Abstract
OBJECTIVES
To characterize the magnitude and duration of risks of rehospitalization by age following hospitalization for heart failure (HF), acute myocardial infarction (AMI), or pneumonia.
DESIGN
Retrospective cohort study.
SETTING
4,767 US hospitals.
PARTICIPANTS
All Medicare fee-for-service beneficiaries ≥65 years-old surviving hospitalization for HF, AMI, or pneumonia between October 2012 and December 2013.
MEASUREMENTS
We calculated daily risks of first rehospitalization for 1 year after hospital discharge by age category (65–74 years, 75–84 years, 85+ years) after adjustment for sex, race, comorbidities, and median zip code income. We identified (1) the time required for adjusted rehospitalization risks to decline 50% from maximum values after discharge; (2) the time required for adjusted risks to approach plateau periods of minimal day-to-day change; and (3) the degree to which adjusted risks are higher among recently hospitalized patients compared with the general elderly population.
RESULTS
We identified 414,720, 177,752, and 568,304 hospitalizations for HF, AMI, and pneumonia, respectively. The adjusted risk of rehospitalization declined with increasing age after HF hospitalization (p<0.001), rose with increasing age after AMI hospitalization (p<0.001), and was slightly lower with increasing age after pneumonia hospitalization (p=0.002). Adjusted risks of rehospitalization were elevated beyond 30 days after hospitalization for all ages.
CONCLUSION
While older age has heterogeneous relationships with rehospitalization risk, the risk of readmission remains elevated for an extended time after discharge regardless of age or admitting condition. Condition-specific data on risk can be used to guide discussions on advanced care planning and strategies for longitudinal follow-up after hospitalization.
Keywords: readmission, geriatrics, cardiovascular disease, pulmonary diseases, quality of care
INTRODUCTION
For older patients, the period after hospital discharge is marked by an elevated risk of adverse events leading to rehospitalization. Data from Medicare beneficiaries has shown that rehospitalization risk peaks soon after discharge, declines gradually over time, and remains elevated for many weeks.1 Both absolute risks of readmission and the rate of decline in these risks vary by admitting condition.1 Yet the influence of other factors on risk trajectories in the year after hospitalization is not known.
Age is an important variable that may influence the absolute risk of rehospitalization soon after discharge and the manner in which this risk decays over time. Previous work has shown that age is not clearly associated with short-term rehospitalization rates.2,3 Yet none of these studies have characterized complex relationships between age and dynamic risk trajectories that change daily after hospital discharge. This knowledge is critical to identifying the influence of increasing age on the extent and timing of vulnerability after hospitalization. This information can help guide conversations with older patients of different ages regarding their expected course of recovery after hospitalization. Findings can also help clinicians tailor post-acute care interventions to the periods of highest absolute risk for older patients. Finally, results can illuminate whether drivers of rehospitalization may be age-related.
There are several specific questions worthy of attention. First, are absolute risks of rehospitalization elevated beyond 30 days after discharge for older patients of all ages? If so, this finding would suggest that all older patients could benefit from risk reduction strategies that persist beyond the 30-day period currently used to measure hospital readmission performance.4 Second, do absolute risks of rehospitalization differ by age? The demonstration of higher risk with increasing age would suggest that diminished physiologic reserves or other factors associated with aging significantly contribute to readmission; in contrast, a lack of relationship between outcomes and age would suggest that other complex variables besides age and its correlates primarily determine outcomes. Finally, does age contribute to the higher magnitude of risk experienced by recently hospitalized patients compared with the general elderly population? These results will describe the extent to which age influences prognosis after hospitalization.
Accordingly, we characterized the magnitude and duration of risks of rehospitalization by age in the year after hospitalization among Medicare beneficiaries admitted with heart failure (HF), acute myocardial infarction (AMI), or pneumonia. The study of older persons hospitalized for three different medical conditions will provide insight into the generalizability of findings relating age to distinctive patterns of risk. We hypothesized that older age would be associated with higher readmission risk and slower declines in risk after hospitalization.
METHODS
Study Sample
We used Medicare Standard Analytic and Denominator files to identify hospitalizations from 10/2012–12/2013 with a principal discharge diagnosis of HF, AMI, or pneumonia. These dates followed passage of the federal Hospital Readmissions Reduction Program, which penalized hospitals with higher-than-expected 30-day readmission rates for HF, AMI, and pneumonia starting October 2012. Study cohorts were defined with International Classification of Diseases, Ninth Revision, Clinical Modification codes used in the Centers for Medicare & Medicaid Services (CMS) publicly reported readmission measures.5–7 We included hospitalizations among patients ≥65 years-old. We excluded hospitalizations with in-hospital death, inter-hospital transfer, and discharge against medical advice. We excluded hospitalizations of patients with <1 year of Medicare fee-for-service (FFS) enrollment prior to hospitalization, as previous claims are needed to identify comorbidities for risk-adjustment. We also excluded hospitalizations with <1 year of Medicare FFS enrollment following hospitalization in the absence of death to permit risk calculations for one year after hospitalization. Like the CMS measures, we used all available hospitalizations for HF, AMI, or pneumonia.
Study Endpoints
For the year following hospitalization, we identified the occurrence of first rehospitalization on each day after discharge. Like the CMS measures, we excluded rehospitalizations for procedures or diagnoses that are typically scheduled such as chemotherapy infusion or organ transplantation.
Comparator Population
To compare the risks of rehospitalization following hospitalization for HF, AMI, or pneumonia to the risks of all-cause hospitalization in the general elderly population, we constructed a comparator population using 2013 Medicare Provider Analysis and Review and Denominator files comprised of all Medicare FFS beneficiaries who were ≥65 years-old with >1 year of enrollment in Medicare FFS in the absence of death. We did not exclude persons hospitalized with HF, AMI, or pneumonia since our aim was to compare rehospitalization risk following discharge with hospitalization risk in the general elderly population, which includes persons hospitalized for all conditions.
Outcomes
Daily Risk of Rehospitalization by Age
We estimated adjusted daily risks of first rehospitalization by age after hospitalization for HF, AMI, or pneumonia. Adjustment was made for sex, race, median income of the zip code of residence, and comorbidities found to be predictive of readmission in CMS 30-day readmission models.5–7 As has been done previously,8,9 we categorized age into 3 categories (65–74 years, 75–84 years, 85+ years). To characterize the length of time required for adjusted risks of first rehospitalization to decline by a clinically significant extent, we calculated the number of days required for these risks to decline 50 percent from their maximum values after discharge. To characterize the length of time required for adjusted risks of first rehospitalization to approach periods of minimal day-to-day change, we calculated the number of days required for daily changes in risk to decline 95% from their maximum daily declines after discharge.
Relative Risks of Hospitalization in Study Populations Compared with Medicare FFS Population by Age
To compare the magnitude of rehospitalization risk experienced by recently hospitalized patients compared with the general elderly population for each age category, we first calculated the cumulative incidence of hospitalization by day (1–365) after discharge following hospitalization for HF, AMI, or pneumonia for each age category. We then compared these results with the cumulative incidence of hospitalization in the similarly aged subset of the comparator population of Medicare FFS beneficiaries after adjusting for sex, race, and median zip code income.
Statistical Analyses
Daily Risk of Rehospitalization by Age
For each age category, we fit separate survival models for the adjusted risk of first rehospitalization. We considered death prior to first rehospitalization as a competing risk. For each age category, we therefore calculated the adjusted subdistribution hazard for first rehospitalization derived from the cumulative incidence function for each day (1–365) after discharge using the approach by Fine and Gray that corrects for competing risk.10 Adjustment was made for sex, race, median zip code income, and comorbidities using Cox proportional hazard regression. We used 3 race categories (white, black, other) and 3 income categories (≤3rd decile, 3rd to 7th decile, ≥7th decile of US median zip code income). Income data was identified using the 2007–2011 American Community Survey. The difference in adjusted hazard rates between age categories was tested by Gray’s test.11 Data were censored at death, planned readmission, or 1 year after the index hospitalization, whichever occurred first. For each age category, we used the bootstrap method with 500 iterations to construct 95% confidence intervals for the time required for these adjusted hazard rates to decline by 50% from their maximum hazards after discharge.
To calculate the daily change in adjusted risk of first rehospitalization with time after discharge, we calculated differences in 3-day averaged kernel-smoothed hazard estimates12 between each day and its preceding day for each age category. For each day after maximum hazard, we divided the daily change in risk by its maximum daily decline after discharge. We used the bootstrap method with 500 iterations to construct 95% confidence intervals for the number of days required for the daily change in risk to decline 95% from its maximum daily decline after discharge for each age category.
Relative Risks of Hospitalization in Study Populations Compared with Medicare FFS Population by Age
For each age category in the comparator population, we first calculated the 1-year cumulative incidence of hospitalization among all Medicare FFS beneficiaries in 2013. We then prorated 1-year incidence rates over days and compared them with the cumulative incidence of rehospitalization by day (1–365) after hospitalization for HF, AMI, or pneumonia for each age category. The cumulative incidence of rehospitalization was estimated using the Fine and Gray method. To make study and comparator populations more similar in sex, race, and median zip code income, we estimated the cumulative incidence of rehospitalization for sex-race-income strata within each age category. We then directly standardized study populations to the Medicare FFS population for each age category to calculate the relative risk of sex-race-income standardized hospitalization. Direct standardization is a form of frequency matching. We calculated 95% confidence intervals of the relative risk of hospitalization between sex-race-income standardized study cohorts and the Medicare FFS population over the first 30, 60, 90, 180, and 365 days after discharge.
Analyses were conducted using SAS 9.3 (SAS Institute, Cary, North Carolina). We obtained Institutional Review Board approval, including waiver of the requirement for participant informed consent, through the Yale University Human Investigation Committee.
RESULTS
We identified 414,720 hospitalizations for HF (4,522 hospitals), 177,752 hospitalizations for AMI (3,758 hospitals), and 568,304 hospitalizations for pneumonia (4,651 hospitals). The comparator population of Medicare FFS beneficiaries included 21,372,446 persons. The characteristics of study cohorts stratified by age are described in Table 1. Within 1 year of discharge, readmission occurred after 63.0%, 44.2%, and 44.3% of HF, AMI, and pneumonia hospitalizations, respectively.
Table 1.
Demographic Characteristics and Representative Time Points Describing Trajectories of Readmission Risk after Hospitalization for Each Age Group in Heart Failure, Acute Myocardial Infarction, and Pneumonia Cohorts
| Characteristic | Age group | ||
|---|---|---|---|
| Age 65–74 | Age 75–84 | Age 85+ | |
| Heart Failure Cohort | n=114,380 | n=156,466 | n=143,874 |
| Mean Age in Years (SD) | 69.96 (2.75) | 79.71 (2.86) | 89.52 (3.61) |
| Mean Income of the Zip Code of Residence in Dollars | $51,599.13 | $52,663.60 | $54,256.40 |
| Female | 41.79% | 44.65% | 53.43% |
| White Race | 76.35% | 85.13% | 90.02% |
| Black Race | 18.79% | 11.41% | 7.09% |
| Days of Highest Risk | 2 | 2 | 2 |
| Days for Level of Risk to Decline 50% (95% CI) | 43 (37–47) | 43 (37–47) | 43 (37–47) |
| Days for Daily Change in Risk to Decline 95% (95% CI) | 55 (31–94) | 55 (31–94) | 55 (31–94) |
| Acute Myocardial Infarction Cohort | n=68,552 | n=65,517 | n=43,683 |
| Mean Age in Years (SD) | 69.78 (2.69) | 79.35 (2.87) | 89.36 (3.59) |
| Mean Income of the Zip Code of Residence in Dollars | $51,707.99 | $52,445.43 | $53,830.43 |
| Female | 35.36 | 40.82 | 51.14 |
| White Race | 85.94 | 89.28 | 90.98 |
| Black Race | 9.19 | 7.09 | 5.83 |
| Days of Highest Risk | 2 | 2 | 2 |
| Days for Level of Risk to Decline 50% (95% CI) | 14 (13–16) | 14 (13–16) | 14 (13–16) |
| Days for Daily Change in Risk to Decline 95% (95% CI) | 31 (25–47) | 31 (25–47) | 31 (25–47) |
| Pneumonia Cohort | n=171,581 | n=217,537 | n=179,186 |
| Mean Age in Years (SD) | 69.97 (2.72) | 79.60 (2.87) | 89.53 (3.70) |
| Mean Income of the Zip Code of Residence in Dollars | $51,209.34 | $51,892.36 | $53,178.90 |
| Female | 46.62 | 45.20 | 50.83 |
| White Race | 86.41 | 89.83 | 91.67 |
| Black Race | 9.28 | 6.44 | 5.02 |
| Days of Highest Risk | 2 | 2 | 2 |
| Days for Level of Risk to Decline 50% (95% CI) | 28 (24–30) | 28 (24–30) | 28 (24–30) |
| Days for Daily Change in Risk to Decline 95% (95% CI) | 38 (32–60) | 38 (32–60) | 38 (32–60) |
FFS, Fee-for-Service; SD, Standard Deviation
Daily Risk of Rehospitalization by Age
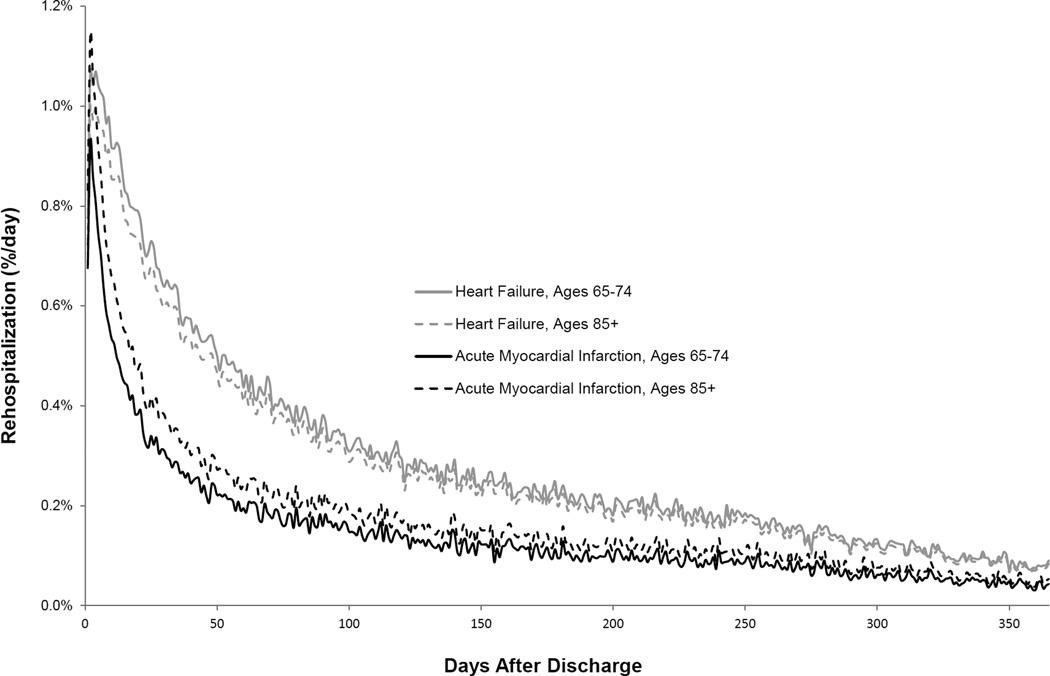
The adjusted risk of first rehospitalization inconsistently varied by age across admitting conditions. The adjusted risk of rehospitalization declined with increasing age after HF hospitalization (p<0.001), rose with increasing age after AMI hospitalization (p<0.001), and was slightly lower with increasing age after pneumonia hospitalization (p=0.002) (Supplemental Figures S1 and S2). Illustrative data for HF and AMI are presented in Figure 1. Adjusted risks of first rehospitalization were highest after 2 days for HF, 2 days for AMI, and 2 days for pneumonia for all age groups and then declined 50% after 43 days, 14 days, and 28 days, respectively (Table 1).
Figure 1. Adjusted Risk of First Rehospitalization by Age after Hospitalization for Heart Failure or Acute Myocardial Infarction.
Risk is described using daily hazard rates that have been adjusted for sex, race, median income of the zip code of residence, and comorbidities. To illustrate the heterogeneous relationship of rehospitalization risk with age across admitting conditions, data is shown for older patients 65–74 years-old and 85+ years-old who were discharged after hospitalization for heart failure or acute myocardial infarction. Adjusted readmission risk trajectories are shown for the full year after hospitalization.
Adjusted risks of first rehospitalization approached periods of minimal day-to-day change by 55 days after hospitalization for all age categories and conditions (Table 1). Specifically, the number of days required for daily changes in risk of first rehospitalization to decline 95 percent from their maximum daily declines was 55 days, 31 days, and 38 days for both the youngest and oldest age categories in HF, AMI, and pneumonia cohorts, respectively.
Relative Risks of Hospitalization in Study Populations Compared with Medicare FFS Population by Age
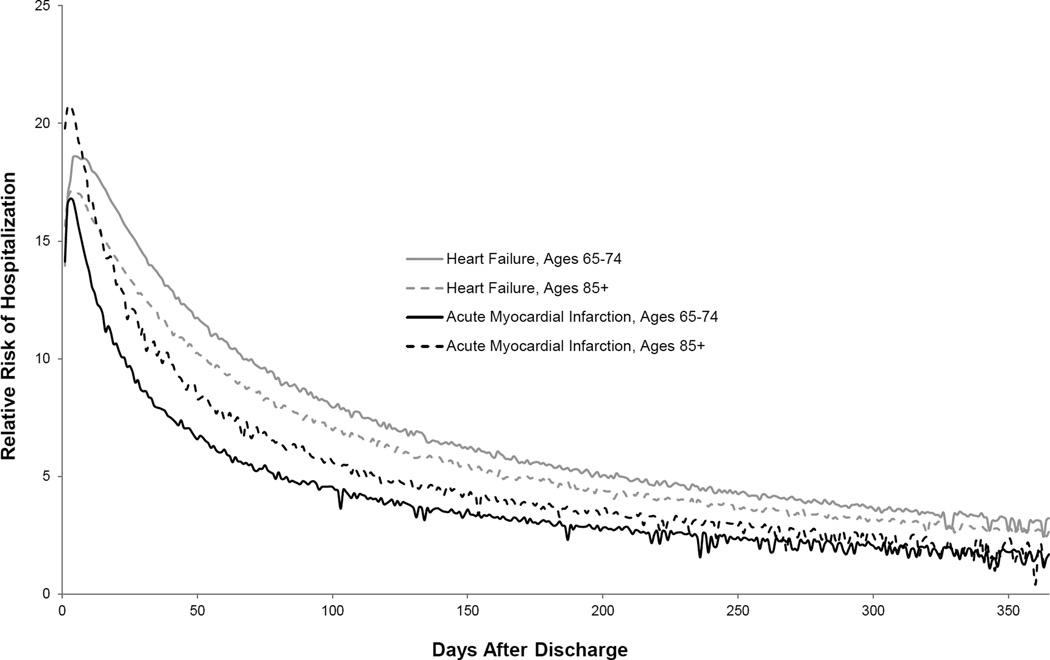
Risk of hospitalization was markedly higher for recently hospitalized patients compared with the comparator population of Medicare FFS beneficiaries for all age categories (Supplemental Figure S3). Illustrative data for HF and AMI are presented in Figure 2. For example, following hospitalization for HF, the sex-race-income standardized relative risk of hospitalization for the oldest age category was 12.6 times, 9.4 times, 7.5 times, 4.9 times, and 2.7 times greater over the first 30, 60, 90, 180, and 365 days after discharge.
Figure 2. Relative Risk of First Hospitalization by Age after Hospitalization for Heart Failure or Acute Myocardial Infarction Compared with the General Elderly Population.
Post-discharge populations have been directly standardized to the Medicare FFS population with respect to sex, race, and median income of the zip code of residence. Illustrative data is shown for older patients 65–74 years-old and 85+ years-old who were discharged after hospitalization for heart failure or acute myocardial infarction. In 2013, the 30-day and 1-year cumulative incidence of hospitalization among Medicare FFS beneficiaries was 1.6% and 19.6%, respectively. Relative risks of first rehospitalization by age are shown for the full year after hospitalization.
DISCUSSION
Through study of a national population of Medicare beneficiaries hospitalized with HF, AMI, or pneumonia, we found that age has variable relationships with readmission risk in the year after hospital discharge. These results suggest that readmission is not an age-related phenomenon and may more strongly relate to other variables such as the quality of transitional care and complex social factors. However, we also found that readmission risk is elevated beyond the 30-day period used by the federal government to measure hospital readmission performance for all age groups. The implication of this finding is that efforts to reduce readmissions in older persons of all ages may be useful beyond the initial post-discharge month.
Our findings extend the literature on readmission risk and demonstrate that vulnerability after hospital discharge is not a clear function of age. We found that while rehospitalization risk rose with age following hospitalization for AMI, it declined with age following hospitalization for HF, and was minimally lower with age following hospitalization for pneumonia. These findings contrast with what is known about mortality risk after hospitalization, which has been strongly linked to age for multiple admitting conditions.13–17 Factors closely associated with aging such as reduced physiologic reserves,18,19 geriatric syndromes, and functional impairments may therefore be less important to readmission than mortality.
The variability in readmission risk by age across conditions may be explained by disease- and non-disease related factors. For example, it is possible that readmission risk is higher among younger patients with HF because these persons predominantly have heart failure with reduced ejection fraction rather than heart failure with preserved ejection fraction.20 These two syndromes have distinct pathogenesis, cardiac functional impairments, and coexisting chronic conditions.21,22 Younger patients with HF may also be more likely to have behavioral health disorders that increase susceptibility to rehospitalization in ways that are not captured by our risk adjustment.23 In contrast, the higher risk of rehospitalization with age following AMI may relate to the fact that older patients are more likely to have non-ST elevation myocardial infarction24 with larger burdens of residual obstructive coronary artery disease and higher risks of future coronary events.25 Older patients with AMI are also more likely to have complications from treatment, including significant bleeding,26 that may cause rehospitalization.
We extend our previous work1 by finding that rehospitalization risks remain elevated well after hospital discharge for older patients of all ages. For example, after hospitalization for heart failure, the relative risks of hospitalization over the first 2 months after discharge for the youngest and oldest age categories are almost 11 and 9 times that of persons in the general elderly population matched for sex, race, and median zip code income. This persistently elevated risk among recently hospitalized older patients should motivate routine discussions about prognosis and advanced care planning. Currently, these discussions are frequently missed27 with the greatest gaps among the youngest elderly patients.28
Our study has the following potential limitations. First, we used administrative data rather than chart review to identify hospitalizations. However, administrative data is highly specific for diagnosing acute cardiopulmonary conditions.29,30 Second, we did not exclude patients receiving hospice care and may have therefore biased our readmission estimates upward. However, our intent was to identify general relationships of age with post-hospital outcomes and therefore did not want beneficiary status for a discretionary benefit to bias outcomes differences between age groups. In addition, findings were not materially different after hospice patients were removed (results available by request). Third, we could not ascertain other outcomes important to older persons such as function, symptoms, and quality of life following hospitalization, as this information is not available in administrative data.
In summary, we found that age has heterogeneous relationships with readmission risk that varies by admitting condition. In all cases, however, readmission risks remain elevated for an extended time regardless of age. All older patients should therefore remain vigilant for deterioration in health well beyond the immediate time after hospital discharge. In addition, clinicians caring for recently discharged older patients should consider using condition-specific data on risk to guide discussions on advanced care planning and strategies for longitudinal follow-up after hospitalization.
Supplementary Material
Acknowledgments
Funding sources: Dr. Dharmarajan is supported by grant K23AG048331 from the National Institute on Aging and the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. He is also supported by grant P30AG021342 via the Yale Claude D. Pepper Older Americans Independence Center. Dr. Krumholz is supported by grant 1U01HL105270 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not represent the official views of the National Institute on Aging, American Federation for Aging Research, or the National Heart, Lung, and Blood Institute.
Appendix
Conflict of interest disclosures (details below):
| Elements of Financial/Pers onal Conflicts |
K. Dharmarajan |
A. Hsieh | R. Dreyer |
J. Welsh |
L. Qin | H. Krumholz | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | N o |
Yes | N o |
Ye s |
N o |
Ye s |
N o |
Yes | N o |
Yes | N o |
|
|
Employment or Affiliation |
X | X | X | X | X | X | ||||||
| Centers for Medicare and Medicaid Services |
Centers for Medicare and Medicaid Services |
Centers for Medicare and Medicaid Services |
||||||||||
| Grants/Funds | X | X | X | X | X | X | ||||||
| Medtronic and Johnson & Johnson |
||||||||||||
| Honoraria | X | X | X | X | X | X | ||||||
|
Speaker Forum |
X | X | X | X | X | X | ||||||
| Consultant | x | X | X | X | X | X | X | |||||
| Clover Health |
||||||||||||
| Stocks | X | X | X | X | X | X | ||||||
| Royalties | X | X | X | X | X | X | ||||||
|
Expert Testimony |
X | X | X | X | X | X | ||||||
|
Board Member |
X | X | X | X | X | X | ||||||
| Clover Health |
UnitedHealt h |
|||||||||||
| Patents | X | X | X | X | X | X | ||||||
|
Personal Relationship |
X | X | X | X | X | X | ||||||
Footnotes
Prior Presentation: No prior presentation.
- Kumar Dharmarajan: Employment or affiliation: works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures; Grants/funds: no; Honoraria: no; Speaker forum: no; Consultant: consultant for Clover Health; Stocks: no; Royalties: no; Expert testimony: no; Board member: member of a scientific advisory board for Clover Health; Patents: no; Personal relationship: no
- Angela Hsieh: Employment or affiliation: no; Grants/funds: no; Honoraria: no; Speaker forum: no; Consultant: no; Stocks: no; Royalties: no; Expert testimony: no; Board member: no; Patents: no; Personal relationship: no
- Rachel P. Dreyer: Employment or affiliation: no; Grants/funds: no; Honoraria: no; Speaker forum: no; Consultant: no; Stocks: no; Royalties: no; Expert testimony: no; Board member: no; Patents: no; Personal relationship: no
- Jack Welsh: Employment or affiliation: no; Grants/funds: no; Honoraria: no; Speaker forum: no; Consultant: no; Stocks: no; Royalties: no; Expert testimony: no; Board member: no; Patents: no; Personal relationship: no
- Li Qin: Employment or affiliation: works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures; Grants/funds: no; Honoraria: no; Speaker forum: no; Consultant: no; Stocks: no; Royalties: no; Expert testimony: no; Board member: no; Patents: no; Personal relationship: no
- Harlan M. Krumholz: Employment or affiliation: works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures; Grants/funds: recipient of research grants from Medtronic and Johnson & Johnson, through Yale University, to develop methods of clinical trial data sharing; Honoraria: no; Speaker forum: no; Consultant: no; Stocks: no; Royalties: no; Expert testimony: no; Board member: chair of a cardiac scientific advisory board for UnitedHealth; Patents: no; Personal relationship: no
Authors’ contributions:
Study concept and design: Dharmarajan, Krumholz
Acquisition of data: Krumholz
Analysis and interpretation of data: Dharmarajan, Hsieh, Dreyer, Welsh, Qin, Krumholz
Drafting of the manuscript: Dharmarajan
Critical revision of the manuscript for important intellectual content: Dharmarajan, Hsieh, Dreyer, Welsh, Qin, Krumholz
Statistical analysis: Hsieh, Qin
Obtained funding: Dharmarajan, Krumholz
Administrative, technical, or material support: Krumholz
Study supervision: Krumholz
Non-author contributors: none
Sponsor’s role: The sponsors had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
REFERENCES
- 1.Dharmarajan K, Hsieh AF, Kulkarni VT, et al. Trajectories of risk after hospitalization for heart failure, acute myocardial infarction, or pneumonia: retrospective cohort study. BMJ. 2015;350:h411. doi: 10.1136/bmj.h411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross JS, Mulvey GK, Stauffer B, et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med. 2008;168:1371–1386. doi: 10.1001/archinte.168.13.1371. [DOI] [PubMed] [Google Scholar]
- 3.Desai MM, Stauffer BD, Feringa HH, et al. Statistical models and patient predictors of readmission for acute myocardial infarction: a systematic review. Circ Cardiovasc Qual Outcomes. 2009;2:500–507. doi: 10.1161/CIRCOUTCOMES.108.832949. [DOI] [PubMed] [Google Scholar]
- 4.Kocher RP, Adashi EY. Hospital readmissions and the Affordable Care Act: paying for coordinated quality care. JAMA. 2011;306:1794–1795. doi: 10.1001/jama.2011.1561. [DOI] [PubMed] [Google Scholar]
- 5.Keenan PS, Normand SL, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 6.Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4:243–252. doi: 10.1161/CIRCOUTCOMES.110.957498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindenauer PK, Normand SL, Drye EE, et al. Development, validation, and results of a measure of 30-day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6:142–150. doi: 10.1002/jhm.890. [DOI] [PubMed] [Google Scholar]
- 8.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander KP, Newby LK, Cannon CP, et al. Acute coronary care in the elderly, part I: Non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–2569. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 10.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94:496–509. [Google Scholar]
- 11.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 12.Gasser T, Müller HG. Kernel estimation of regression functions. Smoothing techniques for curve estimation. Lecture Notes in Mathematics. 1979;757:23–68. [Google Scholar]
- 13.Lee DS, Austin PC, Rouleau JL, et al. Predicting mortality among patients hospitalized for heart failure: Derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 14.Antman EM, Cohen M, Bernink PM, et al. The timi risk score for unstable angina/non–st elevation mi: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 15.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. Jama. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 16.Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 17.Fine MJ, Auble TE, Yealy DM, et al. A Prediction Rule to Identify Low-Risk Patients with Community-Acquired Pneumonia. New England Journal of Medicine. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 18.Creditor MC. Hazards of Hospitalization of the Elderly. Annals of Internal Medicine. 1993;118:219–223. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 19.Beer RJ, Teasdale TA, Ghusn HF, et al. Estimation of severity of illness with APACHE II: age-related implications in cardiac arrest outcomes. Resuscitation. 1994;27:189–195. doi: 10.1016/0300-9572(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 20.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 21.Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–1431. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 22.Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–286. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranasinghe I, Wang Y, Dharmarajan K, et al. Readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia among young and middle-aged adults: a retrospective observational cohort study. PLoS Med. 2014;11:e1001737. doi: 10.1371/journal.pmed.1001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta RH, Rathore SS, Radford MJ, et al. Acute myocardial infarction in the elderly: differences by age. J Am Coll Cardiol. 2001;38:736–741. doi: 10.1016/s0735-1097(01)01432-2. [DOI] [PubMed] [Google Scholar]
- 25.Genereux P, Palmerini T, Caixeta A, et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol. 2012;59:2165–2174. doi: 10.1016/j.jacc.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehran R, Pocock SJ, Nikolsky E, et al. A Risk Score to Predict Bleeding in Patients With Acute Coronary Syndromes. Journal of the American College of Cardiology. 2010;55:2556–2566. doi: 10.1016/j.jacc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 27.Teno J, Lynn J, Wenger N, et al. Advance directives for seriously ill hospitalized patients: effectiveness with the patient self-determination act and the SUPPORT intervention. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. J Am Geriatr Soc. 1997;45:500–507. doi: 10.1111/j.1532-5415.1997.tb05178.x. [DOI] [PubMed] [Google Scholar]
- 28.Dunlay SM, Swetz KM, Mueller PS, et al. Advance directives in community patients with heart failure. Circ Cardiovasc Qual Outcomes. 2012;5:283–289. doi: 10.1161/CIRCOUTCOMES.112.966036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birman-Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 30.Skull SA, Andrews RM, Byrnes GB, et al. ICD-10 codes are a valid tool for identification of pneumonia in hospitalized patients aged > or = 65 years. Epidemiol Infect. 2008;136:232–240. doi: 10.1017/S0950268807008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.