Abstract
Objectives
Preeclampsia, a pregnancy-specific hypertensive disorder, has been associated with cardiovascular risk factors and vascular changes, such as acute atherosis in placental blood vessels, which are similar to early-stage atherosclerosis. The objective of this study was to determine whether women with preeclampsia have increased atherosclerotic burden, as determined by carotid intima-media thickness (CIMT), when compared to women who did not have preeclampsia.
Methods
We conducted a systematic review and meta-analysis of studies that reported CIMT, a non-invasive, ultrasound-based measure of subclinical atherosclerosis, in women who did vs. did not have preeclampsia. Studies were eligible if they were conducted during pregnancy or during the first decade postpartum, and if CIMT was measured in the common carotid artery. Studies published before March 7, 2016 were identified through PubMED, EMBASE and Web of Science. Two reviewers used predefined forms and protocols to independently evaluate the eligibility of all titles and abstracts, perform full text screening, data abstraction and quality assessment. Heterogeneity was assessed using the I2 statistic. Standardized mean difference was used as a measure of effect size.
Results
Fourteen studies were included in the meta-analysis; 7 studies at the time of preeclampsia and 10 studies up to 10 years postpartum. Three studies included measurements at both time periods. Women who had preeclampsia had significantly higher CIMT compared to those who did not, either at the time of diagnosis (SMD: 1.10, 95% CI: 0.73-1.48, p<0.001), or in the first decade postpartum (SMD: 0.58, 95% CI: 0.36-0.79, p<0.001
Conclusions
Atherosclerotic load is present at the time of preeclampsia and may be one mechanism associated with preeclampsia. CIMT may offer an opportunity for early recognition of premenopausal women with atherosclerotic burden after preeclamptic pregnancies.
Keywords: preeclampsia, cardiovascular risk, meta-analysis, intima-media thickness, subclinical atherosclerosis
Introduction
Preeclampsia is a pregnancy-specific hypertensive disorder, clinically characterized by hypertension (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg) and commonly proteinuria (≥300 mg in 24 h) that occur after 20 weeks gestation. 1 Preeclampsia remains one of the leading causes of maternal and fetal morbidity and mortality worldwide. The underlying cellular and molecular mechanisms of preeclampsia are not well understood and are under active investigation by several laboratories. However, there is general agreement that, similar to cardiovascular disease, endothelial dysfunction plays a crucial role in its pathogenesis. On a clinical level, preeclampsia and atherosclerotic cardiovascular disease share common risk factors, and most of the severe complications of preeclampsia include those that typically are seen with the latter, namely, ischemic heart disease, stroke, and heart failure, further supporting the notion that preeclampsia is a state of increased atherosclerotic burden. These clinical observations are supported further by histological studies of placental vascular changes in preeclampsia. Acute atherosis of the placental blood vessels in preeclampsia, consisting of subendothelial lipid-filled foam cells, fibrinoid necrosis of the arterial wall, and perivascular lymphocytic infiltration, is similar to early-stage atherosclerosis. 2
Carotid intima-media thickness (CIMT) increasingly is used as a measure of preclinical atherosclerosis, and is evaluated using non-invasive, ultrasound-based imaging of the combined thickness of the intimal and medial arterial wall components. 3 Despite increasing recognition of CIMT as a valuable marker of preclinical atherosclerosis, only a few studies have investigated CIMT at the time of the diagnosis of preeclampsia. Furthermore, these studies have provided conflicting evidence regarding the association between preeclampsia and CIMT elevations. We hypothesized, in the current study, that pregnant women at the time of their preeclampsia diagnoses will have increased atherosclerotic burden, as defined by CIMT, compared to pregnant women without preeclampsia. We conducted a systematic review and meta-analysis of studies that reported CIMT as a measure of subclinical atherosclerosis, both at the time of pregnancies affected by preeclampsia and in the first decade postpartum. The latter was evaluated to determine whether subclinical atherosclerosis is present/persists during premenopausal years in women after preeclamptic pregnancies.
Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews 4 and Meta-analysis of Observational Studies in Epidemiology, 5 following a standardized protocol that is available from the study authors. A vascular physiologist (TLW) and biostatistician with expertise in conducting systematic reviews and meta-analyses (NMM) developed the search strategy. Searches of Pub MED, EMBASE and Web of Science through March 7, 2016 were performed for studies that compared CIMT among women who had preeclampsia and women who had pregnancies that were not complicated by preeclampsia. Studies were included if they compared CIMT between these groups before preeclampsia, at the time of active disease or if CIMT was measured in non-pregnant women months to 10 years after delivery. Studies were eligible for inclusion if CIMT was measured in the common carotid artery; studies that measured IMT in other arteries were excluded.
Studies that were identified abased on the above criteria were stratified into ≥ 1 of the following time periods:
Before preeclampsia: CIMT was measured in pregnant women who were followed until delivery to determine whether they developed preeclampsia. No participants had preeclampsia at the time of CIMT measurement.
At the time of active disease: CIMT was measured after 20 weeks of gestation to compare CIMT in pregnant women, with versus without preeclampsia, at the time of the CIMT measuring.
Postpartum: CIMT was measured in non-pregnant women, hours up to 10 years after delivery.
There were no restrictions on publication language or status. Authors of relevant abstracts were contacted to identify eligible unpublished datasets and obtain any missing data, as well as information on the study design and methodology. Studies that combined preeclampsia with gestational hypertension and/or chronic hypertension in pregnancy were only eligible if data for the subset of women who developed preeclampsia could be obtained. Two reviewers (NMM and TLW) independently evaluated the eligibility of all titles and abstracts, and performed full text screening to select articles for inclusion. Disagreements were resolved by consensus. Two reviewers (TLW and JML) independently abstracted the data and evaluated the quality of selected manuscripts using an adapted version of the Newcastle-Ottawa tool {G, 2000 #1298}for observational studies (available from authors upon request). Independent reviewers used standardized forms and protocols when selecting and abstracting data. Authors were contacted to clarify and confirm the accuracy of abstracted data.
The Supplemental Material contains detailed methodology for diagnostic criteria, search strategy, article screening and selection, and missing information.
Statistical Analysis
The primary outcome was CIMT, expressed as means with standard deviations. Methodologies for measuring CIMT vary among labs; therefore we used the standardized mean difference (SMD) to examine differences between the preeclampsia and non-preeclampsia groups. .A recent study also suggested that the SMD may be preferable than the mean difference (MD) from the viewpoint of generalizability.{Takeshima, 2014 #1271} This measurement of effect size expresses the difference between group means in units of standard deviations, and was estimated by pooling individual trial results using random-effects models via the Der Simonian-Laird method. Heterogeneity was assessed using the Chi-square Q and I2 statistic. I2 presents the inconsistency between the study results and quantifies the proportion of observed dispersion that is real, i.e., due to between-study differences and not due to random error. The categorization of heterogeneity was based on the Cochrane Handbook6 and states that I2<30%, 30% to 60% or >60%, corresponds to low, moderate and high heterogeneity, respectively. A separate forest plot was constructed for each analysis showing the SMD (box), 95% confidence interval (lines), and weight (size of box) for each trial. The overall effect size is represented by a diamond. Sensitivity analyses were conducted to examine the effects of: 1) inclusion of studies that presented CIMT as median (IQR) or median (range), 2) inclusion of measurements performed in less severe vs. more severe forms of preeclampsia, if pooled measurements were not available, 3) exclusion of studies that included women with chronic hypertension at the time of pregnancy, 4) replacement of the results of the study performed in 2006 with the follow up results in a subgroup of the same participants who were re-tested 4 years later, and 5) the inclusion of studies for which the exact postpartum time periods are unknown, and 6) the exclusion of studies that were published as abstracts only. A p value <0.05 was considered to be statistically significant. Analyses were performed in R. 8,9
Results
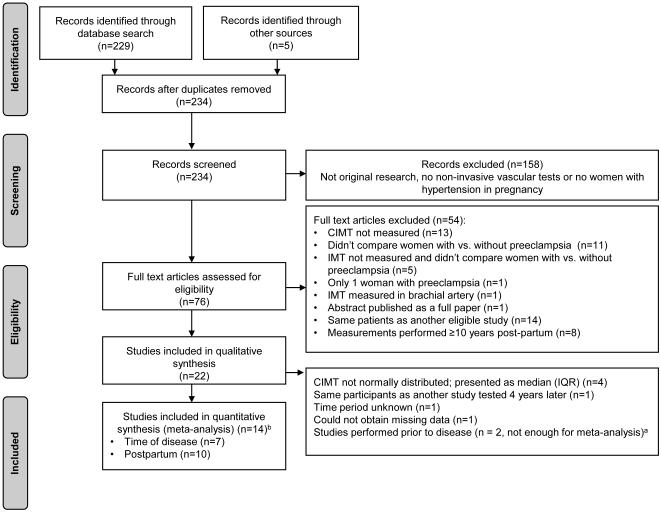
We identified 234 potentially eligible articles. The study selection process is presented in Figure 1. Of the 76 full text articles that were reviewed, 22 were selected for the systematic review (qualitative synthesis). 10–31 Fourteen studies were included in the meta-analysis (quantitative synthesis) and included studies that measured CIMT: 1) at the time of preeclampsia (n=7),17–23 and 2) up to 10 years postpartum (n=10). 19,21,23–30 Three studies included measurements both at the time of the preeclampsia diagnosis and postpartum. 19,21,23 Only two studies examined CIMT prior to the diagnosis of preeclampsia 19,31; therefore this time period was not included in the meta-analysis. Detailed explanations for excluding studies in every step of the study selection process are presented in Figure 1. All 17 full text publications and 5 abstracts included in systematic review are presented in details in the Supplemental Material which includes tables describing summary characteristics for the included studies (Table S1-3); diagnostic criteria for studies examining women with more severe and less severe forms of preeclampsia (Table S4); exclusion criteria (Table S5-7); detailed information about CIMT methodology (Table S8); and summary statistics used in sensitivity analyses (Table S9). Detailed information regarding the quality of the included studies is also provided (Table S10-12).
Figure 1.
Flow chart showing selection of studies for meta-analysis (a one study that performed measurements prior to disease was included in the meta-analysis as it also included measurements at the time of disease and post-partum, b three studies included measurements both at the time of disease and postpartum)
At the time of preeclampsia:
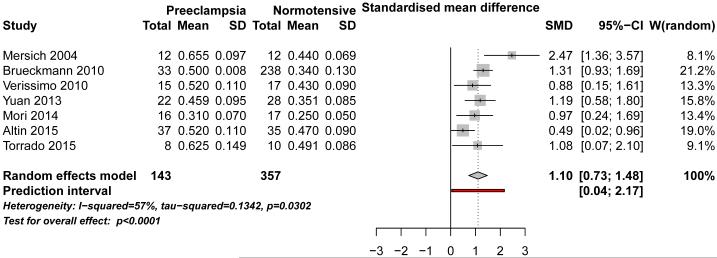
Seven cross-sectional studies 17–23 that were eligible for the meta-analysis included 143 preeclamptic women and 357 non-preeclamptic pregnant women. Two additional studies 10,13 were included in the qualitative synthesis. CIMT was significantly higher in women at the diagnosis of preeclampsia (SMD: 1.10, 95% CI: 0.73-1.48, p<0.001), compared to those who did not have preeclampsia (Figure 2). The analysis revealed moderate heterogeneity among the results of the respective studies (I2=57%, p=0.030). The presented effect remained significant in sensitivity analyses that included two studies that reported medians, with IQRs 10,13 (SMD:0.89, 95% CI: 0.50-1.28, p<0.001), and in a sensitivity analysis that excluded women with chronic hypertension at the time of their pregnancies (SMD: 1.50, 95% CI: 0.73-2.26, p<0.001). 20,21,23 Results were not different after inclusion of measurements performed in less severe vs. more severe forms of preeclampsia, 13,18 where pooled measurements were not available (SMD: 0.97, 95% CI: 0.55-1.40, p<0.001). Exclusion of two studies that were published as abstracts only did not alter estimated effect size (SMD: 1.22, 95% CI: 0.77-1.66, p<0.001).
Figure 2.
Meta-analysis of differences in CIMT between pregnant women with vs. without preeclampsia at the time of diagnosis
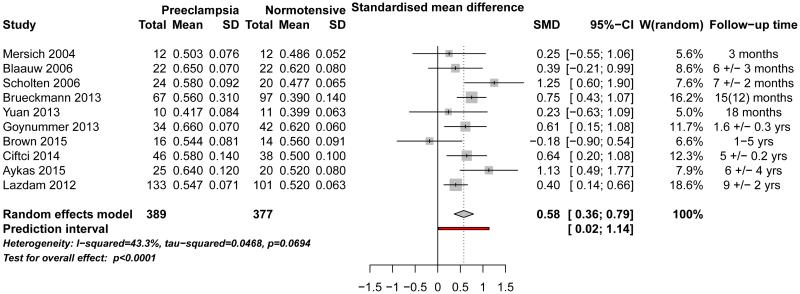
Postpartum period (up to 10 years postpartum): Six cross-sectional studies, 21,24,26–29 3 cohort studies 19,23,30 and pre-randomization baseline data from one randomized controlled trial 25 were eligible for meta-analysis. These studies included 389 women who had histories of preeclampsia and 377 women who did not. Four additional studies (3 cross-sectional studies 11,12,15 and one cohort study 10) were included in the qualitative synthesis. CIMT was significantly higher among women with histories of preeclampsia in the studies conducted in the first decade postpartum, with a SMD of 0.58, and 95% CI of 0.36-0.79, p<0.001 (Figure 3). The analysis revealed moderate heterogeneity among the results of the respective studies (I2=43%, p=0.069). This effect remained significant in sensitivity analyses that included three studies that reported medians 10–12 (SMD: 0.40, 95% CI: 0.15-0.64, p=0.002), and in a sensitivity analysis that excluded women with chronic hypertension at the time of their pregnancies (SMD: 0.53, 95% CI: 0.24-0.81, p<0.001). 21,23–26,28–30 Results were not different after inclusion of study for which the exact postpartum time period is unknown 15 (SMD: 0.67, 95% CI: 0.40-0.94, p<0.001) or the replacement of the results of the study performed in 2006 24 with the follow up results in a subgroup of the same participants who were re-tested 4 years later 14 (SMD: 0.57, 95% CI: 0.35-0.79, p<0.001). Exclusion of two studies that were published as abstracts only did not alter estimated effect size (SMD: 0.69, 95% CI: 0.49-0.90, p<0.001).
Figure 3.
Meta-analysis of differences in CIMT between women with vs. without histories of preeclampsia in the first decade postpartum
. G W, B S, D Oc, J P, V. W. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analyses. 2000
2. Takeshima N, Sozu T, Tajika A, Ogawa Y, Hayasaka Y, Furukawa TA. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Medical Research Methodology. 2014;14:30
3. Gabbay-Benziv R, Oliveira N, Baschat AA. Optimal first trimester preeclampsia prediction: A comparison of multimarker algorithm, risk profiles and their sequential application. Prenatal Diagnosis. 2016;36:34-39
4. Baschat AA. First-trimester screening for pre-eclampsia: Moving from personalized risk prediction to prevention. Ultrasound in Obstetrics & Gynecology. 2015;45:119-129
5. Zheng J, Shan PF, Gu W. The efficacy of metformin in pregnant women with polycystic ovary syndrome: A meta-analysis of clinical trials. J Endocrinol Invest. 2013;36:797-802
6. Charlton F, Tooher J, Rye KA, Hennessy A. Cardiovascular risk, lipids and pregnancy: Preeclampsia and the risk of later life cardiovascular disease. Heart Lung Circ. 2014;23:203-212
Discussion
The goal of this study was to conduct a systematic review and meta-analysis to determine whether CIMT, a sensitive measure for quantifying subclinical atherosclerosis, is elevated in pregnancies affected by preeclampsia, both at the time of delivery and up to 10 years thereafter. We found that CIMT was significantly higher among women at the time of preeclampsia diagnosis (SMD: 1.10, 95% CI: 0.73-1.48), as well as in the first decade postpartum (SMD: 0.58, 95% CI: 0.36-0.79), compared to women who did not have preeclampsia. The reported effects remained significant in sensitivity analyses performed to: 1) exclude women with chronic hypertension at the time of pregnancy, 2) include measurements performed in less severe vs. more severe forms of preeclampsia, and 3) include studies for which any approximation of the main outcome or assumption about the study design was used. There were not enough studies to determine whether women who develop preeclampsia have higher CIMT values prior to preeclampsia diagnosis. Our results indicate that there is an increased atherosclerotic burden in women with preeclamptic compared to normotensive pregnancies, both at the time of delivery and 10 years postpartum, which may contribute to an increased risk to cardiovascular disease (CVD) in the affected women.
In normal pregnancy, the invasive activity of cytotrophoblast leads to important changes in the spiral arteries, causing them to lose muscle and elastic tissue and undergo transformation into flaccid, large capacitance uteroplacental arteries. {Pijnenborg, 1991 #165}’ {Brosens, 2002 #164}’ {Khong, 1986 #145} The net result of these changes, frequently referred to as “physiological change” or “spiral artery remodeling,” is increased blood supply that meets the increased metabolic demands of the developing fetus and placenta. In preeclampsia, placental spiral arteries fail to lose their musculoelastic layers ultimately leading to decreased placental perfusion. {Meekins, 1994 #182}’ {Khong, 1986 #145} These non-transformed arteries are susceptible to acute atherosis, vessel lesions that closely resemble early-stage atherosclerosis. {De Wolf, 1975 #1303} The pro-atherosclerotic milieu of preeclampsia is supported further by studies that reported the role of classical cardiovascular risk factors (such as hypertension, diabetes, and hyperlipidemia) in prediction of preeclampsia as early as in the first trimester, {Gabbay-Benziv, 2016 #1268} {Baschat, 2015 #1269} Clinical corollaries of these observations relate to increased risks for atherosclerotic disease both at the time of pregnancy, and postpartum. One notable example is an increase of 54% in pregnancy related-hospitalizations for stroke from 1994-1995 to 2006-2007 in the USA, with hypertensive disorders as a leading cause. 39 With respect to post-pregnancy outcomes, patients with severe forms of preeclampsia are at greater risk for cardiovascular death as early as the first decade after their affected pregnancies. 40 Taken together, these studies further support the concept of preeclampsia as a state of increased atherosclerotic burden that is associated with an increased risk for cardiovascular events, both immediately and long-term. The overall atherosclerotic burden may be further increased by advanced age at first pregnancy (a trend increasingly described in many countries), and by increased usage of the sophisticated techniques of assisted reproduction, such as in vitro fertilization (IVF), that have made pregnancy possible for women with infertility conditions that are associated with cardiovascular disease risk factors (such as polycystic ovary syndrome). {Meekins, 1994 #182}’ {Khong, 1986 #145}Despite this convincing evidence of atherosclerotic burden in preeclamptic pregnancies, only a small number of studies have investigated CIMT at the time of the diagnosis of preeclampsia and thereafter.
CIMT increasingly is used as a measure of preclinical atherosclerosis. Nevertheless, current guidelines do not support routine measurements of CIMT in risk assessment for a first atherosclerotic cardiovascular disease event for the general population. This recommendation is based on the available evidence indicating that the addition of CIMT measurements to the Framingham Risk Score was associated with a small and clinically non-significant improvement in 10-year prediction of the first atherosclerotic cardiovascular disease event. 42 Recent studies of the role of CIMT for risk prediction in highly selected patient subgroups have provided encouraging results. Meta-analyses of the studies that included patients with anti-phospholipid syndrome, 43 diabetes mellitus type 1, 44 and systemic lupus erythematosus, 45 have provided evidence that CIMT may serve as a good marker of cardiovascular risk in highly selected patient populations. Our current meta-analysis indicates that a greater atherosclerotic burden, as measured by CIMT, is present at the time of preeclampsia diagnosis, compared to women without such a diagnosis, and up to 10 years postpartum, when most of them are premenopausal. Conceivably, this pro-atherosclerotic milieu can contribute to cardiovascular complications during and after preeclamptic pregnancies.
The limitations of our study are those that are inherent to meta-analyses in general. Discrepant data regarding the association between preeclampsia and CIMT reported by individual studies may be due to differences in their designs, CIMT methodology, and patient recruitment strategies. However, the meta-analysis of published studies clearly indicates that atherosclerotic load is present at the time of preeclamptic pregnancies and that it may be one of the mechanisms leading to this disease, which is increasingly viewed as a heterogeneous disorder, with different clinical subtypes possibly reflecting distinct underlying pathological mechanisms. 46 Consistent with this notion are data indicating that statins may decrease endothelial dysfunction in primary human tissues, thus supporting their role as a candidate therapy for preeclampsia. 47 As to the impact of preeclampsia on future health, CIMT may offer an opportunity for early recognition of women with atherosclerotic burdens after preeclamptic pregnancies, even before menopause, when cardiovascular risk in women tends to increase. Our study cannot support the use of CIMT in the clinical practice neither for identification of pregnant women at risk for preeclampsia, nor for CVD risk stratification after preeclamptic pregnancies. However, our results support the role of atherosclerotic process as one of the mechanisms that is associated with preeclampsia, and set the stage for longitudinal, adequately powered studies that will use a standardized CIMT technique to validate the role of CIMT for diagnosing vascular disease at the time of pregnancy, and for screening of vascular disease thereafter.
Supplementary Material
Acknowledgments
Funding: This study was supported by National Institute of Health P50 AG044170 (VDG). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Footnotes
Competing interests: None of the authors declare competing financial interests.
References
- 1.Milne F, Redman C, Walker J, Baker P, Bradley J, Cooper C, de Swiet M, Fletcher G, Jokinen M, Murphy D, Nelson-Piercy C, Osgood V, Robson S, Shennan A, Tuffnell A, Twaddle S, Waugh J, Waterstone M, Bewley S, Wolfe C, Duckitt K, Harrington D, Stettler R, Cunningham F, Koike T, Minakami H, Izumi A, Watanabe T, Matsubara S, Sato I, Carroli G, Villar J, Piaggio G, Khan-Neelofur D, Gulmezoglu M, Mugford M, Said DA, Annegers J, Cantrell DC, Frankowski R, Willmore L, Henderson J, Roberts T, Sikorski J, Wilson J, Clement S, Banias B, Devoe L, Nolan T, Douglas K, Redman C, Sibai B, Mercer B, Schiff E, Friedman S, Barton J, O’Brien J, Bergauer N, Jacques D, Sibai B, Stamilio D, Sehdev H, Morgan M, Propert K, Macones G, North R, Taylor R, Schellenberg J, Seshadri L, Venkataraman I, Ferrazzani S, Caruso A, Carolis S De, Martino I, Mancuso S, Durnwald C, Mercer B, Martin J, May W, Magann E, Terrone D, Rinehart B, Blake P, Witlin A, Saade G, Mattar F, Sibai B. The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community. BMJ. 2005;330(7491):576–580. doi: 10.1136/bmj.330.7491.576. doi:10.1136/bmj.330.7491.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J-Y, Kim YM. Acute Atherosis of the Uterine Spiral Arteries: Clinicopathologic Implications. J Pathol Transl Med. 2015;49(6):462–471. doi: 10.4132/jptm.2015.10.23. doi:10.4132/jptm.2015.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart J-C, Kastelein JJP. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109(23 Suppl 1):III33–III38. doi: 10.1161/01.CIR.0000131516.65699.ba. doi:10.1161/01.CIR.0000131516.65699.ba. [DOI] [PubMed] [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. doi:10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. http://www.ncbi.nlm.nih.gov/pubmed/10789670. Accessed August 10, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Ltd; Chichester, UK: 2008. Chapter 22: Overview of Reviews. doi:10.1002/9780470712184.fmatter. [Google Scholar]
- 7.IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247. doi: 10.1136/bmjopen-2015-010247. doi:10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.R Core Team R A Lang Environ Stat Comput. 2016 https://www.r-project.org/
- 9.Schwarzer G, Maintainer Package “meta” Title General Package for Meta-Analysis. 2016 [Google Scholar]
- 10.Akhter T, Wikström A-K, Larsson M, Naessen T. Individual common carotid artery wall layer dimensions, but not carotid intima-media thickness, indicate increased cardiovascular risk in women with preeclampsia: an investigation using noninvasive high-frequency ultrasound. Circ Cardiovasc Imaging. 2013;6(5):762–768. doi: 10.1161/CIRCIMAGING.113.000295. doi:10.1161/CIRCIMAGING.113.000295. [DOI] [PubMed] [Google Scholar]
- 11.Gaugler-Senden IPM, Berends AL, de Groot CJM, Steegers EAP. Severe, very early onset preeclampsia: subsequent pregnancies and future parental cardiovascular health. Eur J Obstet Gynecol Reprod Biol. 2008;140(2):171–177. doi: 10.1016/j.ejogrb.2008.03.004. doi:10.1016/j.ejogrb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Berends AL, de Groot CJM, Sijbrands EJ, Sie MPS, Benneheij SH, Pal R, Heydanus R, Oostra BA, van Duijn CM, Steegers EAP. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertens (Dallas, Tex 1979) 2008;51(4):1034–1041. doi: 10.1161/HYPERTENSIONAHA.107.101873. doi:10.1161/HYPERTENSIONAHA.107.101873. [DOI] [PubMed] [Google Scholar]
- 13.Stergiotou I, Crispi F, Valenzuela-Alcaraz B, Bijnens B, Gratacos E. Patterns of maternal vascular remodeling and responsiveness in early-versus late-onset preeclampsia. Am J Obstet Gynecol. 2013;209(6):558.e1–e558.e14. doi: 10.1016/j.ajog.2013.07.030. doi:10.1016/j.ajog.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Blaauw J, Souwer ETD, Coffeng SM, Smit AJ, van Doormaal JJ, Faas MM, van Pampus MG. Follow up of intima-media thickness after severe early-onset preeclampsia. Acta Obstet Gynecol Scand. 2014;93(12):1309–1316. doi: 10.1111/aogs.12499. doi:10.1111/aogs.12499. [DOI] [PubMed] [Google Scholar]
- 15.Sharashkina N, Runikhina N, Tkacheva O, Novikova I. PP101. Preeclampsia and pregnancy induced hypertension and carotid artery atherosclerosis. Pregnancy Hypertens. 2012;2(3):294–295. doi: 10.1016/j.preghy.2012.04.212. doi:10.1016/j.preghy.2012.04.212. [DOI] [PubMed] [Google Scholar]
- 16.Nasr G, Nasr A, Eleraki A. Myocardial performance index, aortic root diameter and carotid intima-media thickness in pregnancy-induced hypertension. Eur J Echocardiogr Suppl. 2003:S21. S E. [Google Scholar]
- 17.Altin C, Ozsoy H, Geymis E, Yilmaz M, Sade L, Muderrisoglu H. Assesment of epicaridal fat thickness and carotid intima media thickness in preeclampsia. European Heart J Cardiovascular Imaging. 2015;16:ii144. [Google Scholar]
- 18.Mori T, Watanabe K, Iwasaki A, Kimura C, Matsushita H, Shinohara K, Wakatsuki A. Differences in vascular reactivity between pregnant women with chronic hypertension and preeclampsia. Hypertens Res. 2014;37(2):145–150. doi: 10.1038/hr.2013.131. doi:10.1038/hr.2013.131. [DOI] [PubMed] [Google Scholar]
- 19.Brueckmann A, Seeliger C, Schlembach D, Schleussner E. Carotid intima-media-thickness in the first trimester as a predictor of preeclampsia. Pregnancy Hypertens. 2013;3(2):84. doi: 10.1016/j.preghy.2013.04.075. [DOI] [PubMed] [Google Scholar]
- 20.Torrado J, Farro I, Zócalo Y, Farro F, Sosa C, Scasso S, Alonso J, Bia D. Preeclampsia Is Associated with Increased Central Aortic Pressure, Elastic Arteries Stiffness and Wave Reflections, and Resting and Recruitable Endothelial Dysfunction. Int J Hypertens. 2015;2015:720683. doi: 10.1155/2015/720683. doi:10.1155/2015/720683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan LJ, Xue D, Duan YY, Cao TS, Yang HG, Zhou N. Carotid arterial intima–media thickness and arterial stiffness in pre-eclampsia: analysis with a radiofrequency ultrasound technique. Ultrasound Obstet Gynecol. 2013;42(6):644–652. doi: 10.1002/uog.12409. doi:10.1002/uog.12409. [DOI] [PubMed] [Google Scholar]
- 22.Verissimo C. [Intima-media thickness and hypertensive disorders of pregnancy: a prospective study] Rev Port Cir cardio-torácica e Vasc órgão Of da Soc Port Cir Cardio-Torácica e Vasc. 17(2):123–128. http://www.ncbi.nlm.nih.gov/pubmed/21298125. Accessed August 9, 2016. [PubMed] [Google Scholar]
- 23.Mersich B, RigO J, LEnArd Z, Studinger P, Visontai Z, Kollai M. Carotid artery stiffening does not explain baroreflex impairment in pre-eclampsia. Clin Sci (Lond) 2004;107(4):407–413. doi: 10.1042/CS20040137. doi:10.1042/CS20040137. [DOI] [PubMed] [Google Scholar]
- 24.Blaauw J, van Pampus MG, Van Doormaal JJ, Fokkema MR, Fidler V, Smit AJ, Aarnoudse JG. Increased intima-media thickness after early-onset preeclampsia. Obstet Gynecol. 2006;107(6):1345–1351. doi: 10.1097/01.AOG.0000218097.22464.b4. doi:10.1097/01.AOG.0000218097.22464.b4. [DOI] [PubMed] [Google Scholar]
- 25.Scholten RR, Thijssen DJH, Lotgering FK, Hopman MTE, Spaanderman MEA. Cardiovascular effects of aerobic exercise training in formerly preeclamptic women and healthy parous control subjects. Am J Obstet Gynecol. 2014;211(5):516.e1–e516.e11. doi: 10.1016/j.ajog.2014.04.025. doi:10.1016/j.ajog.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Goynumer G, Yucel N, Adali E, Tan T, Baskent E, Karadag C. Vascular risk in women with a history of severe preeclampsia. J Clin Ultrasound. 41(3):145–150. doi: 10.1002/jcu.21962. doi:10.1002/jcu.21962. [DOI] [PubMed] [Google Scholar]
- 27.Ciftci FC, Caliskan M, Ciftci O, Gullu H, Uckuyu A, Toprak E, Yanik F. Impaired coronary microvascular function and increased intima-media thickness in preeclampsia. J Am Soc Hypertens. 2014;8(11):820–826. doi: 10.1016/j.jash.2014.08.012. doi:10.1016/j.jash.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Aykas F, Solak Y, Erden A, Bulut K, Dogan S, Sarli B, Acmaz G, Afsar B, Siriopol D, Covic A, Sharma S, Johnson RJ, Kanbay M. Persistence of cardiovascular risk factors in women with previous preeclampsia: a long-term follow-up study. J Investig Med. 2015;63(4):641–645. doi: 10.1097/JIM.0000000000000189. doi:10.1097/JIM.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 29.Lazdam M, de la Horra A, Diesch J, Francis J, Kenworthy Y, Shore A, Neubauer S, Kharbanda R, Alp N, Redman C, Kelly B, Leeson P. PP032. Unique features of long-term cardiovascular phenotype in young women with early-onset pre-eclampsia. Pregnancy Hypertens. 2012;2(3):259–260. doi: 10.1016/j.preghy.2012.04.143. doi:10.1016/j.preghy.2012.04.143. [DOI] [PubMed] [Google Scholar]
- 30.Brown CE, Flynn J, Carty DM, Scotland G, Delles C. LB01.05: VASCULAR CONSEQUENCES OF PRE-ECLAMPSIA. J Hypertens. 2015;33(Suppl 1):e46. doi:10.1097/01.hjh.0000467467.39257.dd. [Google Scholar]
- 31.Anastasakis E, Paraskevas KI, Papantoniou N, Daskalakis G, Mesogitis S, Mikhailidis DP, Antsaklis A. Association between abnormal uterine artery Doppler flow velocimetry, risk of preeclampsia, and indices of arterial structure and function: a pilot study. Angiology. 59(4):493–499. doi: 10.1177/0003319708316008. doi:10.1177/0003319708316008. [DOI] [PubMed] [Google Scholar]
- 32.Guddat C, Grouven U, Bender R, Skipka G. A note on the graphical presentation of prediction intervals in random-effects meta-analyses. Syst Rev. 2012;1:34. doi: 10.1186/2046-4053-1-34. doi:10.1186/2046-4053-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham PL, Moran JL. Robust meta-analytic conclusions mandate the provision of prediction intervals in meta-analysis summaries. J Clin Epidemiol. 2012;65(5):503–510. doi: 10.1016/j.jclinepi.2011.09.012. doi:10.1016/j.jclinepi.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Ronsmans C, Graham WJ, Lancet Maternal Survival Series steering group J. McArthur J, WHO UCFUPF. Mahler H, Secretariat PD of the D of E and SA of the U. Van Lerberghe W, De Brouwere V, Loudon I, De Brouwere V, Van Lerberghe W, Hord C, David H, Donnay F, Wolf M, Dieltiens G, Dewan H, Botlero R, Alam N, Chowdhury E, Ronsmans C, Seneviratne H, Rajapaksa L, Liljestrand J, Pathmanathan I, Campbell O, Gipson R, Issa A, Shiffman J, Stanton C, Salazar A, AbouZahr C, Wardlaw T, Campbell O, Graham W, Li X, Fortney J, Kotelchuck M, Glover L, WHO. Pradhan E, West K, Katz J, Høj L, da Silva D, Hedegaard K, Sandstrom A, Aaby P, Ronsmans C, Walraven G, Etard J, Khan K, Wojdyla D, Say L, Gulmezoglu A, Van Look P, WHO. Thonneau P, Goyaux N, Goufodji S, Sundby J, Bartlett L, Mawji S, Whitehead S, Crouse C, Dalil S, Ionete D, Salama P, Team the AMMS. Kodio B, de Bernis L, Ba M, Ronsmans C, Pison G, Etard J, Mswia R, Lewanga M, Moshiro C, Fauveau V, Koenig M, Chakraborty J, Chowdhury A, Ahmed Y, Mwaba P, Chintu C, Grange J, Ustianowski A, Zumla A, Khan M, Pillay T, Moodley J, Connolly C, Sewankambo N, Gray R, et al. Maternal mortality: who, when, where, and why. Lancet (London, England) 2006;368(9542):1189–1200. doi: 10.1016/S0140-6736(06)69380-X. al. et. al. et. al. et. doi:10.1016/S0140-6736(06)69380-X. [DOI] [PubMed] [Google Scholar]
- 35.Villar J, Say L, Gulmezoglu AM, Meraldi M, Lindheimer MD, Betran AP, Critchly H. Eclampsia and pre-eclampsia: a health problem for 2000 years. In: MacLean A, Poston LWJ, editors. Pre-Eclampsia. RCOG Press; London: 2003. pp. 189–207. PG. [Google Scholar]
- 36.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564. doi: 10.1136/bmj.f6564. http://www.ncbi.nlm.nih.gov/pubmed/24201165. Accessed August 29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306. doi: 10.1097/AOG.0b013e3181a45b25. doi:10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- 38.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97(4):533–538. doi: 10.1016/s0029-7844(00)01223-0. http://www.ncbi.nlm.nih.gov/pubmed/11275024. Accessed August 29, 2016. [DOI] [PubMed] [Google Scholar]
- 39.Kuklina EV, Tong X, Bansil P, George MG, Callaghan WM. Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007: reasons for concern? Stroke. 2011;42(9):2564–2570. doi: 10.1161/STROKEAHA.110.610592. doi:10.1161/STROKEAHA.110.610592. [DOI] [PubMed] [Google Scholar]
- 40.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertens (Dallas, Tex 1979) 2010;56(1):166–171. doi: 10.1161/HYPERTENSIONAHA.110.150078. doi:10.1161/HYPERTENSIONAHA.110.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartsch E, Medcalf KE, Park AL, Ray JG, High Risk of Pre-eclampsia Identification Group Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Den Ruijter HM, Peters SAE, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, Engström G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O’Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CDA, Witteman JC, Moons KG, Bots ML. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308(8):796–803. doi: 10.1001/jama.2012.9630. doi:10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 43.Ambrosino P, Lupoli R, Di Minno A, Iervolino S, Peluso R, Di Minno MND. Markers of cardiovascular risk in patients with antiphospholipid syndrome: a meta-analysis of literature studies. Ann Med. 2014;46(8):693–702. doi: 10.3109/07853890.2014.959559. doi:10.3109/07853890.2014.959559. [DOI] [PubMed] [Google Scholar]
- 44.Sun Y-P, Cai Y-Y, Li H-M, Deng S-M, Leng R-X, Pan H-F. Increased carotid intima–media thickness (CIMT) levels in patients with type 1 diabetes mellitus (T1DM): A meta-analysis. J Diabetes Complications. 2015;29(5):724–730. doi: 10.1016/j.jdiacomp.2015.03.018. doi:10.1016/j.jdiacomp.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Wu G-C, Liu H-R, Leng R-X, Li X-P, Li X-M, Pan H-F, Ye D-Q. Subclinical atherosclerosis in patients with systemic lupus erythematosus: A systemic review and meta-analysis. Autoimmun Rev. 2016;15(1):22–37. doi: 10.1016/j.autrev.2015.10.002. doi:10.1016/j.autrev.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Powers RW, Roberts JM, Plymire DA, Pucci D, Datwyler SA, Laird DM, Sogin DC, Jeyabalan A, Hubel CA, Gandley RE. Low placental growth factor across pregnancy identifies a subset of women with preterm preeclampsia: type 1 versus type 2 preeclampsia? Hypertens (Dallas, Tex 1979) 2012;60(1):239–246. doi: 10.1161/HYPERTENSIONAHA.112.191213. doi:10.1161/HYPERTENSIONAHA.112.191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brownfoot FC, Tong S, Hannan NJ, Binder NK, Walker SP, Cannon P, Hastie R, Onda K, Kaitu’u-Lino TJ. Effects of Pravastatin on Human Placenta, Endothelium, and Women With Severe Preeclampsia. Hypertens (Dallas, Tex 1979) 2015;66(3):687–697. doi: 10.1161/HYPERTENSIONAHA.115.05445. discussion 445. doi:10.1161/HYPERTENSIONAHA.115.05445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.