Abstract
Arsenic trioxide (As2O3) can induce apoptosis in many tumors. However, the associated mechanisms are not clearly understood. We found that As2O3 significantly inhibited the proliferation of WSU-CLL cells and induced apoptosis in dose- and time-dependent manners. WSU-CLL cells treated with 2 μM As2O3 showed survivin down-regulation and p53 up-regulation. Survivin siRNA combined with As2O3 further inhibited the proliferation of WSU-CLL cells. p53 inhibition by siRNA prevented the down-regulation of survivin by As2O3 and prevented the As2O3-induced cytotoxicity of WSU-CLL cells. These results suggest that As2O3 may be of therapeutic value for chronic lymphocytic leukemia.
Keywords: Arsenic trioxide, WSU-CLL, Apoptosis, Survivin, P53 and CLL
1. Introduction
B-cell chronic lymphocytic leukemia (CLL) is characterized by the extensive accumulation of monoclonal, relatively mature CD5+ B cells in lymphoid organs, bone marrow, and peripheral blood. CLL cells accumulate because of defective apoptosis, which extends survival [1].
Arsenic trioxide (As2O3) has been shown to be useful in the treatment of patients with acute promyelocytic leukemia (APL). Preclinical studies have demonstrated that As2O3 can induce apoptosis and inhibit tumor cell growth in a wide variety of tumors [2–5]. As2O3 is also being used to treat other hematologic malignancies, such as myelodysplastic syndrome [6]. Few studies have addressed the effect of As2O3 on B-CLL cells. It was recently reported that As2O3 preferentially induced apoptosis in B-CLL cases with unfavorable prognoses [7–11], and in analyses of a small number of B-CLL samples, As2O3 was shown to induce B-CLL apoptosis [8]. These previous studies suggest that As2O3 could be a possible therapeutic agent for B-CLL. The mechanism by which As2O3 induces B-CLL apoptosis has not been established.
Previous studies have shown that survivin expression is closely related to apoptosis resistance [12,13]. Survivin is a member of the inhibitor of apoptosis protein (IAP) family [14]. It has been shown that survivin is overexpressed in CLL patients, particularly those with poor prognostic factors [15]. A previous study demonstrated that survivin inhibits apoptosis, enhances proliferation, and promotes angiogenesis [16], and it is regarded as a promising target for the treatment of cancer.
The induction of apoptosis by As2O3 in tumors such as acute promyelocytic leukemia, Burkitt’s lymphoma and human glioma is thought to occur through the down-regulation of survivin [2,17,18]. Li et al. [2] found that As2O3 inhibited the proliferation of Burkitt’s lymphoma cell lines, and after treatment with different concentrations of As2O3, survivin gene expression decreased. Ghaffari et al. [17] demonstrated that As2O3 might inhibit cell growth and proliferation and induce apoptosis in NB4 cells through the transcriptional repression of survivin.
As2O3 has previously been shown to induce apoptosis in many malignancies, such as multiple myeloma, neuroblastoma and human pre-monocytic leukemia, depending on the p53 status in the cells [19–21]. p53 plays a central role in the activation of apoptosis via the mitochondrial apoptosis pathway [20]. Previous studies have shown that p53 is one of the most important components in the apoptosis pathway, which is affected by As2O3, and that it may be a target of As2O3 [22,23]. Kircelli et al. [19] proposed that As2O3 could induce apoptosis in multiple myeloma in a p53-dependent manner.
Cell growth and cell death are usually determined by a balance between the activities of oncogenes and tumor-suppressor genes. Survivin over-expression and loss of p53 function occur in many cancers [24–27]. Shao et al. [28] suggested a correlation between the levels of p53 and survivin proteins expressed in prostate tumors. Their data suggest that p53 may be directly altered conformationally in the presence of survivin. Nabilsi et al. [29] proved that the balanced regulation of the p53-survivin signaling pathway is necessary for cell survival in mammalian cells. They suggested that p53, a negative regulator of survivin, could decrease the expression of survivin protein through transcriptional regulation.
Given these results, we hypothesized that As2O3 can induce cytotoxic effects in WSU-CLL cells, a CLL cell line that is refractory to fludarabine and combination therapies, including cyclophosphamide, vincristine and prednisone (CVP) and vincristine, adriamycin and dexamethasone (VAD) [30–32]. The mechanism may be modulated through the induction of apoptosis due to the p53-dependent down-regulation of survivin. Therefore, in the present study, we evaluated whether As2O3 could inhibit the proliferation of WSU-CLL cells. Furthermore, we examined whether this inhibition was due to decreased survivin expression through the p53 signaling pathway.
2. Materials and methods
2.1. Cell culture and reagents
WSU-CLL is a human CLL cell line, and the cells were generously provided by Dr. Bo Huang (Department of Immunology, Institute of Basic Medical Sciences, Chinese Academy of Medical Science, China). The cells were cultured in 5% CO2 at 37 °C in RPMI 1640 (Life Technologies, Inc.) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Life Technologies, Inc.), 2 mM L-glutamine, 100 units/ml penicillin G, and 100 mg/ml streptomycin (Life Technologies, Inc.). The characteristics of this cell line have been described in detail elsewhere [30–32].
A stock solution of arsenic trioxide (As2O3) was kindly supplied by Harbin Yida Pharmaceutical Company (Harbin, China). It was diluted in RPMI-1640 medium to a concentration range of 1–8 μM. Antibodies against survivin, myc and p53 were purchased from BD Biosciences Pharmingen.
2.2. Cell viability assay
The in vitro effect of As2O3 on WSU-CLL cell growth was determined by counting the viable cells with trypan blue staining. Briefly, cells were seeded in 96-well plates at a density of 5 × 104 cells/ml and pre-incubated overnight. After varying lengths of exposure to various concentrations of As2O3, each cell suspension (0.02 ml) was mixed well with 0.02 ml trypan blue solution. After 1 or 2 min, each solution was placed on a hemocytometer, and the blue-stained cells were counted as non-intact.
2.3. Assessment of apoptosis
WSU-CLL cell apoptosis was assessed by observing the translocation of phosphatidyl serine to the cell surface, as detected with an Annexin V-FITC/PI apoptosis detection kit (BD Biosciences Pharmingen, USA). The cells were collected, washed with cold PBS and suspended in binding buffer. The cells were stained with 5 μl annexin V-FITC and 10 μl PI and analyzed with a FACScan flow cytometer.
2.4. Western blotting analysis
After various treatments, the cells were lysed by adding lysis buffer (1 M Tris–HCl, pH 6.8, 2 M NaCl, 0.1 M EDTA, 0.05 M EGTA, 0.5% NP-40 and 10 mM PMSF). Then, samples were vortexed briefly and incubated in lysis buffer for 30 min on ice and centrifuged at 15,000 × g for 15 min. The protein concentration was determined using the Bradford method. Samples of 40 μg of total protein were subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane (Roche). The membranes were incubated with primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies, and the immunoblots were visualized by enhanced chemiluminescence procedures according to the manufacturer’s recommendations. β-actin was used as the protein loading control. Each immunoblot was repeated at least three times.
2.5. RT-PCR analysis
Total RNA was isolated from WSU-CLL cells treated with or without As2O3 using TRIzol reagent. RNA was quantified using a UV spectrometer, and cDNA was synthesized with 2 μg of total RNA (Fermentas Inc.). PCR was performed in a final volume of 20 μl containing 10 μl SYBR-Green master mix (including TaqE), 0.5 μl of each forward and reverse primer (10 pmol), 2 μl cDNA samples and 7 μl nuclease-free water. The thermal cycling conditions were as follows: 95 °C for 5 min; 40 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s, and 90 °C for 5 s; and an annealing/extension step at 72 °C for 5 min.
The specific PCR primers were as follows: [33]
survivin: 5′-GGACCACCGCATCTCTAC-3′ and 5′-CAGCCTTCCAGCTCCTTG-3′;
β-actin: 5′-GGATTCCTATGTGGGCGACAG-3′ and 5′-CGCTCGGTGAGGATCTTCATG-3′.
The primers were synthesized by Shanghai GenePharma Co. (Shanghai, China).
2.6. Small interfering RNA design
We used http://www.jura.wi.mit.edu/bioc/siRNA to construct a series of expression plasmids containing small interfering RNA (siRNA) specific to survivin, p53 or negative control siRNA expression elements.
The following primers were used:
survivin siRNA: 5′-AGCAUUCGUCCGGUUGCGCdTdT-3′ and 5′-GCGCAACCGGACGAAUGCUTT-3′;
p53 siRNA: 5′-GCAUGAACCGGAGGCCCAUdTdT-3′ and 5′-AUGGGCCUCCGGUUCAUGCTT-3′
negative control siRNA: 5′-UUCUCCGAACGUGUCACGUdTdT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′.
After these siRNAs were designed, they were compared with the sequence in the human expressed sequence tag (EST) database to confirm that no other genes were targeted, and they were then synthesized by Shanghai GenePharma Co. (China).
2.7. Transient transfection and reporter assay
The survivin luciferase reporter and Myc-tagged survivin were a kind gift from Dr. Bo Huang (Department of Immunology, Institute of Basic Medical Sciences, Chinese Academy of Medical Science, China). The cells were incubated in fresh media without antibiotics for 24 h before transfection. On the following day, the cells were transfected with plasmid DNA for 20 h.
For the survivin reporter assay, the cells were transfected with a survivin-Luc reporter and pCMV-β-gal, and reporter transcription was measured with a luciferase assay. The relative luciferase activity was calculated by normalizing the total luciferase activity by the β-galactosidase activity. The results were presented as the fold increase in activity relative to control.
For siRNA transfection, the cells were incubated in fresh media without antibiotics in 96- and 6-well plates for 24 h. Then, they were transfected with 0, 25, 50, 100, 200, or 400 nmol/L (final concentration) of siRNA per well for 20 h using lipofectamine 2000 transfection reagent (Invitrogen, USA), according to the manufacturer’s instructions. Western blots were used to detect the efficiency of siRNA transfection. The results of preliminary experiments indicated that 100 nmol/L siRNA could successfully inhibit target gene expression (data not shown). We therefore used 100 nmol/L as the siRNA concentration in this study.
Cells were treated with either As2O3 (2 μM) or solvent control for an additional 24 h after transfection. Treated cells were collected and used for measurement. Cell viability was measured using trypan blue exclusion as described above.
2.8. Statistical analysis
The data are expressed as the mean ± standard deviation (SD). All experiments were performed in triplicate. Statistical analyses were performed using SPSS software (SPSS, Version 16.0, Chicago, IL, USA). Differences among treatment groups were analyzed by one-way analysis of variance (ANOVA) with Dunnett’s post hoc analysis. Differences were considered significant when P < 0.05.
3. Results
3.1. Effect of As2O3 on WSU-CLL cell viability
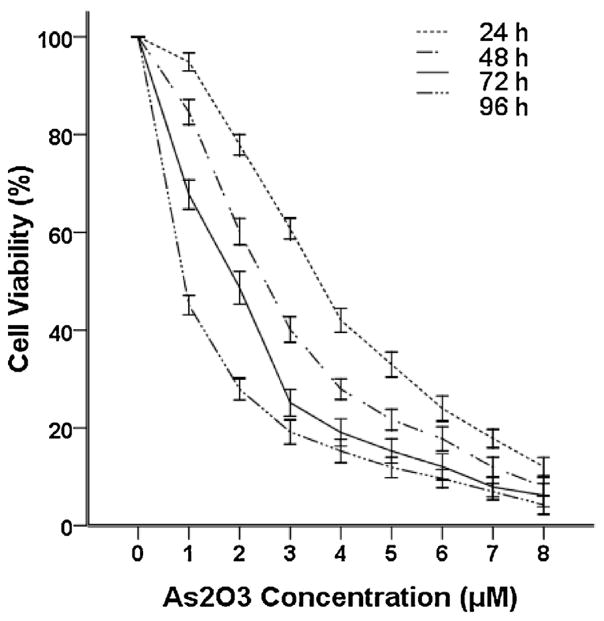
Previous studies have shown that As2O3 exerts cytotoxic effects on cancer cells [2,3,17]. To evaluate the inhibitory effects of As2O3 on the growth of the WSU-CLL cell line, WSU-CLL cells were incubated with 0–8 μM As2O3 for 24, 48, 72 and 96 h. The inhibition rates and 50% inhibitory concentration (IC50) were evaluated by trypan blue dye exclusion. WSU-CLL cell growth was inhibited by As2O3 in time- and dose-dependent manners. The IC50 after 72 h of As2O3 treatment in CLL cells was 1.96 μM.
As shown in Fig. 1, As2O3 was cytotoxic to WSU-CLL cells at concentrations at or above 2 μM. The therapeutic range of As2O3 in treating APL is 1–2 μM [17]. On the basis of our observations as well as others [11,17], we examined the effect of 2 μM As2O3 in subsequent experiments.
Fig. 1.
Cytotoxicity of As2O3 to WSU-CLL cells. WSU-CLL cells were cultured without or with different concentrations of As2O3 for 24, 48, 72 and 96 h. Cell viability was determined based on trypan blue exclusion. Control cell viability was set at 100%, and the survival relative to the control is presented. Each point represents the mean ± SD of three experiments with nine replicates per dose.
3.2. Apoptosis induction by As2O3 in WSU-CLL cells
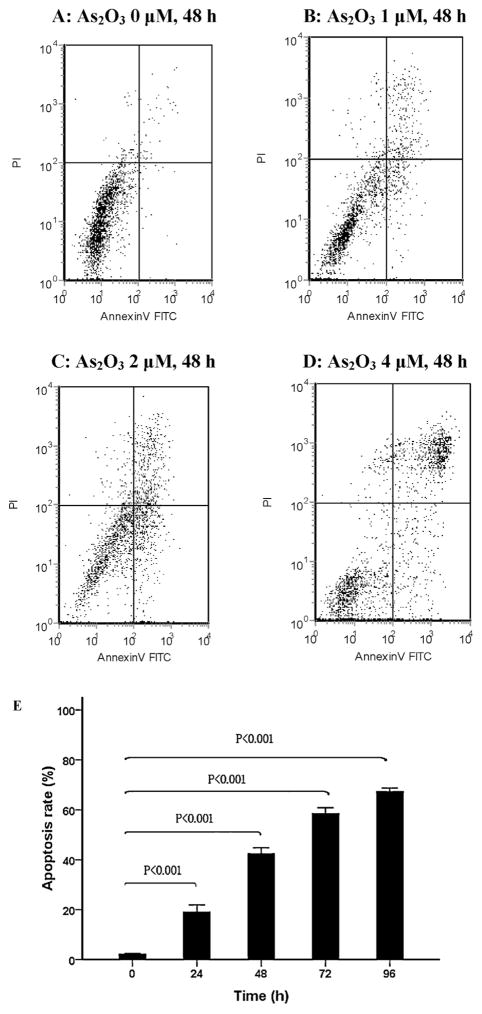
To explore whether the cytotoxic effect of As2O3 in WSU-CLL cells was the result of apoptosis induction, Annexin V-FITC/PI staining and flow cytometry were performed. WSU-CLL cells were treated with different concentrations of As2O3 for 0, 24, 48, 72 and 96 h. There was a dose- and time-dependent increase in the number of apoptotic cells, as shown in Fig. 2. The apoptosis rates of WSU-CLL cells in the presence of 2 μM As2O3 at 0, 24, 48, 72 and 96 h were 2.0%, 18.9%, 42.3%, 58.3% and 67.2%, respectively.
Fig. 2.
Apoptosis of WSU-CLL cells after As2O3 treatment. (A–D) Apoptosis of WSU-CLL cells after treatment with different concentrations of As2O3 for 48 h. (A) 0 μM, (B) 1 μM, (C) 2 μM, (D) 4 μM and (E) WSU-CLL cells were treated with 2 μM As2O3 for 0, 24, 48, 72 and 96 h. Cell apoptosis was evaluated by flow cytometry after Annexin V and PI staining. The data are presented as the mean ± SD of three independent experiments.
3.3. Down-regulation of survivin by As2O3
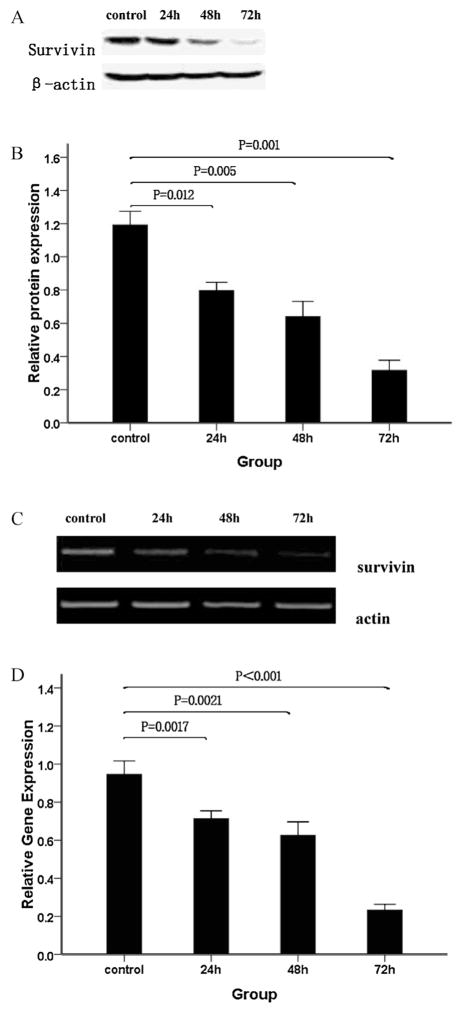
Survivin was recently reported to modulate the balance between cell death and viability in cancer [33]. It is regarded as one of the most important anti-apoptosis proteins in malignant proliferation. It has been shown that survivin is overexpressed in WSU-CLL cells [30]. We hypothesized that the cytotoxic effects of As2O3 on WSU-CLL cells may be due, in part, to survivin down-regulation. We therefore examined the effects of As2O3 on survivin expression in WSU-CLL cells. Western blotting of whole-cell extracts revealed that As2O3 strongly decreased the level of survivin protein (Fig. 3A and B). Our results demonstrated that As2O3 induced a time-dependent down-regulation of survivin protein in WSU-CLL cells.
Fig. 3.
Analyses of survivin protein and mRNA levels. WSU-CLL cells were exposed to 2.0 μM As2O3 for 0, 24, 48 and 72 h. Survivin was analyzed by Western blotting. (A) Representative immunoblots of the expression level of survivin in cells treated with 2.0 μM As2O3 for 0, 24, 48 and 72 h. (B) β-actin expression was used as a loading control, and the relative survivin protein levels (means ± SD, n = 3) were determined. Survivin mRNA levels were measured by RT-PCR. (C) RT-PCR images of survivin in WSU-CLL cells treated with As2O3 for different lengths of time. (D) Quantification of the survivin mRNA level in WSU-CLL cells treated with As2O3 for different lengths of time in three separate experiments. As2O3 reduced survivin transcriptional activity.
To investigate whether As2O3 exposure triggered a decrease in survivin protein levels at the transcriptional level, we evaluated the impact of As2O3 on survivin mRNA levels. As shown in Fig. 3C and D, RT-PCR revealed that 2 μM As2O3 caused a large reduction in the survivin mRNA level.
3.4. Involvement of survivin expression in As2O3-induced cytotoxicity
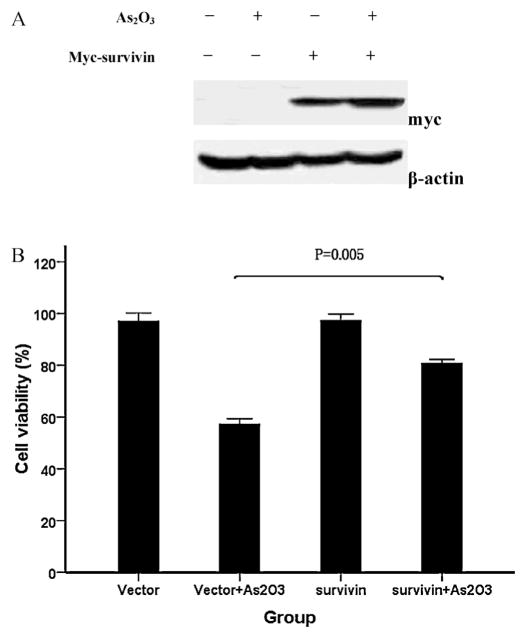
To determine whether survivin down-regulation was responsible for As2O3-induced cytotoxicity, we examined the effects of As2O3 in cells transiently transfected with a plasmid encoding myc-survivin or pc-DNA. As shown in Fig. 4A, cells pre-treated with myc-survivin showed a high level of survivin protein. The cell viability of WSU-CLL cells treated with As2O3 was increased in the survivin group compared with the vector group (Fig. 4B). These observations suggest that the overexpression of survivin in CLL cells may help to overcome As2O3-induced cytotoxicity.
Fig. 4.
Involvement of survivin in As2O3-induced cytotoxicity. WSU-CLL cells were transfected with myc-survivin or pcDNA (vector) for 20 h and then incubated with or without 2 μM As2O3 for 48 h. (A) Survivin protein expression was analyzed by Western blotting. Survivin protein was overexpressed in WSU-CLL cells transfected with survivin. (B) Cell viability was determined by trypan blue exclusion. Survivin overexpression reduced As2O3-induced cytotoxicity. The viability of control cells was set at 100%, and the viability relative to the control is shown.
We next investigated the role of survivin in As2O3-induced cell death using survivin siRNA. Western blotting demonstrated that survivin siRNA successfully inhibited survivin expression in WSU-CLL cells (Fig. 5A). As shown in Fig. 5B, increased cytotoxicity was observed in cells transfected with survivin siRNA compared with cells treated with control siRNA. Moreover, treatment with survivin siRNA and As2O3 resulted in a greater extent of cell death than control siRNA.
Fig. 5.
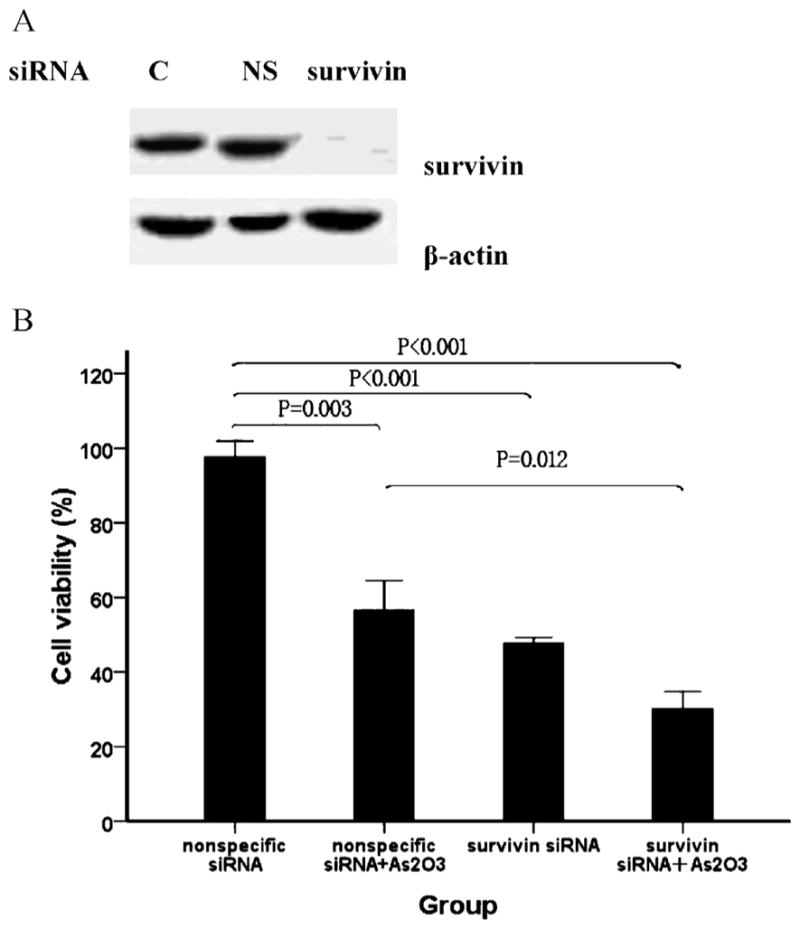
Survivin inhibition by siRNA sensitizes WSU-CLL cells to As2O3-induced cell death. WSU-CLL cells were transfected with survivin or nonspecific siRNA for 20 h and then treated with or without 2 μM As2O3 for 48 h. (A) Cells that were pre-treated with survivin siRNA (survivin) showed a significant decrease in survivin expression compared with the control group (C) and the nonspecific siRNA group (NS). (B) Cell viability was determined by trypan blue exclusion. Control cell viability was set at 100%, and the viability relative to the control is shown.
These results demonstrate that survivin can counteract the cytotoxicity induced by As2O3 in WSU-CLL cells. The induction of cell death by As2O3 is due, at least in part, to the down-regulation of survivin.
3.5. The reduction of survivin expression by As2O3 treatment is dependent on p53 accumulation
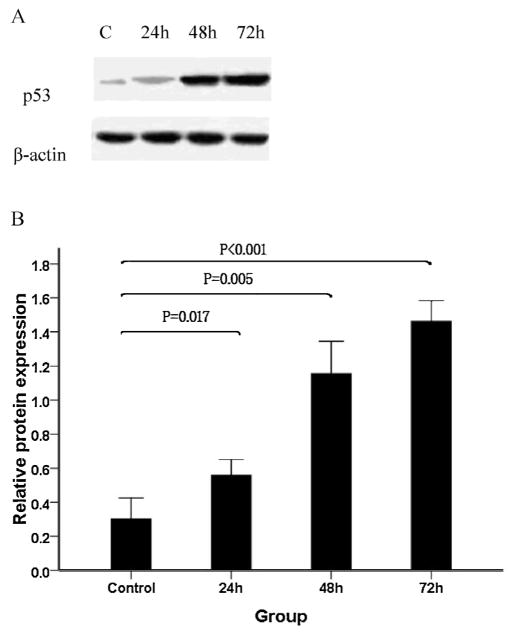
Previous reports have suggested that p53 down-regulates the expression of survivin in some cell models and cancer cell lines [29,34]. To examine the effect of As2O3 on p53 expression and determine the relationship between survivin and p53, we first monitored the p53 protein levels of WSU-CLL cells treated with As2O3. We found that p53 expression was increased 24 h after exposure to As2O3 and that it was sustained for at least 72 h (Fig. 6).
Fig. 6.
Effect of As2O3 on p53 protein expression in WSU-CLL cells. WSU-CLL cells were exposed to 2.0 μM As2O3 for 0, 24, 48, and 72 h. Western blot analyses were performed. (A) Representative immunoblots of the p53 expression level following treatment with 2 μM As2O3 for 0, 24, 48 and 72 h. (B) β-actin expression was used as a loading control. Relative p53 protein levels (means ± SD, n = 3) were determined.
Previous studies have suggested that survivin overexpression inhibits the p53-dependent apoptosis pathway [34]. Therefore, survivin inhibition may allow for the re-activation of this p53-mediated apoptosis program.
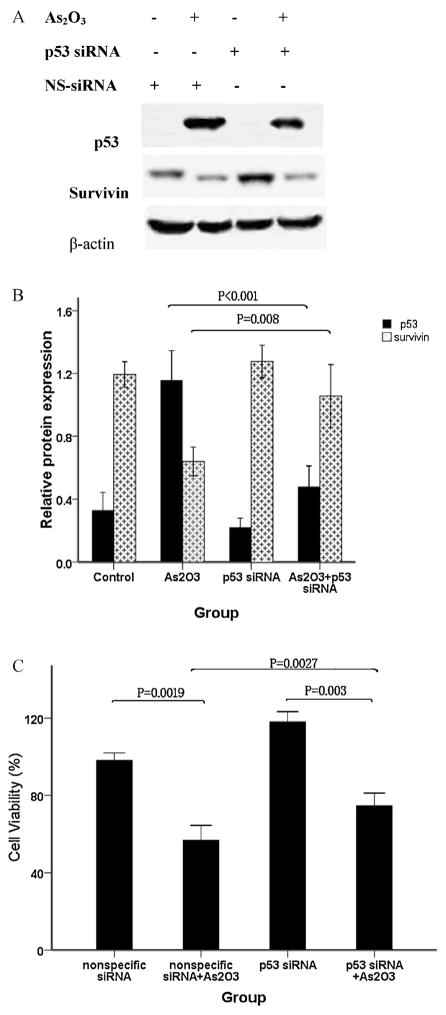
We next examined whether p53 up-regulation by As2O3 was responsible for survivin down-regulation using an siRNA targeting p53. Western blot analysis revealed that the siRNAs were highly effective at decreasing the p53 protein level (Fig. 7A). As shown in Fig. 7B, survivin protein expression increased as p53 protein decreased. These results indicate that survivin down-regulation by As2O3 is mediated by p53 activation.
Fig. 7.
Knockdown of p53 with siRNA targeting p53 can prevent cell death due to As2O3. WSU-CLL cells were transfected with p53 siRNA or nonspecific siRNA for 20 h and then incubated with or without 2 μM As2O3 for 48 h. Western blot and cell viability analyses were performed. (A) Representative immunoblots of p53 and survivin. The p53 siRNAs were highly effective at decreasing the level of p53 protein and simultaneously increased survivin protein expression. (B) The relative protein expression of p53 and survivin (means ± SD, n = 3) was determined. p53 siRNA decreased p53 protein expression, which was up-regulated by As2O3. The decrease in the survivin protein level caused by As2O3 was partially rescued by p53 siRNA. (C) Cell viability was determined by trypan blue exclusion. Control cell viability was set at 100%, and the viability relative to the control is shown. The knockdown of p53 using siRNA resulted in the partial rescue of cell viability.
To further investigate the role of p53 in As2O3-induced WSU-CLL cell cytotoxicity, we used trypan blue exclusion to examine cell viability in the presence of As2O3 and/or p53 siRNA. As shown in Fig. 7C, As2O3 significantly decreased cell viability. Furthermore, when cultured with p53 siRNA, As2O3-induced cytotoxicity was partially rescued. Our data suggest that the p53-survivin pathway is involved in the effects of As2O3-induced apoptosis in WSU-CLL cells.
4. Discussion
Studies have demonstrated that As2O3 slows cancer cell growth, including cells from leukemias and solid tumors [10,35]. Although As2O3 influences numerous signal transduction pathways, cell cycle progression, and programmed cell death, the detailed mechanisms of how programmed cell death is induced by As2O3 are complex and not entirely delineated [36]. The current study found that As2O3 reduced WSU-CLL cell viability in dose- and time-dependent manners. Our findings support the results of previous studies showing similar effects on various types of human cancer cell lines [17,20,29,33]. Pharmacokinetic analyses of APL patients successfully treated with As2O3 have shown that the peak plasma concentration is 5.5–7.3 μM and that the steady state is believed to be between 1 and 2 μM [37]. In our study, the IC50 value of As2O3 in WSU-CLL cells after 72 h of treatment was 1.96 μM, which is similar to those reported previously [8]. Taken together, the results of our study suggest that As2O3 may be clinically useful in patients with malignant chronic lymphoblastic leukemia.
The mechanisms of As2O3-induced cell growth inhibition have been extensively investigated. Apoptosis appears to be the primary phenomenon resulting in significant cell death [2,7,20]. In our experiments using dual staining with Annexin V and PI, we clearly demonstrated that WSU-CLL cells treated with As2O3 underwent apoptosis.
The balance between apoptosis and survival signals plays an important role in the pathogenesis of a variety of cancers [14,18]. Survivin is a unique member of the inhibitor of apoptosis protein (IAP) family. It is expressed in most human tumors, but it is barely detectable in terminally differentiated normal cells/tissues [14]. The differential expression of survivin in cancer versus normal tissues suggests that it is a promising therapeutic target. Disruption of the survivin induction pathway resulted in an increase in apoptosis and the inhibition of tumor growth.
Survivin has been shown to inhibit apoptosis, promote tumor-associated angiogenesis, and serve as a resistance determinant to various anti-cancer therapies [38]. Survivin expression inhibits cell death induced by various apoptotic stimuli in vitro and in vivo [28,38]. In the current study, we demonstrated that As2O3 is a potent down-regulator of survivin expression at both the protein and mRNA levels and a potent inducer of apoptosis in WSU-CLL cells. Thus, As2O3 may serve as a novel chemotherapeutic method for the treatment of CLL.
Overall, previous studies have reported two major findings. First and foremost is that a decrease in the survivin level plays an important role in CLL. Second, these studies clearly suggest that survivin is an important molecular factor in CLL cell survival as well as in resistance to apoptosis and that a decrease in survivin expression leads to the apoptosis of CLL cells. Several studies have concluded that there is a strong association between increased survivin expression and the progression of human CLL [14,15].
In our study, we found that the down-regulation of survivin expression by siRNA sensitized cells to As2O3-induced apoptosis. This finding further supports the hypothesis that survivin modulates the sensitivity of WSU-CLL cells to apoptosis. Our result indicated that the effect of this treatment was specific.
In mammalian cells, p53, the “guardian of the genome,” is involved in the maintenance of genomic stability. As a downstream factor that is highly expressed in cancer and regulated by p53 [39], survivin is a dual mediator of resistance to apoptosis and cell cycle progression [40]. Thus, regulation of the p53-survivin signaling pathway is important for cell survival [29,41]. Because p53 binds to the survivin promoter and suppresses its transcription, we examined the role of p53 in the down-regulation of survivin by As2O3. We found that p53 is activated in WSU-CLL cells by As2O3. p53 inhibition by transfection with p53 siRNA prevented the down-regulation of survivin by As2O3. Further studies are needed to determine whether additional survivin-independent pathways participate in the induction of apoptosis by As2O3.
In conclusion, this study demonstrated that As2O3 induced apoptosis in the WSU-CLL cell line, which was associated with the up-regulation of p53 and down-regulation of survivin. Our data indicate that As2O3 plays an important role in apoptosis induction in WSU-CLL cells. These results demonstrate that As2O3 may be a novel therapeutic strategy for treating CLL. Future studies are needed to verify these results in primary patient samples and identify patients who are suitable for this therapeutic strategy.
Acknowledgments
Role of the funding source
This work was supported by National Natural Science Foundation of China (No. 81270643), The National Key Technology Support Program (No. 2012BAI38B03), National Natural Science Foundation of China (No. 81070449), and Natural Science Foundation of Beijing (No. 7112139).
We thank Bo Huang from Department of Immunology, Institute of Basic Medical Sciences, Chinese Academy of Medical Science, China, for excellent technical support in the study
The authors thank Huang Xiao-Jun for his technical assistance and for critically reading the manuscript.
Footnotes
Conflict of interest
All authors declare no conflict of interest.
Contributions. Xiao-Hui Zhang and Ru Feng share first authorship. X.L. Zheng and X.H. Zhang designed the study, reviewed the data analysis, reviewed the manuscript; R. Feng helped with the design of the study and writing the manuscript; X.H. Zhang and R. Feng performed the laboratory testing and analysis of the experimental work and final submission of the manuscript. M.L., Q.J., H.H.Z., and Y.Z.Q. contributed to the acquisition of data of F.C.S. and P.C.R. and performed the statistical analysis. J.L.B. and X.L.Z. co-ordinated the research.
References
- 1.Caligaris-Cappio F, Hamblin TJ. B-cell chronic lymphocytic leukemia: a bird of a different feather. J Clin Oncol. 1999;17:399–408. doi: 10.1200/JCO.1999.17.1.399. [DOI] [PubMed] [Google Scholar]
- 2.Li HM, Long Y, Qing C, Yu M, Li ZH, Zhang XM, et al. Arsenic trioxide induces apoptosis of Burkitt lymphoma cell lines through multiple apoptotic pathways and triggers antiangiogenesis. Oncol Res. 2011;19:149–63. doi: 10.3727/096504011x12935427587885. [DOI] [PubMed] [Google Scholar]
- 3.Jutooru I, Chadalapaka G, Sreevalsan S, Lei P, Barhoumi R, Burghardt R, et al. Arsenic trioxide downregulates specificity protein (Sp) transcription factors and inhibits bladder cancer cell and tumor growth. Exp Cell Res. 2010;316:2174–88. doi: 10.1016/j.yexcr.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens JJ, Graham-Evans B, Walker AM, Armstead B, Tchounwou PB. Cytotoxic effect of arsenic trioxide in adenocarcinoma colorectal cancer (HT-29) cells. Met Ions Biol Med. 2008;10:458–62. [PMC free article] [PubMed] [Google Scholar]
- 5.Pettersson HM, Pietras A, Munksgaard Persson M, Karlsson J, Johansson L, Shoshan MC, et al. Arsenic trioxide is highly cytotoxic to small cell lung carcinoma cells. Mol Cancer Ther. 2009;8:160–70. doi: 10.1158/1535-7163.MCT-08-0595. [DOI] [PubMed] [Google Scholar]
- 6.Vey N, Bosly A, Guerci A, Feremans W, Dombret H, Dreyfus F, et al. Arsenic trioxide in patients with myelodysplastic syndromes: a phase II multicenter study. J Clin Oncol. 2006;24:2465–71. doi: 10.1200/JCO.2005.03.9503. [DOI] [PubMed] [Google Scholar]
- 7.Merkel O, Heyder C, Asslaber D, Hamacher F, Tinhofer I, Holler C, et al. Arsenic trioxide induces apoptosis preferentially in B-CLL cells of patients with unfavourable prognostic factors including del17p13. J Mol Med (Berl) 2008;86:541–52. doi: 10.1007/s00109-008-0314-6. [DOI] [PubMed] [Google Scholar]
- 8.Bairey O, Vanichkin A, Shpilberg O. Arsenic-trioxide-induced apoptosis of chronic lymphocytic leukemia cells. Int J Lab Hematol. 2010;32:e77–85. doi: 10.1111/j.1751-553X.2008.01134.x. [DOI] [PubMed] [Google Scholar]
- 9.Redondo-Munoz J, Escobar-Diaz E, Hernandez Del Cerro M, Pandiella A, Terol MJ, Garcia-Marco JA, et al. Induction of B-chronic lymphocytic leukemia cell apoptosis by arsenic trioxide involves suppression of the phosphoinositide 3-kinase/Akt survival pathway via c-jun-NH2 terminal kinase activation and PTEN upregulation. Clin Cancer Res. 2010;16:4382–91. doi: 10.1158/1078-0432.CCR-10-0072. [DOI] [PubMed] [Google Scholar]
- 10.Goussetis DJ, Platanias LC. Arsenic trioxide and the phosphoinositide 3-kinase/akt pathway in chronic lymphocytic leukemia. Clin Cancer Res. 2010;16:4311–2. doi: 10.1158/1078-0432.CCR-10-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biswas S, Zhao X, Mone AP, Mo X, Vargo M, Jarjoura D, et al. Arsenic trioxide and ascorbic acid demonstrate promising activity against primary human CLL cells in vitro. Leuk Res. 2010;34:925–31. doi: 10.1016/j.leukres.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sisson TH, Maher TM, Ajayi IO, King JE, Higgins PD, Booth AJ, et al. Increased survivin expression contributes to apoptosis-resistance in IPF fibroblasts. Adv Biosci Biotechnol. 2012;3:657–64. doi: 10.4236/abb.2012.326085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng R, Zhou S, Liu Y, Song D, Luan Z, Dai X, et al. Sox2 protects neural stem cells from apoptosis via up-regulating survivin expression. Biochem J. 2013;450(3):459–68. doi: 10.1042/BJ20120924. [DOI] [PubMed] [Google Scholar]
- 14.Smolewski P, Robak T. Inhibitors of apoptosis proteins (IAPs) as potential molecular targets for therapy of hematological malignancies. Curr Mol Med. 2011;11:633–49. doi: 10.2174/156652411797536723. [DOI] [PubMed] [Google Scholar]
- 15.Grzybowska-Izydorczyk O, Cebula B, Robak T, Smolewski P. Expression and prognostic significance of the inhibitor of apoptosis protein (IAP) family and its antagonists in chronic lymphocytic leukaemia. Eur J Cancer. 2010;46:800–10. doi: 10.1016/j.ejca.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Ryan BM, O’Donovan N, Duffy MJ. Survivin: a new target for anti-cancer therapy. Cancer Treat Rev. 2009;35:553–62. doi: 10.1016/j.ctrv.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Ghaffari SH, Momeny M, Bashash D, Mirzaei R, Ghavamzadeh A, Alimoghaddam K. Cytotoxic effect of arsenic trioxide on acute promyelocytic leukemia cells through suppression of NFkbeta-dependent induction of hTERT due to down-regulation of Pin1 transcription. Hematology. 2012;17:198–206. doi: 10.1179/1607845412Y.0000000008. [DOI] [PubMed] [Google Scholar]
- 18.Chiu HW, Ho YS, Wang YJ. Arsenic trioxide induces autophagy and apoptosis in human glioma cells in vitro and in vivo through downregulation of survivin. J Mol Med (Berl) 2011;89:927–41. doi: 10.1007/s00109-011-0763-1. [DOI] [PubMed] [Google Scholar]
- 19.Kircelli F, Akay C, Gazitt Y. Arsenic trioxide induces p53-dependent apoptotic signals in myeloma cells with SiRNA-silenced p53: MAP kinase pathway is preferentially activated in cells expressing inactivated p53. Int J Oncol. 2007;30:993–1001. [PubMed] [Google Scholar]
- 20.Keim A, Rossler OG, Rothhaar TL, Thiel G. Arsenite-induced apoptosis of human neuroblastoma cells requires p53 but occurs independently of c-Jun. Neuroscience. 2012;206:25–38. doi: 10.1016/j.neuroscience.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Ho SY, Chen WC, Chiu HW, Lai CS, Guo HR, Wang YJ. Combination treatment with arsenic trioxide and irradiation enhances apoptotic effects in U937 cells through increased mitotic arrest and ROS generation. Chem Biol Interact. 2009;179:304–13. doi: 10.1016/j.cbi.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Zhao S, Zhang J, Zhang X, Dong X, Sun X. Arsenic trioxide induces different gene expression profiles of genes related to growth and apoptosis in glioma cells dependent on the p53 status. Mol Biol Rep. 2008;35:421–9. doi: 10.1007/s11033-007-9102-6. [DOI] [PubMed] [Google Scholar]
- 23.Mathieu J, Besancon F. Clinically tolerable concentrations of arsenic trioxide induce p53-independent cell death and repress NF-kappa B activation in Ewing sarcoma cells. Int J Cancer. 2006;119:1723–7. doi: 10.1002/ijc.21970. [DOI] [PubMed] [Google Scholar]
- 24.Ling X, Cao S, Cheng Q, Keefe JT, Rustum YM, Li F. A novel small molecule FL118 that selectively inhibits survivin, Mcl-1, XIAP and cIAP2 in a p53-independent manner, shows superior antitumor activity. PLoS ONE. 2012;7:e45571. doi: 10.1371/journal.pone.0045571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo L, Tang M, Yang L, Xiao L, Bode AM, Li L, et al. Epstein-Barr virus oncoprotein LMP1 mediates survivin upregulation by p53 contributing to G1/S cell cycle progression in nasopharyngeal carcinoma. Int J Mol Med. 2012;29:574–80. doi: 10.3892/ijmm.2012.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyner JW, Jemal AM, Thayer M, Druker BJ, Chang BH. Targeting survivin and p53 in pediatric acute lymphoblastic leukemia. Leukemia. 2012;26:623–32. doi: 10.1038/leu.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bongiovanni L, Mazzocchetti F, Malatesta D, Romanucci M, Ciccarelli A, Buracco P, et al. Immunohistochemical investigation of cell cycle and apoptosis regulators (survivin, beta-catenin, p53, caspase 3) in canine appendicular osteosarcoma. BMC Vet Res. 2012;8:78. doi: 10.1186/1746-6148-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao Y, Liu Y, Shao C, Hu J, Li X, Li F, et al. Enhanced tumor suppression in vitro and in vivo by co-expression of survivin-specific siRNA and wild-type p53 protein. Cancer Gene Ther. 2010;17:844–54. doi: 10.1038/cgt.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nabilsi NH, Broaddus RR, Loose DS. DNA methylation inhibits p53-mediated survivin repression. Oncogene. 2009;28:2046–50. doi: 10.1038/onc.2009.62. [DOI] [PubMed] [Google Scholar]
- 30.Wall NR, Beck FW, Al-Katib AM, Mohammad RM. Treatment-induced expression of anti-apoptotic proteins in WSU-CLL, a human chronic lymphocytic leukemia cell line. J Drug Target. 2001;9:329–39. doi: 10.3109/10611860108998769. [DOI] [PubMed] [Google Scholar]
- 31.Moon EY, Lerner A. Benzylamide sulindac analogues induce changes in cell shape, loss of microtubules and G(2)-M arrest in a chronic lymphocytic leukemia (CLL) cell line and apoptosis in primary CLL cells. Cancer Res. 2002;62:5711–9. [PubMed] [Google Scholar]
- 32.Mohammad RM, Mohamed AN, Hamdan MY, Vo T, Chen B, Katato K, et al. Establishment of a human B-CLL xenograft model: utility as a preclinical therapeutic model. Leukemia. 1996;10:130–7. [PubMed] [Google Scholar]
- 33.Jin HO, Yoon SI, Seo SK, Lee HC, Woo SH, Yoo DH, et al. Synergistic induction of apoptosis by sulindac and arsenic trioxide in human lung cancer A549 cells via reactive oxygen species-dependent down-regulation of survivin. Biochem Pharmacol. 2006;72:1228–36. doi: 10.1016/j.bcp.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Jiang R, Zhao Y, Xu Y, Ling M, Pang Y, et al. Opposed arsenite-mediated regulation of p53-survivin is involved in neoplastic transformation, DNA damage, or apoptosis in human keratinocytes. Toxicology. 2012;300:121–31. doi: 10.1016/j.tox.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Stevens JJ, Graham B, Walker AM, Tchounwou PB, Rogers C. The effects of arsenic trioxide on DNA synthesis and genotoxicity in human colon cancer cells. Int J Environ Res Public Health. 2010;7:2018–32. doi: 10.3390/ijerph7052018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker AM, Stevens JJ, Ndebele K, Tchounwou PB. Arsenic trioxide modulates DNA synthesis and apoptosis in lung carcinoma cells. Int J Environ Res Public Health. 2010;7:1996–2007. doi: 10.3390/ijerph7051996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu XH, Shen YL, Jing YK, Cai X, Jia PM, Huang Y, et al. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772–8. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]
- 38.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 39.Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277:3247–57. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- 40.Wang K, Brems JJ, Gamelli RL, Holterman AX. Survivin signaling is regulated through nuclear factor-kappa B pathway during glycochenodeoxycholate-induced hepatocyte apoptosis. Biochim Biophys Acta. 2010;1803:1368–75. doi: 10.1016/j.bbamcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Raj D, Liu T, Samadashwily G, Li F, Grossman D. Survivin repression by p53, Rb and E2F2 in normal human melanocytes. Carcinogenesis. 2008;29:194–201. doi: 10.1093/carcin/bgm219. [DOI] [PMC free article] [PubMed] [Google Scholar]