Abstract
Objective
To explore barriers to anticoagulation among older atrial fibrillation (AF) patients at high risk for stroke and identify opportunities where interventions might increase use of oral anticoagulants (OAC).
Design
Retrospective cohort study
Setting
Two large community-based AF cohorts
Participants
1405 patients (mean age 79 years) with ischemic stroke surviving hospitalization.
Measurements
Using structured chart review, we identified reasons for non-use of OACand assessed one-year post-stroke survival. Logistic regression identified correlates of OAC non-use.
Results
The median CHA2DS2-VASc score was 5, yet 44% of patients were not prescribed OAC at discharge. The most frequent (non-mutually exclusive) physician reasons for non-prescription of OAC included fall risk (26.7%), poor prognosis (19.3%), bleeding history (17.1%), patient/family refusal (14.9%), older age (11.0%) and dementia (9.4%). Older age (OR 8.96, 95% CI 5.01–16.04 for age ≥85 vs. age <65 years) and increased disability (OR 12.58, 95% CI 5.82–27.21 for severe vs. no deficit) were the most important independent predictors of non-use of OAC. By one year, 42.5% of those not receiving OAC at discharge had died versus 19.1% of those receiving OAC (p<0.0001), far higher than recurrent stroke rates.
Conclusion
Despite very high stroke risk, over 40% of patients were not discharged on OAC. Dominant reasons included fall risk, poor prognosis, older age, and dementia. These patients’ elevated 1-year mortality rate confirmed their high level of comorbidity. Future work to improve outcomes and clinical decisions regarding anticoagulation in this patient population should focus on: mitigation of fall risk, better assessment and decision tools for determining risk/benefit in individual patients, and determining whether newer anticoagulants are safer in complex elderly and/or frail patients.
Keywords: Ischemic stroke, atrial fibrillation, oral anticoagulants, decision-making
Introduction
Prior ischemic stroke is one of the most important risk factors for recurrent ischemic stroke in patients with atrial fibrillation (AF).1 Oral anticoagulant (OAC) therapy can reduce the risk of ischemic stroke by two-thirds in AF patients with prior ischemic stroke.2 Despite this, a large proportion of patients with AF are not prescribed OAC following ischemic stroke.3 There is a lack of understanding of the reasons why OAC therapy is not prescribed for such patients at very high risk of recurrent ischemic stroke. Previous studies have primarily included lower stroke risk patients without prior stroke4 and have been limited by small sample sizes4–6. Greater insights into non-use of OAC therapy in high-risk secondary prevention populations may enable targeted interventions to increase appropriate use of OAC therapy in suitable candidates, including AF patients without prior stroke but who are at otherwise high stroke risk. In this study, we describe the reasons for non-prescription of OAC therapy following acute ischemic stroke in two large community-based cohorts of patients with AF.
Methods
Study Design
We report on the clinical course of all patients sustaining an ischemic stroke during follow-up of two separate cohorts of patients with AF, the ATRIA (Anticoagulation and Risk factors in Atrial Fibrillation) and ATRIA-CVRN (Anticoagulation and Risk factors in Atrial Fibrillation Cardiovascular Research Network).
Population
The first cohort, the ATRIA nonvalvular AF cohort has been described in detail previously.7 In brief, members of Kaiser Permanente of Northern California between July 1, 1996, and December 31, 1997 aged ≥18 years old with either 2 or more outpatient AF diagnoses (ICD-9 code 427.31) or 1 outpatient AF diagnosis with ECG validation, were included, resulting in a 13,559 member cohort. Patients with mitral stenosis, or an aortic or mitral valve replacement were excluded. Follow-up continued through September 2003. Clinical data were collected from inpatient, outpatient, laboratory, pharmacy and administrative databases as well as a longitudinal diabetes registry.8, 9,10 The current study focuses on cohort members who sustained an ischemic stroke during follow-up. Potential stroke events were identified via hospitalization and billing databases using ICD-9 codes for ischemic stroke in the primary discharge position (ICD-9 codes: 433.00-.01, 433.10-.11, 433.20-.21, 433.30-.31, 434.00-.01, 434.10-.11, 434.90-.91, and 436). Medical records of potential events were abstracted using a formal protocol with each event adjudicated by two physicians with a third available to resolve disagreements. In rare cases a consultant neurologist provided the final diagnosis. Patients who presented to non-Kaiser institutions with stroke were identifiable in Kaiser Permanente databases. A valid IS was defined as the sudden onset of a neurologic deficit fitting a vascular distribution persisting for at least 24 hours and not explained by other etiologies. The modified Rankin (mRankin) score11 of functional disability at discharge was estimated from medical chart notes. Such estimated mRankin scores are highly correlated with post-hospitalization mortality rates.12 Post-stroke mortality was ascertained via medical chart review, hospital databases, health plan member reporting, Social Security Administration files and the California state death certificate registry.13 The sole OAC prescribed was warfarin. Warfarin use at the time of admission was determined from medical chart review. Warfarin prescription at the time of hospital discharge was determined from medical chart review, supplemented by a validated warfarin use algorithm assessing warfarin use following hospitalization.7
The second study cohort ATRIA-CVRN, has also been described in detail previously14. Briefly, the ATRIA-CVRN cohort consists of 33,247 patients from Kaiser Permanente Northern California and Southern California aged ≥21 years with incident AF or atrial flutter first diagnosed between January 2006 and June 2009 and confirmed by ECG or physician diagnosis in the electronic medical record. A valid diagnosis of AF included ≥1 inpatient diagnosis or ≥2 outpatient diagnoses. ATRIA-CVRN included patients with mitral stenosis or a valve replacement in the mitral or aortic positions (1.5% of the cohort). Emergency Department visits for stroke not resulting in hospital admission were included as ATRIA-CVRN outcome events. We adopted the same approach as for ATRIA for reviewing charts, determining mRankin score and warfarin use following hospitalization or Emergency Department visit, and for validating ischemic stroke events and death. Follow-up continued through June 2009.
We confined our analysis to patients alive at hospital or Emergency Department discharge following acute ischemic stroke. For the current study, the date of diagnosis of the first ischemic stroke in the cohort was considered the patient’s index date.
Outcome Measures
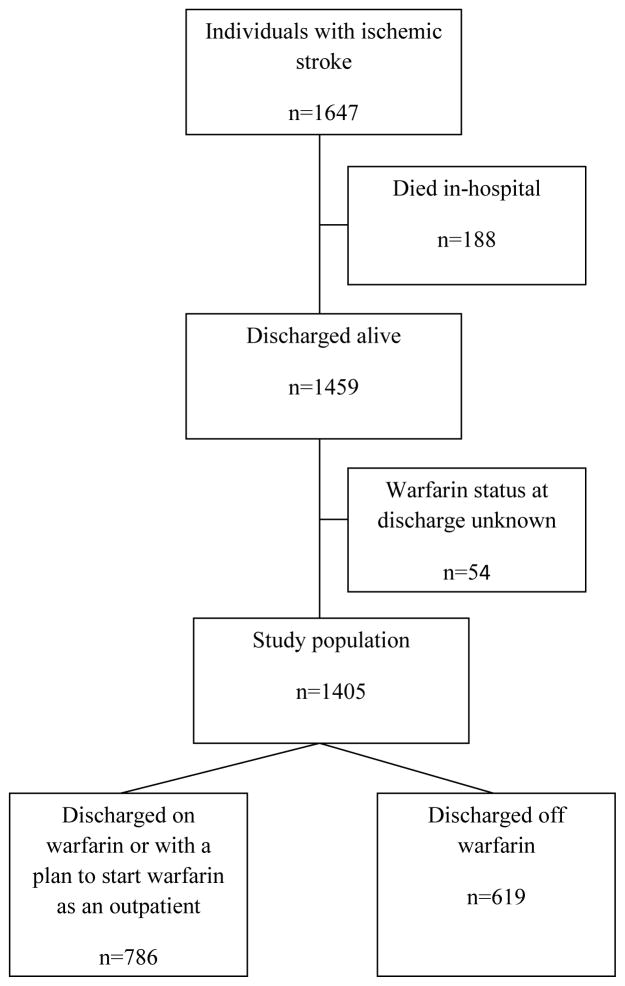
The primary outcome was prescription of warfarin at the time of hospital discharge following acute ischemic stroke. Patients for whom use of warfarin was planned after discharge were counted as having been discharged on warfarin (Figure 1). Secondary outcomes included time to death and recurrent ischemic stroke following discharge for the index stroke.
Figure 1.
Flowchart of Ischemic Stroke Patients in ATRIA and ATRIA-CVRN
Covariates
We included variables hypothesized to be associated with OAC use following ischemic stroke: 1) ischemic stroke risk factors, including prior ischemic stroke, history of heart failure, hypertension, age, diabetes mellitus, vascular disease (coronary artery and peripheral artery disease), female sex and renal impairment; and 2) potential contraindications to use of OAC, including dementia, prior gastrointestinal or intracranial hemorrhage, post-stroke mRankin disability score, as well as race. Age and race were obtained from administrative databases. For the ATRIA cohort, the remaining covariates were obtained from structured chart review. For the ATRIA-CVRN cohort, disability was obtained from chart review but the remaining covariates were obtained from outpatient and inpatient diagnostic codes.7, 14, 15
For patients not discharged on OAC, medical record reviewers recorded the specifically stated or clinically apparent reason(s) why OAC was not prescribed from a list of reasons provided to the reviewer in both the ATRIA and ATRIA-CVRN cohorts. If necessary, a free text field could be used by the reviewer for reasons not listed (see table 3 for list of reasons). In addition, an option indicated a plan to prescribe warfarin at some period post discharge. Six hundred and nineteen patients were discharged off warfarin, of whom 95 did not have an explicitly documented nor apparent clinical reason for nonprescription of warfarin at discharge. In addition, 22 patients did not have a documented history of AF in the medical chart during the stroke admission. Although all 22 met study entry criteria for AF, the absence of mention of AF in the record indicated that anticoagulation for AF was not part of the physicians’ discharge decision. As a result, we included 502 patients with clearly documented reasons for nonuse of warfarin on discharge in the analysis of reasons why OAC was not prescribed at discharge.
Table 3.
Physician Reasons for Non-prescription of OAC Therapy on Dischargea
| Reason | All patients discharged off OAC N=502 n (%) |
|---|---|
| Fall risk | 134 (26.7) |
| Poor prognosis/comfort care only | 97 (19.3) |
| Prior bleed | 86 (17.1) |
| Patient/family refusal | 75 (14.9) |
| Increased age | 55 (11.0) |
| Poor mental status/dementia | 47 (9.4) |
| Risk of hemorrhagic conversion of IS | 44 (8.8) |
| No AF captured during hospitalization, paroxysmal AF, or prior cardioversion of AF | 35 (7.0) |
| Hemorrhagic conversion of IS | 34 (6.8) |
| Current bleed | 30 (6.0) |
| Increased risk of bleeding | 17 (3.4) |
| Source of stroke thought to be non cardio-embolic and unrelated to AF | 16 (3.2) |
| Warfarin status to be determined as an outpatient | 16 (3.2) |
| Allergy or intolerance to warfarin | 9 (1.8) |
| History of medication non-adherence | 9 (1.8) |
| Elevated INR/difficulty controlling INR | 6 (1.2) |
| Planned procedure/surgery/dentistry | 2 (0.4) |
| Underlying coagulopathy | 2 (0.4) |
| Otherb | 13 (2.6) |
Patients could have more than one reason cited for non-prescription of OAC therapy at discharge. 345 (69%) patients had reasons for non-use of OAC specifically stated in the medical chart. For 157 (31%), reasons were clinically apparent but not specifically stated in the medical chart.
In ATRIA, specific reasons listed under ‘other’ included: ‘improving exam’, ‘visual disturbance’, ‘no evident benefit to anticoagulation’, ‘not a good candidate’, ‘not an anticoagulation candidate’ and ‘rare episodes of paroxysmal AF’. In ATRIA-CVRN, reasons listed under ‘other’ included: ‘outpatient physicians had previously decided not to anticoagulate’ and ‘aspirin alone recommended by neurology consult for unknown reasons’.
Statistical Analysis
A similar percentage of patients were prescribed warfarin at hospital discharge in the two cohorts (43% in the ATRIA cohort and 46% in the ATRIA-CVRN cohort) and so the results of the two cohorts were pooled to enhance statistical power. No patients were shared between the two cohorts. For descriptive analyses, χ2 tests compared categorical variables and student’s t-tests compared continuous variables. Univariate and multivariable logistic regression models assessed the likelihood of not prescribing OAC therapy on discharge given the presence of clinical features. Warfarin status following discharge was missing in only 54 (3.7%) of patients (Figure 1). These patients were excluded from the analysis. We included variables in the multivariable models based on clinical and statistical (P<0.05) significance. Warfarin use at the time of admission for ischemic stroke was included in the analyses. Unadjusted Kaplan-Meier curves were generated for time to recurrent ischemic stroke and time to death, according to OAC therapy status on discharge, and statistical significance was assessed using the logrank test. For all analyses, a two-sided P-value <0.05 was considered to be statistically significant. All analyses were conducted using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC).
The study was approved by the institutional review boards of the collaborating institutions. Waiver of informed consent was obtained due to the nature of the study.
Results
In the combined ATRIA and ATRIA-CVRN cohorts (n=46,806), a total of 1647 (3.5%) patients were admitted with acute ischemic stroke, 1459 (88.6%) of whom were discharged alive, 897 patients from the ATRIA cohort and 562 from the ATRIA-CVRN cohort (Figure 1). Warfarin status at discharge was known for 1405 (96.3%). The majority were age 75 years or older (72.6%), caucasian (80.4%) and women (54.0%). A large proportion of patients had significant comorbidities, including 30.4% with diabetes, 78.1% with hypertension, 32.7% with coronary disease, and 37.9% with heart failure. (Table 1) Because of the patients’ comorbidities and having sustained a stroke, the AF ischemic stroke risk scores were very high with median (IQR) values as follows: ATRIA: 10 (9–11) (range 0–15) , CHA2DS2-VASc: 5 (5–6), (range 0–9); and CHADS2: 4 (4–5), (range 0–6). Fifty-one percent were discharged with major or severe disability.
Table 1.
Baseline Features of Patients with AF and IS Discharged Alive with Known Warfarin Statusa
| Variable | All patients | OAC at discharge | No OAC at discharge |
|---|---|---|---|
| N (%) | 1405 | 786 | 619 |
| Age, mean (SD), years | 79 (9) | 76 (9) | 82 (9) |
| Age <65 years | 132 (9.4) | 109 (13.9) | 23 (3.7) |
| Age 65–74 years | 253 (18.0) | 168 (21.4) | 85 (13.7) |
| Age 75–84 years | 616 (43.8) | 383 (48.7) | 233 (37.6) |
| Age ≥ 85 years | 404 (28.8) | 126 (16.0) | 278 (44.9) |
| Women | 759 (54.0) | 404 (51.4) | 355 (57.4) |
| White race | 1130 (80.4) | 626 (79.6) | 504 (81.4) |
| Diabetes mellitus | 427 (30.4) | 251 (31.9) | 176 (28.4) |
| Hypertension | 1097 (78.1) | 635 (80.8) | 462 (74.6) |
| Coronary artery disease | 460 (32.7) | 268 (34.1) | 192 (31.0) |
| Chronic heart failure | 532 (37.9) | 293 (37.3) | 239 (38.6) |
| Peripheral artery disease | 124 (8.8) | 75 (9.5) | 49 (7.9) |
| Renal impairmentb | 328 (23.3) | 159 (20.2) | 169 (27.3) |
| Diagnosed dementia | 195 (13.9) | 67 (8.5) | 128 (20.7) |
| Prior gastrointestinal hemorrhage | 136 (9.7) | 50 (6.4) | 86 (13.9) |
| Prior intracranial hemorrhage | 54 (3.8) | 15 (1.9) | 39 (6.3) |
| Warfarin use at admission | 456 (32.5) | 403 (51.3) | 53 (8.6) |
| ATRIA score c | |||
| 7–9 | 528 (37.6) | 333 (42.4) | 195 (31.5) |
| 10–11 | 594 (42.3) | 337 (42.9) | 257 (41.5) |
| 12–15 | 283 (20.1) | 116 (14.8) | 167 (27.0) |
| Median score (IQR) | 10 (9–11) | 10 (9–11) | 10 (9–12) |
| CHA2DS2VASc scored | |||
| 2–3 | 83 (5.9) | 59 (7.5) | 24 (3.9) |
| 4–5 | 742 (52.8) | 406 (51.7) | 336 (54.3) |
| 6–8 | 580 (41.3) | 321 (40.8) | 259 (41.8) |
| Median score (IQR) | 5 (5–6) | 5 (4–6) | 5 (5–6) |
| CHADS2 scoree | |||
| 2–3 | 306 (21.8) | 196 (24.9) | 110 (17.8) |
| 4 | 591 (42.1) | 308 (39.2) | 283 (45.7) |
| 5–6 | 508 (36.2) | 282 (35.9) | 226 (36.5) |
| Median score (IQR) | 4 (4–5) | 4 (4–5) | 4 (4–5) |
| Aspirin prescribed at discharge | 689 (49.0) | 240 (30.5) | 449 (72.5) |
| Disability at dischargef | |||
| No disability (mRankin 0) | 81 (5.8) | 59 (7.5) | 22 (3.6) |
| Minor (mRankin 1–2) | 593 (42.2) | 402 (51.1) | 191 (30.9) |
| Major (mRankin 3–4) | 575 (40.9) | 290 (36.9) | 285 (46.0) |
| Severe (mRankin 5) | 142 (10.1) | 25 (3.2) | 117 (18.9) |
Warfarin status at discharge was missing for 54 (3.7%) of patients.
estimated glomerular filtration rate <45 ml/min/1.73m2 or end-stage renal disease
ATRIA score components: Stroke, Age, Female, Diabetes mellitus, Congestive heart failure, Hypertension, Proteinuria, and eGFR<45ml/min/1.73m2 or end stage renal disease, range 0–15.
CHA2DS2VASc score components: Congestive heart failure, Hypertension, Age, Diabetes mellitus, Stroke,
Vascular disease, Age and Sex category (female), range 0–9.
CHADS2 score components: Congestive heart failure, Hypertension, Age, Diabetes mellitus, Stroke, range 0–6.
Data were missing for disability at discharge in 14 (1.0%) patients.
Forty-four percent (619/1405) of patients were not discharged on OAC therapy. Discharge off OAC therapy was much more likely among patients who were not on OAC at admission: 59.6% (566/949) of patients who were not on OAC therapy on admission versus 11.6% (53/456) who were on OAC therapy on admission (P<0.0001). A higher proportion of patients with major or severe disability were not discharged on OAC therapy (56.1%) compared to those with no or mild disability (31.6%) (P<0.0001). Seventy-three percent of patients not discharged on OAC therapy were prescribed aspirin (Table 1).
There were strong independent associations of older age, dementia, and disability on non-use of OAC therapy at discharge. On multivariable analysis, the odds ratio (OR) for non-use of OAC at discharge was 3.25 (95% CI 1.79–5.89) for age 65–74, 3.43 (95% CI 1.98–5.94) for age 75–84, and 8.96 (95% CI 5.01–16.04) for age 85 compared to age <65 years. The OR was 1.69 (95% CI 1.12–2.57) for diagnosed dementia, and 1.39 (95% CI 0.77–2.51) for minor disability, 2.78 (95% CI 1.53–5.05) for major disability, and 12.58 (95% CI 5.82–27.21) for severe disability compared to no disability. Large associations were also seen for patients with prior GI or intracranial hemorrhage. Even after controlling for multiple clinical features, patients who were admitted off OAC were far more likely to also be discharged off OAC (OR 11.25, 95% CI 7.95–15.92) (Table 2).
Table 2.
Clinical Features associated with Non-use of OAC on Discharge following IS
| Variable | Percent not discharged on OAC | Univariate OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|---|
| Age | |||
| <65 years | 17.4 | reference | |
| 65–74 years | 33.6 | 2.40 (1.43–4.03) | 3.25 (1.79–5.89) |
| 75–84 years | 37.8 | 2.88 (1.79–4.65) | 3.43 (1.98–5.94) |
| ≥ 85 years | 68.8 | 10.46 (6.36–17.18) | 8.96 (5.01–16.04) |
| Gender | |||
| Male | 40.9 | reference | |
| Female | 46.8 | 1.27 (1.03–1.57) | 0.79 (0.60–1.05) |
| Diabetes mellitus | |||
| No | 45.3 | reference | |
| Yes | 41.2 | 0.85 (0.67–1.07) | 1.20 (0.89–1.62) |
| Hypertension | |||
| No | 51.0 | reference | |
| Yes | 42.1 | 0.70 (0.54–0.90) | 0.69 (0.50–0.95) |
| Coronary artery disease | |||
| No | 45.2 | reference | |
| Yes | 41.7 | 0.87 (0.69–1.09) | 0.90 (0.67–1.20) |
| Chronic heart failure | |||
| No | 43.5 | reference | |
| Yes | 44.9 | 1.06 (0.85–1.31) | 0.84 (0.63–1.12) |
| Peripheral artery disease | |||
| No | 44.5 | reference | |
| Yes | 39.5 | 0.81 (0.56–1.19) | 0.72 (0.44–1.15) |
| Renal impairment | |||
| No | 41.8 | reference | |
| Yes | 51.5 | 1.48 (1.16–1.90) | 1.38 (1.00–1.90) |
| Diagnosed dementia | |||
| No | 40.6 | reference | |
| Yes | 65.6 | 2.80 (2.04–3.84) | 1.69 (1.12–2.57) |
| Prior gastrointestinal hemorrhage | |||
| No | 42.0 | reference | |
| Yes | 63.2 | 2.38 (1.65–3.42) | 1.95 (1.25–3.04) |
| Prior intracranial hemorrhage | |||
| No | 42.9 | reference | |
| Yes | 72.2 | 3.45 (1.89–6.33) | 3.76 (1.74–8.12) |
| No OAC at time of admission for ischemic stroke | |||
| No | 59.6 | reference | |
| Yes | 11.6 | 11.24 (8.21–15.39) | 11.25 (7.95–15.92) |
| Disability at dischargea | |||
| No disability | 27.2 | reference | |
| Minor | 32.2 | 1.27 (0.76–2.14) | 1.39 (0.77–2.51) |
| Major | 49.6 | 2.64 (1.57–4.42) | 2.78 (1.53–5.05) |
| Severe | 82.4 | 12.55 (6.53–24.11) | 12.58 (5.82–27.21) |
Disability at discharge was missing for 14 patients.
Reasons for non-use of oral anticoagulant therapy
The most commonly cited reasons for non-prescription of OAC therapy on discharge included a perceived increased risk of falls (26.7%), poor prognosis (19.3%), prior history of bleeding (17.1%), patient or family refusal (14.9%), older age (11.0%), poor cognitive status (9.4%, n=47) and risk of hemorrhagic conversion of ischemic stroke (8.8%, n=44) (Table 3). In all, 72% (360/502) of patients not receiving OAC at discharge had one or more of the following long-term strong contraindications cited: risk of falls, poor prognosis/comfort care only, prior history of bleeding, patient or family refusal, and dementia/poor cognitive status. We excluded “increased age” and “risk of hemorrhagic conversion” as not strong long-term contraindications.
Post-discharge death or recurrent ischemic stroke
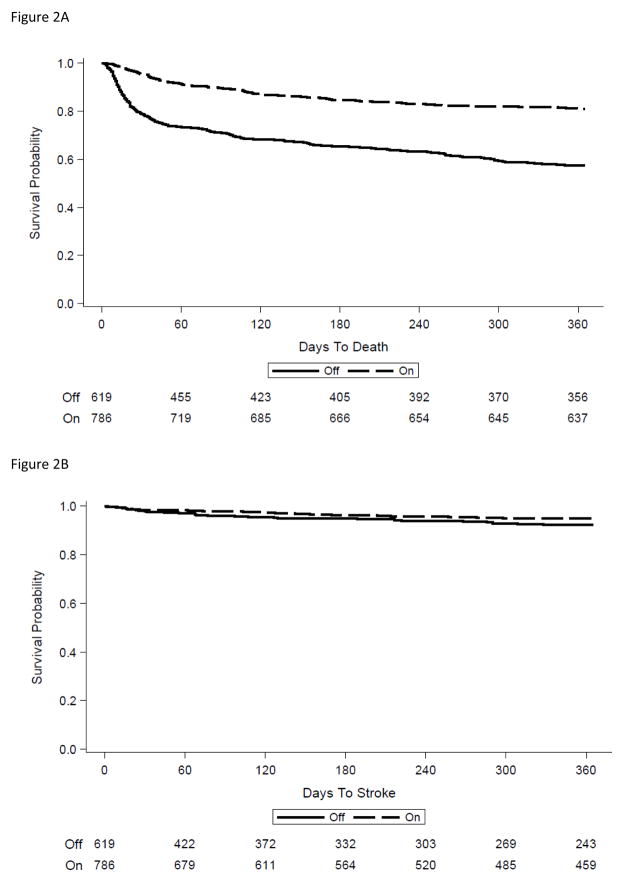
Non-prescription of OAC therapy on discharge was strongly associated with subsequent mortality. Among those patients not receiving OAC at discharge, 21% had died by 30 days post-admission versus 4.6% of those discharged on OAC (Figure 2a). By one year, the Kaplan-Meier estimate for mortality was 42.5% of those not receiving OAC at discharge versus 19.1% of those receiving OAC at discharge (p<0.0001 comparing overall survival curves). The rates of recurrent ischemic stroke were much smaller than those for mortality and were moderately higher among patients not receiving OAC at discharge (2.1% vs 1.7% at 30 days; 7.7% versus 4.9% at 1 year) (p=0.071 comparing unadjusted survival curves over one year follow-up) (Figure 2b). Among patients not receiving OAC at discharge, those categorized as contraindicated because of risk of falls, poor prognosis/comfort care only, prior history of bleeding, patient or family refusal, or dementia/poor cognitive status had a 30-day mortality rate of 26%. By one year, nearly one-half (49%) had died.
Figure 2.
Figure 2a. Mortality according to Anticoagulant Therapy on Discharge
Figure 2b. Recurrent Stroke according to Anticoagulant Therapy on Discharge
Discussion
Atrial fibrillation patients who have had an acute ischemic stroke are at the highest risk of a recurrent ischemic stroke.14 Nonetheless, over 40% of acute IS patients in our study were discharged without OAC therapy. Impressively, of patients who suffered an ischemic stroke while not on anticoagulation, almost 60% were discharged still off OAC therapy. This large proportion of non-use of warfarin in these high stroke risk patients occurred in a health care system with excellent supports for management of warfarin therapy through dedicated anticoagulation management services.8 Further, review of medical charts made clear that physicians were aware that OAC was indicated to prevent stroke in patients with AF. Our results demonstrate that strong contraindications dominate the anticoagulation decision in many older and/or debilitated patients with AF, even those at the highest risk for future ischemic stroke. The most commonly cited ongoing reasons for non-use of OAC therapy on discharge included perceived increased risk of falls, poor prognosis, prior bleeding, patient/family refusal, and dementia, as well as the less specific contraindication of “increased age.” Fully 72% (360/502) of patients not receiving OACs at discharge had one or more of these cited contraindications as the basis for the decision. Our multivariable analyses confirmed the strong independent associations of increased age, disability, prior bleeding, as well as dementia inhibiting prescription of OACs at hospital discharge. Interestingly, non-use of OAC therapy at admission remained a major determinant of non-use at discharge, even after accounting for other features related to OAC at discharge. This finding indicates that the pre-admission contraindications still dominated the OAC decision at discharge and suggests that other unmeasured factors added to the documented contraindications. The main reasons cited for non-use of OACs were highly related to mortality and, indeed, over 40% of patients discharged off OAC died by one year. Notably, the rate of recurrent stroke at one year in patients discharged off OAC was 7.7%, indicating that the vast majority of these patients died from non-stroke related comorbidities. Such patients would have had little opportunity to benefit from the stroke preventive effects of OAC.
The most common reasons for not using OAC in our study have also been cited in other studies. 16–19 It is worth considering whether they should serve as strong contraindications and whether they can be mitigated. Certainly, poor prognosis, if assessed accurately, is a reasonable contraindication; a short remaining lifespan would make benefit from OAC unlikely. Prior bleeding with risk of recurrence is another common strong contraindication for OAC. While the expected stroke preventive benefit from OAC treatment outweighs the harm from nearly all extracranial hemorrhages, most patients do not re-start OAC after a major bleeding event.20
The most commonly cited reason for non-prescription of OAC therapy in our cohort was an increased risk of falls. Physicians are concerned that anticoagulants will aggravate trauma following falls, particularly head trauma.21 Indeed, a recent large, database study highlighted the high incidence of anticoagulant-associated intracranial hemorrhage, fall-related and otherwise, among older U.S. veterans with AF and another recent study reported a mortality rate of 6% for AF patients on OAC following a ground level fall.22, 23 In contrast, one older modeling study has estimated that an individual patient would have to fall up to 295 times per year before the risks of anticoagulation outweigh the benefits.24 In any case, physician reluctance to use OAC in patients who are at high risk of falling is understandable. Formal fall risk assessment and interventions to reduce fall risk might increase the use of OACs in frail elders. 25, 26
Old patients with AF are less likely to be treated with OAC despite good evidence that they gain substantial net benefit from anticoagulants.27, 28 Bleed risk clearly increases with age but so does the risk of ischemic stroke and the potential benefits of OAC.28–30 “Advanced age” as a contraindication may be a synonym for frailty. However, for robust elders, age alone should not be considered a valid contraindication to OAC therapy.
Cognitive impairment was a reason for non-use of OAC therapy in 9% of patients. Previous studies have reported similar underuse of OAC therapy in patients with cognitive impairment.16, 31 Use of anticoagulant therapy in this population can be challenging. However, in the ACTIVE-W trial, Mini Mental State Examination score was not associated with increased risk of vascular events or major hemorrhage.32 Such findings indicate that warfarin therapy, and presumably newer anticoagulants, can be administered to patients with dementia under appropriate supervision.
The proportion of AF patients treated with OAC has not increased substantially in recent years despite the introduction of novel anticoagulants that are easier to take and have a reduced risk of intracranial hemorrhage33 as well as the substantial attention to AF stroke prevention in lay and professional media.34–36 In a recent report from an AF registry based in cardiology practices, less than half of high-risk patients received OAC37. A recent Swedish study from administrative databases reported similarly low rates of OAC uptake in patients with AF after ischemic stroke; only 35% of patients received OAC within 3 months of discharge. Our study demonstrates that, even among AF patients at the highest risk of ischemic stroke, there are large subgroups with major and complex contraindications to anticoagulant therapy consistent with the predominantly older age of patients with AF.38. While some of these contraindications may be addressable in individual patients, going forward it is likely that a significant fraction of AF patients will remain untreated with anticoagulants.
Our study benefits from its large size, a detailed chart review protocol that included questions explicitly addressing contraindications to OAC use at discharge, follow-up for stroke and death post-discharge, all within well-studied community-based, cohorts of patients with AF with high quality ascertainment of use of anticoagulants. A real-time survey exploring physicians’ reasons for not prescribing OAC therapy would have been preferable to our retrospective chart review. However, such a study would be difficult to implement on a large scale and at risk for a poor response rate. Finally, our data reflect OAC decisions before the era of novel anticoagulants. It is conceivable that some patients not treated with warfarin might now be treated with a novel agent, although recent prescription data suggest that novel anticoagulants are replacing warfarin but not increasing the proportion of AF patients treated with anticoagulants.35 Novel anticoagulants led to fewer intracranial hemorrhages in randomized trials versus warfarin.33, 39 However, these trials were less likely to enroll the very old, frail, and fall-prone. As a result, the relative safety of novel anticoagulants in such individuals with AF is currently not clearand is an important area for future investigation.22, 33, 39, 40
CONCLUSION
Despite the very high risk of recurrent stroke faced by AF patients who have suffered an acute ischemic stroke, over 40% of our study patients were not discharged on OAC therapy. The dominant reasons for non-use of OAC were risk of falls, poor prognosis/comfort care only, prior history of bleeding, patient or family refusal, older age, and dementia/poor cognitive status. These data suggest that more work is needed in order to improve outcomes in this high risk patient population in order to improve outcomes and individual care decisions regarding anticoagulation. Future work should focus on strategies to mitigate fall risk, develop and validated formal assessment and decision tools for determining risk/benefit in individual patients, and determining whether newer anticoagulants are safer in complex elderly and/or frail patients.
Acknowledgments
Funding Source
This study was supported by the National Institute on Aging (R01 AG15478), the National Heart, Lung and Blood Institute (RC2HL101589 and U19 HL091179) and the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital (Boston, MA).
The funding sources had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript.
COI Checklist: All authors have approved the final version of the manuscript.
| Elements of Financial/Personal Conflicts | Author 1Emer McGrath | Author 2Alan Go | Author 3Yuchiao Chang | Author 4Leila Borowsky | Author 5 Margaret Fang | Author 6 Kristi Reynolds | Author 7 Daniel Singer | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Grants/Funds | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Honoraria | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Speaker Forum | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Consultant | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Stocks | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Royalties | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Expert Testimony | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Board Member | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Patents | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Personal Relationship | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
Dr. Go received research support from iRhythm.
Dr. Singer receives research support from Bristol-Myers Squibb, Boehringer Ingelheim, and Medtronic. He serves as a consultant or advisory board member for Boehringer Ingelheim, Bristol-Myers Squibb, Johnson and Johnson, Medtronic, Merck, Pfizer, and CVS Health.
Funding: This study was supported by the National Institute on Aging (R01 AG15478), the National Heart, Lung, and Blood Institute (RC2HL101589 and U19 HL091179) and the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital (Boston, MA).
The funding sources had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript.
Footnotes
Authors’ Contributions
Dr. McGrath contributed to the design of the study, interpretation of data, and drafting and revision of the manuscript. Dr. Go contributed to acquisition and interpretation of the data and provided critical review of the manuscript. Dr. Chang contributed to interpretation of the data and drafting and revision of the manuscript. Ms. Borowsky contributed to interpretation of the data and drafting and revision of the manuscript. Dr. Fang provided critical review of the manuscript Dr. Reynolds contributed to acquisition and interpretation of the data and provided critical review of the manuscript. Dr. Singer contributed to the design of the study, interpretation of data, and drafting and revision of the manuscript All authors have approved the final version of the manuscript.
Sponsor’s Role
The funding sources had no role in the study concept and design; acquisition of data, analysis and interpretation of the data; and preparation of the manuscript.
References
- 1.Stroke Risk in Atrial Fibrillation Working Group. Independent predictors of stroke in patients with atrial fibrillation: A systematic review. Neurology. 2007;69:546–554. doi: 10.1212/01.wnl.0000267275.68538.8d. [DOI] [PubMed] [Google Scholar]
- 2.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 3.McGrath ER, Kapral MK, Fang J, et al. Antithrombotic therapy after acute ischemic stroke in patients with atrial fibrillation. Stroke. 2014;45:3637–3642. doi: 10.1161/STROKEAHA.114.006929. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava A, Hudson M, Hamoud I, et al. Examining warfarin underutilization rates in patients with atrial fibrillation: Detailed chart review essential to capture contraindications to warfarin therapy. Thromb J. 2008;6:6. doi: 10.1186/1477-9560-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid AA, Ofner S, Shorr RI, et al. Bleeding risk, physical functioning and non-use of anticoagulation among patients with stroke and atrial fibrillation. QJM. 2015;108:189–196. doi: 10.1093/qjmed/hcu176. [DOI] [PubMed] [Google Scholar]
- 6.Partington SL, Abid S, Teo K, et al. Pre-admission warfarin use in patients with acute ischemic stroke and atrial fibrillation: The appropriate use and barriers to oral anticoagulant therapy. Thromb Res. 2007;120:663–669. doi: 10.1016/j.thromres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: The anticoagulation and risk factors in atrial fibrillation (atria) study. Annals of Internal Medicine. 1999;131:927–934. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: How well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–2692. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: The anticoagulation and risk factors in atrial fibrillation (atria) study. Ann Intern Med. 1999;131:927–934. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 10.Selby JV, Ray GT, Zhang D, et al. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes care. 1997;20:1396–1402. doi: 10.2337/diacare.20.9.1396. [DOI] [PubMed] [Google Scholar]
- 11.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 12.Fang MC, Go AS, Chang Y, et al. Long-term survival after ischemic stroke in patients with atrial fibrillation. Neurology. 2014;82:1033–1037. doi: 10.1212/WNL.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arellano MG, Petersen GR, Petitti DB, et al. The california automated mortality linkage system (camlis) American journal of public health. 1984;74:1324–1330. doi: 10.2105/ajph.74.12.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer DE, Chang Y, Borowsky LH, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: The atria study stroke risk score. Journal of the American Heart Association. 2013;2:e000250. doi: 10.1161/JAHA.113.000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Go AS, Hylek EM, Phillips JA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The anticoagulation and risk factors in atrial fibrillation (atria) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 16.Holt TA, Hunter TD, Gunnarsson C, et al. Risk of stroke and oral anticoagulant use in atrial fibrillation: A cross-sectional survey. Br J Gen Pract. 2012;62:e710–717. doi: 10.3399/bjgp12X656856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monette J, Gurwitz JH, Rochon PA, et al. Physician attitudes concerning warfarin for stroke prevention in atrial fibrillation: Results of a survey of long-term care practitioners. J Am Geriatr Soc. 1997;45:1060–1065. doi: 10.1111/j.1532-5415.1997.tb05967.x. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien EC, Holmes DN, Ansell JE, et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: Findings from the outcomes registry for better informed treatment of atrial fibrillation (orbit-af) registry. Am Heart J. 2014;167:601–609. e601. doi: 10.1016/j.ahj.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Perera V, Bajorek BV, Matthews S, et al. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing. 2009;38:156–162. doi: 10.1093/ageing/afn293. [DOI] [PubMed] [Google Scholar]
- 20.Fang MC, Go AS, Chang Y, et al. Warfarin discontinuation after starting warfarin for atrial fibrillation. Circulation Cardiovascular quality and outcomes. 2010;3:624–631. doi: 10.1161/CIRCOUTCOMES.110.937680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612–617. doi: 10.1016/j.amjmed.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Dodson JA, Petrone A, Gagnon DR, et al. Incidence and determinants of traumatic intracranial bleeding among older veterans receiving warfarin for atrial fibrillation. JAMA Cardiol. 2016;1:65–72. doi: 10.1001/jamacardio.2015.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inui TS, Parina R, Chang DC, et al. Mortality after ground-level fall in the elderly patient taking oral anticoagulation for atrial fibrillation/flutter: A long-term analysis of risk versus benefit. The journal of trauma and acute care surgery. 2014;76:642–649. doi: 10.1097/TA.0000000000000138. discussion 649–650. [DOI] [PubMed] [Google Scholar]
- 24.Man-Son-Hing M, Nichol G, Lau A, et al. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med. 1999;159:677–685. doi: 10.1001/archinte.159.7.677. [DOI] [PubMed] [Google Scholar]
- 25.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 26.Panel on Prevention of Falls in Older Persons. Summary of the updated american geriatrics society/british geriatrics society clinical practice guideline for prevention of falls in older persons. Journal of the American Geriatrics Society. 2011;59:148–157. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- 27.Mant J, Hobbs FDR, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in atrial fibrillation in the elderly community population: The birmingham atrial fibrillation treatment of the aged study (bafta), a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 28.Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151:297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation. 2011;123:2562–2570. doi: 10.1161/CIRCULATIONAHA.110.985655. [DOI] [PubMed] [Google Scholar]
- 30.Casciano JP, Singer DE, Kwong WJ, et al. Anticoagulation therapy for patients with non-valvular atrial fibrillation: Comparison of decision analytic model recommendations and real-world warfarin prescription use. American journal of cardiovascular drugs : drugs, devices, and other interventions. 2012;12:313–323. doi: 10.1007/BF03261840. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher AM, Rietbrock S, Plumb J, et al. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: Do the appropriate patients receive stroke prophylaxis? J Thromb Haemost. 2008;6:1500–1506. doi: 10.1111/j.1538-7836.2008.03059.x. [DOI] [PubMed] [Google Scholar]
- 32.Flaker GC, Pogue J, Yusuf S, et al. Cognitive function and anticoagulation control in patients with atrial fibrillation. Circulation Cardiovascular quality and outcomes. 2010;3:277–283. doi: 10.1161/CIRCOUTCOMES.109.884171. [DOI] [PubMed] [Google Scholar]
- 33.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 34.Shroff GR, Solid CA, Herzog CA. Atrial fibrillation, stroke, and anticoagulation in medicare beneficiaries: Trends by age, sex, and race, 1992–2010. Journal of the American Heart Association. 2014;3:e000756. doi: 10.1161/JAHA.113.000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai NR, Krumme AA, Schneeweiss S, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation- quality and cost implications. Am J Med. 2014;127:1075–1082. e1071. doi: 10.1016/j.amjmed.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 36.January CT, Wann LS, Alpert JS, et al. 2014 aha/acc/hrs guideline for the management of patients with atrial fibrillation: A report of the american college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: Insights from the ncdr pinnacle registry. JAMA Cardiology. 2016;1:55–62. doi: 10.1001/jamacardio.2015.0374. [DOI] [PubMed] [Google Scholar]
- 38.Friberg L, Rosenqvist M, Lindgren A, et al. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014;45:2599–2605. doi: 10.1161/STROKEAHA.114.006070. [DOI] [PubMed] [Google Scholar]
- 39.Sardar P, Chatterjee S, Chaudhari S, et al. New oral anticoagulants in elderly adults: Evidence from a meta-analysis of randomized trials. J Am Geriatr Soc. 2014;62:857–864. doi: 10.1111/jgs.12799. [DOI] [PubMed] [Google Scholar]
- 40.Sharma M, Cornelius VR, Patel JP, et al. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: Systematic review and meta-analysis. Circulation. 2015;132:194–204. doi: 10.1161/CIRCULATIONAHA.114.013267. [DOI] [PMC free article] [PubMed] [Google Scholar]