Abstract
Objective
The aim of this study was to assess the methods to conduct a substantive clinical trial to evaluate the effects of accessory joint mobilization (AJM) vs neural mobilization (NM) techniques for shoulder motion restriction after breast cancer surgery.
Methods
This pilot study was a prospective randomized and double-blind clinical trial in which 18 women who underwent unilateral breast cancer surgery and axillary lymph node dissection participated. The study was conducted at the Women’s Health Research Group at the Physical Therapy Department of Alcalá University, Madrid, Spain. The intervention was AJM vs NM, with a 6-month follow-up. Primary outcomes included recruitment, adherence to treatment and retention rates, assessment procedures, and implementation of the 2 manual therapy techniques. Secondary outcomes included range of motion, sensory disturbance, pain, and upper limb functionality.
Results
All participants accepted to be randomly assigned to study groups. One hundred percent retention was attained with all participants attending the 3-month and 6-month assessments. Adherence with treatment attendance was excellent. At 6-month follow-up, flexion range of motion had a mean change of 38.4° (±28.9) (P = .002) in the AJM group and a mean change of 36.8° (±21.8) (P = .002) in the NM group. Abduction range of motion had a median change of 52.4° (±43.6) (P = .004) in AJM group and a median change of 44° (±17.5) (P = .012).
Conclusions
These preliminary results of the effects of AJM and NM techniques in breast cancer survivors indicate that a full clinical trial will be worthwhile. The research methods tested and the modifications proposed within this pilot study offer a suitable foundation to conduct a substantive clinical trial.
Key Indexing Terms: Breast Cancer, Physical Therapy, Accessory Joint Mobilization, Neural Mobilization, Shoulder, Joint Range of Motion
Introduction
The incidence of breast cancer (BC) among women has continued to increase within the last decade in spite of screening mammography and the reduction of mortality.1 The annual incidence is 110 in 100 000 women in all age groups in Europe2; similarly, BC affects 1 in 8 US women in their lifetime.3 Although 5-year survival rates now approximate 90% of women diagnosed,1 quality of life after treatment is a key concern and carries a significant burden of disease.2
Approximately 40% of BC survivors have metastasis of the axillary lymph nodes,4 indicating that cancer has possibly spread beyond the breast.5 Axillary lymph node dissection (ALND) is commonly employed as a procedure for diagnosing and treating positive lymph nodes.4 However, several consequences may develop after breast surgery and ALND, such as axillary web syndrome,6, 7, 8 frozen shoulder,9 numbness, shoulder pain and range of motion (ROM) restriction,5, 6, 10 lymphostasis,9 and lymphedema.8 Shoulder ROM restriction has previously been recorded in 21% to 30% of survivors11, 12 and is a significant impairment after ALND.12 More recently, a less aggressive axillary approach termed sentinel lymph node biopsy can be undertaken where possible, such as for tumors smaller than 5 cm.3 Despite the fact that sentinel lymph node biopsy involves less morbidity than ALND10 and the postoperative consequences are less common,6, 9, 10 postoperative impairments can still be present.7, 13 The subsequent effects of surgical scarring and the radiation-induced fibrosis may disturb the mechanics of the shoulder region through tethering of soft tissue or pain-inhibited movement, leading to shoulder ROM restriction.14
Besides ROM restriction, pain is one of the most common and recurrent symptoms after BC surgery with ALND, being reported by 30% to 40% of BC survivors.11 One common source of pain is the peripheral nervous system. The intercostobrachial, medial brachial cutaneous, and third and fourth intercostal nerves may be injured or become inflamed during surgery, leading to a sensory disturbance in the medial aspect of the arm.2, 15, 16, 17 This may contribute to persistent pain after BC surgery, identified in 20% to 65% of patients.5, 18 This condition tends to spread through the breast, axilla, and upper limb, and neuropathic pain is a commonly type of pain in BC19 that may lead to increased neural mechanosensitivity20, 21 and functional impairment.18 However, according to Nijs et al,22 cancer treatment may lead not only to neuropathic pain but also to predominant nociceptive pain or central sensitization pain. Indeed, the pathophysiology of pain after BC is multifactorial and may include psychological factors, such as depression, pain catastrophizing, and psychological distress,17, 18 and treatment factors, such as radiotherapy18 and ALND.17, 18
Physical therapy consistently has been reported to be an effective approach for dealing with postoperative BC impairments as well as with quality of life in these BC survivors.5, 9, 15, 23, 24, 25, 26, 27, 28 The aims are to address impairments such as shoulder ROM restriction, pain, and weakness10 and deal with likely lymphatic impairments.8 Physical therapy treatments to improve shoulder ROM restriction comprise general physical therapy5, 15, 28 and exercise programs,23, 26, 27, 29, 30, 31, 32 and both have proven beneficial.5, 15, 23, 26, 27, 29, 30, 31, 32 To date, manual therapy has not been evaluated for its effects on shoulder ROM restriction after BC surgery despite the potential to alleviate pain,33 reduce neural tissue sensitivity,34 and improve movement.33 Therefore, we suggest studying the effects of 2 manual therapy techniques, accessory joint mobilization (AJM) and neural mobilization (NM). Accessory joint mobilization is delivered to relieve pain by stimulating peripheral mechanoreceptors and modulating nociceptors and to improve joint mobility by enabling exchange between synovial fluid and cartilage matrix.35 Its positive effects on shoulder ROM restriction were reported within other conditions.35, 36, 37
Peripheral nerve damage after BC surgery has been widely reported,2, 15, 16, 20, 21 but no physical treatment for subsequent impairments has been described yet. Two observational studies described an increased neural mechanosensitivity using the upper limb neurodynamic test 1 (ULNT1) in BC survivors.20, 21 This increased neural mechanosensitivity may arise after nerve damage20, 21 and lead to shoulder ROM restriction as a protective neural response to movement or traction.38 Neural mobilization is a manual therapy technique to reduce neural mechanosensitivity and resolve pain and functional impairment39, 40 and has been reported to be effective in other conditions.34, 37, 41, 42
Our hypotheses is that manual therapy may provide greater and quicker shoulder ROM improvement than current BC treatments because of its effects in modulating neurophysiological mechanisms33, 43 that seem to be disturbed after BC surgery.14
The purpose of this pilot study was to test the feasibility for a full clinical trial to evaluate the effectiveness of AJM vs NM techniques for shoulder ROM restriction after BC surgery. The primary aims were to evaluate the feasibility of recruitment, randomization, retention, and assessment procedures and implementation of AJM and NM techniques. The secondary aim was to undertake a preliminary analysis of the effects of AJM and NM techniques on shoulder movement among BC survivors and to estimate the variability of the clinical outcomes (ROM, presence or absence of sensory disturbance, pain, and upper limb functionality) to facilitate future sample size calculation.
Methods
Design and Participants Recruitment
A pilot double-blind randomized study was conducted by the Women’s Health Research Group of Alcala University. Twenty-five women who underwent unilateral breast carcinoma surgery with ALND were recruited from September 2014 to June 2015 at Príncipe de Asturias Hospital, Madrid, Spain. The study was approved by the Local Ethical Committee, C.E.I.C. Hospital Príncipe de Asturias (Ethics Approval number 12/2009). The sample of study was made up of 18 women who fulfilled the inclusion criteria of unilateral BC diagnosis and ALND approach and provided informed consent. The exclusion criteria were bilateral BC diagnosis, locoregional recurrence, systemic disease, had not undergone ALND, or presenting with any contraindication for physical therapy. The research was conducted in accordance with the 1975 Helsinki Declaration. Protocols in human research personal data protection were fulfilled (Organic Law 15/99).
This study was registered on http://clinicaltrials.gov (NCT): 02366793.
Randomization
Women were randomly assigned to 2 groups by EPIDAT 3.1 software (Dirección Xeral de Saúde Pública de la Consellería de Sanidade, Xunta de Galicia, 2004): AJM was applied to one group, and the other group received the NM technique. Randomization was conducted by an independent physical therapist (M.T.L.).
Blinding
The assessor (I.D.L.R.D.) and the statistician (C.G.O.) remained blinded to treatment arm. Participants were also blinded to the intervention arm, because of the difficulty of distinguishing between techniques, and were simply informed that they would receive a manual therapy intervention.
Follow-up
After baseline assessment (A0), performed 1 or 2 days before surgery, 4 follow-up assessments were scheduled: the second assessment (A1) was before the physical therapy intervention (the day of hospital discharge: 3-6 days after surgery); the third assessment (A2) was performed after the physical therapy intervention; and the fourth (A3) and fifth assessments (A4) were performed at 3 and 6 months after the physical therapy intervention.
Study Interventions
Each participant received 9 sessions of physical therapy commencing on the discharge day (the third to sixth day after surgery) over the course of 3 weeks. Every session lasted 30 minutes. There were 2 treating physical therapists with 10 years of experience. One treating physical therapist (E.C.T.) delivered the AJM technique to one group, and the other physical therapist (C.D.D.C.G.R.) delivered the NM technique to the other group. The blinded assessor (I.D.L.R.D.) was another independent physical therapist with 9 years of experience.
The AJM technique consisted of 3 kinds of glenohumeral glides, described by Kalterborn44: anterior, posterior, and caudal glides. Participants remained in the supine position during the whole treatment. The techniques were delivered in a rhythmic way, with 2 seconds of glide/distraction and then a 2-second break. Each technique was carried out for 2 minutes.
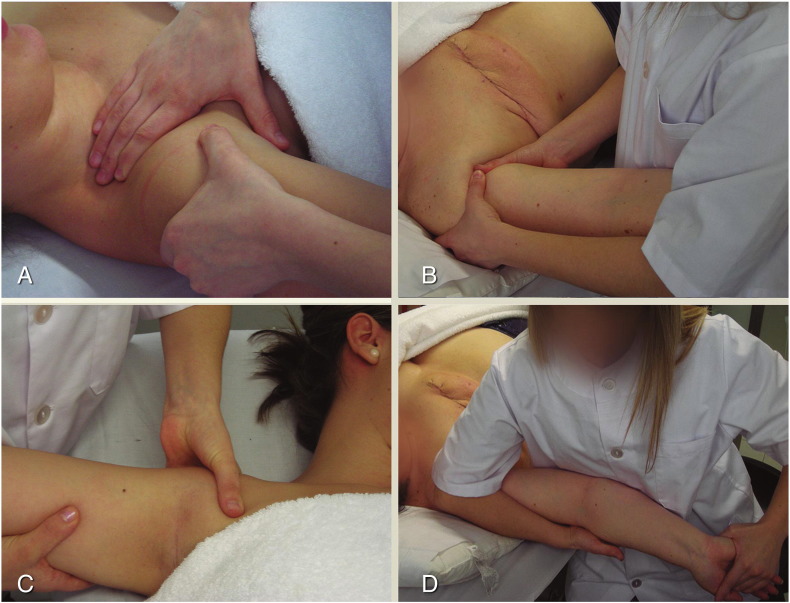
Another group received the NM technique. This consisted of neural tissue longitudinal glide using the ULNT1, the neurodynamic test sequence for the median nerve, described by Butler.40, 45, 46 With participants in the supine position, the shoulder was abducted and externally rotated, the scapula depressed, the forearm supinated, and the wrist and fingers extended. Mobilization was applied by depressing the scapula, flexing the elbow, and elevating the scapula, extending the elbow, within a pain-free range (Fig 1). The mobilization was applied for 2 minutes.
Fig 1.
Manual therapy techniques. AJM: anterior glenohumeral slide (A); AJM: posterior glenohumeral slide (B); AJM: caudal glenohumeral slide (C); NM: longitudinal median neural mobilization (ULNT1) (D). AJM, accessory joint mobilization; NM, neural mobilization; ULNT, upper limb neural mobilization test.
In addition, a lymphedema prevention physiotherapy protocol8 was delivered to the whole sample. This consisted of manual lymphatic drainage for postoperative edema (thorax, breast, axilla, proximal area of the affected upper limb) using the following techniques: scar massage; proprioceptive neuromuscular facilitation in diagonal patterns, progressing from passive movement to active assisted and subsequently active movement; and therapeutic education.
Outcomes of Study and Measurements
The primary outcomes were feasibility of recruitment, randomization, retention, intervention adherence, assessment procedures, and implementation of AJM vs NM.
Secondary outcomes were shoulder ROM, presence or absence of a sensory disturbance, pain, and upper limb functionality.
Recruitment and Randomization Data Collection
Reasons for exclusion, such as refusing to participate, not fulfilling inclusion criteria, and being unwilling to be randomly assigned to a treatment group, were gathered.
Intervention Adherence and Retention Data Collection
Adherence was measured by the number of sessions attended by participants and retention, by the number of assessments attained. Any complication within the treatment and assessment processes were recorded. Within the first assessment visit (preoperative), the assessor attempted to encourage participants to fulfill the 9 sessions of physical therapy and to attain the follow-up visits to confirm the duration of the intervention effect.
Clinical Outcomes Data Collection
The secondary outcomes were the clinical outcomes. The main clinical outcome was ROM of flexion, extension, abduction, adduction, and external and internal rotation shoulder movements, measured by inclinometry47 using the digital inclinometer baseline model (Fabrication Enterprises Inc., White Plains, NY). The presence or absence of a sensory disturbance, such as hypoesthesia, hyperesthesia, and dysesthesia, was assessed by subjective assessment. The affected area was determined using a body map.
Pain was measured using a visual analog scale (VAS) from 0 cm (no pain) to 10 cm (the most intense pain imaginable).48 Upper limb functionality was assessed by the Wingate Daily Life Activities Table,49 as used by other Spanish studies to measure shoulder impairments in BC,8 because this table measures the upper limb function related to shoulder impairment specifically.49 The Wingate Daily Life Activities Table assesses the degree of difficulty in performing each activity, from 0 points (no difficulty) to 3 points (unable). Glenohumeral joint capsule movement was assessed by the anterior, posterior, and caudal glenohumeral glides.44 The manual examination was performed to detect the presence or absence of glenohumeral joint dysfunction based on pain provocation.50 Cirtometry has gathered to identify changes in the ipsilateral upper limb volume, as measured by Torres et al.8 Diagnosis of lymphedema was conducted using previously published criteria.8
Statistical Analyses
Recruitment, retention, and adherence rates and percentage of participants randomly assigned were described. The intraparticipant effects were analyzed by the paired Student t test for parametric data and Wilcoxon test for nonparametric data. Between-group differences were analyzed using an unpaired Student t test for parametric data or the Mann-Whitney U test for nonparametric data.
The level of significance was fixed with α = .05. All statistical analyses were carried out using SPSS software, version 15.0 for Windows. The sample homogeneity was studied to determine differences between the groups related to age, body mass index, adjuvant treatment administration (hormonal treatment, chemotherapy, radiotherapy), and handedness of the affected upper limb.
Results
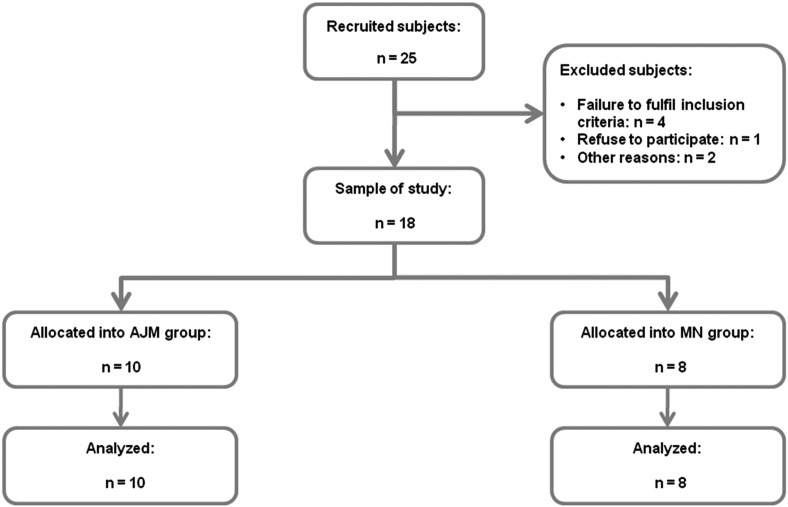
Figure 2 shows the flow of the participants throughout the study. Fifty BC survivors were screened for eligibility, and 25 of them (50%) were potentially eligible for the study. After excluding 7 people (14%), 18 participants (36%) were randomly assigned to AJM group or NM group: 10 in the AJM group and 8 in the NM group. Overall, all participants attained the 9 physical therapy sessions. Thus, adherence to interventions was 100%. Retention was 100% because the 18 participants completed all assessments. No complications were observed within implementation of AJM or NM. However, the assessment of pain by VAS seemed to be insufficient to assess sensory disturbance changes within the follow-up.
Fig 2.
Participants’ flow throughout the study process. AJM, accessory joint mobilization; NM, neural mobilization.
Descriptive statistics are represented in Table 1. Groups were similar at baseline.
Table 1.
Participant Characteristics
| AJM (n = 10) | NM (n = 8) | P | |
|---|---|---|---|
| Age mean (SD) | 54.8 (15.6) | 45.9 (8.6) | .167a |
| BMI mean (SD) | 27.5 (6.5) | 25.6 (6.2) | .537a |
| Chemotherapy, yes/no | 9/1 | 8/0 | 1.000b |
| Radiotherapy, yes/no | 6/4 | 6/2 | .638b |
| Hormonal treatment, yes/no | 1/9 | 4/4 | .118b |
| Affected arm, right/left | 4/6 | 4/4 | 1.000b |
AJM, accessory joint mobilization; BMI, body mass index; NM, nerve mobilization.
Unpaired Student t test.
Fisher’s exact test.
Clinical Outcomes
Between-group differences for each time point are presented in Table 2, Table 3. Both groups had significant shoulder ROM restriction after surgery for flexion ROM (mean change of 33.4° in the AJM group, P = .001; mean change of 37.9° in the NM group, P < .001) and abduction ROM (median change of 46.5° in the AJM group, P = .005; median change of 50.5° in the NM group, P = .012) compared with baseline. Significant increases were identified at post-treatment assessment compared with postoperative assessment in flexion ROM (mean change of 27.5° in the AJM group, P = .003; mean change of 24.75° in the NM group, P < .001) and also in abduction ROM (mean change of 42° in the AJM group, P = .001; median change of 35.5° in the NM group, P = .025). At 6-month follow-up, flexion ROM had a mean change of 38.4° (±28.9; P = .002) in the AJM group and a mean change of 36.8° (±21.8; P = .002) in the NM group. Abduction ROM had a mean change of 52.4° (±43.6; P = .004) in the AJM group and a median change of 44° (±17.5; P = .012).
Table 2.
Differences Between Preoperative and Postoperative Assessments
| Accessory Joint Mobilization (n = 10) |
Neural Mobilization (n = 8) |
|||||||
|---|---|---|---|---|---|---|---|---|
| A0 | A1 | A0 – A1 (95% CI) | P | A0 | A1 | A0 – A1 (95% CI) | P | |
| ROM, degrees | ||||||||
| Flexion mean (SD) | 167.3 (14.4) | 133.9 (27) | 33.4 (17.8, 49) | .001a | 174.3 (6) | 136.4 (21.8) | 37.9 (23.5, 52.3) | <.001a |
| Abduction median (IQR) | 171 (155, 177.25) | 124.5 (96, 154.75) | 31 (9.5, 67.5) | .005b | 175 (173, 176.75) | 124.5 (110.25, 140.25) | 45.5 (33, 61.75) | .012b |
| Pain, 0-10 cm | ||||||||
| VAS median (IQR) | 0 (0, 2.25) | 5 (0, 6) | –2(–6, 0) | .033b | 0 (0, 2.25) | 4.5 (3.25, 5.75) | –4 (–5, –0.75) | .034b |
| UL Functionality (0-3 points), median (IQR) | ||||||||
| To comb | 0 (0, 0) | 1 (0, 1) | –1 (–1, 0) | .020b | 0 (0, 0) | 1 (0.25, 1) | –1 (–1, –0.25) | .014b |
| To get washed | 0 (0, 0) | 1 (0, 1) | –0.5 (–1, 0) | .025b | 0 (0, 0) | 1 (0.25, 1) | –1 (–1, –0.25) | .014b |
| To button the bra | 0 (0, 0.25) | 1 (0, 1) | 0 (–1, 0) | .046b | 0 (0, 0) | 1 (0, 1) | –1 (–1, 0) | .025b |
| To mop the floor | 0 (0, 0) | 1 (0.75, 1) | –1 (–1, 0) | .008b | 0 (0, 0) | 0.5 (0, 1) | –0.5 (–1, 0) | .046b |
| To clean windows | 0 (0, 0) | 1 (1, 1) | –1 (–1, –1) | .003b | 0 (0, 0) | 1 (0.25, 1) | –1 (–1, 0) | .034b |
| To hang up | 0 (0, 0) | 1 (1, 1) | –1 (–1, –1) | .003b | 0 (0, 0) | 1 (1, 1) | –1 (–1, –1) | NSb |
| To make the bed | 0 (0, 0) | 1 (0, 1) | –1 (–1, 0) | .014b | 0 (0, 0) | 1 (0, 1) | –1 (–1, 0) | .034b |
| To carry shopping cart | 0 (0, 0) | 1 (0, 1) | –1 (–1, 0) | .014b | 0 (0, 0) | 1 (0.25, 1) | –1 (–1, –0.25) | .020b |
| To put on the pullover | 0 (0, 0.25) | 1 (1, 1.25) | –1 (–1, –1) | .004b | 0 (0, 0) | 1 (1, 1) | –1 (–1, –1) | .008b |
| To take off the pullover | 0 (0, 0.25) | 1 (1, 1.25) | –1 (–1, –1) | .004b | 0 (0, 0) | 1 (1, 1) | –1 (–1, –1) | .011b |
| Leisure | 0 (0, 0) | 0 (0, 1) | 0 (–1, 0) | .046b | 0 (0, 0) | 0.5 (0, 1) | –0.5 (–1, 0) | .046b |
| Glenohumeral Glides | ||||||||
| PG (±) | 2/8 | 5/5 | — | NSc | 1/7 | 5/3 | — | NSc |
| AG (±) | 0/10 | 1/9 | — | NSc | 0/8 | 0/8 | — | — |
| CAU (±) | 1/9 | 2/8 | — | NSc | 1/7 | 0/8 | — | — |
Data presented as mean (SD) or median (IQR).
A0, preoperative assessment; A1, postoperative assessment; AG, anterior glenohumeral glide; CAU, caudal glenohumeral glide; CI, confidence interval; Glenohumeral Glides (±), number of participants who showed painful glide/normal glide; IQR, interquartile range; NS, not significant; PG, posterior glenohumeral glide; ROM, range of motion; SD, standard deviation; UL functionality, upper limb functionality, from 0 points (no difficulty) to 3 points (unable); VAS, visual analog scale, from 0 cm (no pain) to 10 cm (the most intense pain imaginable).
Paired Student’s t test.
Wilcoxon test.
Fisher’s exact test.
Table 3.
Between-Group Differences for Mean Change (SD) or Median Change (IQR) Scores: Accessory Joint Vs Neural Mobilization
| Accessory Joint Mobilization (n = 10) |
Neural Mobilization (n = 8) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 – A2 | P | A1 – A3 | P | A1 – A4 | P | A1 – A2 | P | A1 – A3 | P | A1 – A4 | P | |
| ROM, degrees | ||||||||||||
| Flexion, mean (SD) | –27.5 (21) | .003a | –34.6 (27.5) | .003a | –38.4 (28.9) | .002a | –24.75 (12.8) | .001a | –36 (19.4) | .001a | –36.8 (21.8) | .002a |
| Abd, mean (SD)/median (IQR) | –42 (28.47) | .001a | –49.6 (41.2) | .004a | –52.4 (43.6) | .004a | –35.5 (–50.25, 6.25) | .025b | –48 (–59.75, –37.75) | .025b | –44 (–55.5, –38) | .012b |
| Pain Relief, 0-10 cm | ||||||||||||
| VAS, median (IQR)/mean (SD) | 2.5 (0, 4.25) | .034b | 1.5 (0, 4.25) | .028b | 0.5 (0, 5.25) | .041b | 2.75 (2.5) | .034a | 1.87 (4.9) | .310a | 2.37 (2.67) | .043a |
| Functionality (0-3 points), median (IQR) | ||||||||||||
| To comb | 1 (0, 1) | .020b | 1 (0, 1) | .020b | 1 (0, 1) | .020b | 1 (0.25, 1) | .014b | 1 (0.25, 1) | .014b | 1 (0.25, 1) | .014b |
| To get washed | 1 (0, 1) | .014b | 1 (0, 1) | .014b | 1 (0, 1) | .014b | 1 (0.25, 1) | .014b | 1 (0.25, 1) | .014b | 1 (0.25, 1) | .014b |
| To button the bra | 1 (0, 1) | 0.020b | 1 (0, 1) | 0.020b | 0.5 (0, 1) | 0.236b | 1 (0, 1) | 0.025b | 1 (0, 1) | 0.025b | 1 (0, 1) | 0.025b |
| To mop the floor | 0.5 (0, 1) | .025b | 1 (0, 1) | .034b | 1 (0.75, 1) | .005b | 0 (0, 0.75) | .157b | 0.5 (0, 1) | .046b | 0.5 (0, 1) | .046b |
| To clean the windows | 0.5 (0, 1) | .025b | 1 (0, 1) | .008b | 1 (1, 1) | .002b | 0 (0, 1) | .257b | 0.5 (0, 1) | .059b | 1 (0.25, 1) | .020b |
| To hang up | 1 (0.75, 1) | .005b | 1 (0.75, 1) | .005b | 1 (1, 1) | .002b | 0.5 (0, 1) | .059b | 1 (0.25, 1) | .059b | 1 (1, 1) | .011b |
| To make the bed | 1 (0, 1) | .014b | 1 (0, 1) | .034b | 1 (0, 1) | .008b | 0.5 (0, 1) | .059b | 1 (0, 1) | .096b | 1 (0, 1) | .034b |
| To carry shopping cart | 1 (0, 1) | .008b | 1 (0, 1) | .034b | 1 (0, 1) | .008b | 1 (0, 1) | .034b | 1 (0.25, 1) | .020b | 1 (0.25, 1) | .020b |
| To put on the pullover | 1 (0.75, 1.25) | .008b | 1 (1, 1.25) | .005b | 1 (1, 1.25) | .003b | 1 (0.25, 1) | .014b | 1 (1, 1) | .008b | 1 (1, 1) | .008b |
| To take off the pullover | 1 (0.75, 1.25) | .008b | 1 (1, 1.25) | .005b | 1 (1, 1.25) | .003b | 1 (1, 1) | .008b | 1 (1, 1) | .011b | 1 (1, 1) | .011b |
| Leisure | 0 (0, 1) | .046b | 0 (0, 1) | .046b | 0 (0, 1) | .460b | 0.5 (0, 1) | .046b | 0.5 (0, 1) | .046b | 0.5 (0, 1) | .046b |
| Glenohumeral Glides | Pc | Pc | Pc | Pc | Pc | Pc | ||||||
| PG ± | 5/5 – 9/1 | NS | 5/5 – 8/2 | NS | 5/5 – 10/0 | – | 3/5 – 6/2 | NS | 3/5 – 6/2 | NS | 3/5 – 6/2 | NS |
| AG ± | 9/1 – 10/0 | — | 9/1 – 10/0 | — | 9/1 – 10/0 | — | 8/0 – 8/0 | — | 8/0 – 8/0 | — | 8/0 – 8/0 | — |
| CAU ± | 8/2 – 9/1 | NS | 8/2 – 10/0 | — | 8/2 – 10/0 | — | 8/0 – 8/0 | — | 8/0 – 8/0 | — | 8/0 – 8/0 | — |
A1, postoperative assessment; A2, post-treatment assessment; A3, 3-month follow-up assessment; A4, 6-month follow-up assessment; Abd median (IQR), median change (interquartile range) of abduction degrees in neural mobilization group; Abd mean (SD), mean change (standard deviation) of abduction degrees in accessory joint mobilization group; AG, anterior glenohumeral glide; CAU, caudal glenohumeral glide; flexion mean (SD), mean change (standard deviation) of flexion degrees; functionality median (IQR), median change (interquartile range) of points in upper limb functionality; glenohumeral glides (±), number of participants who had normal slide/altered glide; NS, not significant; PG, posterior glenohumeral head glide; ROM, range of motion; VAS median (IQR), median change (interquartile range) of centimeters of pain relief on visual analog scale.
Paired Student’s t test.
Wilcoxon test.
χ2 test.
Pain relief was statistically significant in both groups within the follow-up except in the NM group 3-month assessment. The median change in VAS was 2.5 cm (P = .034) in the AJM group, and 2.75 cm (P = .034) was the mean change obtained in the NM group. Significant improvements were identified for upper limb functionality in both groups (Table 3).
No between-group differences were identified for any of these outcomes.
Cirtometry measurements determined that no participant developed lymphedema because of the lack of difference in upper limb volume throughout the follow-up assessments.
With regard to sensory disturbance, no within- or between-group differences were identified. Nevertheless, the hypoesthesia of the medial aspect of the arm was determined to be the most common kind of sensory disturbance presented by these participants. It was reported by 34% of participants in AJM group and by 50% of participants in the NM group. The second most common kind of sensory disturbance was the dysesthesia of the same area.
Data for glenohumeral glides were not consistently related to a significant pattern of painful glide. Nevertheless, the posterior glide seemed to be the most commonly altered, with 10 participants having painful movement on application of the glide (Table 2).
Sample Size Calculation for a Substantive Clinical Trial
The standard deviations of ROM obtained by this pilot study enabled estimation of the sample size for a substantive clinical trial.
Sample size estimation to detect a clinically significant difference of at least 10°15 determined that, in a unilateral contrast at the 95% level with a power of 80, a sample of 60 participants is enough to determine the effect of AJM (a standard deviation of ±30.9 was assumed). To determine the effect of NM technique, 62 participants will be required (a standard deviation of ±31.6 was assumed). The replacement percentage that has been foreseen will be 0%.
Discussion
To our knowledge, this is the first pilot randomized trial on the effect of 2 manual therapy techniques on shoulder ROM restriction among BC survivors.
The primary aim of this study was to pilot the research methods, assessment procedures, and implementation of AJM vs NM mobilization proposed for a full randomized controlled trial (RCT). Although recruitment was achieved—that is, 25 participants who were eligible for the study accepted to be randomly assigned to a group—50% of the possible sample population were excluded because they did not fulfill the inclusion criteria. The fact that the 2 groups were interventional possibly improved willingness of participants to enroll in the study. Interventions adherence was excellent, and assessment procedures were adequate, with the exception of the sensory disturbance assessment. It seemed to be insufficient to assess the presence or absence of a sensory disturbance. Retention rates were excellent because all participants attended all follow-up assessments. High levels of adherence and retention might be related to flexibility afforded to scheduling of the visits according to the participants’ preferences.
Shoulder flexion and abduction were the most noticeably impaired movements after surgery, similar to findings of previous studies.6, 10, 15, 16, 23 Regarding ROM improvements at 6-month follow-up, flexion improved by a mean change of 38.4° in the AJM group and 36.8° in the NM group. Shoulder abduction demonstrated a mean change of 52.4° in the AJM group, and 44° of median change was obtained in the NM group. Both flexion and abduction improvements were clinically significant—that is, they increased >10% ROM.23 Compared with previous studies,2, 15, 23, 25, 26, 27, 28 this study has identified greater shoulder ROM improvement than that achieved after 20 complex decongestive physical therapy sessions, where flexion and abduction ROM improvement were, respectively, 34.7° and 36.6°.25 That difference is particularly significant because our BC participants attained a greater shoulder ROM improvement within 9 physical therapy sessions. The same can be observed in the results published by Rezende et al.23 They achieved 31° and 38° of flexion and abduction ROM improvement after 42 sessions of guided-exercises program.23 Similar findings were reported by Kilgour et al26 within 11 sessions. However, certain studies achieved similar shoulder ROM improvement compared with the current study, though further physical therapy sessions were needed as well.5, 15, 28 In addition, all of these studies5, 15, 23, 24, 25, 26, 27, 28 had certain biases, such as a nondetailed intervention procedure,5, 15, 24 short-term follow-up,5, 24, 25, 26, 28 and lack of blinding.23, 24, 25 In cases of exercise approach, difficulties in adherence to the treatment have been noticeably discouraging.32
Concerning the NM group data, we reported that NM may enable the recovery of ROM,39, 40 agreeing with the results of other studies.34, 37, 41, 42 Neural mobilization may improve restricted nerve gliding, reduce scar formation, increase intraneural blood flow,39 and diminish intraneural edema42 and spinal hypersensitivity.43 These effects may normalize neural mechanosensitivity and increase the nerve membrane threshold under compressive or traction stimuli, enabling a nonpainful and larger ROM.38
Regarding upper limb functionality and pain, both techniques produced significant improvement, which was likely clinically significant—that is, a decrease of 1.5 cm in VAS score,51 similar to findings from other conditions.35, 37
Considerations and Limitations
The preliminary results of this study are limited by the small sample size; however, given the pilot nature of this study, this sample was sufficient to test the feasibility of a larger RCT. The lack of a control group is also a limitation, and a control group would be employed in a larger trial. This absence was acceptable because the main objective was to pilot the methods proposed, to estimate the variability of the participants’ reported outcomes, and to pilot the effects of these manual therapy approaches in BC survivors to know if a full RCT is worthwhile. Further, compared with control data from other studies, the improvements observed in this study were greater than control groups in other studies,5, 15, 23, 26, 27, 28 further justifying a larger trial. Given the limitations of the sensory disturbance assessment, future trials should include other measures of neuropathic sensitization, such as a neuropathic screening questionnaire, or follow the International Association for the Study of Pain’s neuropathic pain classification system.52 Additionally, it is suggested to include the neural mechanosensitivity assessment using the ULNT1, as previously reported in BC research.20, 21
Conclusions
The effects of AJM and NM techniques on shoulder ROM restriction among BC participants offered a suitable foundation to conduct a full RCT to determine whether manual therapy can achieve greater ROM, reduce BC rehabilitation times, and improve patient outcomes. More specifically, by comparing joint mobilization with NM, further elucidation of the most effective techniques within this population can be achieved. The research methods, assessment procedures, and implementation of the manual therapy approaches proposed in this pilot study, with some considerations here suggested, may define the full RCT design.
Funding Sources and Conflicts of Interest
The following provided support for this study: Gynecology Service of Principe de Asturias University Hospital (Madrid, Spain): referral of patients; Physical Therapy Research Unit at Alcala University (Madrid, Spain): provision of material and space to treat patients; Physical Therapy Department of Alcala University (Madrid, Spain and Principe de Asturias Hospital (Madrid, Spain): facility the performance of the study. No conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): I.D.L.R.D., M.T.L.
Design (planned the methods to generate the results): I.D.L.R.D., M.T.L.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): I.D.L.R.D., M.T.L.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): I.D.L.R.D., E.C.T., C.D.D.C.G.R.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): C.G.O., I.D.L.R.D.
Literature search (performed the literature search): I.D.L.R.D.
Writing (responsible for writing a substantive part of the manuscript): I.D.L.R.D.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): I.D.L.R.D., M.T.L., E.C.T., C.D.D.C.G.R., C.G.O.
Provision of patients and randomization conduction: M.T.L.
Grammar revision: N.M.
Practical Applications
-
•
This pilot study is the first to evaluate the application of manual therapy to improve shoulder motion in BC survivors.
-
•
We wished to test if manual therapy would provide greater and quicker shoulder motion improvement than current BC treatments because of its effects in modulating neurophysiological mechanisms that seem to be disturbed after BC surgery.
-
•
This study provided information about feasibility of recruitment, randomization, retention, assessment procedures, and implementation of AJM and NM techniques.
-
•
The preliminary results of the effects of AJM and NM techniques on shoulder motion restriction among our BC participants offered a suitable foundation to conduct a full randomized clinical trial to determine whether manual therapy can achieve greater ROM and shorten BC rehabilitation times.
Alt-text: Image 1
References
- 1.Sánchez MC, López N. ERGON; Madrid: 2008. Cáncer de Mama. Temas Actuales. [Google Scholar]
- 2.Vetter M, Rochlitz C. Breast cancer: treatment guidelines and new treatment options in 2012, a medical oncology prospective. Ther Umsch. 2012;69(10):577–854. doi: 10.1024/0040-5930/a000333. [DOI] [PubMed] [Google Scholar]
- 3.Rashid OM, Takabe K. Sentinel lymph node biopsy for breast cancer: our technique and future directions in lymph node staging. J Nucl Med Radiat Ther. 2012;2012(S2) doi: 10.4172/2155-9619.S2-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Roozendaal LM, de Wilt JH, van Dalen T, van der Hage JA, Strobbe LJ, Boersma LJ. The value of completion axillary treatment in sentinel node positive breast cancer patients undergoing a mastectomy: a Dutch randomized controlled multicentre trial (BOOG 2013-07) BMC Cancer. 2015;15:610. doi: 10.1186/s12885-015-1613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beurskens CH, van Uden CJ, Strobbe LJ, Oostendorp RA, Wobbes T. The efficacy of physiotherapy upon shoulder function following axillary dissection in breast cancer, a randomized controlled study. BMC Cancer. 2007;7:166. doi: 10.1186/1471-2407-7-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leidenius M, Leppanen E, Krogerus L, von Smitten K. Motion restriction and axillary web syndrome after sentinel node biopsy and axillary clearance in breast cancer. Am J Surg. 2003;185(2):127–130. doi: 10.1016/s0002-9610(02)01214-x. [DOI] [PubMed] [Google Scholar]
- 7.O'Toole J, Miller CL, Specht MC, Skolny MN, Jammallo LS, Horick N. Cording following treatment for breast cancer. Breast Cancer Res Treat. 2013;140(1):105–111. doi: 10.1007/s10549-013-2616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres Lacomba M, Yuste Sanchez MJ, Zapico Goni A, Prieto Merino D, Mayoral del Moral O, Cerezo Tellez E. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ. 2010;340:b5396. doi: 10.1136/bmj.b5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheville AL, Tchou J. Barriers to rehabilitation following surgery for primary breast cancer. J Surg Oncol. 2007;95(5):409–418. doi: 10.1002/jso.20782. [DOI] [PubMed] [Google Scholar]
- 10.Lauridsen MC, Overgaard M, Overgaard J, Hessov IB, Cristiansen P. Shoulder disability and late symptoms following surgery for early breast cancer. Acta Oncol. 2008;47(4):569–575. doi: 10.1080/02841860801986627. [DOI] [PubMed] [Google Scholar]
- 11.Dahl AA, Nesvold IL, Reinertsen KV, Fossa SD. Arm/shoulder problems and insomnia symptoms in breast cancer survivors: cross-sectional, controlled and longitudinal observations. Sleep Med. 2011;12(6):584–590. doi: 10.1016/j.sleep.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Devoogdt N, Van Kampen M, Christiaens MR, Troosters T, Piot W, Beets N. Short- and long-term recovery of upper limb function after axillary lymph node dissection. Eur J Cancer Care (Engl) 2011;20(1):77–86. doi: 10.1111/j.1365-2354.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 13.Schrenk P, Rieger R, Shamiyeh A, Wayand W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer. 2000;88(3):608–614. doi: 10.1002/(sici)1097-0142(20000201)88:3<608::aid-cncr17>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 14.Crosbie J, Kilbreath SL, Dylke E, Refshauge KM, Nicholson LL, Beith JM. Effects of mastectomy on shoulder and spinal kinematics during bilateral upper-limb movement. Phys Ther. 2010;90(5):679–692. doi: 10.2522/ptj.20090104. [DOI] [PubMed] [Google Scholar]
- 15.Box RC, Reul-Hirche HM, Bullock-Saxton JE, Furnival CM. Shoulder movement after breast cancer surgery: results of a randomised controlled study of postoperative physiotherapy. Breast Cancer Res Treat. 2002;75(1):35–50. doi: 10.1023/a:1016571204924. [DOI] [PubMed] [Google Scholar]
- 16.Blanchard DK, Donohue JH, Reynolds C, Grant CS. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg. 2003;138(5):482–487. doi: 10.1001/archsurg.138.5.482. discussion 487-488. [DOI] [PubMed] [Google Scholar]
- 17.Bruce J, Thornton AJ, Powell R, Johnston M, Wells M, Heys SD. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155(2):232–243. doi: 10.1016/j.pain.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Andersen KG, Duriaud HM, Jensen HE, Kroman N, Kehlet H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain. 2015;156(12):2413–2422. doi: 10.1097/j.pain.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 19.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725–746. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Smoot B, Boyd BS, Byl N, Dodd M. Mechanosensitivity in the upper extremity following breast cancer treatment. J Hand Ther. 2014;27(1):4–11. doi: 10.1016/j.jht.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caro-Morán E, Díaz-Rodríguez L, Cantarero-Villanueva I, Galiano-Castillo N, Arroyo-Morales M, Fernández-Lao C. Nerve pressure pain hypersensitivity and upper limb mechanosensitivity in breast cancer survivors: a case–control study. Pain Med. 2014;15(10):1715–1723. doi: 10.1111/pme.12567. [DOI] [PubMed] [Google Scholar]
- 22.Nijs J, Leysen L, Adriaenssens N, Aguilar Ferrandiz ME, Devoogdt N, Tassenoy A. Pain following cancer treatment: guidelines for the clinical classification of predominant neuropathic, nociceptive and central sensitization pain. Acta Oncol. 2016;55(6):659–663. doi: 10.3109/0284186X.2016.1167958. [DOI] [PubMed] [Google Scholar]
- 23.de Rezende LF, Franco RL, de Rezende MF, Beletti PO, Morais SS, Gurgel MS. Two exercise schemes in postoperative breast cancer: comparison of effects on shoulder movement and lymphatic disturbance. Tumori. 2006;92(1):55–61. doi: 10.1177/030089160609200109. [DOI] [PubMed] [Google Scholar]
- 24.Gosselink R, Rouffaer L, Vanhelden P, Piot W, Troosters T, Christiaens MR. Recovery of upper limb function after axillary dissection. J Surg Oncol. 2003;83(4):204–211. doi: 10.1002/jso.10271. [DOI] [PubMed] [Google Scholar]
- 25.Atalay OT, Ozkir A, Calik BB, Baskan E, Taskin H. Effects of phase I complex decongestive physiotherapy on physical functions and depression levels in breast cancer related lymph edema. J Phys Ther Sci. 2015;27(3):865–870. doi: 10.1589/jpts.27.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilgour RD, Jones DH, Keyserlingk JR. Effectiveness of a self-administered, home-based exercise rehabilitation program for women following a modified radical mastectomy and axillary node dissection: a preliminary study. Breast Cancer Res Treat. 2008;109(2):285–295. doi: 10.1007/s10549-007-9649-x. [DOI] [PubMed] [Google Scholar]
- 27.Bendz I, Fagevik Olsen M. Evaluation of immediate versus delayed shoulder exercises after breast cancer surgery including lymph node dissection—a randomised controlled trial. Breast. 2002;11(3):241–248. doi: 10.1054/brst.2001.0412. [DOI] [PubMed] [Google Scholar]
- 28.Na YM, Lee JS, Park JS, Kang SW, Lee HD, Koo JY. Early rehabilitation program in postmastectomy patients: a prospective clinical trial. Yonsei Med J. 1999;40(1):1–8. doi: 10.3349/ymj.1999.40.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Anderson RT, Kimmick GG, McCoy TP, Hopkins J, Levine E, Miller G. A randomized trial of exercise on well-being and function following breast cancer surgery: the RESTORE trial. J Cancer Surviv. 2012;6(2):172–181. doi: 10.1007/s11764-011-0208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan DN, Lui LY, So WK. Effectiveness of exercise programmes on shoulder mobility and lymphoedema after axillary lymph node dissection for breast cancer: systematic review. J Adv Nurs. 2010;66(9):1902–1914. doi: 10.1111/j.1365-2648.2010.05374.x. [DOI] [PubMed] [Google Scholar]
- 31.Kilbreath S. Early postoperative exercise improves shoulder range of motion in women with breast cancer compared with delayed exercise, but increases wound drainage volume and duration. Evid Based Nurs. 2011;14(1):2. doi: 10.1136/ebn.14.1.2. [DOI] [PubMed] [Google Scholar]
- 32.Latka RN, Alvarez-Reeves M, Cadmus L, Irwin ML. Adherence to a randomized controlled trial of aerobic exercise in breast cancer survivors: the Yale exercise and survivorship study. J Cancer Surviv. 2009;3(3):148–157. doi: 10.1007/s11764-009-0088-z. [DOI] [PubMed] [Google Scholar]
- 33.Page MJ, Green S, Kramer S, Johnston RV, McBain B, Chau M. Manual therapy and exercise for adhesive capsulitis (frozen shoulder) Cochrane Database Syst Rev. 2014;(8):CD011275. doi: 10.1002/14651858.CD011275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nee RJ, Vicenzino B, Jull GA, Cleland JA, Coppieters MW. Neural tissue management provides immediate clinically relevant benefits without harmful effects for patients with nerve-related neck and arm pain: a randomised trial. J Physiother. 2012;58(1):23–31. doi: 10.1016/S1836-9553(12)70069-3. [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen HM, Rozing PM, Obermann WR, le Cessie S, Vliet Vlieland TP. Comparison of high-grade and low-grade mobilization techniques in the management of adhesive capsulitis of the shoulder: randomized controlled trial. Phys Ther. 2006;86(3):355–368. [PubMed] [Google Scholar]
- 36.Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;38(1):114–119. doi: 10.1177/0363546509346050. [DOI] [PubMed] [Google Scholar]
- 37.Tal-Akabi A, Rushton A. An investigation to compare the effectiveness of carpal bone mobilisation and neurodynamic mobilisation as methods of treatment for carpal tunnel syndrome. Man Ther. 2000;5(4):214–222. doi: 10.1054/math.2000.0355. [DOI] [PubMed] [Google Scholar]
- 38.Schmid AB, Brunner F, Luomajoki H, Held U, Bachmann LM, Kunzer S. Reliability of clinical tests to evaluate nerve function and mechanosensitivity of the upper limb peripheral nervous system. BMC Musculoskelet Disord. 2009;10:11. doi: 10.1186/1471-2474-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coppieters MW, Butler DS. Do ‘sliders' slide and ‘tensioners' tension? An analysis of neurodynamic techniques and considerations regarding their application. Man Ther. 2008;13(3):213–221. doi: 10.1016/j.math.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Butler D. Noigroup Publications; Adelaida: 2000. The Sensitive Nervous System. [Google Scholar]
- 41.Fernandez-de-Las-Penas C, Cleland JA, Ortega-Santiago R, de-la-Llave-Rincon AI, Martinez-Perez A, Pareja JA. Central sensitization does not identify patients with carpal tunnel syndrome who are likely to achieve short-term success with physical therapy. Exp Brain Res. 2010;207(1-2):85–94. doi: 10.1007/s00221-010-2436-7. [DOI] [PubMed] [Google Scholar]
- 42.Schmid AB, Elliott JM, Strudwick MW, Little M, Coppieters MW. Effect of splinting and exercise on intraneural edema of the median nerve in carpal tunnel syndrome—an MRI study to reveal therapeutic mechanisms. J Orthop Res. 2012;30(8):1343–1350. doi: 10.1002/jor.22064. [DOI] [PubMed] [Google Scholar]
- 43.Lim EC, Sterling M, Pedler A, Coombes BK, Vicenzino B. Evidence of spinal cord hyperexcitability as measured with nociceptive flexion reflex (NFR) threshold in chronic lateral epicondylalgia with or without a positive neurodynamic test. J Pain. 2012;13(7):676–684. doi: 10.1016/j.jpain.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Kalterborn FM. 10th ed. McGraw-Hill Interamericana; Madrid: 1999. Fisioterapia Manual. Extremidades. [Google Scholar]
- 45.Shacklock M. Butterworth-Heinemann; Edinburgh: 2005. Clinical Neurodynamics. A New System of Musculoskeletal Treatment. [Google Scholar]
- 46.Zamorano ZE. Médica Panamericana; Madrid: 2013. Movilización Neuromeníngea. Tratamiento de los Trastornos Mecanosensitivos del Sistema Nervioso. [Google Scholar]
- 47.Teschke K, Trask C, Johnson P, Chow Y, Village J, Koehoorn M. Measuring posture for epidemiology: comparing inclinometry, observations and self-reports. Ergonomics. 2009;52(9):1067–1078. doi: 10.1080/00140130902912811. [DOI] [PubMed] [Google Scholar]
- 48.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 49.Wingate L. Efficacy of physical therapy for patients who have undergone mastectomies. A prospective study. Phys Ther. 1985;65(6):896–900. doi: 10.1093/ptj/65.6.896. [DOI] [PubMed] [Google Scholar]
- 50.Schneider GM, Jull G, Thomas K, Smith A, Emery C, Faris P. Intrarater and interrater reliability of select clinical tests in patients referred for diagnostic facet joint blocks in the cervical spine. Arch Phys Med Rehabil. 2013;94(8):1628–1634. doi: 10.1016/j.apmr.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 51.Kovacs FM, Abraira V, Royuela A, Corcoll J, Alegre L, Tomas M. Minimum detectable and minimal clinically important changes for pain in patients with nonspecific neck pain. BMC Musculoskelet Disord. 2008;9:43. doi: 10.1186/1471-2474-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haanpaa M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]