Abstract
Patients who have undergone a Fontan procedure face an increased risk for thromboembolic complications. This study aimed to evaluate whether thromboelastography, a global whole-blood assay of coagulation, can be used to detect hypercoagulability in pediatric Fontan patients compared with healthy children. This prospective, cross-sectional study investigated 25 Fontan patients and 51 healthy children in three age groups: 1–5 years, 6–10 years, and 11–16 years. Kaolin-activated thromboelastography was performed on citrated samples. No statistically significant differences in thromboelastography parameters were found among the different age groups of the 51 healthy children. None of the 25 Fontan patients demonstrated evidence of hypercoagulability on thromboelastography (95% confidence interval, 0–7%), as defined by two standard deviations above or below the normal mean. The findings suggest that the percentage of Fontan patients demonstrating hypercoagulability on thromboelastography is substantially lower than the reported incidence of thromboembolic complications. Whether thromboelastography could be helpful in predicting patients at increased risk for thromboembolic complications or not still is not known. Further studies comparing the thromboelastography of Fontan patients with the thromboembolic complications of those without Fontan are needed to delineate these issues.
Keywords: CHD, Fontan, Hematology, Thrombosis
Thromboembolic complications are a major cause of early and late mortality in children with single-ventricle congenital heart defects who have undergone a Fontan procedure [6, 12, 14, 21, 26]. The reported incidence of thromboembolic complications in these patients varies from 3% to 25% depending on the study design (cross-sectional, retrospective cohort, prospective cohort), the imaging technique (transthoracic vs. transesophageal echocardiogram), and the duration of the follow-up period [6, 12, 14, 21, 23, 26]. Studies with longer follow-up periods and more sensitive imaging studies suggest an incidence of at least 20%, with a mortality rate of 25% [6, 12, 14, 21, 23, 26]. Although the etiology for this high risk is not well defined, possible explanations include venous stasis, coagulation abnormalities, dehydration, protein-losing enteropathy, and arrhythmias.
In an attempt to understand the increased risk for thrombosis in the Fontan population, multiple studies of coagulation have been conducted. These studies have focused primarily on measuring the plasma concentrations of isolated coagulation factors. Altered plasma concentrations of procoagulants (factors II, V, VII, VIII, and X and fibrinogen), anticoagulants (protein C, protein S, and antithrombin), and fibrinolytic protein (plasminogen) have been described [3, 4, 6, 7, 10, 15, 16, 19]. In addition to these quantitative differences, increased ex vivo platelet reactivity has been reported [20]. However, the overall effect of these abnormalities on the balance between hemostasis and thrombosis is not well characterized.
Given the complexity of the reported hemostatic derangements observed in Fontan patients, we sought to perform a more comprehensive evaluation of coagulation using thromboelastography (TEG), which uses whole blood to evaluate global hemostatic and fibrinolytic function. The overall function of circulating plasma clotting factors and their inhibitors and platelet function can be determined, as well as the ultimate clot strength and efficacy of fibrinolysis [22].
Studies have proved TEG useful for monitoring hemostatic and fibrinolytic derangements in patients undergoing cardiac surgery or liver transplantation [11, 24]. Modified versions of TEG have been used to evaluate preoperative coagulation in neonates and infants with complex congenital heart disease. They have shown that these infants demonstrate decreased clot formation compared with healthy control subjects [9, 18]. In addition, TEG also has been used to evaluate hypercoagulability, and prospective studies have shown that TEG parameters in adult surgical patients can predict whether patients are at increased risk of deep vein thrombosis or not [13, 25].
We hypothesized that if the coagulation abnormalities in Fontan patients are responsible for the high rate of thrombotic events, then at least a subset of these patients would demonstrate hypercoagulability on TEG. Because there are no published data on TEG performed with kaolin-activated citrated samples in a pediatric population, we also established normal ranges in a cohort of healthy children.
Materials and Methods
Approval to conduct this study was obtained from the Institutional Review Board of the Children’s Hospital of Philadelphia. Written informed consent was obtained from the parent or legal guardian for all the subjects, and patients older than 7 years were asked to assent.
Study Design
In this prospective cross-sectional study, patients who had undergone a Fontan procedure were compared with healthy children.
Healthy Children
Blood samples were collected from 51 healthy children in three age groups (1–5 years, 6–10 years, and 11–16 years) undergoing minor elective surgical procedures. These age categories were chosen because they have been used in several other clinical studies of coagulation proteins in children [2, 5]. The inclusion criteria specified an American Society of Anesthesia Physical Status of P1 (normal healthy patient). Patients were excluded if they had a known underlying medical illness or a clinical history of a known bleeding disorder. Whole blood was collected into 3.2% sodium citrate glass tubes at the time of intravenous (IV) catheter placement.
Fontan Patients
Blood samples were collected from children ages 1 to 16 who had undergone a Fontan procedure. Patients were excluded if they had received an anticoagulant (vitamin K antagonist or heparin) within the preceding 2 weeks or had received a blood or plasma product within the preceding month. Patients receiving aspirin were not excluded because the antiplatelet effect of aspirin does not affect TEG [17].
The majority of patients were recruited when undergoing anesthesia for elective operative procedures. For patients undergoing elective surgery, whole blood was collected at the time of IV catheter placement into 3.2% sodium citrate tubes. Blood was collected via peripheral venipuncture from patients who were not undergoing surgical procedures. Additional data collection included age, sex, ethnicity, surgical history, current medications, and whether the patient had experienced a known thrombotic event or not.
Thromboelastography
Kaolin-activated TEG was performed according to the manufacturer’s recommendations using Thromboelastograph Coagulation Analyzer Model 5000 (Haemoscope Corp., Skokie, IL, USA). Samples were processed in duplicate 60–120 min after collection. The kaolin vials were warmed to room temperature, and 1 ml of the citrated blood sample was pipetted into the vial. Blood and kaolin were mixed by inverting the vial five times, after which 360 μl of this treated blood was pipetted into the preheated cup containing 20 μl of 0.2 mol/l CaCl as an activator.
Figure 1 shows a typical TEG tracing, in which assay time is represented on the x-axis, and amplitude on the y-axis. This curve shows the typical initial flat segment that then splays into two curves. The reaction time (R) is the time from the start of the run until the first detectable levels of fibrin clot formation when the sample begins to splay. The time from the end of R until the tracing reaches 20 mm is a measure for the speed of clot strengthening, the K time (K). The angle (a) is the size, in degrees, of the angle formed by the line tangential to the tracing measured at R and measures the dynamics of clot strengthening. The maximum amplitude (MA) is the width of the widest gap in the tracing, which reflects the maximum strength of the fibrin clot. It is a function of the maximum dynamic properties of fibrin and platelet bonding via GP2b/3a. Lysis 30 (Ly 30) measures the rate of amplitude reduction 30 min after MA and reflects clot stability. Indicators of hypercoagulability from TEG include a shortened R time and an increased a and MA [8].
Fig. 1.
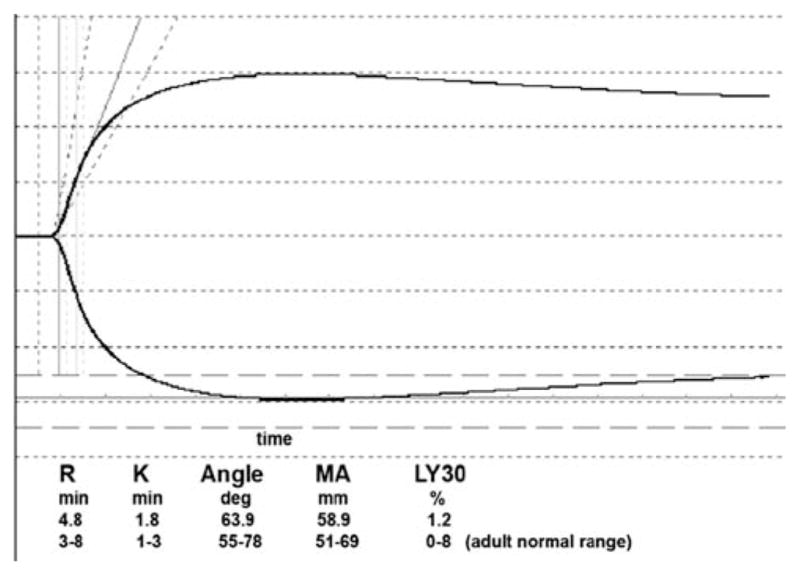
Representative thromboelastography (TEG) tracing. This study investigated a healthy 5-year-old child using the TEG 5000 (Haemoscope Corp., Skokie, IL, USA)
The R, K, a, and MA variables also can be combined to calculate the coagulation index (CI), an overall assessment of coagulation,. The CI is defined by the equation CI = −0.6516R – 0.3772K + 0.1223MA + 0.0759α – 7.7922 (TEG 5000 Manual; Haemoscope Corp. IL, USA). A prolonged CI is indicative of hypercoagulability.
Statistical Analysis
In this study, a positive test for a hypercoagulable state was defined as any measurement (described earlier) exceeding the upper bound of the 95% confidence interval for the normal value. A sample size of at least 15 children in each of the three age groups was chosen to establish the age-related normal values. This was based on the manufacturer’s published adult normal ranges (mean and standard error of the mean) of the kaolin-activated TEG MA. Assuming a normal distribution, there is little variation in the 95% confidence interval around the standard error of the mean after 14 patients. A sample size of 25 Fontan subjects would have a power of 0.9 to detect a 7% incidence of hypercoagulability at a significance level of 0.05.
The data were analyzed using STATA, Release 8 (Stata Corp., College Station, TX, USA). The differences among age groups were evaluated using one-way analysis of variance. The Student’s t test was used to compare continuous variables, and the chi-square test was used to compare dichotomous variables. Significance was defined as a P value less than 0.5.
Results
Healthy Children
Samples were collected from a total of 51 healthy children undergoing minor elective surgery. The blood sample for one patient clotted, preventing analysis for that patient. For nine patients, one of the duplicate TEG tracings was excluded due to a technical error in obtaining a result or a poor sample (i.e., early splitting of the TEG tracing, instrument error, clotted sample). For patients with two TEG tracings, the results were averaged.
Table 1 shows the TEG results for the 50 healthy children according to age group. There was no statistical difference between different coagulation parameters across the age groups.
Table 1.
Kaolin-activated citrated thromboelastography (TEG) parameters in healthy children by age groupa
| 1–6 years (n = 19) | 6–10 years (n = 16) | 10–16 years (n = 15) | P value | |
|---|---|---|---|---|
| R (min) | 6.4 (2.4–10.4) | 5.7 (1.9–9.5) | 6.9 (2.9–10.9) | 0.96 |
| K (min) | 2.0 (1.0–3.0) | 1.8 (1.0–2.6) | 2.1 (1.1–3.1) | 0.82 |
| a (°) | 61.2 (47.6–74.8) | 65.4 (54.8–76.0) | 61.2 (46.8–75.6) | 0.49 |
| MA (mm) | 62.5 (54.3–70.7) | 64.3 (57.5–71.1) | 62.7 (53.1–72.3) | 0.37 |
| Ly30 (%) | 0.6 (0–2.0) | 0.5 (0–1.7) | 0.5 (0–1.5) | 0.33 |
| CI | −0.5 (−4.5–3.5) | 0.6 (−3.6–4.6) | −0.8 (−5.4–3.8) | 0.82 |
R reaction time, K coagulation time, a angle, MA maximum amplitude, Ly30 lysis at 30 min, CI coagulation index
Results are expressed as the mean, with a boundary encompassing the 95% confidence interval in parenthesis
Post-Fontan Subjects
Blood samples were collected from 25 children who had undergone a Fontan procedure and met the eligibility criteria. Of these 25 patients, 18 had hypoplastic left heart syndrome (HLHS), three had a double-outlet right ventricle, and four had tricupsid atresia. These patients had undergone the Fontan procedure a mean of 6.4 years (range, 1.5 months to 13 years) before specimen collection. Most of the patients (20/25) were receiving daily aspirin therapy.
Duplicate TEG tracings were completed for 24 patients. For one patient, one tracing was reliable. Table 2 shows the TEG results for the Fontan patients by age category as well as the pooled results. As with the healthy children, no statistical difference in any TEG parameter was found among these groups. The results for one patient, a 15-year-old with HLHS undergoing cardiac catheterization, are not included in this analysis. The TEG results for this patient demonstrated extremely poor coagulation (R = 21.5, K = 6.6, a = 28, MA = 45), and we could not be absolutely sure that this sample had not been contaminated with heparin.
Table 2.
Kaolin-activated thromboelastography (TEG) parameters in Fontan patients by age groupa
| 1–6 years (n = 7) | 6–10 years (n = 7) | 10–16 years (n = 10) | P value | |
|---|---|---|---|---|
| R (min) | 7.0 (3.8–10.2) | 6.5 (3.3–9.7) | 5.5 (3.9–7.1) | 0.15 |
| K (min) | 2.1 (1.3–2.9) | 2.1 (1.5–2.7) | 2.0 (1.2–2.8) | 0.53 |
| a (°) | 61.8 (52.6–71.0) | 61.0 (55.0–67.0) | 61.6 (53.2–70.0) | 0.61 |
| MA (mm) | 63.5 (64.9–75.9) | 62.4 (57.4–67.4) | 62.0 (51.2–72.8) | 0.11 |
| Ly30 (%) | 0.8 (−2.4–4.0) | 1.0 (−1.8–3.4) | 0.7 (−1.7–3.1) | 0.75 |
| CI | −0.6 (−3.6–2.4) | −0.6 (−3.0–1.8) | 0.1 (−2.5–2.7) | 0.91 |
R reaction time, K coagulation time, a angle, MA maximum amplitude, Ly30 lysis at 30 min, CI coagulation index
Results are expressed as the mean, with a boundary encompassing the 95% confidence interval in parenthesis
Only one patient in this cohort had a known history of a thrombotic event. This 7-year-old boy with HLHS had experienced right upper extremity weakness 1 week after Fontan and showed a new left thalamic infarct on magnetic resonance imaging (MRI). There was no evidence of hypercoagulability during the boy’s TEG, which was performed 5 years after his stroke. No Fontan patient had an abnormal R, a, MA, or CI that suggested hypercoagulability.
Because there was no difference between age categories in either the Fontan cohort or the healthy control group, the results from all the age categories were combined, and the two groups are compared in Table 3. As shown in Table 3, the demographics (age, sex, and ethnicity) of the two groups were similar. There was no difference among TEG parameters between the Fontan patients and the healthy children.
Table 3.
Comparison between healthy children and Fontan patients
| Healthy children (n = 50) | Fontan patients (n = 24) | P value | |
|---|---|---|---|
| Age (years) | 8.3 ± 4.7 | 9.5 ± 4.5 | 0.15 |
| Male sex: n (%) | 30 (60) | 17 (71) | 0.36 |
| Ethnicity: n (%) | |||
| Caucasian | 31 (62) | 17 (71) | 0.22 |
| African-American | 18 (36) | 5 (21) | |
| Other | 1 (2) | 2 (8) | |
| R (min)a | 6.3 (2.3–10.3) | 6.2 (3.4–9.0) | 0.60 |
| K (min)a | 1.9 (0.9–2.9) | 2.0 (1.2–2.8) | 0.17 |
| a (°)a | 62.6 (49.4–75.8) | 61.5 (53.7–69.3) | 0.76 |
| MA (mm)a | 63.1 (55–71.3) | 62.5 (52.9–72.1) | 0.71 |
| Ly30 (%)a | 0.5 (0–1.7) | 0.8 (0–3.2) | 0.20 |
| CIa | −0.2 (−4.6–4.3) | −0.3 (−2.9–2.3) | 0.56 |
R reaction time, K coagulation time, a angle, MA maximum amplitude, Ly30 lysis at 30 min, CI coagulation index
Results are expressed as the mean, with a boundary encompassing the 95% confidence interval in parenthesis
Discussion
This is the first study to report the results of TEG for a cohort of Fontan patients. Surprisingly, there were no differences in the TEG parameters between the healthy children and the Fontan patients in this cross-sectional study. Given the well-documented differences in the concentrations of multiple clotting factors for the Fontan patients and the high incidence of thromboembolic complications, we hypothesized that at least a subset of our Fontan cohort would demonstrate hypercoagulability by TEG. Not a single Fontan patient was classified as “hypercoagulable” based on the TEG results.
Unfortunately, we did not have adequate plasma samples to perform a comprehensive battery of coagulation protein assays. This would have been useful for correlation with the TEG findings. Nonetheless, given that numerous studies of Fontan patients have confirmed alterations in coagulation protein concentrations, we expect that our cohort was similar. Therefore, although the concentrations of many procoagulant and anticoagulant factors are altered in the Fontan patients, the overall balance between hemostasis and thrombosis may be maintained, as reflected by the normal TEGs. This may suggest that thromboembolic complications in Fontan patients are related to these alterations in circulating coagulation protein concentrations. This is consistent with the findings of retrospective studies that have been unable to demonstrate a clear link between abnormal coagulation studies and thrombosis in Fontan patients. The only study to suggest an association between coagulation abnormalities and thrombosis reported elevated factor VIII levels in 6 of 20 children after Fontan, 2 of whom had experienced a thromboembolic event [16]. Importantly, our study was not designed to correlate TEG results with clinical outcomes. This would need to be performed in a much larger prospective study.
This also is the first study to provide pediatric reference values for TEG performed on kaolin-activated citrated blood. The advantage to using citrated samples is that the blood does not need to be tested immediately after collection, which often is a logistical challenge. Similar to a prior study of kaolin-activated noncitrated samples, we found no differences among the pediatric age groups tested [5]. Although the concentrations of coagulation proteins in children differ significantly from those in adults, these differences are greatest in children younger than 1 year [1]. Because we aimed to study Fontan patients usually at least 2–3 years old, we did not evaluate children younger than 1 year. These pediatric reference values will be useful for interpreting TEG results of patients tested for clinical indications as well as for future research studies.
Admittedly, this was a relatively small cross-sectional study, and our findings may not be generalizable to larger groups of Fontan patients. It also is possible that TEG may fluctuate over time for a given patient. Interestingly, there is no clustering of thromboembolic complications around a specific period after Fontan. They start to occur during the postoperative period, and the risk appears to be persistent throughout a patient’s lifetime, with no plateau [21]. We did not observe any correlation of TEG values with patient age or time since the Fontan procedure.
Whether TEG can be helpful in predicting patients at increased risk for thromboembolic complications or not still is not known. However, our findings suggest that the percentage of Fontan patients who demonstrate hypercoagulability on TEG is substantially lower than the incidence of thromboembolic complications. Alternatively, it is possible that TEG is not a sufficiently sensitive assay to detect hypercoagulability. Further studies, comparing thromboelastography and coagulation protein assays between Fontan patients with thromboembolic complications and those without such complications are needed to delineate these issues and to understand the etiology of the thrombotic complications experienced by these patients.
Acknowledgments
This study was funded by an NIH/NHLBI K12 Mentored Career Development Award in Benign Hematology.
Contributor Information
Leslie Raffini, Division of Hematology, Department of Pediatrics, The Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, 34th Street and Civic Center Boulevard, Philadelphia, PA 19104-4399, USA.
Alexander Schwed, University of Pennsylvania School of Medicine, 34th Street and Civic Center Boulevard, Philadelphia, PA 19104-4399, USA.
X. Long Zheng, Department of Pathology and Laboratory Medicine, The Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, 34th Street and Civic Center Boulevard, Philadelphia, PA 19104-4399, USA.
Maria Tanzer, Department of Pathology and Laboratory Medicine, The Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, 34th Street and Civic Center Boulevard, Philadelphia, PA 19104-4399, USA.
Susan Nicolson, Division of Cardiac Anesthesia, Department of Anesthesia and Critical Care, The Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, 34th Street and Civic Center Boulevard, Philadelphia, PA 19104-4399, USA.
J. William Gaynor, Division of Cardiothoracic Surgery, Department of Surgery, The Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, 34th Street and Civic Center Boulevard, Philadelphia, PA 19104-4399, USA.
David Jobes, Division of Cardiac Anesthesia, Department of Anesthesia and Critical Care, The Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, 34th Street and Civic Center Boulevard, Philadelphia, PA 19104-4399, USA.
References
- 1.Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, et al. Development of the human coagulation system in the full-term infant. Blood. 1987;70:165–172. [PubMed] [Google Scholar]
- 2.Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood. 1992;80:1998–2005. [PubMed] [Google Scholar]
- 3.Barnes C, Monagle P. Haemostatic changes following the modified Fontan procedure. Thromb Haemost. 2001;86:1341. author reply 2. [PubMed] [Google Scholar]
- 4.Chaloupecky V, Svobodova I, Hadacova I, Tomek V, Hucin B, Tlaskal T, et al. Coagulation profile and liver function in 102 patients after total cavopulmonary connection at midterm follow-up. Heart. 2005;91:73–79. doi: 10.1136/hrt.2003.026419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan KL, Summerhayes RG, Ignjatovic V, Horton SB, Monagle PT. Reference values for kaolin-activated thromboelastography in healthy children. Anesth Analg. 2007;105:1610–1613. doi: 10.1213/01.ane.0000287645.26763.be. table of contents. [DOI] [PubMed] [Google Scholar]
- 6.Cromme-Dijkhuis AH, Henkens CM, Bijleveld CM, Hillege HL, Bom VJ, van der Meer J. Coagulation factor abnormalities as possible thrombotic risk factors after Fontan operations. Lancet. 1990;336:1087–1090. doi: 10.1016/0140-6736(90)92568-3. [DOI] [PubMed] [Google Scholar]
- 7.Francis JL, Francis DA, Gunathilagan GJ. Assessment of hypercoagulability in patients with cancer using the Sonoclot Analyzer and thromboelastography. Thromb Res. 1994;74:335–346. doi: 10.1016/0049-3848(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 8.Goobie SM, Soriano SG, Zurakowski D, McGowan FX, Rockoff MA. Hemostatic changes in pediatric neurosurgical patients as evaluated by thromboelastograph. Anesth Analg. 2001;93:887–892. doi: 10.1097/00000539-200110000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Haizinger B, Gombotz H, Rehak P, Geiselseder G, Mair R. Activated thromboelastogram in neonates and infants with complex congenital heart disease in comparison with healthy children. Br J Anaesth. 2006;97:545–552. doi: 10.1093/bja/ael206. [DOI] [PubMed] [Google Scholar]
- 10.Jahangiri M, Shore D, Kakkar V, Lincoln C, Shinebourne E. Coagulation factor abnormalities after the Fontan procedure and its modifications. J Thorac Cardiovasc Surg. 1997;113:989–992. doi: 10.1016/S0022-5223(97)70283-6. discussion 92–93. [DOI] [PubMed] [Google Scholar]
- 11.Kang YG, Martin DJ, Marquez J, Lewis JH, Bontempo FA, Shaw BW, Jr, et al. Intraoperative changes in blood coagulation and thromboelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–896. [PMC free article] [PubMed] [Google Scholar]
- 12.Kaulitz R, Luhmer I, Bergmann F, Rodeck B, Hausdorf G. Sequelae after modified Fontan operation: postoperative haemo-dynamic data and organ function. Heart. 1997;78:154–159. doi: 10.1136/hrt.78.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCrath DJ, Cerboni E, Frumento RJ, Hirsh AL, Bennett-Guerrero E. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg. 2005;100:1576–1583. doi: 10.1213/01.ANE.0000155290.86795.12. [DOI] [PubMed] [Google Scholar]
- 14.Monagle P, Cochrane A, McCrindle B, Benson L, Williams W, Andrew M. Thromboembolic complications after Fontan procedures: the role of prophylactic anticoagulation. J Thorac Cardiovasc Surg. 1998;115:493–498. doi: 10.1016/s0022-5223(98)70310-1. [DOI] [PubMed] [Google Scholar]
- 15.Odegard KC, McGowan FX, Jr, Zurakowski D, DiNardo JA, Castro RA, del Nido PJ, et al. Coagulation factor abnormalities in patients with single-ventricle physiology immediately prior to the Fontan procedure. Ann Thorac Surg. 2002;73:1770–1777. doi: 10.1016/s0003-4975(02)03580-4. [DOI] [PubMed] [Google Scholar]
- 16.Odegard KC, McGowan FX, Jr, Zurakowski D, DiNardo JA, Castro RA, del Nido PJ, et al. Procoagulant and anticoagulant factor abnormalities following the Fontan procedure: increased factor VIII may predispose to thrombosis. J Thorac Cardiovasc Surg. 2003;125:1260–1267. doi: 10.1016/s0022-5223(02)73605-2. [DOI] [PubMed] [Google Scholar]
- 17.Orlikowski CE, Payne AJ, Moodley J, Rocke DA. Thromboelastography after aspirin ingestion in pregnant and nonpregnant subjects. Br J Anaesth. 1992;69:159–161. doi: 10.1093/bja/69.2.159. [DOI] [PubMed] [Google Scholar]
- 18.Osthaus WA, Boethig D, Johanning K, Rahe-Meyer N, Theilmeier G, Breymann T, et al. Whole blood coagulation measured by modified thromboelastography (ROTEM) is impaired in infants with congenital heart diseases. Blood Coagul Fibrinolysis. 2008;19:220–225. doi: 10.1097/MBC.0b013e3282f54532. [DOI] [PubMed] [Google Scholar]
- 19.Rauch R, Ries M, Hofbeck M, Buheitel G, Singer H, Klinge J. Hemostatic changes following the modified Fontan operation (total cavopulmonary connection) Thromb Haemost. 2000;83:678–682. [PubMed] [Google Scholar]
- 20.Ravn HB, Hjortdal VE, Stenbog EV, Emmertsen K, Kromann O, Pedersen J, et al. Increased platelet reactivity and significant changes in coagulation markers after cavopulmonary connection. Heart. 2001;85:61–65. doi: 10.1136/heart.85.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenthal DN, Friedman AH, Kleinman CS, Kopf GS, Rosenfeld LE, Hellenbrand WE. Thromboembolic complications after Fontan operations. Circulation. 1995;92(9):287–293. doi: 10.1161/01.cir.92.9.287. [DOI] [PubMed] [Google Scholar]
- 22.Salooja N, Perry DJ. Thromboelastography. Blood Coagul Fibrinolysis. 2001;12:327–337. doi: 10.1097/00001721-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Shirai LK, Rosenthal DN, Reitz BA, Robbins RC, Dubin AM. Arrhythmias and thromboembolic complications after the extracardiac Fontan operation. J Thorac Cardiovasc Surg. 1998;115:499–505. doi: 10.1016/S0022-5223(98)70311-3. [DOI] [PubMed] [Google Scholar]
- 24.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312–319. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Traverso Cl AJ, Gomez E, Luna D, Lopez-Cantarero M, Garcia JM. Prospective assessment of the risk of deep vein thrombosis in elective abdominal surgery: predictive role of thromboelastography. Thromb Haemorrh Disord. 1993;71:15. [Google Scholar]
- 26.van den Bosch AE, Roos-Hesselink JW, Van Domburg R, Bogers AJ, Simoons ML, Meijboom FJ. Long-term outcome and quality of life in adult patients after the Fontan operation. Am J Cardiol. 2004;93:1141–1145. doi: 10.1016/j.amjcard.2004.01.041. [DOI] [PubMed] [Google Scholar]


