Abstract
The recent advent of genome and epigenome editing technologies has provided a new paradigm in which the landscape of the human genome and epigenome can be precisely manipulated in their native context. Genome and epigenome editing technologies can be applied to many aspects of aging research and offer the potential to development novel therapeutics against age-related diseases. Here, we discuss the latest technological advances in the CRISPR-based genome and epigenome editing toolbox, and provide an insight into how these synthetic biology tools could facilitate aging research by establishing in vitro cell- and in vivo animal-models to dissect genetic and epigenetic mechanisms underlying aging and age-related diseases. We discuss recent developments in the field with the aims to precisely modulate gene expression and dynamic epigenetic landscapes in a spatial- and temporal- manner in cellular and animal models, by complementing the CRISPR-based editing capability with conditional genetic manipulation tools, including chemically inducible expression system, optogenetics, logic gate genetic circuits, tissue-specific promoters, and serotype-specific adeno-associated virus. We also discuss how the combined use of genome and epigenome editing tools permits investigators to uncover novel molecular pathways involved in pathophysiology and etiology conferred by risk variants associated with aging and aging-related disease. A better understanding of the genetic and epigenetic regulatory mechanisms underlying human aging and age-related disease will significantly contribute to the developments of new therapeutic interventions for extending healthspan and lifespan, ultimately improving the quality of life in the elderly populations.
Keywords: Aging, aging-related disease, CRISPR, epigenetics, genetics, genome editing, epigenome editing
Introduction
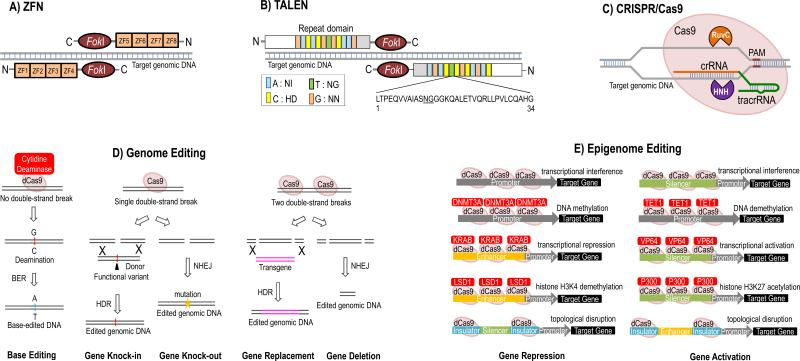
The recent advent of genome and epigenome editing technologies has enabled a precise manipulation of the human genome and epigenome by introducing specific changes in the native states. Targeted genome editing tools based on zinc finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN) and clustered regulatory interspaced short palindromic repeat (CRISPR)/Cas9-based RNA-guided DNA endonuclease have become powerful approaches for modeling human disease by creating isogenic cells and transgenic animals (Figure 1). These efficient programmable chimeric nucleases have provided a breakthrough to generate genetically engineered cell and animals much faster and more economically compared to the traditional homologous recombination-based mutagenesis approach [1].
Figure 1. Targeted genome and epigenome editing by ZFN, TALEN and CRISPR/Cas9 systems.
(A) Zinc finger nuclease (ZFN). ZFN consists of two functional domains: a DNA-binding domain that recognizes target genomic DNA sequences by multiple zinc finger modules (ZFs) and a nuclease domain that cleaves target DNA. Each ZF recognizes a unique 3-base pair of DNA and is designed to bind specific sequences flanking the desired cleavage site. Two nucleases act as a dimer and introduce a double-strand break (DSB). In the example, 12 nucleotides are targeted by 4 ZFNs and FokI generates a DSB at the target sites. (B) Transcription activator-like effector nuclease (TALEN). A TALEN consists of a nuclear localization signal (NLS) at the N-terminal, a tandem amino acid repeat domain, and a nuclease (FokI in this figure) fused to the C-terminal. Typically each repeat contains 33- to 35-amino acids including two unique residues that recognize a specific DNA sequence. Two TALENs form a complex by binding to genomic DNA in the opposite polarity to ZFNs. (C) Clustered regulatory interspaced short palindromic repeat (CRISPR)/Cas9-based RNA-guided DNA endonuclease (CRISPR/Cas9). A CRISPR/Cas9 complex is a ribonuclear protein comprising crRNA, tracrRNA, and an endonuclease Cas9. crRNA binds with Cas9 and guides the complex to the target DNA. Cas9 creates a DSB through nicking each strand of DNA by HNH and Ruv domains. (D) CRISPR-mediated genome editing for diverse DNA modifications. Upon CRISPR-induced single DSB, the restoration of two DNA ends by non-homologous end joining repair (NHEJ) gives rise to small indels in the target DNA and results in gene knockout. The DSB can be repaired by homology-directed recombination (HDR) when donor DNA containing the putative functional variant is provided, leading to a desired sequence knock-in. Large genomic DNA deletion and replacement can be generated by the introduction of two CRISPR sgRNAs with Cas9 to induce repair of the resultant two DSBs by NHEJ and HDR, respectively. Base editing strategy with a fusion of CRISPR/dCas9 and a cytidine deaminase enzyme allows programmable editing and irreversible conversion of a target DNA base into another without double-stranded DNA cleavage. Cytidine deamination induces conversion of G:C into an A:T base pair following DNA replication or base excision DNA repair (BER). (E) CRISPR/dCas9-based precise epigenome engineering for modulating gene transcriptional activities. Gene activation can be achieved by blocking the silencer and insulator regions using dCas9 (catalytically inactive dCas9, D10A;H840A). A stronger gene activation can be achieved by fusing the dCas9 to a minimal catalytic domain of chromatin-modifying enzyme such as VP64 transactivation domain, TET1 DNA demethylase, or P300 histone acetyltransferase. For targeted gene repression, dCas9 can be used to block the insulator and promoter activities. To achieve gene silencing effect, dCas9 can be fused with the KRAB transcriptional repressor, DNMT3A DNA methyltransferases, or LSD1 histone demethylase to repress the enhancer and promoter activities of a specific gene.
The recent breakthrough in the CRISPR-based genome editing tool further revolutionize gene editing technique due to its simplicity in target design, affordability, high efficiency, versatility and multiplexing capability [2]. The commonly used CRISPR system can be implemented in mammalian cells by co-expressing the Cas9 nuclease along with the single guide RNA (sgRNA), which is derived from a synthetic fusion of the CRISPR RNA array (crRNA) and the trans-activating crRNA (tracrRNA) [2] (Figure 1C). The 20bp target site sequence of sgRNA needs to be followed by a 3bp NGG protospacer adjacent motif (PAM) sequence on the 3′ end immediately and an additional guanine nucleotide to the 5’ can increase targeting efficiency [2]. The repertoire of CRISPR-mediated tools is expanding fast and we are now at the dawn of the gene-editing age. Substantial improvement of the CRISPR's efficiency, specificity and flexibility following the discovery of CRISPR's variants [3-5], chemical modification to the sgRNA [6] and more efficient delivery methods [5, 7] has greatly expanded the CRISPR toolbox. More recently, the application of CRISPR/Cas9 system has expanded beyond genome editing (Figure 1D) to epigenome engineering (Figure 1E) to modulate endogenous transcription and epigenetic states [8]. The complement of optogenetics [9] and multi-input logic gate genetic circuits [10] with the CRISPR-based targeted epigenome editing capability enables precise modulation of gene expression and epigenetic landscape in a spatial and temporal manner in specific tissues and cells. The epigenome engineering has created another wave of excitement across the scientific research community. Biologists are embracing the power of gene-editing tools, particularly CRISPR/Cas9 system to explore the landscape of genomes and epigenomes in their native context.
The background and development of ZFN, TALEN and CRISPR-based targeted genome and epigenome editing toolbox have been extensively reviewed elsewhere [1, 11]. Here, we discuss the latest major advances in the CRISPR-based genome and epigenome editing tools, and the potential application of these programmable chimeric enzymes in aging research. More specifically, we provide an insight on how these synthetic biology tools could facilitate the aging research by establishing in vitro and in vivo models to dissect genetic and molecular mechanisms underlying age-related diseases.
Recent Major Advances in Genome Engineering
CRISPR is transforming biomedical science research and has quickly become the preferred tool for genetic manipulation, and shows incredible promise as a versatile genome-editing platform for interrogating endogenous gene function in vitro and in vivo. One of the major addition to the CRISPR toolbox was the discovery of multiple Cas9 variants with expanded capabilities and minimized molecular weight for genetic manipulation to further advance genome and epigenome engineering (Table 1 and Figure 2). Two smaller-size Cas9 orthologues, Streptococcus thermophilus Cas9 (St1Cas9) and Staphylococcus aureus Cas9 (SaCas9) could edit the genome with efficiency and specificity similar to those of commonly used Streptococcus pyogenes (SpCas9) [5, 12]. The SaCas9 and its single guide RNA expression cassette can be packaged into a single adeno-associated virus (AAV) delivery vehicle for efficient and specific in vivo genome editing [5]. Nevertheless, due to exceeding maximal viral genome packaging capacity, addition of tag markers such as commonly used fluorescent reporter to the downstream of fused SaCas9 and its single guide RNA expression cassette results in no production of functional AAV. To circumvent this issue, the previously reported similar dual vector system can be adopted [7], which uses one vector to express fusion of SaCas9 and fluorescent reporter genes, and another to express multiple sgRNAs. In fact, the SpCas9-based dual vector system was successfully used to interrogate gene function in the mammalian brain by editing multiple genes (Dnmt1, Dnmt3a and Dnmt3b) in the adult mouse brain in vivo [7]. As illustrated in Figure 2B, dual vector system is particularly useful to deliver large fusion transgene comprising of CRISPR and chromatin catalytic domains, as well as including tag markers, multiple guide RNA expression cassette, optogenetics- or doxycycline-inducible element. To our knowledge, strategies for in vivo delivery of CRISPR-based epigenome editing using AAV vectors have yet to be established.
Table 1.
Recent Advances in CRISPR-based Genome Editing
| Advancement | Reference |
|---|---|
| Cas variants (other than wild-type SpCas9) | |
| Smaller nuclease size | |
| Staphylococcus aureus Cas9 (SaCas9) | [12] |
| Streptococcus thermophilus Cas9 (St1Cas9) | [12] |
| Controllable sticky end generated | |
| Prevotella and Francisella 1 Cas (Cpf1) | [3] |
| Class 2 Cas (C2c1) | [13] |
| More relaxed PAM | |
| Francisella novicida Cas9 (FnCas9) | [16] |
| Higher specificity | |
| Enhanced specificity SpCas9 (eSpCas9) | [17] |
| High-fidelity SpCas9 (SpCas9-HF1) | [4] |
| Double nicking Cas9 (Cas9 nickase) | [18] |
| Targeting single-stranded RNA | |
| Leptotrichia shahii Cas (C2c2) | [14] |
|
Altered PAM specificities to broaden the Cas9 application | |
| Streptococcus pyogenes Cas9 (SpCas9) | [12] |
| Staphylococcus aureus Cas9 (SaCas9) | [12] |
|
Altered sgRNA and Cas9 to improve genome-editing efficiency and specificity | |
| Chemically modified sgRNA | [6] |
| Truncated sgRNA | [19] |
|
Improved homologous recombination-mediated DNA replacement and gene knockin | |
| Asymmetric donor DNA | [20] |
| Homology-directed repair (HDR) enhancer | [21] |
| Inhibit non-homologous end joining (NHEJ) activity | [22] |
| NHEJ homology-independent knock-in | [24] |
| Silent CRISPR/Cas-blocking mutations | [23] |
| Base editing approach | [25] |
|
Efficient delivery vehicles | |
| Serotype-specific AAV viral vector | [5] |
| Combine use of lipid nanoparticle and AAV viral vector | [55] |
Figure 2. Expanding applications with CRISPR variants and combined use of conditional genetic manipulation techniques.
(A) Alternative ways of genetic modification with CRISPR variants. The sticky-end DNA fragments generated by staggered cutting of Cpf1 allow a precise insertion of donor DNA in the proper orientation into the genome via non-homology-directed repair mechanisms such as NHEJ. C2c2 is a RNA-guided RNA-targeting CRISPR effector that can be programmed to knock down specific mRNAs by cleaving single-stranded RNA targets carrying complementary protospacers. (B) In vivo genome and epigenome editing by adeno-associated viruses (AAV). The SaCas9 and its single guide RNA expression cassette can be packaged into a single AAV delivery vehicle for efficient and specific in vivo genome editing. In dual AAV vectors system, dSaCas9-based chromatin modifiers can be used for multiplex epigenome editing by co-transduction of a dSaCas9-VP64 vector and an expression vector with three U6-sgRNA cassettes in tandem. (C) CRISPR/dCas9-based photoactivatable targeted epigenome engineering. In response to blue light irradiation, fusing of sgRNA-dCas9-CIB1 with the light-sensitive cryptochrome 2 (CRY2) bearing VP64 induces targeted gene activation through VP64 transactivation domain. VP64 co-localizes with dCas9 via CRY2-CIBN interactions and induce transcription only in the presence of blue light. Gene activation is reversible through simple removal of illumination. (D) CRISPR/dCas9-based AND logic gate genetic circuits. CRISPR/dCas9-based AND gate circuits integrate cellular information from two promoters as inputs and activate the output gene only when both inputs are active in the tested disease-relevant cell/tissue types. One promoter (tissue-specific) drives the transcription of dCas9-VP64 mRNA and another promoter is linked to the transcription of sgRNA targeting a specific gene. The expression of sgRNA is mediated by two hammerhead ribozymes placed at both ends of sgRNA. When the primary transcript is generated, it undergoes self-catalyzed cleavage to release the designed sgRNA. The effector (target gene) can be expressed only when both dCas9-VP64 protein and sgRNA are presented. (E) CRISPRainbow and CRISPR-Multicolor for studying nuclear architecture and higher order chromatin organization. Different fluorescent-tagged dCas9-sgRNAs (red, green and blue) allows multiplexed labeling of chromatin loci for tracking gene and chromatin dynamics by spatial and temporal visualizing endogenous genomic loci in live cells. Co-localization of red, green and blue fluorescents indicates a close physical interaction between topologically associated domains (TADs) from different chromosomes. In the case of studying cis/trans-acting regulatory elements within a TAD, CRISPRainbow and CRISPR-Multicolor allow tracking dynamics interaction of enhancers or silencers with a specific promoter for transcriptional regulation. When the dCas9-Blue labeled enhancer close in contact with the dCas9-Red labeled promoter, gene A is activated and pink fluorescent can be visualized. Similarly, when the dCas9-Green labeled silencer close in contact with the dCas9-Red labeled promoter, gene B is activated and yellow fluorescent can be visualized.
Recent studies reveal the potential of another two distinct Class 2 CRISPR/Cas systems, Cpf1 [3] and C2c1 [13] proteins, which could expand, complement, and extend the existing CRISPR/Cas9 genome-editing tools. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR/Cas system and could mediate robust and efficient genome-editing activity with features distinct from Cas9 [3]. Cpf1 lacks tracrRNA, utilizes a T-rich protospacer-adjacent motif for DNA recognition and requires only one RNA molecule to cleave DNA via a staggered DNA double-stranded break [3]. Instead of ‘blunt’ ends resulted by Cas9 cutting both strands in a DNA molecule at the same position, the DNA cleavage activity of Cpf1 could create a ‘sticky’ end [3]. The sticky ends carry information that can direct the insertion of the DNA, hence, it makes the insertion much more controllable and this distinct advantage could offer researchers more options when selecting a genome site to edit. This feature allow a better alternative way to precisely introduce DNA in the proper orientation into the genome via non-homology-directed repair mechanisms especially in non-dividing cells (Figure 2A). Apart from Cas9 and Cpf1, C2c1 is another distinct Class 2 CRISPR/Cas system and mediates DNA interference in a 5′-protospacer adjacent motif (PAM)-dependent fashion analogous to Cpf1 [13]. Unlike Cpf1, C2c1 depends on both crRNA and tracrRNA for DNA cleavage [13]. Compared to the RNA-guided DNA targeting enzymes such as Cpf1 and C2c1, C2c2 was a newly discovered RNA-guided RNA-targeting CRISPR effector, which can be programmed to knock down specific mRNAs by cleaving single-stranded RNA targets carrying complementary protospacers [14].
Engineered CRISPR/Cas9 nucleases with altered PAM specificities eliminate the constraint that only a specific PAM can be recognized by Cas9 [12]. These Cas9 variants with altered PAM specificities enable robust editing of desired endogenous gene sites which were not targetable by wild-type SpCas9, while maintaining the genome-wide specificities comparable to those of wild-type SpCas9 [12]. These Cas9 variants provide the feasibility of engineering a wide range of Cas9s with altered and improved PAM specificities [12]. The SaCas9 PAM sequence for optimal on-target cutting is 5’-NNGRRT-3’ which is longer than the 5’-NGG-3’ PAM recognition sequence of SpCas9. This short PAM has limited the range of sequences that Cas9 orthologs can target [5]. By using molecular evolution to modify the PAM of SaCas9 to relax the PAM recognition specificity, SaCas9 targeting range can be increased while preserving the robustness of genome editing activities at endogenous loci [15]. Recently, Cas9 from Francisella novicida (FnCas9), one of the largest Cas9 orthologs, was successfully microinjected into mouse zygotes to edit endogenous sites with the more relaxed 5′-YG-3′ PAM, thus expanding the target space of the CRISPR/Cas9 toolbox [16].
Using targeted deep sequencing and unbiased whole-genome off-target analysis, the newly designed “enhanced specificity” SpCas9 (eSpCas9) variants were shown to reduce off-target effects and maintain robust on-target cleavage in human cells [17]. SpCas9-HF1, another high-fidelity variant of CRISPR/Cas9 nucleases could significantly reduce unwanted off-target mutations as shown by genome-wide break capture and targeted sequencing methods [4]. With the exceptional precision and improved specificity, rationally engineered Cas9 nucleases, including SpCas9-HF1 and eSpCas9 could provide an alternative to wild-type SpCas9 for research and therapeutic applications of numerous inherited disorders [4, 17]. Higher genome editing specificity while retaining on-target cleavage efficiency could be achieved by combining a Cas9 nickase mutant with paired guide RNAs to introduce targeted double-strand breaks [18]. In addition, use of truncated guide RNAs could further reduce off-target effects induced by pairs of Cas9 nickases without sacrificing on-target genome editing efficiencies [19].
For transgene knock-in or DNA replacement, the efficiency of precise sequence replacement by CRISPR-mediated homology-directed repair (HDR) could be significantly increased using asymmetric donor DNA [20]. The utilization of an HDR enhancer, RS-1 (3-((benzylamino)sulfonyl)-4-bromo-N-(4-bromophenyl) benzamide) was shown to enhance CRISPR/Cas9- and TALEN-mediated knock-in efficiency in rabbit embryos both in vitro and in vivo by stimulating RAD51 [21]. To support this notion, abolished non-homologous end joining (NHEJ) activity by suppressing the NHEJ key molecules, Ku70 or DNA ligase IV, could increase the efficiency of HDR for CRISPR/Cas9-induced, precise gene editing in mammalian cells [22]. Additionally, highly efficient introduction of specific homozygous and heterozygous mutations could be achieved by incorporating silent CRISPR/Cas-blocking mutations along with pathogenic mutations to improve HDR accuracy [23]. Interestingly, CRISPR/Cas9-induced NHEJ homology-independent knock-in strategy allows more efficient targeted integration of large reporter genes than HDR-mediated gene targeting knock-in in human cells [24]. In this strategy, NHEJ pathway was used to promote DNA integration into a genome by rejoining of cleaved genome and donor plasmids following CRISPR/Cas9-induced DNA DSBs [24]. NHEJ homology-independent knock-in strategy is particularly useful for genome editing in post-mitotic or non-dividing cells since HDR does not function in G0/G1 phase arrested cells.
More recently, the newly developed base editing approach enables programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. This is achieved by directing irreversible conversion of one target DNA base into another in a programmable manner in the absence of dsDNA backbone cleavage or a donor template [25]. As illustrated in Figure 1D, base editing strategy involves the use of CRISPR/dCas9 and a cytidine deaminase enzyme fusion for cytidine deamination and followed by DNA replication or base excision DNA repair to mediate conversion of G:C into an A:T base pair [25]. This scarless genome editing strategy can avoid the issue of random insertions and deletions (indels) at the target locus resulting from the cellular DNA repair response to dsDNA breaks. Hence, this base editing approach has greatly expanded the scope and efficiency of genome editing of point mutations while minimizing indels formation to favor desired clean base-editing outcomes. Additionally, co-delivering chemically modified sgRNAs [6] or truncated guide RNAs [19] along with Cas9 mRNA or protein could enhance genome editing efficiency and retain high specificity by increasing intracellular stability and minimizing toxicity associated with DNA delivery.
From Genome Engineering to Epigenome Editing
DNA methylation, histone posttranslational modification, ATP-dependent chromatin remodeling, histone subunit exchange, genomic imprinting, RNA-associated silencing, three-dimensional nuclear architecture and higher order chromatin organization can contribute to epigenetic alterations and modulate the activities of cis- and trans-acting regulatory DNA elements. Epigenetic changes can switch genes on or off and determine which genes are transcribed. The epigenetic marks can be influenced by several factors including age, the environment/lifestyle, and disease state. Furthermore, the far majority of genetic variants associated with the risk of common diseases detected by GWAS lie in the non-coding regions of the genome [26], suggesting that gene regulatory changes contribute to inter-individual differences in genetic susceptibility to disease. Thus, epigenetic editing tools would be in demand to understand the causal effects of epigenetic variation and ultimately to counteract the epigenetic changes that functionally contribute to disease risk.
Until recently, traditional approaches such as pharmacological inhibition or genetic perturbation have been used to characterize the functional role of cis-/trans-acting DNA regulatory elements and chromatin regulation. These include pharmacological inhibitors of histone modifying enzymes or epigenetic modifier such as 50-AzaC and valproic acid and ectopic overexpression or knock-down of these factors followed by genome-wide profiling of gene expression and chromatin states. Obviously, these approaches have limitations in resolving the complex interactions in the native genetic context at the target locus due to the genome-wide changes in chromatin state and gene expression induced by the general, non-targeted manipulation [7]. For many specific histone modifications and chromatin remodeling, it remains unclear whether these epigenetic states are the cause or the consequence and these methods do not directly assess causal functional roles for chromatin regulators at specific loci due to the potential pleiotropic effects. Targeted epigenetic editing tools address these issues allowing investigation of the precise roles played by specific epigenetic modulators on cell-type specific chromatin state and gene activity in their native contexts.
Cas9 protein has been repurposed by site-specific mutations (D10A; H840A) in the nuclease domain to make the nuclease-deficient Cas9, known as dCas9. CRISPR/dCas9 has revolutionized the ability to modulate the genomic regulatory elements and epigenetic marks, when fused with minimal catalytic domains of chromatin-modifying enzymes to generate synthetic sequence-specific activators and repressors [8, 27]. The nuclease-based fusions of chromatin-modifying enzymes such as P300 (histone acetyltransferase)[8], LSD1 (histone demethylase or enhancer repressor by removing H3K4 methylation) [27], KRAB (transcriptional repressor) [28], VP64 (transcriptional activator) [29] and TET1 (DNA demethylation) [30] were successfully used to achieve targeted DNA methylation or hydroxymethylation, histone methylation or demethylation, histone acetylation or deacetylation in mammalian cells (Table 2). A similar strategy could also be adopted for other nucleosome-modulating factors, chromatin remodelers and histone chaperones to modulate the chromatin structure of genomic functional elements in order to affect the activity of downstream target genes. Hence, targeting histone-modifying activities to specific loci with dCas9 has been a powerful epigenome editing tool for testing the functions of genomic elements and associated chromatin states in their endogenous context [11]. The advances of these nuclease-based site-specific epigenome editing have been recently extensively reviewed elsewhere [11].
Table 2.
Recent Advances in CRISPR-based Epigenome Editing
| Advancement | Reference |
|---|---|
| Epigenetic marks and chromatin states modulation (dCas9 nuclease-based fusions) | |
| dCas9 (CRISPRi) (transcriptional interference) | [28] |
| dCas9-VP64 (transcriptional activation) | [29] |
| dCas9-VPR (VP64-p65-Rta) (transcriptional activation) | [56] |
| dCas9-KRAB (Krüppel associated box) (transcriptional repression) | [28] |
| dCas9-P300 (histone acetylation) | [8] |
| dCas9-LSD1 (histone demethylation) | [27] |
| dCas9-DNMT3A (DNA methylation) | [57] |
| dCas9-peptide repeat and scFv-TET1 catalytic domain fusions (DNA demethylation) | [30] |
|
mRNA and protein localization tracking | |
| RCas9 (nuclear-localized RNA-targeting dCas9) (mRNA tracking) | [31] |
| SLENDR (single-cell labeling of endogenous proteins by CRISPR/Cas9-mediated homology-directed repair) (protein tracking) | [32] |
|
Multiplexed labeling of chromatin loci for tracking chromatin dynamic | |
| CRISPR-Multicolor (multiple loci labeling) | [34] |
| CRISPRainbow (multiple loci labeling) | [35] |
|
Reverse information flow from RNA to DNA | |
| RT-Cas1(reverse transcriptase-Cas1) (reverse transcription) | [58] |
|
Scarless genome editing | |
| recCas9 (dCas9-serine recombinase) (genetic recombination) | [59] |
|
Orthogonal gene knockout and transcriptional activation | |
| SpCas9 with ‘dead RNAs’ (knock out and activate different genes in the same cell) | [60] |
The establishment and maintenance of the newly introduced epigenetic marks depend on the intrinsic amenability of the target genes, the levels of transcription, the local chromatin context, and dynamic interplay between chromatin writers, readers, and erasers [11]. A possible approach to maintain heritability of the edited epigenetic marks in the nuclei is by adopting a light-inducible CRISPR/dCas9-mediated targeted epigenome engineering. Adeno-associated viruses (AAV) mediated expression of photoactivatable CRISPR/dCas9 vectors can exist long term as concatomers in non-dividing cells, however it will be lost in replicating cells. To establish conditional knock-in permanent cell lines stably expressing CRISPR/dCas9-based photoactivatable transcription system, a copy of sgRNA-dCas9-CIB1 and transactivation domain-CRY2 fusion vectors can be inserted into a safe harbor gene knock-in locus such as human adeno-associated virus integration site 1 (AAVS1) site and mouse Rosa26 by using CRISPR/Cas9-mediated homologous recombination approach. In post-mitotic cells such as primary neuronal cells in mammalian brain, there is no need to introduce these optogenetic vectors into the genome since episomal stability enables long-term transgene expression in non-dividing cells. In fact, the non-integrating and replication defective nature of AAV are effectively used in optogenetics-based neuroscience experiments to stably express optogenetic construct in the brain region without the need to integrate these vectors into the host cell genome. As illustrated in Figure 2C, in the presence of blue light, a transactivation domain would co-localize with the catalytically inactive dCas9 following the fusion of the light-sensitive heterodimerizing proteins CRY2 and CIB1 to modulate transcription [9]. Gene activation is reversible through simple removal of illumination. Increasing the number of chromatin-modifying domains localized to the targeted promoter or enhancer sequence could synergistically enhance gene activation [9]. Thus, the CRISPR/dCas9-based photoactivatable transcription system allows rapid and reversible targeted gene activation by light [9]. As illuminating or extinguishing the blue light irradiation is sufficient to switch on or off the epigenome modulation, this system could be used to precise control of epigenome editing activity in a spatial- and temporal-dependent manner for better understanding of complex gene regulatory networks and chromatin dynamics. Light activation of CRISPR/dCas9 allows for the study of epigenetic regulatory mechanism underlying specific gene function with high precision, and can reduce toxicity from off target epigenetic modulation by restricting the function of dCas9 to certain locations or time points [9].
To achieve more precise spatial control of transcriptional gene activities, CRISPR/dCas9-based logic gate genetic circuits could be considered. As illustrated in Figure 2D, CRISPR/dCas9-based AND gate circuits can integrate cellular information from two promoters as inputs (tissue and disease-specific promoters) and activate the output (target gene expression) only when both inputs are active in the tested cells. Using this chimeric system, the interest gene can be manipulated to be expressed only in specific-tissue origin disease cells, but remain silenced in normal cells or other tissue-origin disease cells. As a proof of principle, synthesizing AND gate genetic circuits based on CRISPR/Cas9 has been successfully used to specifically identify and control bladder cancer cells in vitro [10]. Theoretically, dCas9 and designed sgRNAs can be used to build any type of transcriptional logic gates and connect them to on-target epigenetic manipulation in living cells and computational analysis of gene regulatory network. For example, combination use of the elementary logic gates such as AND, OR, NOT, NAND, NOR, XOR and XNOR (universal symbols for logic gates in electronic fields) allow epigenome manipulation of desired transcriptional regulation at targeted loci. Each of this logic gate use only two inputs (e.g. cell-type-dependent and tissue-specific cis/trans-acting chromatin regulation) to give a distinct output (e.g. epigenetic state and gene expression level). Hence, CRISPR/dCas9-based logic gate genetic circuits could be used to selectively and robustly correct transcriptional misregulation or restore normal epigenetic patterning in disease-relevant cell types.
Recently, nuclear-localized RNA-targeting nuclease-inactive Cas9 (RCas9) has been developed to track endogenous RNA in living cells in a programmable manner without relying on incorporation of genetically encoded exogenous tags [31]. Subsequently, an in vivo genome editing technique, called single-cell labeling of endogenous proteins by CRISPR/Cas9-mediated homology-directed repair (SLENDR) has been developed to allow high-throughput, high-resolution mapping of protein localization in mammalian brain [32]. Additionally, an integrated approach of CRISPR genome editing and single-molecule imaging has been used to track the dynamics of telomerase recruitment to telomeres in nuclei of living human cells [33]. These three modified CRISPR-based strategies enable the real-time localization tracking of mRNAs and proteins in a specific cellular compartment and live-cell dynamic monitoring of gene and pathway activities.
To understand a dynamic 3D organization and interactions of genomic elements that are critical for the spatial and temporal regulation of gene expression, a series of CRISPR-based imaging has been recently developed (Figure 2E). These include CRISPR-Multicolor [34] and CRISPRainbow [35], and are used to determine genomic DNA localization, dynamics and inter-locus interactions in living cells in space and time. In combined use with super-resolution microscopy such as structured illumination microscopy or spectral precision distance/position determination microscopy, a CRISPRainbow system allows simultaneous imaging and tracking of multiple genomic loci in living cells to interrogate the intranuclear dynamics and chromatin architecture [35]. As illustrated in Figure 2E, using pairs of differently fluorescent-tagged dCas9-sgRNAs (red, green and blue), the intranuclear distance between loci on different chromosomes could be determined [34, 35]. CRISPRainbow and CRISPR-Multicolor also allow tracking dynamics interaction between cis/trans-acting regulatory elements and a promoter to rewire transcriptional activity under a specific experimental condition. By correlating the linear intrachromosomal distance and the fluorescence spatial resolution between two loci on the same chromosome, the DNA compaction in the region of interest could be assessed in a live cell [34, 35]. This combination of nanoscale imaging and CRISPR system opens a new avenue to study the 3D nuclear topography of active and inactive regulatory sequences directly on the individual cell level at a single molecule resolution.
Genome and Epigenome Engineering to Investigate the Molecular Mechanisms Underlying Aging and Aging-related Diseases in Humans
Aging is characterized by a decline in the maintenance of homeostatic processes over time that increases the risk for many common chronic diseases and degenerative conditions, ultimately resulting in death. As aging affects multiple organs and systems in humans, the complexity in animal modeling has been a major obstacle in elucidating the mechanisms by which genetic factors, as seen in hereditary longevity and the high recurrence of the same age-related disease within a family, impact the differential progression of aging. Compared to invertebrate models, vertebrate models (e.g. mice or rats) can recapitulate many more important pathological features of human aging and disease phenotypes. However, modeling aging and age-related diseases in the lab is challenging because classical vertebrate models have relatively long lifespans and high costs of maintenance. Luckily, the emerging CRISPR-based genome and epigenome-editing technologies have shorten the lengthy steps in generating multiple transgenic animal models. By enabling the introduction of desired genetic modification in many genes encompassing the hallmarks of aging, the CRISPR/Cas9 technology permits a rapid exploration of their roles in healthspan and lifespan in a high-throughput manner and functional characterization of genetic variants mapped by human genome-wide association studies (GWAS) [36] (Figure 3).
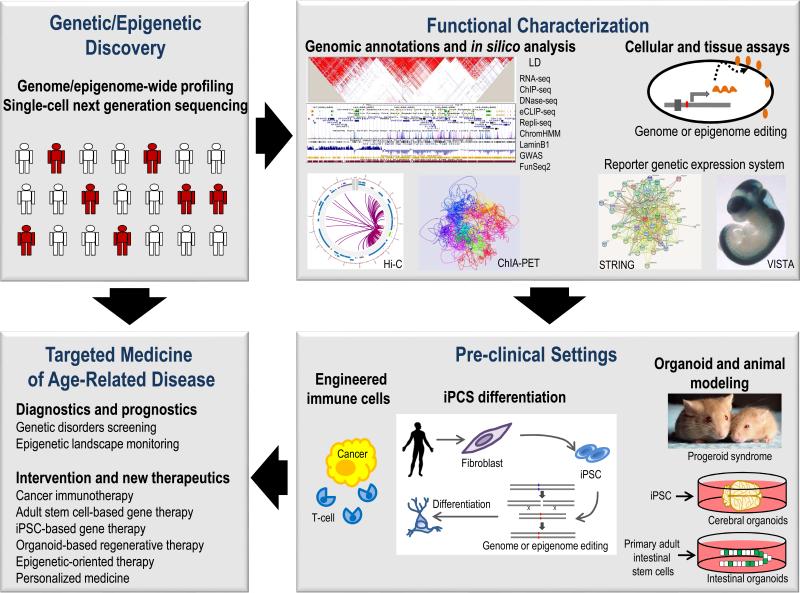
Figure 3. Translational genetics and epigenetics of aging.
A strategy for targeted intervention based on genetic, epigenetic, functional and mechanistic studies is shown in the flow chart. Genome- and epigenome-wide association studies, chromatin states and gene expression profiling lead to identification of genetic variants associated with an aging-related phenotype. Identification of the causal, functional variants underlying association can be further pursued by prioritization through in silico analysis and genome annotations (LD, linkage disequilibrium or haplotype block; RNA-seq, gene expression; ChIP-seq, histone mark enrichment; DNase-seq, open chromatin; eCLIP-seq, RNA binding protein occupancy; Repli-seq, replication timing; ChromHMM, chromatin state segmentation; LaminB1, nuclear lamina associated domains; GWAS, genome-wide association studies; FunSeq2, variant annotation; Hi-C, topologically associating domains and compartments; ChIA-PET, promoter-enhancer links) followed bycellular and tissue assays. Bioluminescent- and fluorescent-based reporter genetic expression system enable high-throughput analyzing of functional regulatory variants and intracellular dynamics, followed by prioritization of variants for establishing transgenic cell line. The VISTA Enhancer Browser enables the identification of distant-acting transcriptional enhancers in the human genome by using mouse transgenesis enhancer assay to characterize noncoding DNA elements with strong evolutionary conservations and epigenomic evidence of putative enhancer marks. Mass spectrometry-based proteomics profiling and functional protein association networks (STRING) analysis allow mapping of protein-protein interaction networks. Genome and epigenome editing in iPSCs and in vivo mouse modeling enable mechanistic studies in the relevant tissue. An iPSC-derived three-dimensional organoid culture system, termed cerebral organoids (miniature 3D brain tissue) can serve as a good in vitro model for studying human brain development and a wide range of complex brain diseases. Human primary intestinal stem cells from cystic fibrosis patients can be expanded in culture as genetically and phenotypically stable epithelial organoids for gene correction by CRISPR-mediated homologous recombination. CRISPR-edited immune cells can be used to target and eradicate tumour cells in cancer immunotherapy. The integrated information gained from genetic, epigenetic, functional and mechanistic studies will ultimately help the development of effective targeted intervention strategies against aging and aging-related diseases.
The importance of epigenetic dysregulation in the etiology of human disease and aging process is increasingly recognized. Diverse epigenetic mechanisms have been implicated in human diseases, where it can affect disease etiology and progression. Incorporates epigenetic variation into genetic studies may help to explain the late onset and progressive nature of most common diseases, the severity of symptoms, the quantitative nature of complex traits and the role of environment in disease development, which a purely sequence-based approach might not. Even monozygotic twins may succumb to the same disease, but often the severity of symptom is different and the age of onset is years apart, and this phenotypic discordance became more noticeable with age, hence, environmental exposure driven heritable changes in the epigenome play a critical role in the modulation of human aging and age-related diseases. A cross-sectional and longitudinal data on monozygotic twin pairs has shown that sustained epigenetic differences arise during the adult lifespan could contribute to an increasing discordance of monozygotic twins during aging [37]. Apart from genetic defects in genome maintenance and mitochondria function, epigenetic dysregulation has recently been highlighted as an important biological hallmark of aging and longevity because transcriptional misregulation resulted by aberrant epigenetic landscape cause a shorter lifespan and premature aging in humans and mice [38-40]. Genome-wide DNA methylation profile in centenarians and centenarians’ offspring revealed the contribution of epigenetic factors in human aging and longevity [40].
From a clinical point of view, targeted editing of aging-related genes offers novel therapeutic avenues for multiple diseases. The feasibility of creating specific genetic variants in human induced pluripotent stem cell (iPSC) derived from patients or engineered de novo has been demonstrated [23]. An iPSC-derived three-dimensional organoid culture system, termed cerebral organoids (miniature 3D brain tissue), closely mimics the endogenous developmental program and can serve as a good in vitro model for studying human brain development and a wide range of complex brain diseases [41]. Cerebral organoids can be used in combination with CRISPR/Cas9-based genome and epigenome editing to unravel genetic and epigenetic mechanisms that cause neurodevelopmental and neurodegenerative disorders. Additionally, human primary intestinal stem cells derived from cystic fibrosis patients can be expanded in culture as genetically and phenotypically stable epithelial organoids for restoring functional activity of CFTR by CRISPR-mediated homologous recombination [42]. CFTR (cystic fibrosis transmembrane conductance regulator) encodes an anion channel essential for fluid and electrolyte homeostasis of epithelia and a hereditary defect of this gene cause cystic fibrosis [42]. Since intestine is an organ with a high turnover and cystic fibrosis is a single-gene hereditary defect disorder, in accompanying with the facts that primary intestinal stem cells can be expanded as cultured organoids and genetically corrected intestinal organoids can be re-implanted in the intestine of patients, the precise correction of point mutations linked to cystic fibrosis in patient-derived organoids might enable effective adult stem cell-based gene therapy of this disease in near future. The combination use of CRISPR/Cas9-based gene correction approach with organoid culture technology enables clonal expansion of single adult patient stem cells and the selection of genetically modified clonal organoid cultures harboring the precise gene correction (Figure 3).
Disease modeling in cell line does not recapitulate the complex interactions between tissues and complex responses to environment or drugs. An increasing number of studies have utilized the CRISPR/Cas9 system for in vivo gene editing. Epigenetic studies of longevity and aging based on diverse animal models has been comprehensively reviewed recently [43]. Of special interest are lifespan extension in various animal models have strongly support an epigenetic role in modulating aging and longevity pathways [43]. Thus far, the majority of evidence suggests that histones loss coupled with chromatin remodeling, an imbalance of activating and repressive histone modifications, transcriptional change, global and local change in DNA methylation, site-specific loss and gain in heterochromatin, and significant nuclear reorganization as a general mechanism of aging in mammalians [43]. For example, to mimic the autism condition in humans, researchers have engineered monkeys to have the MECP2-duplication syndrome [44]. The protein of MECP2 (methyl-CpG binding protein 2) is essential for normal brain function and transcriptional repression through chromatin modification. Severe neurodevelopmental disorders associated with MECP2 include Rett Syndrome and X-linked mental retardation [45]. Additionally, many of the symptoms of autism are found in people who have extra copies of the MECP2 gene being expressed in the brain [44]. To examine the impact on autism-like symptoms in monkeys, the researchers plan to use the CRISPR system to knockout the extra MECP2 copies in specific brain regions [44]. Depletion of DNA methyltransferase MeCP2 leads to lethality and causes a neuronal phenotype, but postnatal, neuron-specific activation of MeCP2 could significantly prolonged the lifespan of mutant mice and delayed the onset of neurologic symptoms [38]. On the other hand, depleting the histone methyltransferase SUV39H1 was shown to improve DNA repair capacity, delay senescence and extend lifespan in a progeria mouse model, providing a potential strategy for aging intervention by targeting SUV39H1-mediated heterochromatin remodeling [39].
The transgenic animal model is useful to recapitulate the premature aging features of the human segmental progeroid syndromes, including Werner syndrome (WS) and Hutchinson-Gilford progeria syndrome (HGPS), which is characterized by defective DNA helicases WRN and aberrant processing of the nuclear envelope protein lamin A LMNA, respectively [46]. Aberrant DNA methylation profiles in WRN and LMNA mutant patients can also impact these premature aging diseases [46]. By generating human induced pluripotent stem cells (iPSC) from fibroblasts obtained from patients with HGPS, in vitro iPSC-based model could recapitulate laminopathy phenotypes such as lack the nuclear envelope and epigenetic alterations normally associated with premature ageing [47]. The CRISPR/Cas9 technology will accelerate the progress in dissecting the contributions of genetic and epigenetic components to lifespan and longevity in animal models. This gene editing technology allow us to uncover principles that determine the spatial organization of chromosomes, to reveal novel layers of chromatin structure and to relate these aberrant structures to gene dysregulation underlying human aging. Modulating chromatin states and cis-/trans-acting DNA regulatory elements may be prime targets for therapeutic intervention to help improve the healthspan and lifespan by correcting transcriptional dysregulation or restore normal epigenetic patterning during aging. Owing to the fact that epigenome editing does not involve genetic changes and the reversible nature of epigenetic mechanisms, it may be less risky with respect to unwanted off-target effects as well as side effects upon healthy cells and tissues.
There have been a number of human clinical trials using the alternative gene-editing techniques but none so far has used CRISPR. The application of ZFN technology for gene therapy has been completed and six early-phase clinical trials are currently undergoing (ClinicalTrials.gov identifiers). Excitingly, the first human trial of CRISPR will start later this year for cancer immunotherapy [48]. A team of researchers from China has announced plans to begin the very first clinical trial with CRISPR-edited immune cells as early as August 2016 to treat patients with lung cancer [48]. Other CRISPR trials may not be far behind. In June 2016, an advisory panel of the US National Institutes of Health (NIH) approved a similar CRISPR-based study to create genetically altered immune cells, which are infused back into a patient with melanoma, sarcoma or myeloma to target and eradicate cancer cells [49]. However, the US proposal has yet to receive a green light from the US Food and Drug Administration (FDA) and a university review board [49]. Although the chimeric antigen receptor (CAR) T-cell therapy was successfully used to treat the patients with leukemia [50] and multiple myeloma [51], they have not worked for solid tumors such as sarcomas and melanomas. Therefore, CRISPR-edited immune cells can be used to overcome the limitation of CAR-modified T-cells to treat patients with solid malignancies.
As higher order chromatin structure and heritable epigenetic marks can directly influence gene transcriptional activity, site-specific epigenome editing provides the opportunity to study the contributions of epigenetic regulation to aging and aging-related disease. CRISPR/Cas9 system can be used to identify and characterize the super-enhancers, which are large clusters of transcriptional enhancers with unusually high levels of mediator binding (e.g. cofactors and chromatin regulators) that drive expression of cell identity genes and when dysregulated play a key role in disease [52]. Disease-associated variation was shown to be especially enriched in the super-enhancers of disease-relevant cell types [52]. Insulator and silencer are another two important cis/trans-acting DNA regulatory elements that may act in concert with enhancer, but with opposing role to regulate gene transcriptional activity. Chromatin insulators can block the action of transcriptional enhancers by forming chromatin loop domains to affect the enhancer-promoter interactions. A recent study revealed CRISPR-mediated disruption of the CCCTC-binding motif (CTCF) in human IDH wild-type gliomas was able to induce the loss of insulation between topological domains and lead to aberrant oncogene activation by permitting a constitutive enhancer to interact aberrantly with a prominent glioma oncogene [53]. Additionally, CRISPR/Cas9 nuclease can be used to disrupt the topologically associating domains (TADs) or chromatin domain clusters (CDCs), and the boundary regions separating them to study the consequences of genomic rearrangements in gene dysregulation and disease. Disruption of TADs was shown to lead to de novo enhancer-promoter interactions and rewire long-range regulatory genome architecture, thereby affecting the pathogenicity of human structural variants, particularly in non-coding regions of the human genome [54].
Conclusions and Perspective
Genome and epigenome editing have revolutionized biomedical research and offers the unprecedented opportunity to study the molecular mechanisms underlying aging and aging-related disease in humans. CRISPR expressed by tissue-specific promoters and serotype-specific AAV enable highly precise editing of genome and epigenome in a specific tissue. Multi-input logic gate genetic circuits can be integrated with optogenetics-based targeted epigenome engineering to precisely modulate gene expression and epigenetic landscape in a spatial and temporal specific manner. Light activation of CRISPR could allow gene functional studies with high precision, and may reduce toxicity from off-target effects by restricting the function of CRISPR to certain locations (e.g. tissue/cell) and time points (e.g. turn on/off the transcriptional activity). While an increasing number of experimental studies have demonstrated the potential and promises of these techniques, methodological and conceptual advances will be needed before genome and epigenome editing can be effectively and routinely applied as a research tool and in clinical settings. Furthermore, strategies for in vivo delivery of CRISPR/dCas9-based epigenome editing tools have yet to be established. Small packaging capacity of AAV has hindered the application of AAV to deliver large fusion transgene comprising of CRISPR/dCas9-based epigenetic modulators.
Together with the genome editing toolbox, site-specific recruitment of engineered chromatin regulators to modulate epigenetic marks and associated chromatin states at proximal promoter or distal DNA regulatory elements will allow us to gain molecular insight into the function of epigenetic marks underlying human aging and aging-related disease. Combined use of genome and epigenome editing tools will help uncover novel molecular pathways that account for association detected by global analysis including GWAS by elucidating genotype-epigenotype interactions. A better understanding of the genetic and epigenetic regulatory mechanisms underlying human aging and age-related disease will significantly contribute to the developments of new therapeutic interventions for extending healthspan and lifespan, ultimately improving the quality of life in the elderly populations.
Acknowledgments
This work was funded by NIH grants AG017242, GM104459, and CA180126.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Gaj T, Gersbach CA, Barbas CF., 3rd Zfn, talen, and crispr/cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using crispr/cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV, Zhang F. Cpf1 is a single rna-guided endonuclease of a class 2 crispr-cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, Joung JK. High-fidelity crisprcas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529:490–495. doi: 10.1038/nature16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FA R, L C, WX Y, DA S, JS G, AJ K, B Z, O S, X W, KS M, EV K, PA S, F Z. In vivo genome editing using staphylococcus aureus cas9. Nature. 2015;520:186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendel A, Bak RO, Clark JT, Kennedy AB, Ryan DE, Roy S, Steinfeld I, Lunstad BD, Kaiser RJ, Wilkens AB, Bacchetta R, Tsalenko A, Dellinger D, Bruhn L, Porteus MH. Chemically modified guide rnas enhance crisprcas genome editing in human primary cells. Nat Biotechnol. 2015;33:985–989. doi: 10.1038/nbt.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, Zhang F. In vivo interrogation of gene function in the mammalian brain using crispr-cas9. Nat Biotechnol. 2015;33:102–106. doi: 10.1038/nbt.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilton IB, D'Ippolito AM, Vockley CM, Thakore PI, Crawford GE, Reddy TE, Gersbach CA. Epigenome editing by a crispr-cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol. 2015;33:510–517. doi: 10.1038/nbt.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nihongaki Y, Kawano F, Nakajima T, Sato M. Photoactivatable crispr-cas9 for optogenetic genome editing. Nat Biotechnol. 2015;33:755–760. doi: 10.1038/nbt.3245. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Zeng Y, Liu L, Zhuang C, Fu X, Huang W, Cai Z. Synthesizing and gate genetic circuits based on crispr-cas9 for identification of bladder cancer cells. Nat Commun. 2014;5:5393. doi: 10.1038/ncomms6393. [DOI] [PubMed] [Google Scholar]
- 11.Keung AJ, Joung JK, Khalil AS, Collins JJ. Chromatin regulation at the frontier of synthetic biology. Nat Rev Genet. 2015;16:159–171. doi: 10.1038/nrg3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleinstiver BP, Prew MS, Tsai SQ, Topkar VV, Nguyen NT, Zheng Z, Gonzales AP, Li Z, Peterson RT, Yeh JR, Aryee MJ, Joung JK. Engineered crispr-cas9 nucleases with altered pam specificities. Nature. 2015;523:481–485. doi: 10.1038/nature14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shmakov S, Abudayyeh OO, Makarova KS, Wolf YI, Gootenberg JS, Semenova E, Minakhin L, Joung J, Konermann S, Severinov K, Zhang F, Koonin EV. Discovery and functional characterization of diverse class 2 crispr-cas systems. Mol Cell. 2015;60:385–397. doi: 10.1016/j.molcel.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abudayyeh OO, Gootenberg JS, Konermann S, Joung J, Slaymaker IM, Cox DB, Shmakov S, Makarova KS, Semenova E, Minakhin L, Severinov K, Regev A, Lander ES, Koonin EV, Zhang F. C2c2 is a single-component programmable rna-guided rna-targeting crispr effector. Science. 2016;353:aaf5573. doi: 10.1126/science.aaf5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinstiver BP, Prew MS, Tsai SQ, Nguyen NT, Topkar VV, Zheng Z, Joung JK. Broadening the targeting range of staphylococcus aureus crispr-cas9 by modifying pam recognition. Nat Biotechnol. 2015;33:1293–1298. doi: 10.1038/nbt.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano H, Gootenberg JS, Horii T, Abudayyeh OO, Kimura M, Hsu PD, Nakane T, Ishitani R, Hatada I, Zhang F, Nishimasu H, Nureki O. Structure and engineering of francisella novicida cas9. Cell. 2016;164:950–961. doi: 10.1016/j.cell.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered cas9 nucleases with improved specificity. Science. 2016;351:84–88. doi: 10.1126/science.aad5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F. Double nicking by rna-guided crispr cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK. Improving crispr-cas nuclease specificity using truncated guide rnas. Nat Biotechnol. 2014 doi: 10.1038/nbt.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson CD, Ray GJ, DeWitt MA, Curie GL, Corn JE. Enhancing homology-directed genome editing by catalytically active and inactive crispr-cas9 using asymmetric donor DNA. Nat Biotechnol. 2016;34:339–344. doi: 10.1038/nbt.3481. [DOI] [PubMed] [Google Scholar]
- 21.Song J, Yang D, Xu J, Zhu T, Chen YE, Zhang J. Rs-1 enhances crispr/cas9- and talen-mediated knock-in efficiency. Nat Commun. 2016;7:10548. doi: 10.1038/ncomms10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR, Ploegh HL. Increasing the efficiency of precise genome editing with crispr-cas9 by inhibition of nonhomologous end joining. Nat Biotechnol. 2015;33:538–542. doi: 10.1038/nbt.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paquet D, Kwart D, Chen A, Sproul A, Jacob S, Teo S, Olsen KM, Gregg A, Noggle S, Tessier-Lavigne M. Efficient introduction of specific homozygous and heterozygous mutations using crispr/cas9. Nature. 2016 doi: 10.1038/nature17664. [DOI] [PubMed] [Google Scholar]
- 24.He X, Tan C, Wang F, Wang Y, Zhou R, Cui D, You W, Zhao H, Ren J, Feng B. Knock-in of large reporter genes in human cells via crispr/cas9-induced homology-dependent and independent DNA repair. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016 doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roadmap Epigenomics C, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, Ziller MJ, Amin V, Whitaker JW, Schultz MD, Ward LD, Sarkar A, Quon G, Sandstrom RS, Eaton ML, Wu YC, Pfenning AR, Wang X, Claussnitzer M, Liu Y, Coarfa C, Harris RA, Shoresh N, Epstein CB, Gjoneska E, Leung D, Xie W, Hawkins RD, Lister R, Hong C, Gascard P, Mungall AJ, Moore R, Chuah E, Tam A, Canfield TK, Hansen RS, Kaul R, Sabo PJ, Bansal MS, Carles A, Dixon JR, Farh KH, Feizi S, Karlic R, Kim AR, Kulkarni A, Li D, Lowdon R, Elliott G, Mercer TR, Neph SJ, Onuchic V, Polak P, Rajagopal N, Ray P, Sallari RC, Siebenthall KT, Sinnott-Armstrong NA, Stevens M, Thurman RE, Wu J, Zhang B, Zhou X, Beaudet AE, Boyer LA, De Jager PL, Farnham PJ, Fisher SJ, Haussler D, Jones SJ, Li W, Marra MA, McManus MT, Sunyaev S, Thomson JA, Tlsty TD, Tsai LH, Wang W, Waterland RA, Zhang MQ, Chadwick LH, Bernstein BE, Costello JF, Ecker JR, Hirst M, Meissner A, Milosavljevic A, Ren B, Stamatoyannopoulos JA, Wang T, Kellis M. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearns NA, Pham H, Tabak B, Genga RM, Silverstein NJ, Garber M, Maehr R. Functional annotation of native enhancers with a cas9-histone demethylase fusion. Nat Methods. 2015;12:401–403. doi: 10.1038/nmeth.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandegar MA, Huebsch N, Frolov EB, Shin E, Truong A, Olvera MP, Chan AH, Miyaoka Y, Holmes K, Spencer CI, Judge LM, Gordon DE, Eskildsen TV, Villalta JE, Horlbeck MA, Gilbert LA, Krogan NJ, Sheikh SP, Weissman JS, Qi LS, So PL, Conklin BR. Crispr interference efficiently induces specific and reversible gene silencing in human ipscs. Cell Stem Cell. 2016 doi: 10.1016/j.stem.2016.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu J, Lei Y, Wong WK, Liu S, Lee KC, He X, You W, Zhou R, Guo JT, Chen X, Peng X, Sun H, Huang H, Zhao H, Feng B. Direct activation of human and mouse oct4 genes using engineered tale and cas9 transcription factors. Nucleic Acids Res. 2014;42:4375–4390. doi: 10.1093/nar/gku109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morita S, Noguchi H, Horii T, Nakabayashi K, Kimura M, Okamura K, Sakai A, Nakashima H, Hata K, Nakashima K, Hatada I. Targeted DNA demethylation in vivo using dcas9–peptide repeat and scfv–tet1 catalytic domain fusions. Nature Biotechnology. 2016 doi: 10.1038/nbt.3658. [DOI] [PubMed] [Google Scholar]
- 31.Nelles DA, Fang MY, O'Connell MR, Xu JL, Markmiller SJ, Doudna JA, Yeo GW. Programmable rna tracking in live cells with crispr/cas9. Cell. 2016;165:488–496. doi: 10.1016/j.cell.2016.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mikuni T, Nishiyama J, Sun Y, Kamasawa N, Yasuda R. High-throughput, high-resolution mapping of protein localization in mammalian brain by in vivo genome editing. Cell. 2016;165:1803–1817. doi: 10.1016/j.cell.2016.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt JC, Zaug AJ, Cech TR. Live cell imaging reveals the dynamics of telomerase recruitment to telomeres. Cell. 2016;166:1188–1197. e1189. doi: 10.1016/j.cell.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma H, Naseri A, Reyes-Gutierrez P, Wolfe SA, Zhang S, Pederson T. Multicolor crispr labeling of chromosomal loci in human cells. Proc Natl Acad Sci U S A. 2015;112:3002–3007. doi: 10.1073/pnas.1420024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma H, Tu LC, Naseri A, Huisman M, Zhang S, Grunwald D, Pederson T. Multiplexed labeling of genomic loci with dcas9 and engineered sgrnas using crisprainbow. Nat Biotechnol. 2016;34:528–530. doi: 10.1038/nbt.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harel I, Benayoun BA, Machado B, Singh PP, Hu CK, Pech MF, Valenzano DR, Zhang E, Sharp SC, Artandi SE, Brunet A. A platform for rapid exploration of aging and diseases in a naturally short-lived vertebrate. Cell. 2015;160:1013–1026. doi: 10.1016/j.cell.2015.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talens RP, Christensen K, Putter H, Willemsen G, Christiansen L, Kremer D, Suchiman HE, Slagboom PE, Boomsma DI, Heijmans BT. Epigenetic variation during the adult lifespan: Cross-sectional and longitudinal data on monozygotic twin pairs. Aging Cell. 2012;11:694–703. doi: 10.1111/j.1474-9726.2012.00835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Partial rescue of mecp2 deficiency by postnatal activation of mecp2. Proc Natl Acad Sci U S A. 2007;104:1931–1936. doi: 10.1073/pnas.0610593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu B, Wang Z, Zhang L, Ghosh S, Zheng H, Zhou Z. Depleting the methyltransferase suv39h1 improves DNA repair and extends lifespan in a progeria mouse model. Nat Commun. 2013;4:1868. doi: 10.1038/ncomms2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gentilini D, Mari D, Castaldi D, Remondini D, Ogliari G, Ostan R, Bucci L, Sirchia SM, Tabano S, Cavagnini F, Monti D, Franceschi C, Di Blasio AM, Vitale G. Role of epigenetics in human aging and longevity: Genome-wide DNA methylation profile in centenarians and centenarians' offspring. Age (Dordr) 2013;35:1961–1973. doi: 10.1007/s11357-012-9463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EE, Beekman JM, Clevers H. Functional repair of cftr by crispr/cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Sen P, Shah PP, Nativio R, Berger SL. Epigenetic mechanisms of longevity and aging. Cell. 2016;166:822–839. doi: 10.1016/j.cell.2016.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cyranoski D. Monkeys genetically modified to show autism symptoms. Nature. 2016;529:449. doi: 10.1038/529449a. [DOI] [PubMed] [Google Scholar]
- 45.Meloni I, Bruttini M, Longo I, Mari F, Rizzolio F, D'Adamo P, Denvriendt K, Fryns JP, Toniolo D, Renieri A. A mutation in the rett syndrome gene, mecp2, causes x-linked mental retardation and progressive spasticity in males. Am J Hum Genet. 2000;67:982–985. doi: 10.1086/303078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heyn H, Moran S, Esteller M. Aberrant DNA methylation profiles in the premature aging disorders hutchinson-gilford progeria and werner syndrome. Epigenetics. 2013;8:28–33. doi: 10.4161/epi.23366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu GH, Barkho BZ, Ruiz S, Diep D, Qu J, Yang SL, Panopoulos AD, Suzuki K, Kurian L, Walsh C, Thompson J, Boue S, Fung HL, Sancho-Martinez I, Zhang K, Yates J, 3rd, Izpisua Belmonte JC. Recapitulation of premature ageing with ipscs from hutchinson-gilford progeria syndrome. Nature. 2011;472:221–225. doi: 10.1038/nature09879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cyranoski D. Chinese scientists to pioneer first human crispr trial. Nature. 2016;535:476–477. doi: 10.1038/nature.2016.20302. [DOI] [PubMed] [Google Scholar]
- 49.First-in-human crispr trial. Nat Biotechnol. 2016;34:796. doi: 10.1038/nbt0816-796a. [DOI] [PubMed] [Google Scholar]
- 50.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified t cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, Zheng Z, Vogl DT, Cohen AD, Weiss BM, Dengel K, Kerr ND, Bagg A, Levine BL, June CH, Stadtmauer EA. Chimeric antigen receptor t cells against cd19 for multiple myeloma. N Engl J Med. 2015;373:1040–1047. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flavahan WA, Drier Y, Liau BB, Gillespie SM, Venteicher AS, Stemmer-Rachamimov AO, Suva ML, Bernstein BE. Insulator dysfunction and oncogene activation in idh mutant gliomas. Nature. 2016;529:110–114. doi: 10.1038/nature16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lupianez DG, Kraft K, Heinrich V, Krawitz P, Brancati F, Klopocki E, Horn D, Kayserili H, Opitz JM, Laxova R, Santos-Simarro F, Gilbert-Dussardier B, Wittler L, Borschiwer M, Haas SA, Osterwalder M, Franke M, Timmermann B, Hecht J, Spielmann M, Visel A, Mundlos S. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell. 2015;161:1012–1025. doi: 10.1016/j.cell.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y, Wu Q, Park A, Yang J, Suresh S, Bizhanova A, Gupta A, Bolukbasi MF, Walsh S, Bogorad RL, Gao G, Weng Z, Dong Y, Koteliansky V, Wolfe SA, Langer R, Xue W, Anderson DG. Therapeutic genome editing by combined viral and non-viral delivery of crispr system components in vivo. Nat Biotechnol. 2016;34:328–333. doi: 10.1038/nbt.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chavez A, Scheiman J, Vora S, Pruitt BW, Tuttle M, E PRI, Lin S, Kiani S, Guzman CD, Wiegand DJ, Ter-Ovanesyan D, Braff JL, Davidsohn N, Housden BE, Perrimon N, Weiss R, Aach J, Collins JJ, Church GM. Highly efficient cas9-mediated transcriptional programming. Nat Methods. 2015;12:326–328. doi: 10.1038/nmeth.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vojta A, Dobrinic P, Tadic V, Bockor L, Korac P, Julg B, Klasic M, Zoldos V. Repurposing the crispr-cas9 system for targeted DNA methylation. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silas S, Mohr G, Sidote DJ, Markham LM, Sanchez-Amat A, Bhaya D, Lambowitz AM, Fire AZ. Direct crispr spacer acquisition from rna by a natural reverse transcriptase-cas1 fusion protein. Science. 2016;351:aad4234. doi: 10.1126/science.aad4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaikind B, Bessen JL, Thompson DB, Hu JH, Liu DR. A programmable cas9-serine recombinase fusion protein that operates on DNA sequences in mammalian cells. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dahlman JE, Abudayyeh OO, Joung J, Gootenberg JS, Zhang F, Konermann S. Orthogonal gene knockout and activation with a catalytically active cas9 nuclease. Nat Biotechnol. 2015;33:1159–1161. doi: 10.1038/nbt.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]