Abstract
The L-type Ca2+ channel Cav1.2 controls multiple functions throughout the body including heart rate and neuronal excitability. It is a key mediator of fight-or-flight stress responses triggered by a signaling pathway involving β-adrenergic receptors (βARs), cyclic adenosine monophosphate (cAMP), and protein kinase A (PKA). PKA readily phosphorylates Ser1928 in Cav1.2 in vitro and in vivo, including in rodents and humans. However, S1928A knock-in (KI) mice have normal PKA-mediated L-type channel regulation in the heart, indicating that Ser1928 is not required for regulation of cardiac Cav1.2 by PKA in this tissue. We report that augmentation of L-type currents by PKA in neurons was absent in S1928A KI mice. Furthermore, S1928A KI mice failed to induce long-term potentiation in response to prolonged theta-tetanus (PTT-LTP), a form of synaptic plasticity that requires Cav1.2 and enhancement of its activity by the β2-adrenergic receptor (β2AR)–cAMP–PKA cascade. Thus, there is an unexpected dichotomy in the control of Cav1.2 by PKA in cardiomyocytes and hippocampal neurons.
INTRODUCTION
Cav1.2 is the most abundant L-type Ca2+ channel in mammalian brain, heart, and vascular smooth muscle (1–4) regulating neuronal excitability, cardiac contractility, and vascular tone. Mutations in Cav1.2 affect many organs, as illustrated by the cardiac arrhythmias, autistic-like behavior, and developmental abnormalities in Timothy syndrome patients, who have gain-of-function mutations in Cav1.2 (5).
In the brain, L-type Ca2+ currents control neuronal excitability (6, 7), gene expression (8–11), and long-term potentiation (LTP) of synaptic strength. LTP refers to the persistent increase in synaptic strength that a synapse undergoes when it experiences a period of high-frequency activation (12–14). LTP is a key cellular correlate of learning and memory. In particular, L-type Ca2+ currents conducted by the voltage-gated calcium channel Cav1.2 are important for LTP induced by the widely used 1-s-long, 200-Hz tetanus (15, 16) or by a 90- to 180-s-long, 5- to 10-Hz tetanus mimicking the endogenous ~7-Hz theta rhythm, which we call prolonged theta-tetanus LTP (PTT-LTP) (17). Ca2+ influx through Cav1.2 is also important for long-term depression (LTD) induced by metabotropic glutamate receptor (mGluR) signaling (18). LTD is a persistent decrease in synaptic strength induced by a prolonged stimulation of a synapse with a frequency in the range of 1 Hz (18).
Norepinephrine is important for wakefulness, behavioral acuity, and various forms of learning, especially in novel or emotionally charged situations (19–23). Norepinephrine activates adenylyl cyclase (AC)–cyclic adenosine monophosphate (cAMP)–protein kinase A (PKA) signaling by the β1- and β2-adrenergic receptors (β1AR and β2AR) (24). This signaling cascade stimulates Ca2+ influx through Cav1.2 into neurons (25–28) and cardiomyocytes (29, 30).
Vertebrate Cav1.2 is composed of the pore-forming α11.2 subunit, a so-called α2-δ subunit, and a β subunit (2, 31). α11.2 consists of a cytosolic N terminus, the four homologous domains I to IV, each with six transmembrane segments, and the long C terminus of ~660 residues. Injection of active PKA into cells exogenously expressing α11.2 without the other subunits increases channel activity (32), suggesting that PKA acts directly on α11.2. In purified preparations of Cav1.2 channels, Ser1928 in the distal C-terminal domain is the most readily detectable site of PKA phosphorylation (33–36). βAR stimulation with isoprote-renol (ISO) results in a pronounced increase in Ser1928 phosphorylation in isolated cardiomyocytes (37) and in the brain in vivo (38) [see also (39, 40)]. However, functional studies in transfected nonmuscle cells indicate that phosphorylation of Ser1700 in the proximal C-terminal domain increases Cav1.2 channel activity, whereas phosphorylation of Ser1928 in the distal C-terminal domain does not (41). Moreover, studies of Cav1.2 regulation in virally transfected cardiac myocytes and in ventricular myocytes dissociated from knock-in (KI) mice in which Ser1928 has been mutated to alanine (S1928A KI mice) show that βAR stimulation of L-type currents is normal when Ser1928 is mutated to alanine (42, 43).
In the brain and heart, the β2AR forms a signaling complex with Cav1.2 that also contains AC, the heterotrimeric guanine nucleotide–binding protein (G protein) Gs, an A-kinase anchoring protein (AKAP), and PKA (10, 25, 28, 29, 35, 38, 41, 44–47). This complex allows highly localized and thereby effective and specific signaling from the β2AR to Cav1.2 in neurons (25) and cardiomyocytes (29, 48) [for a review, see (24)]. The β2AR binds to α11.2 residues 1923 to 1942, and this interaction is strictly required for receptor-mediated regulation of Cav1.2 (17). Furthermore, stimulating the β2AR leads to its temporary displacement from Cav1.2, creating a refractory period of ~5 min, during which neither phosphorylation nor channel activity of Cav1.2 can be augmented again by restimulation of the β2AR (17). When we tested whether such a refractory period for sequential β2AR stimulation would be observed in S1928A KI mice, we discovered that βAR stimulation did not augment neuronal L-type currents in these mice. In further contrast to cardiomyocytes, we found that, in neurons, L-type currents were exclusively augmented by β2AR but not by β1AR stimulation. Finally, we demonstrated that this Ser1928-dependent regulation of L-type Ca2+ currents was a key requirement for the induction of stable PTT-LTP by theta rhythm stimulation in the presence of a βAR agonist. Collectively, our results demonstrated a bifurcation of PKA signaling to Cav1.2 channels and define a new phosphorylation site that is required for LTP. Furthermore, they revealed that phosphorylation of a single site, Ser1928, not only uncouples Cav1.2 regulation from β2AR signaling upon repeated stimulation but also mediates the channel-stimulating response to β2AR signaling in hippocampal neurons.
RESULTS
Increase in L-type Ca2+ currents upon βAR stimulation depends on Ser1928 in neurons
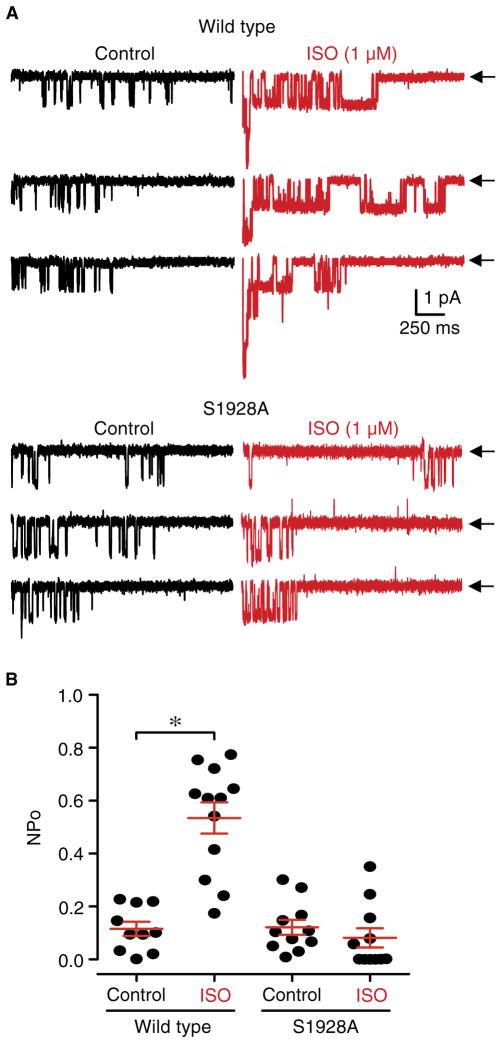
In neurons, phosphorylation of Ser1928 upon βAR stimulation displaces the β2AR from Cav1.2 for ~5 min. A second application of the βAR agonist ISO only increases L-type currents in neurons if added more than 5 min after the first application has ended (17). In neuronal cultures from the hippocampal CA1, CA2, and CA3 regions, augmentation of L-type currents by ISO is much more apparent in cell-attached recordings, which typically yield increases of >200% (17, 25, 27), than in whole-cell recordings, which only show increases of 10 to 20% (27, 28). The weak ISO effect in whole-cell recordings from neurons is much smaller than in whole-cell recordings from cardiomyocytes (43). To functionally test whether Ser1928 phosphorylation mediates this refractory period of L-type current augmentation, we recorded single-channel L-type currents from hippocampal neurons cultured from wild-type and S1928A KI mice by cell-attached patch-clamping (Fig. 1A), using conditions as previously described (17, 25).
Fig. 1. βAR augmentation of L-type currents in neurons requires Ser1928.
(A) Representative single-channel recordings from hippocampal neurons from wild-type and litter-matched S1928A KI mice at 7 to 14 days in vitro upon depolarization from −80 to 0 mV without (left, black traces) and with 1 μM ISO (right, red traces) in the patch pipette. Arrows (←) indicate the zero-current level (that is, the closed channel). (B) Summary plot of NPo recorded from neurons from wild-type and S1928A KI mice with vehicle (H2O) or 1 μM ISO added to the patch pipette solution. For statistical analysis, NPo was determined for each recording and pooled under each condition for comparison (*P < 0.05, unpaired t test for experiments in wild-type cells and Mann-Whitney test for experiments in S1928A cells). Mean NPo values are 0.12 ± 0.03 for wild-type control (n = 10), 0.53 ± 0.06 for wild-type ISO (n = 12), 0.12 ± 0.03 for S1928A control (n = 11), and 0.08 ± 0.04 for S1928A ISO (n = 11) (n, number of cell-attached patch recordings pooled across three to five independent culture experiments).
We determined open probability (Po) for all L-type channels within each patch (NPo) during depolarizing pulses from −80 to 0 mV with ω-conotoxins GVIA and MVIIC in the patch pipette to blocked non–L-type Ca2+ currents (Fig. 1B) (17). In neurons from wild-type mice, the addition of ISO to the patch pipette significantly increased NPo (Fig. 1, A and B). This increase was also evident in neurons prepared from only the hippocampal CA1 region, avoiding the CA2 and CA3 regions, from wild-type mice (fig. S1). In neurons from S1928A KI mice, however, the NPo value under control conditions was not significantly changed by ISO application (Fig. 1). Given that cardiac L-type currents measured in S1928A KI mice were significantly increased by βAR signaling (43), this result in neurons was unexpected. Thus, we confirmed under our experimental conditions that there was no difference in basal L-type currents between cardiomyocytes from wild-type and S1928A KI mice (fig. S2, A and B), and potentiation by ISO was equally strong for both genotypes (fig. S2, C to G).
Signaling by the β2AR but not the β1AR augments L-type currents in neurons
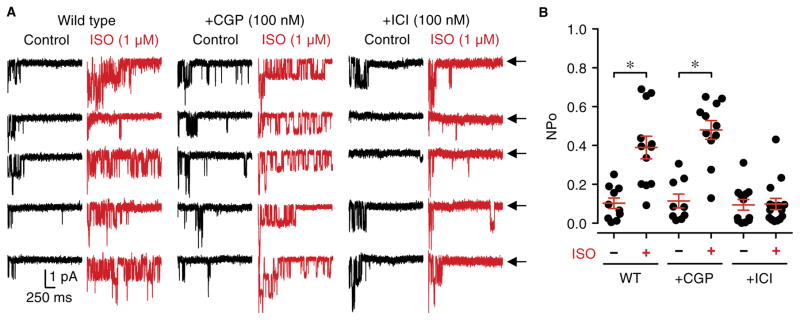
In cardiomyocytes, L-type currents are mainly enhanced by β1ARs with a much smaller contribution by β2ARs (29, 37, 48–52). To test the role of these two receptors in regulating neuronal Cav1.2, we applied ISO (a nonselective βAR agonist) during cell-attached recordings in wild-type rat hippocampal neurons in the absence or presence of CGP20712 (a β1AR-specific antagonist) or ICI118551 (a β2AR-specific antagonist) (53). Blocking β2AR with ICI118551 completely blocked the ISO-induced increase in NPo (Fig. 2, A and B, and fig. S1), whereas blocking β1AR CGP20712 had no effect (Fig. 2, A and B).
Fig. 2. β2AR but not β1AR signaling augments L-type currents in neurons.
(A) Representative single-channel recordings from wild-type (WT) rat hippocampal neurons. The patch pipette solution contained vehicle (H2O; control; −) or 1 μM ISO (+; red traces) plus 100 nM CGP20712 (+CGP) or 100 nM ICI118551 (+ICI). Arrows throughout the figure (←) indicate the zero-current level (closed channel). (B) Summary plot for data in (A). For statistical analysis, the NPo value was determined for each recording and pooled under each condition for comparison (*P < 0.05; unpaired t test was used to compare statistical significance in wild-type cells under control conditions with those treated with CGP. Mann-Whitney test was used in cells treated with ICI). Average NPo values are 0.10 ± 0.03 for wild type – ISO (n = 10), 0.39 ± 0.06 for wild type + ISO (n = 12), 0.11 ± 0.04 for CGP – ISO (n = 9), 0.48 ± 0.05 for CGP + ISO (n = 11), 0.09 ± 0.03 for ICI – ISO (n = 12), and 0.10 ± 0.03 for ICI + ISO (n = 15) (n, number of cell-attached patch recordings from three independent culture experiments).
Signaling by the β2AR but not the β1AR promotes phosphorylation of α11.2 subunits in hippocampal neurons
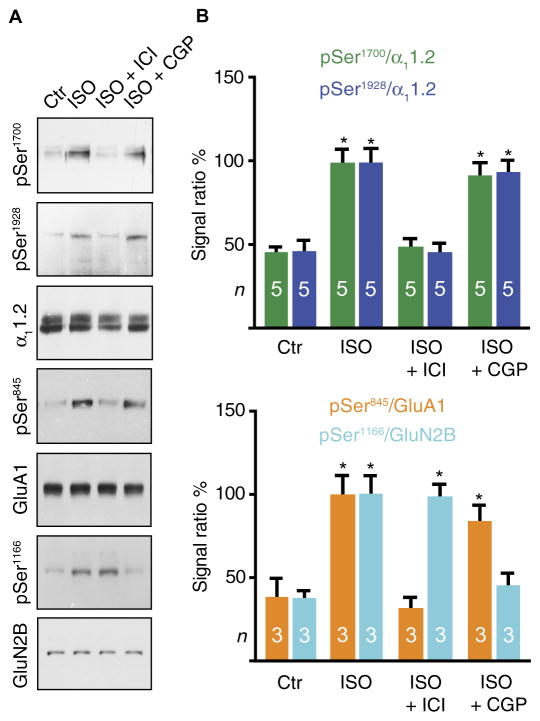
The importance of β2AR for the regulation of Cav1.2 in neurons was further confirmed by biochemical analysis (Fig. 3, A and B, top). Treatment of acutely isolated murine forebrain slices with ISO for 5 min significantly increased phosphorylation of Ser1928 and also Ser1700. These effects were completely blocked by ICI118551 but unaffected by CGP20712. The increase in ISO-induced Ser1928 phosphorylation and its significant inhibition by ICI118551 were also observed when only the hippocampal CA1 region was used for such analysis (fig. S3). These results indicate that, in neurons in the forebrain and hippocampus, and specifically in those from the CA1 region, βAR signaling of Cav1.2 occurs through the β2AR but not through the β1AR.
Fig. 3. β2AR controls phosphorylation of Cav1.2 and GluA1, and β1AR controls phosphorylation of GluN2B.
(A) Forebrain slices from wild-type mice of either sex were treated with ASCF containing vehicle (H2O; Ctr) or 1 μM ISO plus 100 nM ICI118551 (+ICI) or 100 nM CGP20712 (+CGP), as indicated, for 5 min before solubilization, ultracentrifugation, simultaneous immunoprecipitation (IP) of α11.2 plus GluA1, and subsequent immunoprecipitation of GluN2B from supernatants resulting from the α11.2/GluA1 immunoprecipitation. Samples were analyzed by sequential immunoblotting for phosphorylated Ser1928 (pSer1928), pSer1700, and α11.2 (top panels) and for pSer845 and GluA1 (middle panels) from the same blots and sequential immunoblot-ting for pSer1166 and GluN2B of a second blot using corresponding regions of the blots. (B) For quantification of α11.2 phosphorylation, pSer1928 and pSer1700 signals were nor-malized to total α11.2 [*P < 0.05 versus control, analysis of variance (ANOVA) with Bonferroni correction for multiple testing between all experimental conditions; n, number of samples analyzed across three independent experiments]. For quantification of GluA1 phosphorylation, pSer845 signals were normalized to GluA1 (*P < 0.05, ANOVA with Bonferroni’s test; n, number of samples analyzed across three independent experiments). For quantification of GluN2B phosphorylation, pSer1166 signals were normalized to GluN2B (*P < 0.05, ANOVA with Bonferroni correction for multiple testing; n, number of samples analyzed across three independent experiments).
To confirm the specificity of the antagonists for β2AR and β1AR and that both receptors are present and active in the neurons, we also analyzed the effect of these drugs on ISO-induced phosphorylation of AMPA-type glutamate receptor (AMPAR) subunits and N-methyl-D-aspartate (NMDA)–type glutamate receptor (NMDAR) subunits. The AMPAR subunit GluA1 is phosphorylated on Ser845 through a β2AR-dependent pathway (17, 53–55), and the NMDAR subunit GluN2B is phosphorylated on Ser1166 through a β1AR-dependent pathway (Fig. 3).
The AMPAR forms a signaling complex analogous to Cav1.2 containing β2AR, Gs, AC, AKAP, and PKA to enable highly localized signaling by cAMP (54, 55). In this complex, the β2AR is linked to the AMPAR GluA1 subunit through the scaffold protein PSD-95 and AMPAR subunits known as transmembrane AMPAR regulatory proteins (TARPs) (54). Phosphorylation of GluA1 on Ser845 in this complex augments postsynaptic strength by increasing channel activity of the AMPAR (56) and its postsynaptic accumulation (24, 54, 57–67). ICI118551 completely blocked the ISO-induced increase in the phosphorylation of the GluA1 subunit of the AMPAR on Ser845, but there was minimal effect of CGP20712 (Fig. 3, A and B, bottom).
Phosphorylation of the NMDAR subunit GluN2B on Ser1166 by PKA selectively increases Ca2+ permeability of the NMDAR (68, 69). The ISO-induced increase in Ser1166 phosphorylation was not blocked by ICI118551, but it was completely eliminated by CGP20712 (Fig. 3, A and B, bottom). Thus, in contrast to Cav1.2 and GluA1, these data showed that NMDAR phosphorylation is mediated by the β1AR and not by the β2AR. These results could reflect differential β-adrenergic signaling in individual neurons, or the differential effects of β1AR versus β2AR signaling could occur in different subcellular regions or different neuronal subtypes, because our forebrain slices consist of a mixture of cells. Furthermore, these results demonstrated that CGP20172 effectively blocked the β1AR. Therefore, its lack of effect on Cav1.2 and GluA1 phosphorylation reflects the requirement for β2AR activation and not the lack of efficacy of the drug treatment (53).
Induction of PTT-LTP requires channel activity of Cav1.2
Extended stimulation of the Schaffer collateral pathway, which originates in the hippocampal CA3 region and projects to the CA1 region, at 5–10 Hz induces PTT-LTP but only if β-adrenergic stimulation occurs at the same time (22, 53, 70, 71). This stimulation pattern is especially relevant because it mimics the naturally occurring theta rhythm (~7 Hz) (72). During the first 5 min of stimulation, which include 3 min for delivery of the 5-Hz tetanus, synaptic transmission shows an initial depression before it recovers from what might be desensitization of the postsynaptic response, and the potentiation develops during the subsequent 5 min (22, 53, 70).
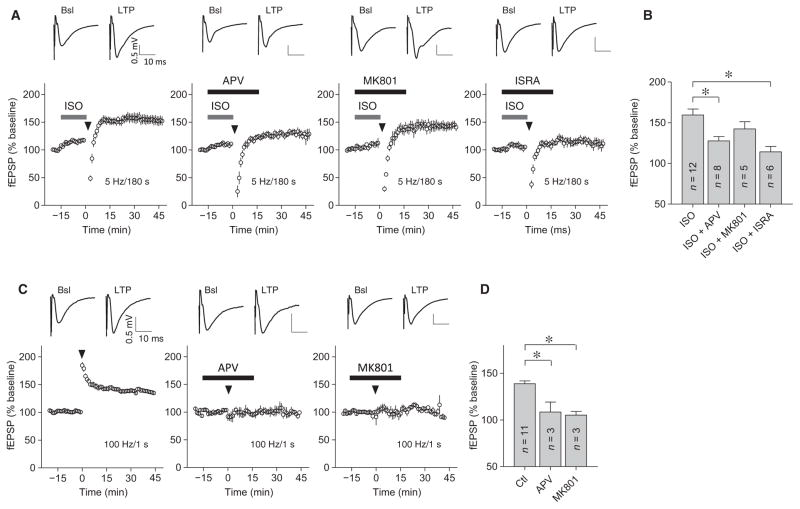
The complete inhibition of PTT-LTP by ICI118551 and not by CGP20712 (53) indicates that ISO acts through β2AR but not through β1AR during PTT-LTP. Because β2AR stimulation prominently augments Ca2+ influx through Cav1.2 at postsynaptic sites (73), we tested whether Cav1.2 and its enhancement by the β2AR are required for PTT-LTP. We recently found that PTT-LTP is absent in conditional forebrain Cav1.2 knockout (KO) mice (17). Cav1.2 KO mice could have altered synaptic signaling due to changes in gene expression rather than electrophysiological changes that acutely contribute to PTT-LTP. We used acute pharmacological inhibition of L-type channels and NMDARs, both of which are main conduits for Ca2+ influx during many forms of synaptic plasticity (12–14), to evaluate their respective contributions. Whereas the competitive glutamate site antagonist aminophosphonovaleric acid (APV) significantly reduced PTT-LTP, the pore blocker MK801 had a more modest, nonsignificant effect (Fig. 4, A and B). Standard LTP induced by a single 100-Hz tetanus, which strictly requires NMDARs, was fully abrogated by either drug, indicating that both drugs effectively blocked the NMDAR in these experiments (Fig. 4, C and D). The L-type Ca2+ channel blocker isradipine significantly reduced PTT-LTP to a degree that appeared to be larger than that by APV or MK801. Although these differences in PTT-LTP did not reach statistical significance (Fig. 4, A and B; P = 0.0806 for ISO + isradipine versus ISO + APV and P = 0.0591 for ISO + isradipine versus ISO + MK801, Mann-Whitney test), they collectively suggested that Ca2+ influx through L-type channels plays a more prominent role in PTT-LTP than Ca2+ influx through NMDARs under our experimental conditions.
Fig. 4. L-type Ca2+ channels mediate PTT-LTP.
Graphs show representative fEPSP initial slopes recorded from hippocampal CA1 and amalgamated data before [basal (Bsl)] and after either a 3-min, 5-Hz tetanus (A and B) or two 1-s, 100-Hz tetani (C and D). Arrowheads mark onset of tetani, gray bars indicate perfusion with 1 μM ISO, and black bars indicate perfusion with 50 μM DL-APV, 100 μM MK801, or 10 μM isradipine (ISRA), as indicated. Insets on top show sample traces immediately before (left) and ~30 min after (right) tetani. Degree of potentiation was determined 30 min after tetanus and compared to baseline before tetanus. PTT-LTP was 159.4 ± 7.4% of baseline for control conditions, 127.6 ± 5.3% for APV, 142.4 ± 8.7% for MK801, and 107.0 ± 4.1% for isradipine. LTP induced by two 100-Hz tetani was 138.9 ± 3.0% for control conditions, 108.6 ± 10.5% for APV, and 105.2 ± 4.0% for MK801 (*P < 0.05; Kruskal-Wallis with Dunn’s test; n, number of slices from three to six mice of either sex used for n number of recordings).
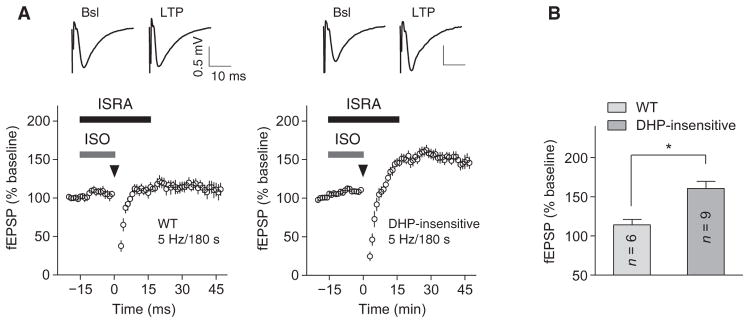
Isradipine, which is also known as PN200-110 when tritiated, is selective for L-type Ca2+ channels but binds multiple members of this class of channels, not only Cav1.2 (1). Hippocampal neurons also contain the L-type Ca2+ channel Cav1.3, albeit at a much lower amount than Cav1.2 (1, 3). Therefore, the effect of isradipine may have resulted from blocking Cav1.3 rather than Cav1.2 or by blocking both channels. Mutating Thr1066 to Tyr in the α11.2 subunit makes Cav1.2 insensitive to dihydropyridines such as isradipine (3). We used T1066Y KI mice (3) to scrutinize whether isradipine inhibited PTT-LTP by blocking Cav1.2 rather than Cav1.3. Isradipine blocked PTT-LTP in litter-matched wild-type mice but not in homozygous dihydropyridine-insensitive T1066Y KI mice (Fig. 5). These results indicated that induction of PTT-LTP depends on Cav1.2 channel activity.
Fig. 5. PTT-LTP requires Cav1.2.
Graphs show fEPSP initial slopes recorded from hippocampal slices from litter-matched wild-type mice and isradipine-insensitive (DHP) T1066Y KI mice (A) and amalgamated data (B). Arrowheads mark onset of the 3-min, 5-Hz tetanus, gray bars indicate perfusion with 1 μM ISO, and black bars indicate perfusion with 10 μM isradipine. Insets on top show sample traces immediately before (left) and ~30 min after (right) tetani. Degree of potentiation was determined 30 min after tetanus and compared to baseline before tetanus. Average fEPSP was 114.2 ± 6.8% of baseline in slices from wild-type mice and 160.4 ± 9.2% of baseline in slices from DHP-insensitive, T1066Y KI mice (*P < 0.05; Mann-Whitney test; n, number of slices for the same number of recordings; wild-type mice: three animals; isradipine-insensitive mice: six animals of either sex).
Phosphorylation of Cav1.2 on Ser1928 is required for PTT-LTP
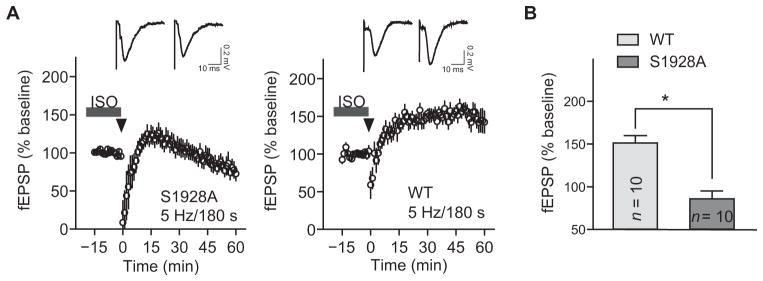
Given that the β2AR and Cav1.2 are required for PTT-LTP and that Ser1928 is needed for augmenting Cav1.2 activity, we hypothesized that PTT-LTP depends on phosphorylation of Ser1928 by PKA downstream of the β2AR. PTT-LTP was absent 30 to 60 min after a theta burst stimulus in slices from S1928A KI mice, whereas it was readily induced in litter-matched control wild-type mouse slices (Fig. 6). In hippocampal slices from mice of each genotype, after the typical initial reversal of the depression of synaptic transmission seen during the theta tetanus, synaptic strength returned to the baseline level seen before PTT-LTP induction and then increased above the baseline up to 15 min after stimulation (Fig. 6A). However, after 15 min, synaptic strength declined to below control values for S1928A mice, whereas it remained increased for at least 60 min in controls (Fig. 6B).
Fig. 6. PTT-LTP requires phosphorylation of Cav1.2 on Ser1928.
Graphs show fEPSP initial slopes recorded from hippocampal slices from litter-matched S1928A KI mice and wild-type mice (A) and amalgamated data (B). Arrowheads mark onset of the 5 Hz, 3 min tetanus, and gray bars indicate perfusion with 1 μM ISO. Insets on top show sample traces immediately before (left) and ~30 min after (right) tetani. Degree of potentiation was determined 30 min after tetanus and compared to baseline before tetanus. Average fEPSP was 151.3 ± 8.6% of baseline in slices from wild-type mice (*P < 0.05 baseline versus tetanized; Mann-Whitney test) and 85.8 ± 9.3% of baseline in slices from S1928A KI mice (*P < 0.05; Mann-Whitney test; n, number of slices for the same number of recordings; wild-type mice: six animals; S1928A KI mice: three animals of either sex)..
Input-output curves for increased stimulus strength versus field excitatory postsynaptic potential (fEPSP) response strength, an indication of postsynaptic glutamate receptor activity, and paired-pulse facilitation, an indication of presynaptic glutamate release function, did not show any differences between slices from S1928A KI mice and those from litter-matched wild-type control mice over a wide range of values (fig. S4). Accordingly, basal synaptic transmission including presynaptic glutamate release and postsynaptic glutamate receptor activity appear unaltered in S1928A KI mice.
Both the PKA-targeted site Ser1700 and the nearby residue Thr1704 are implicated in the regulation of Cav1.2 (41, 74–76). We analyzed PTT-LTP in S1700A/T1704A double KI mice (“STAA mice”). There was no difference in PTT-LTP between slices from STAA mice and litter-matched wild-type mice (fig. S5). In addition, input-output curves for fEPSP responses and paired-pulse facilitation were comparable for these two genotypes (fig. S6). We concluded that induction of PTT-LTP requires increased Cav1.2 activity mediated by β2AR-PKA signaling through Ser1928 phosphorylation but not through Ser1700 or Thr1704 phosphorylation.
DISCUSSION
Regulation of the L-type Ca2+ channel Cav1.2 by βAR-cAMP-PKA signaling plays a prominent role in mediating the fight-or-flight response of our heartbeat, as triggered by norepinephrine (29, 30, 77, 78). Signaling by norepinephrine through this pathway also augments arousal, behavioral acuity, and emotionally motivated learning (19–23). A prominent target of norepinephrine action in the brain is Cav1.2, which forms a complex with the β2AR, Gs, AC, AKAPs, and PKA (24–26, 28, 29). In turn, Cav1.2 controls neuronal excitability through closely associated Ca2+-activated K+ channels (6, 7). Ca2+ influx through Cav1.2 is more effective in regulating gene expression through the cAMP response element–binding protein and nuclear factor of activated T cells than Ca2+ influx through other Ca2+ channels (8–11), and Cav1.2-induced Ca2+ influx is important for various forms of synaptic plasticity (15, 16, 18). Yet, the molecular mechanism by which PKA increases ion flux through Cav1.2 in the brain and heart remains incompletely defined. We identified Ser1928 of Cav1.2 as an essential PKA-regulated site important for PTT-LTP. Furthermore, we found differential regulation of Cav1.2 by the PKA pathway in the brain and heart. Parallel work on the regulation of Cav1.2 by PKA in vascular smooth muscle cells (VSMCs) also shows that this regulation is completely abrogated in S1928A KI mice (79).
The findings in neurons and VSMCs are in striking contrast to cardiomyocytes, in which Ser1928 in the distal C-terminal domain does not seem to be involved; rather, phosphorylation of Ser1700 in the proximal C-terminal domain has been implicated in the increase in channel activity of cardiac Cav1.2 by βAR signaling (41, 74–76). In neurons, we found that the β2AR-specific blocker ICI118551 completely blocked the ISO-induced increase in NPo of Cav1.2, whereas blocking β1AR with CGP20712 had no effect. In contrast, cardiac Cav1.2 channels are primarily regulated by β1AR signaling (29, 37, 48–52). Although β2ARs are present in rodent and human heart, they do not participate in the increase in Cav1.2 channel activity that augments contractility in the flight-or-flight response. Accordingly, the βAR-mediated regulation of Cav1.2 in the heart and brain depends on different βAR subtypes and phosphorylation of different sites on Cav1.2.
β2AR regulation of Cav1.2 channels in the heart is thought to occur mostly in caveolae and to be important for initiation of cardiac hypertrophy in response to hypertrophic signals (80). The β1AR and β2AR engage different downstream effectors in the G protein and arrestin pathways through biased signaling (81, 82), and these different signaling pathways may lead to biased changes in phosphorylation status of Cav1.2 channels in the heart and brain. More work is required to determine how these two signaling pathways can engage regulation of Cav1.2channels through phosphorylation of Ser1928 versus Ser1700 and Thr1704. Some possible mechanisms for the different regulation of Cav1.2 in the brain and heart include differential subunit composition of the channel, differential proteolytic processing of the channel, and differential association of the channel with AKAPs in the brain and heart.
Four different genes encode α2δ subunits (α2δ1 to α2δ4) and β subunits (β1 to β4), which further undergo differential splicing (83, 84). In cardiomyocytes, β2 is functionally the most prominent β subunit in defining the biophysical properties of Cav1.2 (85). β2A is phosphorylated by PKA on Ser459, Ser478, and Ser479, which are not present in the β1, β3, or β4 subunit (86). Although there is evidence that Ser478 and Ser479 are not required for increased Cav1.2 activity in virally infected ventricular myocytes (42), this system does not fully reconstitute β-adrenergic regulation of L-type currents, leaving open the possibility that Ser478 and Ser479 contribute to the increase of L-type currents in the heart. In the brain, all four β subunits are broadly expressed (87–89). Thus, it is conceivable that PKA-regulated phosphorylation of β2 compensates for the loss of Ser1928 phosphorylation in S1928A KI mice in cardiomyocytes but not neurons. It is also possible that differential splicing of α11.2 plays a role, although all known splice variants are predicted to contain Ser1928 (90, 91).
In cardiomyocytes, α11.2 is proteolytically processed, and this processing and subsequent noncovalent association of the cleaved C-terminus with the channel core are required for PKA-dependent regulation (41, 46). Reconstitution of regulation of a cardiac form of Cav1.2 by PKA in cultured nonmuscle cells requires formation of an autoinhibitory signaling complex composed of the core of the Cav1.2 channels with the noncovalently bound cleaved portion (41), which makes analysis of PKA-dependent regulation in this system challenging. Cav1.2 channels are not as extensively proteolytically processed in hippocampal neurons as in the heart, unless massive Ca2+ influx is induced by prolonged activation of NMDA receptors (33, 92). Therefore, differences in proteolytic processing and assembly of the Cav1.2 signaling complex in the heart and brain may contribute to the differences in mechanisms of PKA regulation.
Regulation of Cav1.2 channels in the heart and brain requires AKAPs to anchor PKA (10, 28, 35, 38, 41, 44–47). Differential regulation of Cav1.2 channels can be induced in reconstituted nonmuscle cells by coexpression of different AKAPs (93). Association of Cav1.2 channels with different sets of AKAPs in the brain and heart might contribute to differential channel regulation by PKA phosphorylation of distinct sites.
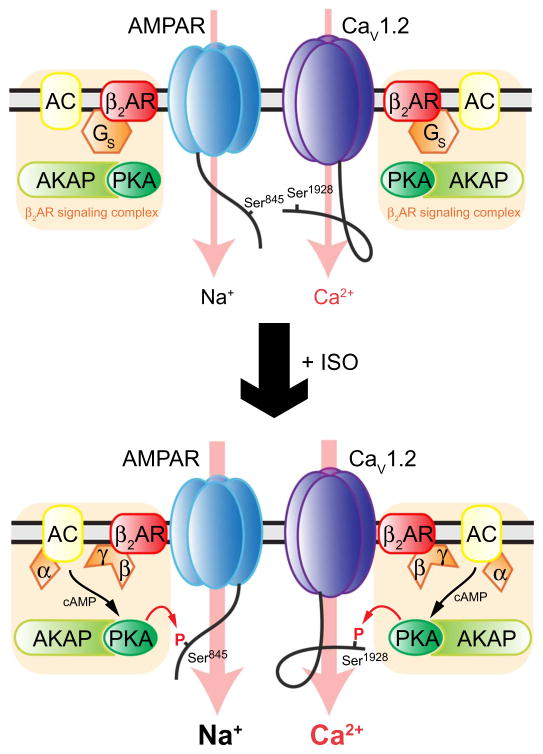
Another important finding was that PTT-LTP depends on Cav1.2 and its phosphorylation of Ser1928 but not Ser1700. PTT-LTP is thought to underlie contextual learning under difficult or stressful situations (22). PTT-LTP requires simultaneous stimulation by prolonged theta tetanus and β2AR signaling (22, 53, 70). The β2AR forms two distinct signaling complexes with postsynaptic ion channels, namely, AMPARs and Cav1.2, and both are now emerging as critical targets for β2AR-PKA signaling during PTT-LTP (17, 53, 63).
The β2AR binds to the scaffold protein PSD-95, which, in turn, binds to the AMPAR (54, 55). In addition, AKAP150, which is associated with GluA1 through SAP97 (94), recruits AC (65, 95), PKA, and the phosphatase PP2B to the AMPAR complex (24, 62, 63, 65, 96–98). The close proximity of all components of this cAMP cascade in this AMPAR complex and the analogous β2AR-Gs-AC-PKA-Cav1.2 complex results in highly localized, selective, and potent augmentation of channel activity of AMPARs and Cav1.2 by cAMP (17, 25, 29, 47, 48, 54). Phosphorylation of Cav1.2 on Ser1928 and of the AMPAR GluA1 subunit on Ser845, which is also required for PTT-LTP (53), might synergistically augment Ca2+ influx into postsynaptic sites through Cav1.2 in PTT-LTP (Fig. 7). During PTT-LTP, stimulation of the β2AR results in phosphorylation of Ser845, which increases AMPAR Po (56, 97) and promotes postsynaptic accumulation of GluA1-containing AMPARs (54, 57, 58, 60). Consequently, Na+ influx increases, resulting in depolarization of postsynaptic sites during PTT-LTP. In addition, we found that phosphorylation of Ser1928 increases the Po of Cav1.2. In other cell types, Cav1.2 phosphorylation at Ser1928 also shifts the activation threshold for Cav1.2 toward more negative membrane potentials (fig. S2) (27, 32, 37, 43, 75, 76). This leftward shift means that less depolarization is required for channel opening. In addition, it further increases Ca2+ influx because the driving force for this Ca2+ influx is larger at these more negative potentials. The combined effect of Ser845 and Ser1928 phosphorylation would thereby be stronger Ca2+ influx through Cav1.2.
Fig. 7. Model for enhancement of Ca2+ influx through Cav1.2 during PTT-LTP.
Under basal conditions, phosphorylation of Ser845 in the AMPAR GluA1 subunit and of Ser1928 in the Cav1.2 α11.2 subunit and Ser1928 is low. Stimulation of AMPAR- and Cav1.2-associated β2ARs activates Gs-mediated AC signaling and thereby cAMP production and PKA. The resulting Ser845 phosphorylation increases Po of the AMPAR, which results in Na+ influx and depolarization during synaptic transmission induced by the theta stimulation. Ser1928 phosphorylation increases Po of Cav1.2 and thereby Ca2+ influx through this channel. This increase in Ca2+ influx through Cav1.2 triggers the potentiation of synaptic strength in PTT-LTP via yet-to-be-defined signaling pathways.
The time course of PTP-LTP after the theta tetanus train in S1928A mice initially showed an increase in synaptic strength above aseline, but eventually, postsynaptic responses fell below those of wild-type controls (Fig. 6). It is possible that, in the absence of the ISO-induced increase in Cav1.2 activity through Ser1928 phosphorylation, PKA phosphorylation of Ser845 in GluA1 in the AMPAR is sufficient to initially enhance synaptic strength. However, without augmented Ca2+ entry through the Cav1.2 channels, the neurons ultimately respond with synaptic depression. Such coincidence detection of depolarization through AMPAR and Ca2+ entry mediated by Cav1.2 channels could be a mechanism to ensure precise capture of incoming signals that lead to LTP.
In contrast to the S1928A KI mice, a decrease in synaptic transmission upon PTT-LTP induction was not seen in slices from S845A KI mice, although PTT-LTP was also impaired in S845A KI slices (53). Perhaps in the absence of increased Ca2+ influx through Cav1.2 in the S1928A slices, mechanisms that induce NMDAR-dependent LTD prevail, which requires Ser845 phosphorylation for temporary insertion of Ca2+-permeable AMPARs into the postsynaptic membrane (63). Another potential explanation is that, in S1928A KI mice, the constitutive absence of the increase in Cav1.2 activity upon βAR receptor signaling affects gene expression in a way that influences postsynaptic signaling during PTT-LTP. The absence of PTT-LTP in S1928A KI mice is consistent with an essential role of Ser1928 phosphorylation in the increase in Cav1.2 channel activity by β2AR signaling because PTT-LTP depends on both β2AR and Cav1.2 (17, 53). In summary, we demonstrate that Ser1928 phosphorylation of Cav1.2 is required for augmentation of Cav1.2 channel activity by β2AR stimulation and that this mechanism constitutes a critical component of the molecular mechanism underlying stable PTT-LTP.
MATERIALS AND METHODS
Animals
All procedures involving animals followed the guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health and had been approved by the Institutional Animal Care and Use committees at the University of California, Davis, and the University of Washington. S1928A KI mice were described in (43), and STAA mice were described in (75).
Reagents, peptides, and antibodies
ISO in the form of ISO bitartrate salt, ICI118551, CGP20712, and microcystin LR were from Sigma-Aldrich; protein A–coated beads were from Repligen; polyvinyldene fluoride (PVDF) membranes were from Millipore; horseradish peroxidase (HRP)–coupled protein A and enhanced chemiluminescence (ECL) reagents were from GE Healthcare; EGTA, EDTA, Tween 20, Triton X-100, and tris were from Thermo Fisher Scientific; and ω-conotoxins GVIA and MCVIIC were from Bachem. (S)-(−)-Bay K8644 was from Millipore, and isradipine was from Enzo Life Sciences. Other reagents were from the typical suppliers and of standard quality. Antibodies against α11.2 (FP1), pSer1700, pSer1928, GluA1, and pSer845 are exactly as detailed (17). Antibodies against GluN2B and pSer1166 are described in (69).
Preparation of brain slices and use for biochemical analysis
Eight- to 12-week-old mice with a genetic background of 50% C57BL/6 and 50% S129/Sv (S1928A KI mice) or mostly C57BL/6 (STAA, back-crossed at least four times with C57BL/6) were decapitated, and brains placed into ice-cold artificial cerebrospinal fluid (ACSF) (127 mM NaCl, 26 mM NaHCO2, 1.2 mM KH2PO4, 1.9 mM KCl, 2.2mMCaCl2,1mM MgSO4, and 10 mM D-glucose; 290 to 300 mosmol/kg; saturated with 95% O2 and 5% CO2; final pH 7.3). About one-third of the rostral and caudal portions of the brain were trimmed off. Forebrain slices (350 μm thick) containing the hippocampus were prepared with a vibratome (Leica VT 1000A) and equilibrated in oxygenated ACSF for 1 hour at 30°C before transfer to incubation or recording chambers.
Biochemistry
Slices were equilibrated for 30 min at 32°C and treated with the various drugs for 5 min. Slices were processed with a glass Teflon homogenizer in a 10-fold excess (v/w) of immunoprecipitation buffer [1% Triton X-100, 150 mM NaCl, 10 mM EDTA, 10 mM EGTA, and 10 mM tris-HCl (pH 7.4)] (35) containing protease inhibitors [pepstatin A (1 μg/ml), leupeptin (10 μg/ml), aprotinin (20 μg/ml), and 200 nM phenylmethylsulfonyl fluoride, the latter being added immediately before homogenization and again at the start of the immunoprecipitation] and phosphatase inhibitors (2 μM microcystin LR, 1 mM p-nitrophenyl phosphate, 50 mM Na-pyrophosphate, and 50 mM NaF) (38, 99). Nonsolubilized material was removed by ultracentrifugation (250,000g for 30 min) before immunoprecipitation (4 hours at 4°C) with antibodies against α11.2 (FP1; 4 μg) plus GluA1 (1 μg) and GluN2B. The protein A beads were washed three times with immunoprecipitation buffer containing 0.05% Triton X-100 plus 0.5% glycerol. SDS–polyacrylamide gel electrophoresis and transfer onto PVDF membranes were followed by blocking [5% dry milk in tris-buffered saline (TBS)], incubation with primary antibodies (5% dry milk in TBS; 1 hour), three washes with 0.05% Tween 20 in TBS, incubation with HRP–protein A (1:10,000 in 5% dry milk in TBS; 1 hour), several washes for 2 hours, and detection of HRP with ECL or ECL plus chemiluminescence reagents. Multiple exposures of increasing length ensured that signals were in the linear range, as previously described (40, 99).
Cell-attached patch-clamp recording
Primary hippocampal neurons were cultured from Sprague-Dawley rats or wild-type and S1928A KI mice, which had a 50% 129/SV and 50% C57BL/6 genetic background, of either sex, as previously described (100–102). Cell-attached patch-clamp recordings were performed after 7 to 14 days in culture, as previously described (17, 25), on an Olympus IX70 inverted microscope at 22°C. Signals were recorded at 10 kHz and low-pass–filtered at 2 kHz with an Axopatch 200B amplifier and Digidata 1440 digitizer (all from Molecular Devices). Recording electrodes were pulled from borosilicate capillary glass (outer diameter, 0.86 mm) with a Flaming micropipette puller (model P-97, Sutter Instruments) and polished (polisher from World Precision Instruments; resistance, 3.5 to 6.5 megohms). The patch transmembrane potential was zeroed by keeping neurons in high K+ extracellular solution containing 145 mM KCl, 10 mM NaCl, and 10 mM Hepes [pH 7.4 (NaOH)] during the recordings. The pipette solution contained 20 mM tetraethylammonium chloride (TEA-Cl), 110 mM BaCl2 (as charge carrier), and 10 mM Hepes [pH 7.3 (TEA-OH)] plus 1 μM ω-conotoxin GVIA and 1 μM ω-conotoxin MCVIIC to block N- and P/Q-type Ca2+ channels, respectively. (S)-(−)-Bay K8644 (500 nM) was added to the pipette solution to promote longer open times. This procedure is routinely applied to augment detection of L-type channels by single-channel recordings. Single-channel activity was recorded during an average of 50 2-s-long pulses from a holding potential of −80 to 0 mV every 5 s for each experimental condition. The single-channel event-detection algorithm of pClamp 10 was used to determine single-channel opening amplitudes and NPo. NPo values were combined for each condition and analyzed with GraphPad Prism 5.
fEPSP recording from brain slices
Recordings were from either sex, as previously described (17, 53). Slices were perfused with a flow rate of 2 ml/min at 30°C, with ACSF equilibrated with 95% O2 and 5% CO2 (final pH 7.3). Schaffer collaterals were stimulated every 15 s with a bipolar tungsten electrode, and resulting fEPSPs in CA1 were recorded with a glass electrode filled with ACSF. Signals were amplified with an Axopatch 2B amplifier, digitized with Digidata 1320A, and analyzed with Clampex 9 (Molecular Devices). Stimulus strength was titrated to define maximal response and input-output curves and adjusted to result in ~50% of maximal response. PTT-LTP was induced by a 3-min, 5-Hz tetanus. The average of fEPSP initial slopes from the 5 min preceding the tetanus was set to equal 100% baseline level. The PTT-LTP strength was defined as the average of fEPSP initial slopes obtained between 15 and 45 min after the tetanus. To determine paired-pulse facilitation, fEPSP initial slopes of two consecutive stimuli with the indicated interevent intervals were recorded.
Whole-cell patch-clamp recording from cardiomyocytes
Ventricular myocytes were isolated from mice of either sex, as previously described (Alliance for Cellular Signaling Procedure Protocol PP00000125) (103), maintained at 37°C, and aerated with 98% O2 and 2% CO2. Calcium currents (ICa) were recorded in whole-cell mode at room temperature from calcium-tolerant myocytes within 1 to 24 hours of isolation. The extracellular solution contained 137 mM NaCl, 1.8 mM CaCl2, 25 mM CsCl, 0.5 mM MgCl2, 10 mM Hepes, and 10 mM glucose (pH 7.4). Patch pipettes (1 to 1.5 megohms) were filled with 120 mM CsCl, 10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid, 1 mM EGTA, 1 mM MgCl2, 1 mM Na2GTP, 5 mM phosphocreatine, and 10 mM Hepes (pH 7.2). ICa was elicited by depolarizing steps. A prepulse from −80 to −40 mV was used to inactivate fast Na+ currents and then stepped to voltages between −40 and +60 mV (10-mV increments). ICa was recorded by an Axopatch 200B amplifier (Axon Instruments) and stored on a computer through an analog-digital converter (DIGIDATA 1332A; Axon Instruments). Protocols were controlled by pClamp 9 software (Axon Instruments). ICa was measured as the difference between the peak inward current and the current after the test pulse ended. After establishing a stable baseline, the effect of 1 μM ISO on ICa was examined.
Statistical analysis
Data are expressed as means ± SEM. Data were analyzed using GraphPad Prism software. Data were assessed for normality of distribution using the Shapiro-Wilk test. Statistical significance was then determined using appropriate paired or unpaired Student’s t test, ANOVA, or nonparametric tests. Statistical significance, denoted by asterisks (*) in figures, was considered at P < 0.05.
Supplementary Material
Acknowledgments
Funding: This work was supported by NIH grants R01 NS078792, R01 MH097887, and R01 AG055357 (to J.W.H.), R01 HL098200 and R01 HL121059 (to M.F.N.), R01 HL085372 (to W.A.C.), and F31 NS086226 (to O.R.B.); DFG HO 579/24-1 (to F.H.); AHA 11POST7020009 (to L.M.) and 16POST26560000 (to D.C.); P20 GM103492 (to M.A.N.); and Brain and Behavior Research Foundation NARSAD Young Investigator Grant 20748 (to L.M.).
Footnotes
Author contributions: J.W.H. developed the research strategy; M.F.N. and J.W.H. oversaw the project and interpretation of the experimental data; H.Q., T.P., J.L.P., L.M., M.N.-C., B.L., O.R.B., D.C., E.N., M.P., and M.F.N. performed the experiments; F.H. provided the S1928A KI mice; W.A.C. provided the STAA mice; H.Q., T.P., J.L.P., M.N.-C., B.L., L.M., E.N., M.P., M.A.N., and M.F.N. analyzed the data; H.Q., T.P., J.L.P., L.M., M.P., and M.F.N. prepared figures; and H.Q., T.P., W.A.C., F.H., M.F.N., and J.W.H. wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: The mutant mice require a material transfer agreement from the University of Munich (S1928A KI mice) or the University of Washington (STAA mice).
Exclusive licensee American Association for the Advancement of Science.
REFERENCES AND NOTES
- 1.Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renström E, Wietzorrek G, Berjukov S, Cavalli M, Walter D, Koschak A, Waldschütz R, Hering S, Bova S, Rorsman P, Pongs O, Singewald N, Striessnig J. Isoform-specific regulation of mood behavior and pancreatic β cell and cardiovascular function by L-type Ca2+ channels. J Clin Invest. 2004;113:1430–1439. doi: 10.1172/JCI20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navedo MF, Amberg GC. Local regulation of L-type Ca2+ channel sparklets in arterial smooth muscle. Microcirculation. 2013;20:290–298. doi: 10.1111/micc.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. CaV1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- 7.Marrion NV, Tavalin ST. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- 8.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Pink MD, Murphy JG, Stein A, Dell’Acqua ML, Hogan PG. Balanced interactions of calcineurin with AKAP79 regulate Ca2+–calcineurin–NFAT signaling. Nat Struct Mol Biol. 2012;19:337–345. doi: 10.1038/nsmb.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall MR, Clark JP, III, Westenbroek R, Yu FH, Scheuer T, Catterall WA. Functional roles of a C-terminal signaling complex of CaV1 channels and A-kinase anchoring protein 15 in brain neurons. J Biol Chem. 2011;286:12627–12639. doi: 10.1074/jbc.M110.175257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma H, Groth RD, Cohen SM, Emery JF, Li B, Hoedt E, Zhang G, Neubert TA, Tsien RW. γCaMKII Shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell. 2014;159:281–294. doi: 10.1016/j.cell.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Collingridge GL, Isaac JTR, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 14.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: The last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990;347:477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- 16.Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Müller J, Stiess M, Marais E, Schulla V, Lacinova L, Goebbels S, Nave KA, Storm DR, Hofmann F, Kleppisch T. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patriarchi T, Qian H, Di Biase V, Malik ZA, Chowdhury D, Price JL, Hammes EA, Buonarati OR, Westenbroek RE, Catterall WA, Hofmann F, Xiang YK, Murphy GG, Chen CY, Navedo MF, Hell JW. Phosphorylation of Cav1.2 on S1928 uncouples the L-type Ca2+ channel from the β2 adrenergic receptor. EMBO J. 2016;35:1330–1345. doi: 10.15252/embj.201593409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- 19.Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: Dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- 20.Cahill L, Prins B, Weber M, McGaugh JL. β-Adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 21.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS. Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science. 2008;322:1700–1702. doi: 10.1126/science.1164908. [DOI] [PubMed] [Google Scholar]
- 24.Sanderson JL, Dell’Acqua ML. AKAP signaling complexes in regulation of excitatory synaptic plasticity. Neuroscientist. 2011;17:321–336. doi: 10.1177/1073858410384740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. A2+ β2 adrenergic receptor signaling complex assembled with the Ca channel Cav1.2. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 26.Dittmer PJ, Dell’Acqua ML, Sather WA. Ca2+/calcineurin-dependent inactivation of neuronal L-Type Ca2+ channels requires priming by AKAP-anchored protein kinase A. Cell Rep. 2014;7:1410–1416. doi: 10.1016/j.celrep.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray R, Johnston D. Noradrenaline and β-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987;327:620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- 28.Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for β2-adrenergic regulation. Proc Natl Acad Sci USA. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301:569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- 31.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 32.Sculptoreanu A, Rotman E, Takahashi M, Scheuer T, Catterall WA. Voltage-dependent potentiation of the activity of cardiac L-type calcium channel alpha 1 subunits due to phosphorylation by cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1993;90:10135–10139. doi: 10.1073/pnas.90.21.10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full length form of the α1 subunit of the cardiac L-type calcium channel by adenosine 3‘,5‘-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 34.Hell JW, Yokoyama CT, Wong ST, Warner C, Snutch TP, Catterall WA. Differential phosphorylation of two size forms of the neuronal class C L-type calcium channel α1 subunit. J Biol Chem. 1993;268:19451–19457. [PubMed] [Google Scholar]
- 35.Davare MA, Dong F, Rubin CS, Hell JW. The A-kinase anchor protein MAP2B and cAMP-dependent protein kinase are associated with class C L-type calcium channels in neurons. J Biol Chem. 1999;274:30280–30287. doi: 10.1074/jbc.274.42.30280. [DOI] [PubMed] [Google Scholar]
- 36.Davare MA, Horne MC, Hell JW. Protein phosphatase 2A is associated with class C L-type calcium channels (Cav1.2) and antagonizes channel phosphorylation by cAMP-dependent protein kinase. J Biol Chem. 2000;275:39710–39717. doi: 10.1074/jbc.M005462200. [DOI] [PubMed] [Google Scholar]
- 37.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during β1-adrenergic regulation. Proc Natl Acad Sci USA. 2006;103:16574–16579. doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, McKnight GS, Hell JW. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry. 2007;46:1635–1646. doi: 10.1021/bi062217x. [DOI] [PubMed] [Google Scholar]
- 39.Hell JW, Yokoyama CT, Breeze LJ, Chavkin C, Catterall WA. Phosphorylation of presynaptic and postsynaptic calcium channels by cAMP-dependent protein kinase in hippocampal neurons. EMBO J. 1995;14:3036–3044. doi: 10.1002/j.1460-2075.1995.tb07306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davare MA, Hell JW. Increased phosphorylation of the neuronal L-type Ca2+ channel Cav1.2 during aging. Proc Natl Acad Sci USA. 2003;100:16018–16023. doi: 10.1073/pnas.2236970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010;3:ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganesan AN, Maack C, Johns DC, Sidor A, O’Rourke B. β-Adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of α1C but not serine 1928. Circ Res. 2006;98:e11–e18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, Hofmann F, Moosmang S. Unchanged β-adrenergic stimulation of cardiac L-type calcium channels in Cav1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283:34738–34744. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, McKnight GS. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ Res. 2010;107:747–756. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy JG, Sanderson JL, Gorski JA, Scott JD, Catterall WA, Sather WA, Dell’Acqua ML. AKAP-anchored PKA maintains neuronal L-type calcium channel activity and NFAT transcriptional signaling. Cell Rep. 2014;7:1577–1588. doi: 10.1016/j.celrep.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu Y, Westenbroek RE, Yu FH, Clark JP, III, Marshall MR, Scheuer T, Catterall WA. Deletion of the distal C terminus of CaV1.2 channels leads to loss of β-adrenergic regulation and heart failure in vivo. J Biol Chem. 2011;286:12617–12626. doi: 10.1074/jbc.M110.175307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. β-Adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci USA. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen-Izu Y, Xiao RP, Izu LT, Cheng H, Kuschel M, Spurgeon H, Lakatta EG. Gi-dependent localization of β2-adrenergic receptor signaling to L-type Ca2+ channels. Biophys J. 2000;79:2547–2556. doi: 10.1016/S0006-3495(00)76495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao RP, Lakatta EG. β1-Adrenoceptor stimulation and β2-adrenoceptor stimulation differ in their effects on contraction, cytosolic Ca2+, and Ca2+ current in single rat ventricular cells. Circ Res. 1993;73:286–300. doi: 10.1161/01.res.73.2.286. [DOI] [PubMed] [Google Scholar]
- 50.Hool LC, Harvey RD. Role of β1- and β2-adrenergic receptors in regulation of Cl− and Ca2+ channels in guinea pig ventricular myocytes. Am J Physiol. 1997;273:H1669–H1676. doi: 10.1152/ajpheart.1997.273.4.H1669. [DOI] [PubMed] [Google Scholar]
- 51.Nagykaldi Z, Kem D, Lazzara R, Szabo B. Canine ventricular myocyte β2-adrenoceptors are not functionally coupled to L-type calcium current. J Cardiovasc Electrophysiol. 1999;10:1240–1251. doi: 10.1111/j.1540-8167.1999.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 52.MacDougall DA, Agarwal SR, Stopford EA, Chu H, Collins JA, Longster AL, Colyer J, Harvey RD, Calaghan S. Caveolae compartmentalise β2-adrenoceptor signals by curtailing cAMP production and maintaining phosphatase activity in the sarcoplasmic reticulum of the adult ventricular myocyte. J Mol Cell Cardiol. 2012;52:388–400. doi: 10.1016/j.yjmcc.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qian H, Matt L, Zhang M, Nguyen M, Patriarchi T, Koval ON, Anderson ME, He K, Lee HK, Hell JW. β2-Adrenergic receptor supports prolonged theta tetanus-induced LTP. J Neurophysiol. 2012;107:2703–2712. doi: 10.1152/jn.00374.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joiner M-lA, Lisé M-F, Yuen EY, Kam AYF, Zhang M, Hall DD, Malik ZA, Qian H, Chen Y, Ulrich JD, Burette AC, Weinberg RJ, Law PY, El-Husseini A, Yan Z, Hell JW. Assembly of a β2-adrenergic receptor—GluR1 signalling complex for localized cAMP signalling. EMBO J. 2010;29:482–495. doi: 10.1038/emboj.2009.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang D, Govindaiah G, Liu R, De Arcangelis V, Cox CL, Xiang YK. Binding of amyloid β peptide to β2 adrenergic receptor induces PKA-dependent AMPA receptor hyperactivity. FASEB J. 2010;24:3511–3521. doi: 10.1096/fj.10-156661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: Requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 58.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 59.Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 60.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 61.Lee HK, Takamiya K, He K, Song L, Huganir RL. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. J Neurophysiol. 2010;103:479–489. doi: 10.1152/jn.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanderson JL, Gorski JA, Gibson ES, Lam P, Freund RK, Chick WS, Dell’Acqua ML. AKAP150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca2+-permeable AMPA receptors. J Neurosci. 2012;32:15036–15052. doi: 10.1523/JNEUROSCI.3326-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanderson JL, Gorski JA, Dell’Acqua ML. NMDA receptor-dependent LTD requires transient synaptic incorporation of Ca2+-permeable AMPARs mediated by AKAP150-anchored PKA and calcineurin. Neuron. 2016;89:1000–1015. doi: 10.1016/j.neuron.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keith DJ, Sanderson JL, Gibson ES, Woolfrey KM, Robertson HR, Olszewski K, Kang R, El-Husseini A, Dell’Acqua ML. Palmitoylation of A-kinase anchoring protein 79/150 regulates dendritic endosomal targeting and synaptic plasticity mechanisms. J Neurosci. 2012;32:7119–7136. doi: 10.1523/JNEUROSCI.0784-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang M, Patriarchi T, Stein IS, Qian H, Matt L, Nguyen M, Xiang YK, Hell JW. Adenylyl cyclase anchoring by a kinase anchor protein AKAP5 (AKAP79/150) is important for postsynaptic β-adrenergic signaling. J Biol Chem. 2013;288:17918–17931. doi: 10.1074/jbc.M112.449462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD, Usachev YM, McKnight GS, Hell JW. Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J. 2007;26:4879–4890. doi: 10.1038/sj.emboj.7601884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu Y, Zhang M, Lim IA, Hall DD, Allen ML, Medvedeva Y, McKnight GS, Usachev YM, Hell JW. AKAP150-anchored PKA activity is important for LTD during its induction phase. J Physiol. 2008;586:4155–4164. doi: 10.1113/jphysiol.2008.151662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MVL, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- 69.Murphy JA, Stein IS, Lau CG, Peixoto RT, Aman TK, Kaneko N, Aromolaran K, Saulnier JL, Popescu GK, Sabatini BL, Hell JW, Zukin RS. Phosphorylation of Ser1166 on GluN2B by PKA is critical to synaptic NMDA receptor function and Ca2+ signaling in spines. J Neurosci. 2014;34:869–879. doi: 10.1523/JNEUROSCI.4538-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomas MJ, Moody TD, Makhinson M, O’Dell TJ. Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron. 1996;17:475–482. doi: 10.1016/s0896-6273(00)80179-8. [DOI] [PubMed] [Google Scholar]
- 71.Gelinas JN, Nguyen PV. β-Adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci. 2005;25:3294–3303. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mizuseki K, Sirota A, Pastalkova E, Buzsáki G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron. 2009;64:267–280. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoogland TM, Saggau P. Facilitation of L-type Ca2+ channels in dendritic spines by activation of β2 adrenergic receptors. J Neurosci. 2004;24:8416–8427. doi: 10.1523/JNEUROSCI.1677-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hell JW. β-Adrenergic regulation of the L-type Ca2+ channel CaV1.2 by PKA rekindles excitement. Sci Signal. 2010;3:pe33. doi: 10.1126/scisignal.3141pe33. [DOI] [PubMed] [Google Scholar]
- 75.Fu Y, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation sites required for regulation of cardiac calcium channels in the fight-or-flight response. Proc Natl Acad Sci USA. 2013;110:19621–19626. doi: 10.1073/pnas.1319421110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu Y, Westenbroek RE, Scheuer T, Catterall WA. Basal and β-adrenergic regulation of the cardiac calcium channel CaV1.2 requires phosphorylation of serine 1700. Proc Natl Acad Sci USA. 2014;111:16598–16603. doi: 10.1073/pnas.1419129111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bean BP, Nowycky MC, Tsien RW. β-Adrenergic modulation of calcium channels in frog ventricular heart cells. Nature. 1984;307:371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- 78.Catterall WA. Regulation of cardiac calcium channels in the fight-or-flight response. Curr Mol Pharmacol. 2015;8:12–21. doi: 10.2174/1874467208666150507103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nystoriak MA, Nieves-Cintrón M, Patriarchi T, Buonarati OR, Prada MP, Morroti S, Grandi E, Fernandes JDS, Forbush K, Hofmann F, Sasse KC, Scott JD, Ward SM, Hell JW, Navedo MF. Ser1928 phosphorylation by PKA stimulates the L-type Ca2+ channel CaV1.2 and vasoconstriction during acute hyperglycemia and diabetes. Sci Signal. 2017;10:eaaf9647. doi: 10.1126/scisignal.aaf9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Makarewich CA, Correll RN, Gao H, Zhang H, Yang B, Berretta RM, Rizzo V, Molkentin JD, Houser SR. A caveolae-targeted L-type Ca2+ channel antagonist inhibits hypertrophic signaling without reducing cardiac contractility. Circ Res. 2012;110:669–674. doi: 10.1161/CIRCRESAHA.111.264028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, Rajagopal S, Strachan RT, Huang TY, Bressler EA, Hara MR, Shenoy SK, Gygi SP, Lefkowitz RJ. Distinct phosphorylation sites on the β2-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Foell JD, Balijepalli RC, Delisle BP, Yunker AM, Robia SL, Walker JW, McEnery MW, January CT, Kamp TJ. Molecular heterogeneity of calcium channel β-subunits in canine and human heart: Evidence for differential subcellular localization. Physiol Genomics. 2004;17:183–200. doi: 10.1152/physiolgenomics.00207.2003. [DOI] [PubMed] [Google Scholar]
- 84.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marban E, Yue DT. Novel functional properties of Ca2+ channel β subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gerhardstein BL, Puri TS, Chien AJ, Hosey MM. Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the β2 subunit of L-type voltage-dependent calcium channels. Biochemistry. 1999;38:10361–10370. doi: 10.1021/bi990896o. [DOI] [PubMed] [Google Scholar]
- 87.Scott VES, De Waard M, Liu H, Gurnett CA, Venzke DP, Lennon VA, Campbell KP. β subunit heterogeneity in N-type Ca2+ channels. J Biol Chem. 1996;271:3207–3212. doi: 10.1074/jbc.271.6.3207. [DOI] [PubMed] [Google Scholar]
- 88.Pichler M, Cassidy TN, Reimer D, Haase H, Kraus R, Ostler D, Striessnig J. β subunit heterogeneity in neuronal L-type Ca2+ channels. J Biol Chem. 1997;272:13877–13882. doi: 10.1074/jbc.272.21.13877. [DOI] [PubMed] [Google Scholar]
- 89.Burgess DL, Biddlecome GH, McDonough SI, Diaz ME, Zilinski CA, Bean BP, Campbell KP, Noebels JL. β subunit reshuffling modifies N- and P/Q-type Ca2+ channel subunit compositions in lethargic mouse brain. Mol Cell Neurosci. 1999;13:293–311. doi: 10.1006/mcne.1999.0748. [DOI] [PubMed] [Google Scholar]
- 90.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 91.Snutch TP, Tomlinson WJ, Leonard JP, Gilbert MM. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron. 1991;7:45–57. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- 92.Hell JW, Westenbroek RE, Breeze LJ, Wang KKW, Chavkin C, Catterall WA. N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons. Proc Natl Acad Sci USA. 1996;93:3362–3367. doi: 10.1073/pnas.93.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fuller MD, Fu Y, Scheuer T, Catterall WA. Differential regulation of CaV1.2 channels by cAMP-dependent protein kinase bound to A-kinase anchoring proteins 15 and 79/150. J Gen Physiol. 2014;143:315–324. doi: 10.1085/jgp.201311075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- 95.Efendiev R, Samelson BK, Nguyen BT, Phatarpekar PV, Baameur F, Scott JD, Dessauer CW. AKAP79 interacts with multiple adenylyl cyclase (AC) isoforms and scaffolds AC5 and -6 to α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors. J Biol Chem. 2010;285:14450–14458. doi: 10.1074/jbc.M110.109769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 97.Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD. Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci. 2002;22:3044–3051. doi: 10.1523/JNEUROSCI.22-08-03044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robertson HR, Gibson ES, Benke TA, Dell’Acqua ML. Regulation of postsynaptic structure and function by an A-kinase anchoring protein-membrane-associated guanylate kinase scaffolding complex. J Neurosci. 2009;29:7929–7943. doi: 10.1523/JNEUROSCI.6093-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hall DD, Feekes JA, Arachchige Don AS, Shi M, Hamid J, Chen L, Strack S, Zamponi GW, Horne MC, Hell JW. Binding of protein phosphatase 2A to the L-type calcium channel Cav1.2 next to Ser1928, its main PKA site, is critical for Ser1928 dephosphorylation. Biochemistry. 2006;45:3448–3459. doi: 10.1021/bi051593z. [DOI] [PubMed] [Google Scholar]
- 100.Chen Y, Stevens B, Chang J, Milbrandt J, Barres BA, Hell JW. NS21: Re-defined and modified supplement B27 for neuronal cultures. J Neurosci Methods. 2008;171:239–247. doi: 10.1016/j.jneumeth.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hall DD, Dai S, Tseng P-Y, Malik ZA, Nguyen M, Matt L, Schnizler K, Shephard A, Mohapatra D, Tsuruta F, Dolmetsch RE, Christel CJ, Lee A, Burette A, Weinberg RJ, Hell JW. Competition between α-actinin and Ca2+-calmodulin controls surface retention of the L-type Ca2+ channel CaV1.2. Neuron. 2013;78:483–497. doi: 10.1016/j.neuron.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Halt AR, Dallapiazza R, Yu H, Stein IS, Qian H, Junti S, Wojcik S, Brose N, Sliva A, Hell JW. CaMKII binding to GluN2B is critical during memory consolidation. EMBO J. 2012;31:1203–1216. doi: 10.1038/emboj.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alliance for Cellular Signaling (AfCS) Procedure Protocol PP00000125; http://afcs.lbl.gov/reports/v1/CM0005/CM0005.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.