Abstract
Objective
The purpose of this case report was to describe chiropractic management of thoracic pain in a patient with a stable thoracic aortic aneurysm.
Clinical Features
An 89-year-old man presented with axial mid- and upper back pain localized predominantly at the T8 and T1 spinal segmental levels. A review of available imaging revealed a stable aneurysmal dilatation of the ascending aorta, which measured 4.3 cm.
Intervention and Outcome
Because the thoracic pain was musculoskeletal in nature and the thoracic aortic aneurysm was stable, mechanical manipulation was provided using the Impulse adjusting instrument. The patient’s pain was measured utilizing a numeric rating scale. The patient’s thoracic pain improved over the course of treatment.
Conclusion
This patient was successfully treated for thoracic spine pain with a course of chiropractic care using a mechanical adjusting instrument.
Key Indexing Terms: Manipulation, Spinal, Aortic Aneurysm, Thoracic, Chiropractic, Back Pain
Introduction
The incidence of thoracic aortic aneurysm (TAA) dissection was estimated in a Swedish population-based study at 16.3 cases per 100 000 men and 9.1 per 100 000 women per year.1 A British population-based study estimated the incidence at 6 cases per 100 000 people.2 Dissection becomes more likely with age and is most common among those between the ages of 50 and 70. Aortic aneurysms are 2 to 4 times more likely to occur in men than women. Risks for TAA include high blood pressure, smoking, atherosclerosis, family history, connective tissue disorders including Marfan syndrome and Ehlers-Danlos syndrome, and deceleration injury.3
An aortic dissection occurs when hypertension, atheroma, trauma, or a connective tissue disorder precipitates a tear in the tunica intima, the innermost layer of the aorta. Blood pools between the intima and media, further separating the layers. The result is an aorta with a true lumen and a false lumen. Usually a re-entrance tear will occur distal to the original tear, allowing blood to pass completely through the false lumen. In other cases, an intramural thrombus may form, altering flow patterns and potentially decreasing maximal shear stress on the artery wall.4, 5 Tearing of the lumen can result in severe intrascapular pain, which cannot be alleviated by changing positions. The mortality rate for elective aneurysm reconstruction is only 5%. Ruptured aneurysms, even those treated with surgical intervention, result in mortality rates as high as 80%.6, 7, 8 Surgical resection is indicated once an ascending aortic aneurysm measures 5.5 cm in diameter. The same is true of a descending aortic aneurysm that measures 6.5 cm in diameter.4, 9 A more proactive approach is suggested for those with connective tissue disorders. For patients with Marfan syndrome or familial TAA, resection should be considered at 5.0 and 6.0 cm for the ascending and descending aorta, respectively.4
Dorsalgia, pain in the upper back, is most commonly of musculoskeletal origin, but can also present as a sequela of visceral pathology. Visceral pain referral to the thoracic spine is typically serious, and may be caused by duodenal or stomach ulceration, vertebral body infection, cholecystitis, cancer, pancreatitis, esophageal disorders, or dissection of the aorta. Each condition presents with clinical nuance and may be discerned based on a thorough history and examination.7
Manipulation has been considered a contraindication in patients with aortic aneurysms even though, to date, there are no published reports associating aortic dissection with spinal manipulative therapy.6, 10, 11 It has been hypothesized that an increase in intraabdominal or intrathoracic pressure could increase the likelihood of dissection or rupture. This view is being called into question based on examples in which a course of spinal manipulative therapy was administered to patients with an aortic aneurysm, but no adverse events were reported.6, 12 Therefore, the purpose of this report is to describe the chiropractic care of a patient with spine pain and a stable TAA.
Case Presentation
An 89-year-old white man was referred to the chiropractic clinic for spinal pain with a provisional diagnosis of chronic back strain. The patient presented with axial mid- and upper back pain localized predominantly at the spinal segments of T1 and T8. He reported transient back pain over previous decades, but stated that his back pain had been exceptionally bad over the last 8 years. Sitting and being sedentary were listed as provocative for his thoracic spine pain. Extension of the patient’s arms and neck, as well as manual traction of the cervical spine, was palliative for his thoracic spine pain. He described his pain as burning and rated the severity at 3/10 at that time, with flare-ups as high as 7/10. The patient’s medical/surgical history was significant for bilateral leg surgeries for shrapnel, repair of bilateral inguinal hernia, hypertension, and TAA. His hypertension was controlled with atenolol. The patient had seen a doctor of chiropractic in the 1960s and 1970s with reportedly favorable results. The patient denied any alcohol or tobacco use. The examination and history were complicated by the patient’s severe hearing and visual impairments, the latter caused by macular degeneration. The patient was only able to hear when the examiner spoke loudly and within a specific vocal range. On subsequent visits, the patient was accompanied by his son, who assisted with communication.
Physical examination revealed the absence of bilateral biceps deep tendon reflex and diminished right patellar reflex, graded +1/4. The left patellar reflex was graded +2/4. Triceps, brachioradialis, and Achilles deep tendon reflexes were graded +2/4 bilaterally. The patient’s vitals were within normal ranges, with a blood pressure of 132/74 mm Hg. Carotid, radial, and posterior tibial pulses were equal with normal rate and rhythm. No abnormalities were detected with heart or lung auscultation. No subclavian or carotid bruits were noted. No bruit of the abdominal aorta or pulsatile mass was detected on examination. Pain in the area of T1 and T8 was reproduced on palpation. Moderate hypertonicity was noted in the trapezius bilaterally. Motion palpation revealed intersegmental hypomobility in the thoracic spine.
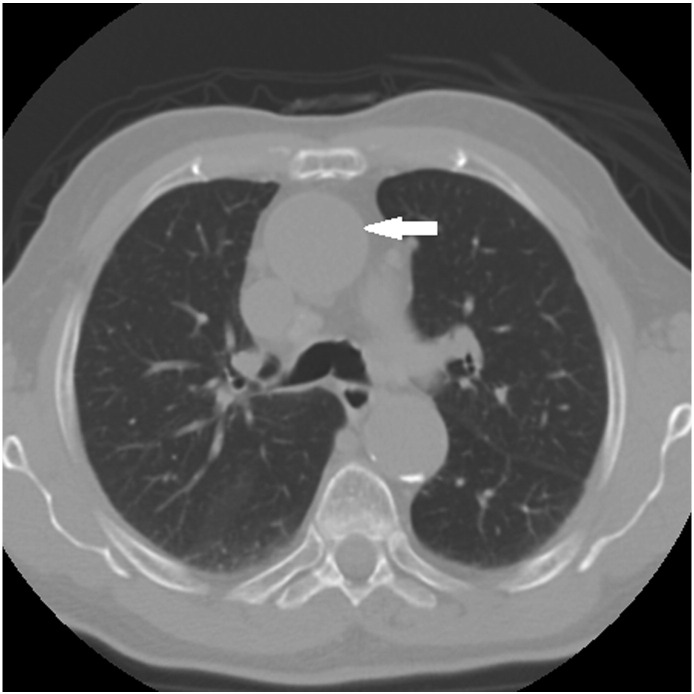
A review of available imaging revealed a stable aneurysmal dilatation of the ascending aorta. The diagnosis of TAA was made with computed tomography by a radiologist on March 15, 2012; the dilatation measured 4.1 cm in diameter. A consult was made to a vascular surgeon by his primary care provider. The vascular surgeon recommended annual surveillance and referral should the aneurysm grow to measure 5 cm. Studies were performed at 6 months, 12 months, and 2 years after the original diagnosis, over which time the aneurysm had increased in diameter by 0.2 cm to measure 4.3 cm. Based on repeat measurements revealing little change in diameter, the radiologist labeled the TAA as stable (Figs 113 and 2). Following examination by the doctor of chiropractic, the patient was diagnosed with midback pain and thoracic somatic dysfunction, as well as TAA without rupture.
Fig 1.
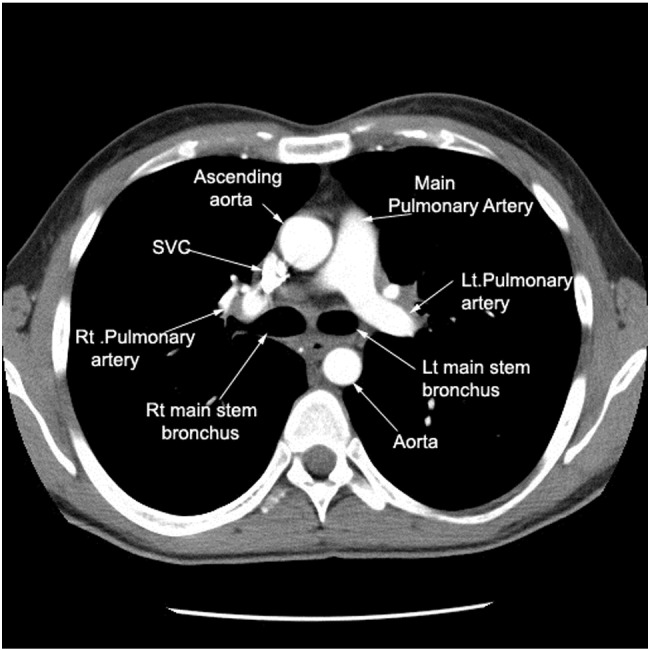
Normalthoracic computed tomogram. SVC, superior vena cava. Courtesy of Chandrasekhar and Chandrasekhar.13
Fig 2.
Patient thoracic computed tomography dated April 23, 2014. Arrow indicating ascending aorta.
Intervention and Outcome
It was decided that because the thoracic pain was characteristically musculoskeletal and the TAA was stable, manipulation was not strictly contraindicated.
Manipulation of the thoracic spine was performed using the Impulse adjusting instrument (Neuromechanical Innovations, Chandler, AZ). The choice of instrument-assisted manipulation of the thoracic spine was made to minimize compressive force in the area and limit increases in intrathoracic pressure. The impulse adjusting instrument was used on its second setting, as recommended by the manufacturer for treatment of the thoracic spine, producing an estimated peak force of 200 N.14
The patient underwent a course of spinal manipulation at a frequency of 1 visit per week for 4 weeks, followed by 3 additional visits over 2 months. Spinal manipulation was accompanied by manual massage of the upper trapezius and cervical paraspinal musculature.
At each visit, the patient was asked to rate his thoracic spine pain on the numeric rating scale before and after treatment. Prior to treatment, the patient reported 3/10 pain with episodes as severe as 7/10. After initial treatment, the patient reported an immediate and marked ameliorative effect. Pretreatment numeric rating scale on the fourth visit was recorded at 1/10, with resolution of pain posttreatment. On the seventh visit, the patient’s thoracic pain was rated at 0/10 pretreatment, and the patient was released from the clinic.
Trapezius hypertonicity improved incrementally with treatment, such that by the third visit the muscles exhibited a normal tonicity.
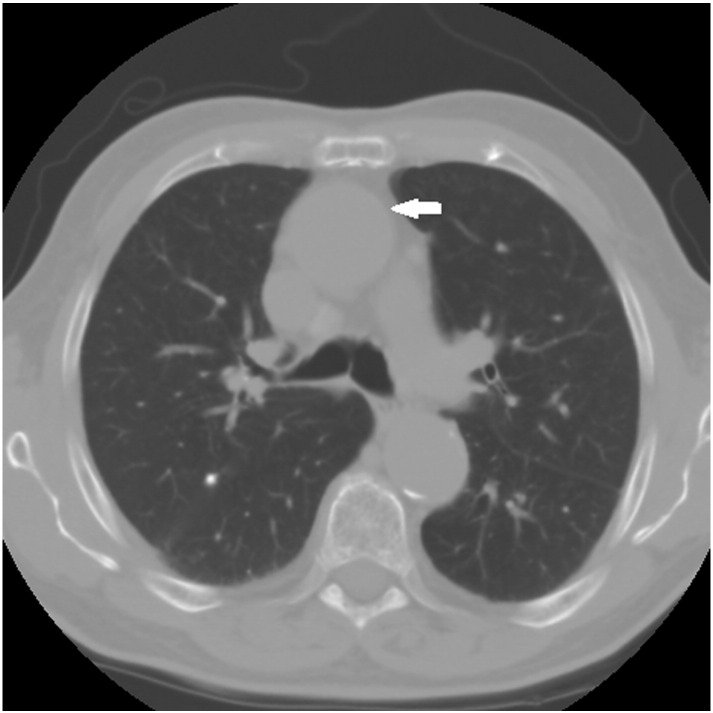
An annual computed tomography scan was performed between the sixth and seventh visits to monitor changes in dimension of the TAA. No changes were detected and the aneurysm was determined to have remained stable (Fig 3). Written consent for publication was obtained from the patient.
Fig 3.
Patient thoracic computed tomography dated October 1, 2014. Arrow indicating ascending aorta.
Discussion
To our knowledge, this is the first case report detailing instrument-assisted spinal manipulation of a patient with a TAA. Previously published reports of chiropractic treatment of patients with abdominal aortic aneurysms (AAA) are available in a limited quantity and feature patients who either presented for care but were referred for surgery or who were treated and the aneurysm was found incidentally.6, 10, 11, 15, 16 In this case, the patient’s aneurysm was known at the outset of treatment, and his pain was determined to be of musculoskeletal origin based on physical examination.
With a growing number of case studies describing the chiropractic management of patients with musculoskeletal pain and thoracic or AAAs, it may be appropriate to re-examine the belief that AAAs and TAAs are strict contraindications to manipulative interventions. Further, these case studies highlight the potential for patients to present to doctors of chiropractic with pain of musculoskeletal origin, as well as underlying pathology such as AAAs or TAAs. Doctors of chiropractic must be prepared to recognize and systematically rule out pain of visceral origin. The differences between pain patterns of visceral and musculoskeletal pathology are not always clear. A thorough physical examination is paramount when discerning the etiology of pain; this is especially true in cases where pain may warn of a life-threatening condition such as aortic dissection. If the pain is the result of aortic dissection, it will not improve with changes in position, and palpation over the area of referral will not exacerbate the pain.3
Once a patient’s pain is determined to be of musculoskeletal origin, considerations can be made with respect to treatment selection. Dorsalgia of musculoskeletal origin has been reported to respond favorably to spinal manipulation.17 A number of mechanisms have been proposed to explain the analgesic effect of spinal manipulation, the most predominant suggesting that targeted mechanotransduction can induce neurophysiologic effects resulting in nociceptive modulation at the periphery, spinal cord, and supraspinal centers. Changes in levels of blood serum cytokines and endocannabinoids, mechanoreceptive bombardment of the dorsal horn, changes in sympathetic tone, and activation of descending inhibition via the periaqueductal gray and rostral ventromedial medulla have all been implicated.18
Limitations
Because this is a single case report, it is not appropriate to generalize the effects from this patient to other patients with TAA and spinal pain. Further research with larger sample sizes is needed to determine the safety of this intervention in patients with thoracic pain and TAA.
Conclusion
This case study describes how a patient with TAA was successfully treated for thoracic spine pain with a course of chiropractic care using a mechanical adjusting instrument.
Funding Sources and Potential Conflicts of Interest
This case study was supported by the Department of Veterans Affairs. The contents do not represent the views of the Department of Veterans Affairs or the U.S. Government.
No conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): S.M.N., C.S.O.
Design (planned the methods to generate the results): S.M.N., C.S.O.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): S.M.N., C.S.O.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): S.M.N., C.S.O.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): S.M.N., C.S.O.
Literature search (performed the literature search): S.M.N., C.S.O.
Writing (responsible for writing a substantive part of the manuscript): S.M.N., C.S.O.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): S.M.N., C.S.O.
Practical Applications
-
•
A thorough history and exam is useful in distinguishing between musculoskeletal and visceral pain generators.
-
•
This patient’s thoracic spine pain was managed using instrument-assisted manipulative therapy without adverse event.
References
- 1.Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114(24):2611–2618. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 2.Howard DP, Banerjee A, Fairhead JF. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013;127(20):2031–2037. doi: 10.1161/CIRCULATIONAHA.112.000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol. 2014;64(16):1725–1739. doi: 10.1016/j.jacc.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74(5):S1877–S1880. doi: 10.1016/s0003-4975(02)04147-4. [DOI] [PubMed] [Google Scholar]
- 5.Bluestein D, Dumont K, De Beule M. Intraluminal thrombus and risk of rupture in patient specific abdominal aortic aneurysm: FSI modelling. Comput Methods Biomech Biomed Eng. 2009;12(1):73–81. doi: 10.1080/10255840903077170. [DOI] [PubMed] [Google Scholar]
- 6.Hadida C, Rajwani M. Abdominal aortic aneurysms: case report. J Can Chiropr Assoc. 1998;42:216–221. [Google Scholar]
- 7.Klineberg E, Demicco R, Mazanec D, Orr D, Bell G. Masquerade: medical causes of back pain. Cleve Clin J Med. 2007;74:905–913. doi: 10.3949/ccjm.74.12.905. [DOI] [PubMed] [Google Scholar]
- 8.Macura KJ, Corl FM, Fisherman EK, Bluemke DA. Pathogenesis in acute aortic syndromes. Am J Roentgenol. 2003;181:309–316. doi: 10.2214/ajr.181.2.1810309. [DOI] [PubMed] [Google Scholar]
- 9.Coady MA, Rizzo JA, Hammond GL. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113(3):476–491. doi: 10.1016/S0022-5223(97)70360-X. [DOI] [PubMed] [Google Scholar]
- 10.Harger BL. Abdominal aortic aneurysm: a case report. ACA J Chiropr. 1993;1993(Dec):69–71. [Google Scholar]
- 11.Weston JP. Chiropractic management of abdominal aortic aneurysm: a case report. J Can Chiropr Assoc. 1995;39(2):75–79. [Google Scholar]
- 12.Tuling JR, Crowther ET, McCord P. Clinical considerations in the chiropractic management of the patient with Marfan syndrome. J Manipulative Physiol Ther. 2000;23:498–502. doi: 10.1067/mmt.2000.108815. [DOI] [PubMed] [Google Scholar]
- 13.Chandrasekhar HV, Chandrasekhar AJ. Radiological anatomy of heart and vessels in thorax. http://www.lumen.luc.edu/lumen/meded/Radio/curriculum/Pulmonary/Image85a.jpg Available at. Accessed March 29, 2016.
- 14.Colloca CJ. Neuromechanical Innovations; Chandler, AZ: 2012. Neuromechanical Innovations: Impulse Adjusting System. [Google Scholar]
- 15.Patel SN, Kettner NW. Abdominal aortic aneurysm presenting as back pain to a chiropractic clinic: a case report. J Manipulative Physiol Ther. 2006;29(5):409.e1–409.e7. doi: 10.1016/j.jmpt.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 16.de Boer NJ, Knaap SF, de Zoete A. Clinical detection of abdominal aortic aneurysm in a 74-year-old man in chiropractic practice. J Chiropr Med. 2010;9(1):38–41. doi: 10.1016/j.jcm.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiller L. Effectiveness of spinal manipulative therapy in the treatment of mechanical thoracic spine pain: a pilot randomized clinical trial. J Manipulative Physiol Ther. 2001;24(6):394–401. doi: 10.1067/mmt.2001.116420. [DOI] [PubMed] [Google Scholar]
- 18.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14(5):531–538. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]