Abstract
Objective
The purpose of this study is to review the effectiveness of the role of whole grain as a therapeutic agent in type 2 diabetes, cardiovascular disease, cancer, and obesity.
Methods
An umbrella review of all published meta-analyses was performed. A PubMed search from January 1, 1980, to May 31, 2016, was conducted using the following search strategy: (whole grain OR whole grains) AND (meta-analysis OR systematic review). Only English language publications that provided quantitative statistical analysis on type 2 diabetes, cardiovascular disease, cancer, and weight loss were retrieved.
Results
Twenty-one meta-analyses were retrieved for inclusion in this umbrella review, and all the meta-analyses reported statistically significant positive benefits for reducing the incidence of type 2 diabetes (relative risk [RR] = 0.68-0.80), cardiovascular disease (RR = 0.63-0.79), and colorectal, pancreatic, and gastric cancers (RR = 0.57-0.94) and a modest effect on body weight, waist circumference, and body fat mass. Significant reductions in cardiovascular and cancer mortality were also observed (RR = 0.82 and 0.89, respectively). Some problems of heterogeneity, publication bias, and quality assessment were found among the studies.
Conclusion
This review suggests that there is some evidence for dietary whole grain intake to be beneficial in the prevention of type 2 diabetes, cardiovascular disease, and colorectal, pancreatic, and gastric cancers. The potential benefits of these findings suggest that the consumption of 2 to 3 servings per day (~45 g) of whole grains may be a justifiable public health goal.
Key Indexing Terms: Whole Grains, Meta-analysis, Diabetes Mellitus, Cardiovascular Diseases, Neoplasms, Obesity
Introduction
Dietary whole grain consumption has been postulated to reduce the risk of type 2 diabetes, cardiovascular disease, colorectal cancer, and obesity.1 A whole grain kernel contains the endosperm, germ, and bran. The bran’s outer coating is rich in fiber and the inner germ contains vitamins, minerals, lignans, and phytochemicals (phenolic acids, polyphenols, and phytosterol compounds). Examples of whole grains include whole wheat, dark bread, brown rice, oats, barley, and rye. In the grain-refining process the most potent protective components of whole grains found in the bran and germ are removed, leaving behind the only the starch-rich endosperm.
There was a significant inverse association between dietary whole grain intake and all-cause mortality when comparing participants with dietary whole grain intakes in the top quintile to those whose intakes were in the bottom quintile.2, 3 Therefore, it is believed that a deficiency in dietary whole grain intake might contribute to the epidemics of type 2 diabetes, cardiovascular disease, cancer, and obesity.3
There is much discrepancy when it comes to randomized controlled studies on the effects of whole grain on clinical endpoints. Several studies on dietary whole grain intake in relation to type 2 diabetes risk have reported inverse associations with higher intake, but some studies found no significant association.4 This discrepancy may be due to differences in study design, selected populations, and the type and amount of whole grain foods consumed. Given the inconsistency of the existing literature and the insufficient statistical power because of small sample sizes, a pooling of information from individual trials could provide a more precise and accurate estimate of whole grain’s role in ameliorating chronic diseases such as type 2 diabetes, cardiovascular disease, cancer, and obesity. To achieve this result, many investigators have turned to performing a powerful statistical method known as meta-analysis. Meta-analyses are fundamental to provide the highest level of evidence to best inform health care decision making. Therefore, the purpose and objective of this paper is to summarize the evidence from previously published meta-analyses regarding the effectiveness of the role of whole grain as a therapeutic dietary agent.
Methods
An umbrella review was selected for this study. An umbrella review provides a summary of existing published meta-analyses and systematic reviews and determines whether authors addressing similar review questions independently observe similar results and arrive at similar conclusions.5
A systematic literature search of PubMed, Cochrane Library, and CINAHL from January 1, 1980, to May 31, 2016, was conducted using the following search strategy: (whole grain OR whole grains) AND (meta-analysis OR systematic review). Only English language publications that provided quantitative statistical analysis on type 2 diabetes, cardiovascular disease, cancer, and weight loss were retrieved. Meta-analyses or systematic reviews that did not present study-specific summary data using a minimum of 4 randomized controlled trials were excluded.
For the published meta-analyses that were accepted into this review, the following information was extracted and entered into an Excel spreadsheet: number of publications included in the meta-analysis, number of total participants, whole grain daily dose, pooled treatment effects for clinical endpoints (such as serum glucose, insulin, or cholesterol concentrations), and summary of relative risks (RRs) and odds ratios (ORs). Because this is a descriptive summary review of meta-analyses, no statistical analyses were performed.
Papers were also assessed for their disclosure of quality assessment, statistical heterogeneity (Cochran’s Q test and I2 statistic), and publication bias (visual inspection of funnel plots and Egger or Begg regression test).
Results
The initial search strategy identified 126 articles and after careful review 22 meta-analyses were retrieved for inclusion into this umbrella review.4, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 One meta-analysis was excluded because it was not published in English; this meta-analysis investigated the use of whole grains on intestinal motility for bowel function disorders.26
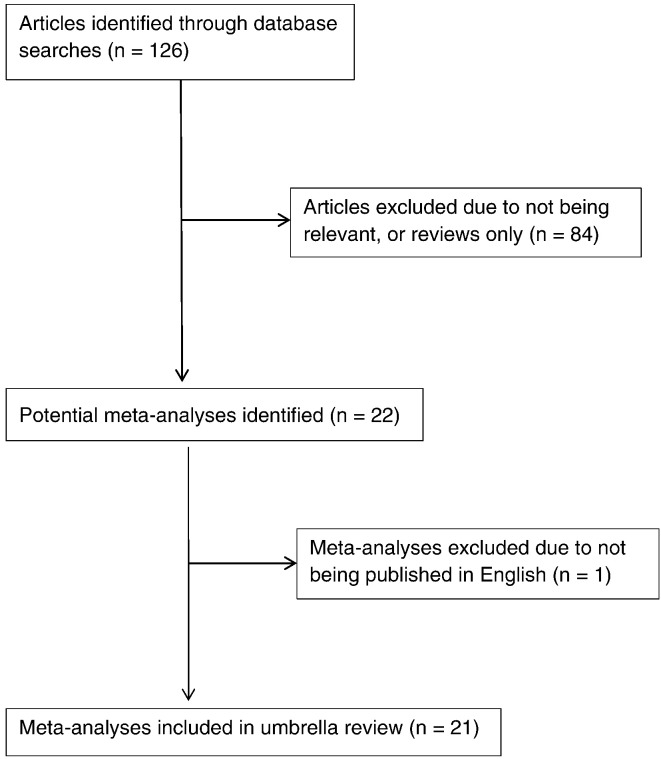
A flow chart of the meta-analyses selection process is provided in Figure 1, and Table 1, Table 2, Table 3, and 4 provide the detailed analysis of the 21 meta-analyses entered into this umbrella review.
Fig 1.
Flow chart of meta-analysis selection.
Table 1.
Summary of Meta-analyses on Glucose and Insulin Concentrations and Type 2 Diabetes Incidence
| Meta-analysis Authors and Date | No. of Studies in Meta-analysis | No. of Participants in Meta-analysis | Average Servings or Amount per Day | Main Findings of Meta-analysis | Q Test P Value | I2 Statistic | Egger or Begg Test P Value |
|---|---|---|---|---|---|---|---|
| Nettleton et al 20106 | 14 | 48 723 | 1.5 | Glucose ↓ 0.02 mmol/L, P < .0001; insulin ↓ 0.02 pmol/L, P < .0001 | NR | 10% | NR |
| de Munter et al 20077 | 6 | 286 125 | 2 or 30 g | Type 2 diabetes RR = 0.79, P < .05 |
.009 | 68% | .30 |
| Aune et al 20134 | 10 | 385 868 | 3 or 45 g | Type 2 diabetes RR = .68, P < .0001 |
.0001 | 82% | .49 |
| Chanson-Rolle et al 20158 | 8 | 316 051 | 2 or 30 g | Type 2 diabetes RR = .80, P < .0001 |
.99 | NR | .70 |
| Ye et al 20129 | 6 | 288 319 | High vs low | Type 2 diabetes RR = 0.74, P < .01 |
.44 | 0% | NS |
NR, not reported; NS, not significant; RR, relative risk.
Table 2.
Summary of Meta-analyses on Plasma Lipids and the Incidence of Cardiovascular Disease and Stroke
| Meta-analysis Authors and Date | No. of Studies in Meta-analysis | No. of Participants in Meta-analysis | Average Servings or Amount per Day | Main Findings of Meta-analysis | Q Test P Value | I2 Statistic | Egger or Begg Test P Value |
|---|---|---|---|---|---|---|---|
| Kelly et al 200710 | 9 | 738 (all with CHD) | 30 g | TC ↓ 7.0 mg/dL, P < .0001 | NS for all 4 | NS for all 4 | NS |
| LDL ↓ 7.7 mg/dL, P < .0001 | |||||||
| HDL 0.0 mg/dL, P = .95 | |||||||
| Trigs ↓ 0.9 mg/dL, P = .84 | |||||||
| Hollænder et al 201511 | 24 | 2275 (2/3 with hypercholesterolemia) | 28 g | TC ↓ 4.6 mg/dL, P < .001 | .01 | 40% | NR |
| LDL ↓ 3.5 mg/dL, P = .003 | .02 | 38% | |||||
| HDL ↓ 0.4 mg/dL, P = .59 | .17 | 21% | |||||
| Trigs ↓ 3.5 mg/dL, P = .10 | .49 | 0% | |||||
| Anderson et al 200012 | 4 | 179 961 | NR | Cardiovascular disease RR = 0.64, P < .05 | .94 | NR | NR |
| Mellen et al 200813 | 7 | 285 376 | 2.5 | Cardiovascular disease RR = 0.63, P < .0001 | .07 | NR | 0.001 |
| Ye et al. 20129 | 10 | 408 605 | High vs low | Cardiovascular disease RR = 0.79, P < .01 | .82 | 0% | .03 |
| Tang et al 201514 | 14 | 400 492 | High vs low | Cardiovascular disease RR = 0.79, P < .001 | .54 | 0% | .63 |
| Fang et al 201515 | 6 | 498 487 | High vs low | Stroke RR = 0.86, P < .05 | NR | 0% | .84 |
| Chen et al 201616 | 5 | 204 895 | High vs low | Stroke RR = 0.92 NS | .08 | 53% | .96 |
| Wei et al 201617 | 9 | 760 637 | High vs low | Cardiovascular mortality RR = 0.81, P < .05 | .01 | 57% | .83 |
| Chen et al 201618 | 12 | 847 024 | High vs low | Cardiovascular mortality RR = 0.82, P < .05 | .58 | 0% | .37 |
CHD, coronary heart disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NR, not reported; NS, not significant; RR, relative risk; TC, total cholesterol; Trigs, triglycerides.
Table 3.
Summary of Meta-analyses on Cancer Incidence
| Meta-analysis Authors and Date | No. of Studies in Meta-analysis | No. of Participants in Meta-analysis | Average Servings or Amount per Day | Main Findings of Meta-analysis | Q Test P Value | I2 Statistic | Egger or Begg Test P Value |
|---|---|---|---|---|---|---|---|
| Jacobs et al 199819 | 8 colorectal cancer | 12 142 | High vs low | Colorectal cancer OR = 0.79, P < .05 |
NR | NR | NR |
| 7 gastric cancer | 8358 | Gastric cancer OR = 0.57, P < .05 |
|||||
| 4 pancreatic cancer | 2659 | Pancreatic cancer OR = 0.70, P < .05 |
|||||
| Haas et al 200920 | 11 | 1 643 188 | High vs low | Colorectal cancer RR = 0.94, NS |
NR | NR | NR |
| Aune et al 201121 | 7 | 774 806 | High vs low | Colorectal cancer RR = 0.79, P < .05 |
.98 | 0% | .54 |
| Lei et al 201622 | 5 | 42 443 | High vs low | Pancreatic cancer OR = 0.76, P < .002 |
.34 | 12% | NR |
| Wang et al 201523 | 8 | 94 506 | High vs low | Prostate cancer RR = 1.13, P = .095 |
.04 | 53% | .48 |
| Wei et al 201617 | 7 | 673 912 | High vs low | Cancer mortality RR = 0.89, P < .05 |
.01 | 64% | .96 |
| Chen et al 201618 | 8 | 684 890 | High vs low | Cancer mortality RR = 0.89, P < .05 |
.04 | 54% | .75 |
NR, not reported; OR, odds ratio; RR, relative risk.
Table 4.
Summary of Meta-analyses on Body Composition
| Meta-analysis Authors and Date | No. of Studies in Meta-analysis | No. of Participants in Meta-analysis | Average Servings or Amount per Day | Main Findings of Meta-analysis | Q Test P Value | I2 Statistic | Egger or Begg Test P Value |
|---|---|---|---|---|---|---|---|
| Harland and Garton 200824 | 15 | 119 829 | 3.3 | BMI ↓ 0.63 kg/m2, P < .0001 Waist circumference ↓ 2.7 cm, P < .03 |
NR | NR | NS |
| Pol et al 201325 | 26 | 2060 | 3 or 45 g | Body weight ↓ 0.06 kg, P = .45 Body fat ↓ 0.48%, P = .04 Waist circumference ↓ 1.2 cm, P < .15 |
NS | NS | NR |
BMI, body mass index; NR, not reported; NS, not significant.
The results in Table 1 indicate that daily whole grain intakes of 2 or 3 servings (30-45 g/day) can significantly reduce the incidence of developing type 2 diabetes, with RRs ranging between 0.68 and 0.80. However, statistically significant heterogeneity was observed in 2 of the 4 meta-analyses. Using either the Egger or Begg test, no significant publication bias was noted. Table 5 shows the individual clinical studies used by these 4 meta-analyses. Functionally, 1.5 servings of whole grain per day significantly reduced both serum glucose and insulin concentrations, and there was no observable heterogeneity or publication bias in this meta-analysis.
Table 5.
Citation Matrix for Meta-analyses on Type 2 Diabetes Incidence
| Chanson-Rolle et al 20158 | Aune et al 20134 | Ye et al 20129 | de Munter et al 20077 | |
|---|---|---|---|---|
| Liu 2000 | Yes | Yes | ||
| Meyer 2000 | Yes | Yes | Yes | Yes |
| Fung 2002 | Yes | Yes | ||
| Montonen 2003 | Yes | Yes | Yes | |
| Esmallzideh 2005 | Yes | Yes | ||
| Van Dam 2006 | Yes | Yes | Yes | |
| de Munter 2007 | Yes | Yes | ||
| Fisher 2009 | Yes | |||
| Sun 2010 | Yes | Yes | ||
| Ericson 2013 | Yes | |||
| Parker 2013 | Yes | Yes | ||
| Wirstrom 2013 | Yes | Yes |
The results in Table 2 show that dietary whole fiber intake can significantly reduce the incidence of developing cardiovascular disease and stroke, with RRs ranging between 0.63 and 0.79 for cardiovascular disease and 0.86 and 0.92 for stroke. No statistically significant heterogeneity was identified in any of these 6 meta-analyses, but significant publication bias, as measured using Egger or Begg tests, was observed in 2 of the 5 (1 meta-analysis did not assess for publication bias). Table 6 shows the individual clinical studies used by the 4 meta-analyses on cardiovascular disease incidence. Functionally, 28 to 30 g/day of whole grain significantly reduced total serum cholesterol and low-density lipoprotein (LDL) cholesterol in 2 separate meta-analyses. However, statistically significant heterogeneity was identified in 1 of these 2 meta-analyses. Finally, 2 meta-analyses both reported a significant inverse association of dietary whole grain intake with cardiovascular mortality (RR = 0.81 and 0.82); however, 1 of the 2 was determined as having statistically significant heterogeneity, but neither had significant publication bias.
Table 6.
Citation Matrix for Meta-analyses on Cardiovascular Disease Incidence
| Tang et al 201514 | Ye et al 20129 | Mellen et al 200813 | Anderson et al 200012 | |
|---|---|---|---|---|
| Fraser 1992 | Yes | |||
| Jacobs 1998 | Yes | Yes | Yes | |
| Jacobs 1999 | Yes | Yes | Yes | Yes |
| Liu 1999 | Yes | Yes | Yes | Yes |
| Lui 2000 | Yes | |||
| Jacobs 2001 | Yes | Yes | ||
| Liu 2003 | Yes | Yes | Yes | |
| Jensen 2004 | Yes | Yes | Yes | |
| Steffen 2004 | Yes | Yes | Yes | |
| Tavani 2004 | Yes | |||
| Sahyoun 2006 | Yes | Yes | ||
| Djoussee 2007 | Yes | |||
| Jacobs 2007 | Yes | |||
| Lockheart 2007 | Yes | |||
| Nettleton 2008 | Yes | |||
| He 2010 | Yes | Yes | ||
| Oliveira 2010 | Yes | |||
| Rautianmem 2012 | Yes |
The results in Table 3 indicate that when comparing populations with the highest to lowest dietary whole grain intakes, there was a statistical significant reduction in the risk of developing colorectal cancer, but this finding was only identified in 2 of the 3 meta-analyses. Heterogeneity was assessed in only 1 of these meta-analyses, and it was not statistically significant. The individual studies used in each of these 3 meta-analyses on colorectal cancer were all unique except for 2 studies, which were shared by 2 of the meta-analyses.
In regard to pancreatic cancer, both meta-analyses determined that the populations with the highest dietary whole grain intakes had significantly reduced incidence of pancreatic cancer, with observed ORs of 0.70 and 0.76. Heterogeneity was assessed in only 1 of these meta-analyses, but it was not statistically significant. Finally, for populations with the highest intake of whole grains, there was a statistically significant reduction in the OR for gastric cancer (OR = 0.57), but the finding was not statistically significant for prostate cancer (RR = 1.13). Publication bias was only assessed and reported in 2 of the 5 meta-analyses on cancer, but neither one observed statistical significance. Finally, 2 meta-analyses observed a significant inverse association of dietary whole grain intake with overall cancer mortality where both presented with an RR of 0.89, but unfortunately, they also both presented with statistically significant heterogeneity.
The results from the 2 meta-analyses on body composition are presented in Table 4. Heterogeneity and publication bias was assessed in only 1 of the 2 meta-analyses, and neither measure was determined to be statistically significant. Dietary whole grain was associated with weight loss and lower body mass index, body fat percentage, and central adiposity, but these changes were not all statistically significant.
In regard to quality assessment, only 7 of the 21 meta-analyses performed such an assessment, with 2 using the Cochrane quality score and 5 using the Newcastle-Ottawa Scale. There was no mention of any studies being excluded because of low quality. Only 5 of the 7 meta-analyses published the numerical details of their quality assessment results, and they found that the majority of the studies scored as high quality (26 of 33).
Seven of the 21 meta-analyses obtained their effect size results using a fixed-effects model given their finding of nonsignificant heterogeneity. For the remaining 14 meta-analyses, 1 did not state which model was used, and the remaining 13 used a random-effects model. In 6 of these 13 meta-analyses, the finding of significant heterogeneity warranted the use of a random-effects model, and for the remaining 5, the assumption of heterogeneity was implied because of the wide differences between studies used in these meta-analyses (differences such as study population characteristics, amounts of whole grain intake, and duration of the study).
Discussion
Almost all meta-analyses in this umbrella review reported positive benefits for dietary whole grain intake in the prevention and management of the most serious health care problems such as type 2 diabetes, cardiovascular disease, stroke, and colorectal, gastric, and pancreatic cancers. However, we must appreciate these positive results with some caution because of statistically significant heterogeneity in the type 2 diabetes meta-analyses and statistically significant publication bias in the cardiovascular meta-analyses. Also, the lack of quality assessment of published studies is problematic because clinical studies of very low quality may have been included in these meta-analyses, which can therefore potentially bias their overall outcomes. Despite the problems of heterogeneity, publication bias, and quality assessment, the potential benefits identified in this umbrella review suggest that the consumption of 2 to 3 servings per day (~45 g) of whole grains, as recommended by the Dietary Guidelines for Americans,27 may continue to be a justifiable public health goal.
The following provides a discussion of the findings in detail. Four meta-analyses4, 7, 8, 9 reported that increased dietary whole grain intake significantly reduces the incidence of developing type 2 diabetes by 20% to 32%; however, it should be noted that 2 of the 4 meta-analyses presented with statistically significant heterogeneity, and this weakens the clinical certainty of this effect.4, 7 Ideally the studies combined into any given meta-analysis should all have used the same experimental protocols; however, increased heterogeneity is inevitable because of the wide variation in study design. Differences in study design include number of participants, duration of the study, age, sex, body mass index, total energy intake for the participants, method of dietary whole grain intake measurements, and source and types of whole grains (eg, whole grain cereals, brown bread, and brown rice).
Defining a “whole grain” food is problematic, with definitions varying between different studies. The most commonly used definition classifies whole grains as products composed of 25% or more of whole grains, whereas others use 50% as the cutoff. Several studies did not state how whole grains were defined; thus, it is difficult to assess whether the differing definitions might have influenced the results.
Although many individual studies adjust for potential confounding factors, not all confounders are adjusted for in every study and hence the potential for statistically significant heterogeneity is increased. On a positive note, no publication bias was found for any of the 4 meta-analyses on the incidence of developing type 2 diabetes.
In regard to whole grain’s mechanism of action on potentially reducing the incidence of developing type 2 diabetes, the insoluble fiber content of whole grains may delay gastric emptying and decrease the rate of glucose absorption, which favorably enhances postprandial glucose and insulin response.28 This mechanism of action is supported by the meta-analysis that identified significant reductions in both serum glucose and insulin levels with 1.5 servings of whole grains per day.6 Constituents of whole grain such as magnesium and chromium may also help to maintain normal glucose and insulin metabolism because they are cofactors for insulin receptor kinase and chromodulin.29, 30
Four meta-analyses9, 12, 13, 14 reported that increased whole grain intake of 2.5 servings, or 33 g/day, reduces the incidence of cardiovascular disease by 21% to 37%. Statistically significant heterogeneity was not identified in any of these 4 meta-analyses; however, 2 of the meta-analyses did have significant publication bias.9, 13 Publication bias occurs because small studies with null results tend not to be published; this is referred to as the “file drawer problem.” Because published studies are more likely than unpublished ones to report positive research outcomes, the significance of the effect size of the weighted average of the published studies is overestimated, and this can potentially bias the results of the meta-analysis. In regard to mechanism of action, the phytosterols found in whole grains can compete with dietary and biliary cholesterol for absorption by the small intestine, which reduces cholesterol absorption and increases excretion.31 Whole grains also contain soluble fiber, which can potentially lower serum LDL cholesterol and hence total serum cholesterol.32 This mechanism of action is supported by the 2 meta-analyses that reported significant reductions in both total serum cholesterol and LDL cholesterol levels with a 30-g serving of whole grains per day.10, 11 Finally, whole grain phytochemical constituents can influence the vascular endothelium directly by promoting vasodilation, which leads to a reduction in blood pressure.33 A reduction in blood pressure could significantly reduce the incidence of stroke, and this was identified in both meta-analyses that reported a reduction in RR of between 8% and 14%; however, only 1 of the 2 meta-analyses was statistically significant.15
Compared with populations with the lowest intakes of dietary whole grains, the populations with the highest intakes observed significant reductions in the incidence of colorectal cancer by 21%, pancreatic cancer by 24% to 30%, and gastric cancer by 43%.19, 21, 22 There was no significant change in the RR of developing prostate cancer between these 2 populations.23 However, although 2 meta-analyses reported significant reductions in the incidence of developing colorectal cancer, 1 meta-analysis identified a nonsignificant reduction in RR of only 6%.20 Except for 2 shared studies between 2 of these 3 meta-analyses on colorectal cancer, all 3 meta-analyses used unique studies to tabulate their effects sizes. Also, of the 5 separate meta-analyses on cancer incidence, only 2 assessed and presented their results on heterogeneity or publication bias. Therefore, the results and conclusion from this set of meta-analyses on cancer should be viewed with caution.
Caution also needs to be applied to the 2 meta-analyses that reported a statistically significant reduction in overall cancer mortality of 11%, mainly because of the statistically significant heterogeneity that was also identified.17, 18 Assuming that increased dietary whole grain intake can significantly reduce the incidence of developing certain types of cancers, the mechanism of action here may involve the antioxidant actions of whole grain’s phenolics and vitamin E, which scavenge free radicals and thereby prevent oxidation damage to DNA bases.34 Trace minerals found in whole grains, such as selenium, zinc, copper, and manganese, are cofactors for enzymes that conduct antioxidant functions such as glutathione peroxidase and superoxide dismutase. The polyphenols typically found in whole grains can also neutralize carcinogenic N-nitrosamines and therefore prevent oxidative DNA damage caused by these compounds.35 Because N-nitrosamine intake is significantly associated with gastric cancer incidence, this mechanism may explain why dietary whole grain intake had the greatest effect on reducing gastric cancer incidence compared with colon and pancreatic cancer.36
The 2 meta-analyses on body composition in Table 4 reported healthful changes in body weight, waist circumference, and body fat mass, but only 1 meta-analysis reported a statistically significant reduction in body weight and waist circumference, whereas the other meta-analysis found that these positive changes were small and not statistically significant.24, 25 The effect sizes in both of these meta-analyses were formulated from unique studies and so any differences between the different studies used by each meta-analysis may have contributed to the inconsistency noted here. For example, it appears that the meta-analysis that reported positive but nonsignificant results did so because the studies in this meta-analysis were not of long enough duration to identify substantial change in body weight (the majority of the studies were only 4-6 weeks’ duration).25 In regard to testing for heterogeneity and publication bias, 1 meta-analysis reported no significant heterogeneity but did not test for publication bias, whereas the other meta-analysis reported no publication bias but did not test for heterogeneity. Because both of these meta-analyses used studies that were 100% completely unique to each meta-analysis, we cannot validly cross over the heterogeneity and publication bias results from one to the other, and therefore the results and conclusions drawn from both meta-analyses need to be viewed with caution.
Assuming dietary whole grain consumption does truly affect body composition in a healthful way, the proposed mechanism of action may be through whole grains’ ability to promote satiety through their dietary fiber content. Also, whole grains promote better insulin function, which aids in reduced lipogenesis and fat storage.27 However, it may be that people who consume more whole grains are likely to have a healthier lifestyle because they exercise more and have lower fat and higher dietary fiber intakes. It has been noted that dietary whole grain intake alone may not contribute to the effects seen here and that the body composition changes are independently a result of hypocaloric diets alone.37
Limitations
One limitation is that only 3 indexing systems were searched and thus it is possible that some meta-analyses were not identified. A second limitation is that only 1 author performed the search and selection of the meta-analyses included in this umbrella review. A third limitation is that the meta-analyses reviewed here represent a heterogeneous group of clinical studies composed of a diverse group of participants of different ages, sexes, genders, races, and ethnic groups, and therefore readers are cautioned against specifying these results to any 1 specific sociodemographic group. And finally, as in all literature reviews, the quality of this umbrella review is directly related to the quality of the included meta-analyses, which are dependent on the quality of the individual studies used to conduct the meta-analysis.
Conclusion
This review suggests that there is some evidence for dietary whole grain intake to be beneficial in the prevention of type 2 diabetes, cardiovascular disease, and colorectal, pancreatic, and gastric cancers. The potential benefits of these findings suggest that the consumption of 2 to 3 servings per day (~45 g) of whole grains may be a justifiable public health goal.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): M.P.M.
Design (planned the methods to generate the results): M.P.M.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): M.P.M.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): M.P.M.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): M.P.M.
Literature search (performed the literature search): M.P.M.
Writing (responsible for writing a substantive part of the manuscript): M.P.M.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): M.P.M.
Practical Applications
-
•
Whole grain consumption has been postulated to reduce the risk for type 2 diabetes, cardiovascular disease, colorectal cancer, and obesity.
-
•
Unfortunately, there is much discrepancy when it comes to randomized controlled studies on the effects of whole grain on these important clinical conditions.
-
•
By combining the meta-analyses on these clinical outcomes as an umbrella review, we can suggest that dietary whole grain intake may be beneficial in the prevention of type 2 diabetes, cardiovascular disease, stroke, and colorectal, gastric, and pancreatic cancers.
Alt-text: Image 1
References
- 1.Seal CJ, Brownlee IA. Whole-grain foods and chronic disease: evidence from epidemiological and intervention studies. Proc Nutr Soc. 2015;74(3):313–319. doi: 10.1017/S0029665115002104. [DOI] [PubMed] [Google Scholar]
- 2.Steffen LM, Jacobs DR, Stevens J, Shahar E, Carithers T, Folsom AR. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78(3):383–390. doi: 10.1093/ajcn/78.3.383. [DOI] [PubMed] [Google Scholar]
- 3.Huang T, Xu M, Lee A, Cho S, Qi L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals. BMC Med. 2015;13:59. doi: 10.1186/s12916-015-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol. 2013;28(11):845–858. doi: 10.1007/s10654-013-9852-5. [DOI] [PubMed] [Google Scholar]
- 5.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 6.Nettleton JA, McKeown NM, Kanoni S. Interactions of dietary whole-grain intake with fasting glucose- and insulin-related genetic loci in individuals of European descent: a meta-analysis of 14 cohort studies. Diabetes Care. 2010;33(12):2684–2691. doi: 10.2337/dc10-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007;4(8):e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanson-Rolle A, Meynier A, Aubin F. Systematic review and meta-analysis of human studies to support a quantitative recommendation for whole grain intake in relation to type 2 diabetes. PLoS One. 2015;10(6):e0131377. doi: 10.1371/journal.pone.0131377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr. 2012;142(7):1304–1313. doi: 10.3945/jn.111.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly SA, Summerbell CD, Brynes A, Whittaker V, Frost G. Wholegrain cereals for coronary heart disease. Cochrane Database Syst Rev. 2007;2:CD005051. doi: 10.1002/14651858.CD005051.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Hollænder PL, Ross AB, Kristensen M. Whole-grain and blood lipid changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. 2015;102(3):556–572. doi: 10.3945/ajcn.115.109165. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JW, Hanna TJ, Peng X, Kryscio RJ. Whole grain foods and heart disease risk. J Am Coll Nutr. 2000;19(Suppl. 3):291S–299S. doi: 10.1080/07315724.2000.10718963. [DOI] [PubMed] [Google Scholar]
- 13.Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis. 2008;18(4):283–290. doi: 10.1016/j.numecd.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Tang G, Wang D, Long J, Yang F, Si L. Meta-analysis of the association between whole grain intake and coronary heart disease risk. Am J Cardiol. 2015;115(5):625–629. doi: 10.1016/j.amjcard.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Fang L, Li W, Zhang W, Wang Y, Fu S. Association between whole grain intake and stroke risk: evidence from a meta-analysis. Int J Clin Exp Med. 2015;8(9):16978–16983. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Huang Q, Shi W, Yang L, Chen J, Lan Q. Meta-analysis of the association between whole and refined grain consumption and stroke risk based on prospective cohort studies [epub ahead of print]. Asia Pac J Public Health. pii: 1010539516650722. Accessed May 1, 2016. [DOI] [PubMed]
- 17.Wei H, Gao Z, Liang R, Li Z, Hao H, Liu X. Whole-grain consumption and the risk of all-cause, CVD and cancer mortality: a meta-analysis of prospective cohort studies. Br J Nutr. 2016;116(3):514–525. doi: 10.1017/S0007114516001975. [DOI] [PubMed] [Google Scholar]
- 18.Chen GC, Tong X, Xu JY. Whole-grain intake and total, cardiovascular, and cancer mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2016;104(1):164–172. doi: 10.3945/ajcn.115.122432. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs DR, Jr., Marquart L, Slavin J, Kushi LH. Whole-grain intake and cancer: an expanded review and meta-analysis. Nutr Cancer. 1998;30(2):85–96. doi: 10.1080/01635589809514647. [DOI] [PubMed] [Google Scholar]
- 20.Haas P, Machado MJ, Anton AA, Silva AS, de Francisco A. Effectiveness of whole grain consumption in the prevention of colorectal cancer: meta-analysis of cohort studies. Int J Food Sci Nutr. 2009;60(Suppl. 6):1–13. doi: 10.1080/09637480802183380. [DOI] [PubMed] [Google Scholar]
- 21.Aune D, Chan DS, Lau R. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei Q, Zheng H, Bi J. Whole grain intake reduces pancreatic cancer risk: a meta-analysis of observational studies. Medicine. 2016;95(9):e2747. doi: 10.1097/MD.0000000000002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang RJ, Tang JE, Chen Y, Gao JG. Dietary fiber, whole grains, carbohydrate, glycemic index, and glycemic load in relation to risk of prostate cancer. Onco Targets Ther. 2015;8:2415–2426. doi: 10.2147/OTT.S88528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harland JI, Garton LE. Whole-grain intake as a marker of healthy body weight and adiposity. Public Health Nutr. 2008;11(6):554–563. doi: 10.1017/S1368980007001279. [DOI] [PubMed] [Google Scholar]
- 25.Pol K, Christensen R, Bartels EM, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. 2013;98(4):872–884. doi: 10.3945/ajcn.113.064659. [DOI] [PubMed] [Google Scholar]
- 26.Zhu J, Ma S, Xiao P, Li L, Yang Y. Meta-analysis on the relationship among fiber of grain and intestinal motility and symptoms. Wei Sheng Yan Jiu. 2015;44(1):1–7. [PubMed] [Google Scholar]
- 27.Ferruzzi MG, Jonnalagadda SS, Liu S. Developing a standard definition of whole-grain foods for dietary recommendations: summary report of a multidisciplinary expert roundtable discussion. Adv Nutr. 2014;5(2):164–176. doi: 10.3945/an.113.005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaline K, Bornstein SR, Bergmann A, Hauner H, Schwarz PE. The importance and effect of dietary fiber in diabetes prevention with particular consideration of whole grain products. Horm Metab Res. 2007;39(9):687–693. doi: 10.1055/s-2007-985811. [DOI] [PubMed] [Google Scholar]
- 29.Dong JY, Xun P, He K, Qin LQ. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care. 2011;34(9):2116–2122. doi: 10.2337/dc11-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin RV, Phung OJ. Effect of chromium supplementation on glycated hemoglobin and fasting plasma glucose in patients with diabetes mellitus. Nutr J. 2015;14:14. doi: 10.1186/1475-2891-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brufau G, Canela MA, Rafecas M. Phytosterols: physiologic and metabolic aspects related to cholesterol-lowering properties. Nutr Res. 2008;28(4):217–225. doi: 10.1016/j.nutres.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Bazzano LA. Effects of soluble dietary fiber on low-density lipoprotein cholesterol and coronary heart disease risk. Curr Atheroscler Rep. 2008;10(6):473–477. doi: 10.1007/s11883-008-0074-3. [DOI] [PubMed] [Google Scholar]
- 33.Biesinger S, Michaels HA, Quadros AS. A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. Eur J Clin Nutr. 2016;70(1):10–16. doi: 10.1038/ejcn.2015.88. [DOI] [PubMed] [Google Scholar]
- 34.Slavin J. Why whole grains are protective: biological mechanisms. Proc Nutr Soc. 2003;62(1):129–134. doi: 10.1079/PNS2002221. [DOI] [PubMed] [Google Scholar]
- 35.Delgado ME, Haza AI, Arranz N, García A, Morales P. Dietary polyphenols protect against N-nitrosamines and benzo(a)pyrene-induced DNA damage (strand breaks and oxidized purines/pyrimidines) in HepG2 human hepatoma cells. Eur J Nutr. 2008;47(8):479–490. doi: 10.1007/s00394-008-0751-6. [DOI] [PubMed] [Google Scholar]
- 36.Song P, Wu L, Guan W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: a meta-analysis. Nutrients. 2015;7(12):9872–9895. doi: 10.3390/nu7125505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thielecke F, Jonnalagadda SS. Can whole grain help in weight management? J Clin Gastroenterol. 2014;48(Suppl. 1):S70–S77. doi: 10.1097/MCG.0000000000000243. [DOI] [PubMed] [Google Scholar]