this study that visual quantification of air trapping was not easy because the borders between areas of gas trapping and those of normal lung are often not clearly demarcated and in patients with emphysema the attenuation difference is much smaller than in those with other diseases resulting in gas trapping (bronchial asthma, etc.).
In conclusion, measurements of gas trapping, such as %LAA-856exp, RVC−856 to −950, and E/I-ratio MLD, seem more feasible than measurements of airway, such as WA% and LD, for estimating the severity of airflow limitation. In mild emphysema, however, the measurements of airway better reflect the degree of airflow limitation. RVC−856 to −950 may be a better predictor of the BODE index than %LAA-950ins.
Acknowledgments
The COPDGene Study is supported by grant awards R01 HL089856 and R01 HL089897 from the NHLBI. The COPDGene project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Siemens and Sunovion.
We thank George Zeman, David Gurka and Joyce Canterbury, respiratory physiology staff in our institution for their assistance in interpreting the results of this study. We also thank Tanya Mann, an administrative assistant of our department, for her help in the preparation of this study.
Footnotes
Conflicts of interest
None.
References
- 1.Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988;94:782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 2.Madani A, Zanen J, de Maertelaer V, Gevenois PA. Pulmonary emphysema: radiation dose and section thickness at multidetector CT quantification—comparison with macroscopic and microscopic morphometry. Radiology. 2007;243(1):250–257. doi: 10.1148/radiol.2431060194. [DOI] [PubMed] [Google Scholar]
- 3.Washko GR, Criner GJ, Mohsenifar Z, et al. Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. COPD. 2008;5(3):177–186. doi: 10.1080/15412550802093025. [DOI] [PubMed] [Google Scholar]
- 4.Heussel CP, Herth FJ, Kappes J, et al. Fully automatic quantitative assessment of emphysema in computed tomography: comparison with pulmonary function testing and normal values. Eur. Radiol. 2009;19:2391–2402. doi: 10.1007/s00330-009-1437-z. [DOI] [PubMed] [Google Scholar]
- 5.D’Anna SE, Asnaghi R, Caramori G, et al. High-resolution computed tomography quantitation of emphysema is correlated with selected lung function values in stable COPD. Respiration. 2012;83(5):383–390. doi: 10.1159/000329871. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed Hoesein FA, de Hoop B, Zanen P, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011;66:782–787. doi: 10.1136/thx.2010.145995. [DOI] [PubMed] [Google Scholar]
- 7.Mets OM, Murphy K, Zanen P, et al. The relationship between lung function impairment and quantitative computed tomography in chronic obstructive pulmonary disease. Eur. Radiol. 2012;22:120–128. doi: 10.1007/s00330-011-2237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han MK, Bartholmai B, Liu LX, et al. Clinical significance of radiologic characterizations in COPD. COPD. 2009;6(6):459–467. doi: 10.3109/15412550903341513. [DOI] [PubMed] [Google Scholar]
- 9.Mair G, Maclay J, Miller JJ, McAllister D, Connell M, Murchison JT, MacNee W. Airway dimensions in COPD: relationships with clinical variables. Respir. Med. 2010 Nov;104(11):1683–1690. doi: 10.1016/j.rmed.2010.04.021. Epub 2010 Jun 11. [DOI] [PubMed] [Google Scholar]
- 10.Grydeland TB, Dirksen A, Coxson HO, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am. J. Respir. Crit. Care Med. 2010;181(4):353–359. doi: 10.1164/rccm.200907-1008OC. [DOI] [PubMed] [Google Scholar]
- 11.Haruna A, Muro S, Nakano Y, et al. CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138(3):635–640. doi: 10.1378/chest.09-2836. [DOI] [PubMed] [Google Scholar]
- 12.Diaz AA, Bartholmai B, San José Estépar R, et al. Relationship of emphysema and airway disease assessed by CT to exercise capacity in COPD. Respir. Med. 2010;104(8):1145–1151. doi: 10.1016/j.rmed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han MK, Kazerooni EA, Lynch DA, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261:274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am. J. Respir. Crit. Care Med. 2000;162(3 Pt 1):1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 15.Aziz ZA, Wells AU, Desai SR, et al. Functional impairment in emphysema: contribution of airway abnormalities and distribution of parenchymal disease. AJR Am. J. Roentgenol. 2005;185(6):1509–1515. doi: 10.2214/AJR.04.1578. [DOI] [PubMed] [Google Scholar]
- 16.Berger P, Perot V, Desbarats P, Tunon-de-Lara JM, Marthan R, Laurent F. Airway wall thickness in cigarette smokers: quantitative thin-section CT assessment. Radiology. 2005;235(3):1055–1064. doi: 10.1148/radiol.2353040121. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa M, Nasuhara Y, Onodera Y, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006 Jun;173(12):1309–1315. doi: 10.1164/rccm.200601-037OC. [DOI] [PubMed] [Google Scholar]
- 18.Mets OM, Zanen P, Lammers JW, et al. Early identification of small airways disease on lung cancer screening CT: comparison of current air trapping measures. Lung. 2012;190(6):629–633. doi: 10.1007/s00408-012-9422-8. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder JD, McKenzie AS, Zach JA, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am. J. Roentgenol. 2013;201:W460–W470. doi: 10.2214/AJR.12.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian DR, Gupta S, Burggraf D, et al. Emphysema- and airway-dominant COPD phenotypes defined by standardised quantitative computed tomography. Eur. Respir. J. 2016:92–103. doi: 10.1183/13993003.01878-2015. [DOI] [PubMed] [Google Scholar]
- 21.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusqasco V, et al. Standardization of spirometry. Eur. Respir. J. 2005;26(3):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 23.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 24.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka S, Kurihara Y, Yagihashi K, Hoshino M, Watanabe N, Nakajima Y. Quantitative assessment of air trapping in chronic obstructive pulmonary disease using inspiratory and expiratory volumetric MDCT. AJR Am. J. Roentgenol. 2008;190:762–769. doi: 10.2214/AJR.07.2820. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell RA, Peebles C, Ward JA, et al. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax. 2004;59:837–842. doi: 10.1136/thx.2003.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paoletti M, Cestelli L, Bigazzi F, Camiciottoli G, Pistolesi M. Chronic obstructive pulmonary disease: pulmonary function and CT lung attenuation do not show linear correlation. Radiology. 2015;276:571–578. doi: 10.1148/radiol.2015141769. [DOI] [PubMed] [Google Scholar]
- 28.Martinez CH, Chen YH, Westgate PM, et al. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012 May;67(5):399–406. doi: 10.1136/thoraxjnl-2011-201185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N. Engl. J. Med. 1968;278:1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 30.Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am. J. Respir. Crit. Care Med. 2005;171(2):142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]